Abstract
Belowground plant responses have received much less attention in climate change experiments than aboveground plant responses, thus hampering a holistic understanding of climate change effects on plants and ecosystems. In addition, responses of plant roots to climate change have mostly been studied in single-factor experiments. In a Danish heathland ecosystem, we investigated both individual and combined effects of elevated CO2, warming and drought on fine root length, net production and standing biomass by the use of minirhizotrons, ingrowth cores and soil coring. Warming increased the net root production from ingrowth cores, but decreased fine root number and length in minirhizotrons, whereas there were no significant main effects of drought. Across all treatments and soil depths, CO2 stimulated both the total fine root length (+44%) and the number of roots observed (+39%), with highest relative increase in root length in the deeper soil layers. Our results suggest that under future climate, plants may allocate considerable resources into roots compared to aboveground biomass. Increased carbon (C) allocation to roots may have a great impact on the overall ecosystem C balance and must be considered in modelling of future ecosystem responses to climate change. To provide models with necessary validation data, more studies are needed to investigate if higher C allocation to roots will lead to long-term C storage in more recalcitrant soil C pools or if this potential increase in soil carbon storage may be offset by increased priming activity and turnover rates for soil organic matter.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The current concentration of CO2 in the atmosphere exceeds by far the natural range of the last 650,000 years, and the increase is expected to continue and even accelerate (IPCC 2013), leading to continuous warming of the Earth’s climate system. Consequently, annual global temperatures have already increased by about 1°C over the last 100 years and will be further complemented by increases in frequency and intensity of extreme events like drought periods (IPCC 2013). These environmental factors are main drivers of many important ecosystem processes, and a critical uncertainty in climate change research is whether terrestrial ecosystems will act as sinks or sources of C under future climate. Consequently, there is need to provide data that can feed models which assess how ecosystem C cycling and storage will be affected by climate change.
Fine root production in terrestrial ecosystems represents roughly 33% of global annual net primary productivity (Jackson and others 1997), and changes in the allocation of C to roots under future climate conditions may therefore significantly affect overall soil C sink strength. Fine root production has been shown to be stimulated by elevated CO2 in ecosystems ranging from forests (Iversen and others 2008; Iversen 2010; Pritchard and others 2008; Johnson and others 2006), to grasslands or steppe (Adair and others 2009; Anderson and others 2010; Edwards and others 2004; Higgins and others 2002; Milchunas and others 2005; Nelson and others 2004), and agricultural crops (Madhu and Hatfield 2013). However, negative or no effects of CO2 on root production are also reported, especially from grasslands (Arnone and others 2000).
Longer-term studies with elevated CO2 have reported no long-lasting effects on fine root biomass in natural ecosystems due to environmental constrains (Day and others 2013; Newingham and others 2013). For example, nitrogen (N) may become increasingly limiting as C and N are sequestered in long-lived plant biomass and soil organic matter (Luo and others 2004), and plants need to compensate for this. Hence, the plant can invest in fine root biomass and deeper rooting distribution, to increase the exploitation of deeper N resources (Finzi and others 2007; Jobbagy and Jackson 2000). Changes in root distribution have been observed especially in forest ecosystems, where elevated CO2 may lead to deeper rooting distributions (Iversen 2010; Iversen and others 2008; Finzi and others 2007; Johnson and others 2006; Pritchard and others 2008). However, in short grass steppe and desert elevated CO2 had no effect on vertical root distribution (LeCain and others 2006; Ferguson and Nowak 2011).
When elevated CO2 is combined with warming, net primary production is often stimulated (Dieleman and others 2012). However, the increased water use efficiency (WUE) under elevated CO2 and the increased evapotranspiration under warming might offset each other (Naudts and others 2013) and together have an intermediate effect on root production (Johnson and others 2006). The direct effect of warming on plants are more difficult to detect, as many ‘side effects’ follow an increased temperature; for example, altered water status (Carrillo and others 2014; Zavalloni and others 2008), nutrient availability (Rustad and others 2001) and a longer growing season length (Reyes-Fox and others 2014). In a meta-analysis of 85 studies, warming alone enhanced the aboveground biomass but did not significantly affect the belowground biomass across different biomes (Wu and others 2011). Warming was, however, found to increase the root turnover (reduced root lifespan) in grasslands (Gill and Jackson 2000; Fitter and others 1999; Edwards and others 2004), and alpine meadow (Wu and others 2014; Wang and others 2016). Reduced root lifespan has also been observed in response to experimental drought (Eissenstat and others 2013), alongside a general decrease in root biomass under drier conditions as shown, for example, for grasslands (Naudts and others 2013). Drought generally has direct negative effects on overall plant photosynthesis and growth as well (Chaves and others 2002) and, similar to warming, drought may affect the response to elevated CO2 in heathlands (Albert and others 2011b), although not always (Selsted and others 2012).
Especially temperate grasslands have a high root biomass (Mokany and others 2006) and hold an order of magnitude greater fine root length compared to other biomes (Jackson and others 1997). In grasslands, as well as heathlands, most of the net primary production occurs belowground (Carrillo and others 2014; Steinaker and Wilson 2005; Aerts and Heil 1993). Still, studies of root dynamics in temperate heathlands exposed to climate change are generally absent. Heathlands and moors account for 7% of the European land cover (European Environmental Agency: http://www.eea.europa.eu) with very different carbon balance characteristics (Beier and others 2009). North Western heathlands are associated with acidic soils of low fertility, and the flora consists primarily of evergreen dwarf shrubs and few grasses. In the UK, more than 100 Tg C is stored in the soil of dwarf shrub heaths (Ostle and others 2009), making this an important ecosystem compartment in the terrestrial carbon balance. Despite the importance, information about root dynamics is lacking.
To reveal the importance of interacting climate factors on ecosystems, some experiments with combined temperature and precipitation manipulations (Wu and others 2011; Leuzinger and others 2011; Beier and others 2012) and eventually also in combination with elevated CO2 (Naudts and others 2013; Pilon and others 2013; Garten and others 2009; Shaw and others 2002) have been initiated. However, the number of experiments with combined climate and elevated CO2 treatments is still very limited and especially long-term manipulation studies of fine roots are lacking. In CLIMAITE (CLIMAte change effects In Terrestrial Ecosystems), the effects of elevated CO2, warming and drought were investigated in a multifactorial experiment in a mixed heathland/grassland ecosystem in Denmark (Mikkelsen and others 2008). The objective of this study was to investigate how fine root length, production and number responded to a realistic future climate scenario anno 2075. Earlier studies from the site have reported interactive effects of the climate factors on plant physiological processes (Albert and others 2011c), belowground processes (Andresen and others 2010a; Andresen and others 2010b) and N cycling (Larsen and others 2011), whereas no long-term effects of aboveground biomass were observed (Kongstad and others 2012). Elevated CO2 did not permanently influence the aboveground standing biomass. Increased biomass was observed in 1 year but was counterbalanced by an increase in litter production. Drought reduced the aboveground growth in 1 year, but no significant long-lasting effects on the aboveground biomass were observed in neither the dominant species Calluna nor Deschampsia. Warming had no effect on aboveground biomass.
We hypothesized that (1) elevated CO2 would stimulate root growth and length, due to increased C assimilation and hence more C being allocated belowground, as we did not observe any effect of the elevated CO2 on the aboveground biomass. We also hypothesized (2) roots to exploit deeper soil layers under elevated CO2, to increase the nutrient uptake in this nutrient-poor environment. Further, we expected (3) warming to increase root growth, but only if there was no decrease in soil water content, as drought was expected to decrease root growth.
Materials and Methods
Site Description
The experimental site is situated in a dry heathland/grassland app. 50 km NW of Copenhagen (55°53′N, 11°58′E), Denmark, on a hilly nutrient-poor acid sandy deposit. The dominant plant species are the evergreen dwarf shrub Calluna vulgaris (L.) Hull (c. 30% cover) and the perennial grass Deschampsia flexuosa (L.) Trin (c. 70% cover), hereafter referred to as Calluna and Deschampsia. All root measurements refer to fine roots below 1 mm in diameter of both species (except the standing fine root biomass which includes roots below 2 mm in diameter).
The soil consists of 71.5% sand, 20.5 % coarse sand, 5.8% silt and 2.2% clay (Nielsen and others 2009). The soil is well-drained with a \( {\text{pH}}_{{{\text{CaCl}}_{2} }} \) in the topsoil of 3.3 increasing to 4.5 in the B-horizon and an organic top layer of 2–5 cm (O-horizon).
The annual mean temperature is 8°C and the annual mean precipitation is 613 mm (Danish Meteorological Institute 2009). The site has relatively low atmospheric N bulk deposition of 1.35 ± 0.04 g N m−2 y−1 in 2007 (Larsen and others 2011).
Prior to the start of our experiment, the site was extensively grazed by deer and managed by occasional cutting and removal of small trees.
Experimental Design
The CLIMAITE manipulation experiment started in October 2005. The treatments were made to match a possible Danish climate scenario in 2075, as predicted by the Danish Meteorological Institute (Danish Meteorological Institute 2009; http://www.DMI.dk), with one important exception: precipitation is forecasted to change with prolonged summer droughts and increased winter precipitation, but with no major changes in annual amounts. The CLIMAITE experiment focused on the prolonged drought only.
The experiment consisted of 12 octagons (7 m in diameter) laid out pair wise in 6 blocks. Each block comprised two octagons, one of which received elevated CO2 (CO2) by FACE (Free Air Carbon Enrichment) to a level of 510 ppm from dawn till dusk, that is, 130 ppm above ambient concentration, and the other receiving ambient CO2 (A). Within each octagon there are four plots, each with one of the following treatments: summer drought (D), that is, exclusion of all rain by automatic shelters for 2–5 weeks during summer, warming (T), that is, passive night-time warming by reflective curtains covering the plots from dusk till dawn, a combination of drought and warming (TD), or untreated control for reference (A). The experiment was made in a full-factorial design (±elevated CO2, ±drought, ±warming) replicated 6 times with the individual treatments CO2, T, D, and their combinations TD, TCO2, DCO2 and TDCO2, and controls (A). Hence, the 8 treatment combinations replicated 6 times resulted in a total of 48 plots. Further details on the experimental setup are given in Mikkelsen and others (2008) and Larsen and others (2011).
The temperature elevation in all warming plots compared to all non-warming plots for the period July 2007–July 2010 during night-time in 20 cm height was 1.3 and 0.6°C during summer (April–August) and winter months (September–March), respectively. In 5-cm soil depth, the corresponding temperature increases were 0.7 and 0.3°C during summer and winter, respectively. Across all times, that is, daytime and night-time, the temperature increases in 5-cm soil depth were 0.4 and 0.2°C during summer and winter, respectively.
The drought periods, during the root scanning measurements, were in 2009 from 18 May to 25 May and again 25 June to 13 July, excluding a total of 38 mm of rain. In 2010, the drought was from 4 May to 3 June excluding 74 mm of rain. The drought treatment was intended to be active until soil water content got below 5 vol% water content in the top 20 cm of the soil. Figure 1 shows the climatic data at the site and effects of treatments on soil temperature and soil water content from July 2007 to July 2010. See Figure 2 for an overview of the experiment and sampling time.
A–D Average precipitation (mm) and air temperature (°C) at the experimental site (A). B The mean temperature difference (Delta T) in 5-cm soil depth for the warming minus non-warmed treatments (T, red line), drought minus non-drought treatments (D, green), and TDCO2 minus the control treatment (TDCO2, black). Delta Soil Water Content (SWC) in 0–20-cm soil depth (C) and 0–60-cm soil depth (D) showing the difference in SWC between warmed plots and non-warmed treatments (T, red), elevated CO2 minus non-CO2 treatments (CO2, black), drought minus non-drought treatments (D, dotted) and the difference between the control treatment and TDCO2 (TDCO2, green). Note different Y-axis in SWC. Depicted in C and D are the experimental drought periods (the black vertical lines) (Color figure online).
Standing Fine Root Biomass
Roots were sampled under mixed Calluna and Deschampsia vegetation in beginning of July 2007, in each experimental plot giving a total of 48 samples. Soil was sampled down to 70-cm soil depth with a soil auger of 6.5 cm in diameter and divided into horizons: organic horizon (O-horizon app. 2–5 cm thick), 0–5, 5–10, 10–30 and 30–70 cm depth. Fine roots (<2 mm) were separated from the soil in the laboratory by use of sieves (2 mm mesh size) and hand picking of fine roots with forceps and then washed carefully. The fine roots were dried in the oven at approximately 60°C and weighed to get the dry weight (DW).
Root Production from Ingrowth Cores
Net root production was estimated by ingrowth cores covering about 8 cm depth from the surface. In each experimental plot, one soil core was sampled in patches of Calluna and Deschampsia, respectively. The soil was sorted to remove roots and was put into mesh bags with a diameter of 5 cm and a mesh size of 1 mm, to capture the fine root production. The soil was placed in the same original order of depth, divided into O-horizon and 0–5 cm depth, separated from each other by a small cloth inside the bag. The 96 ingrowth cores were installed in the soil in January 2009 and retrieved in June 2010, and the time span corresponds to the study period of the minirhizotrons (see below). Shorter ingrowth core studies from the site are reported in Arndal and others (2013).
The fine roots were not separated into species, as the majority of the roots belonged to the species under which the ingrowth cores were placed. However, there was some growth of grass roots in the Calluna samples, while no ingrowth of Calluna roots was seen in Deschampsia samples. The fine roots were dried in the oven at approximately 60°C and weighed to get the biomass dry weight (DW).
Root Length, Number and Seasonal Root Production from Minirhizotrons
Root length and growth were determined from image analysis of roots by 48 minirhizotrons (one per plot) installed under mixed vegetation of Calluna and Deschampsia. The minirhizotron tubes (high-grade acrylic, 6.35 cm inner diameter, 1 m long) were installed in an angle of 45 degrees in pre-cored holes. The aboveground tube parts were blackened with tape to prevent light from entering the tubes, and the tubes were insulated and sealed between imaging periods to exclude moisture and debris.
The image collection started in the summer of 2008, 1 year after installation, to allow time for roots to establish around the tubes prior to start of the measurements. The total standing root length increased in all treatments during the two study years, especially during the first year. The data suggest that the roots around the minirhizotrons had likely not reached equilibrium during the first 12 months of measurements. This corresponds to Milchunas and others (2005) and Anderson and others (2010), who reported equilibrium times of up to 5 years. During the second year of the study, the standing root length stabilized and we therefore assume that the last year of measurement is the most representative in terms of treatment effects.
Minirhizotron scans of 345° were taken to a vertical depth of about 50 cm, and the root imaging was done by using a CI-600 root scanner (CID, Camas, Wash., USA), with colour pictures taken at 300 ppi. Images were collected every 2 weeks during summer and every month during winter from August 2008 to August 2009, and with slightly larger intervals during the following 12 months ending in July 2010 (4 and 5 years after start of treatments).
Due to poor contact between the tubes and the soil/litter in the organic horizon and 0–5 cm soil layer, information from the first subsample was discarded, and only images from 8 cm below the soil surface were analysed. As the minirhizotrons did not adequately sample the O-horizon and the upper 5 cm soil layer due to poor visibility, the upper 8 cm were therefore studied using data from ingrowth cores. We acknowledge that ingrowth cores represent a very different method, but it informs about the relative differences in net root production between the treatments in the upper soil layers.
Due to the large number of roots, we analysed 14 subsamples (called windows in the analysis software) down the soil profile, which were divided into three depth increments (8–15, 15–25 and 25–50 cm vertical depth) and analysed for treatment effects. All subsamples were cut out from the same location in all images, on the side of the tube, as the roots were distributed unevenly around the tube. This is in accordance with Iversen and others (2012), who reported that the tube sides may be the most representative of total tube colonization. The images were later analysed with the free, open-source software program Rootfly (Rootfly Version 1.8.35 Copyright © 2008 Clemson University). The 14 subsamples analysed had an image area of 78 cm2, which is approximately 10% of the tube area that had been scanned.
At each sampling date, we determined the total standing root length per tube by summing all root lengths (mm) present in a window during a sampling date. Growth was defined as new root length that appeared as the result of either the growth of a pre-existing root or the growth of a new root that appeared in a window between sampling dates. The output from Rootfly also provides root length and diameter for each individual root observed during the study, within the image area.
It was not possible to accurately estimate the timing of root death due to lack of changes in root colour or appearance, and we thus only looked at relative differences of root turnover across treatments. Root turnover was estimated as root turnover index: cumulative length growth over periods of 1 year divided by total root length averaged across periods for that year (Milchunas and others 2005). We only looked at turnover from the last year of measurement, that is, from July 2009 to July 2010.
In total, 17,860 images were digitized and 13,476 individual roots were followed and used for analysis of the treatment effects throughout 2 years, that is, 27 sampling dates. Only fine roots below 1 mm were observed in the images during the study period (data not shown).
As we were unable to distinguish among roots of the two dominant species, Calluna and Deschampsia, we considered the root system of the entire plant community as a whole.
Statistical Methods
For the statistical analyses, we used full-factorial analysis of variance including fixed effects of all the two-level main factors drought (D), warming (T), elevated CO2 (CO2) and their interactions (T × D, T × CO2, D × CO2, T × D × CO2). This means, for instance, that with a significant CO2 main effect, all treatments with CO2 (CO2, TCO2, DCO2, TDCO2) are overall different from all treatments with ambient CO2 (A, T, D, TD).
Fixed Effects
For root biomass, root net production from ingrowth cores, and root length and root number measured at the last session in July 2010 (session 27), interactions between treatment factors and sampling depth were included in the model, because treatments may vary with soil depth. Similarly, the root production pattern over the 2009 season was analysed by including an interaction term with sampling time in the model. The root production pattern over the 2009 season was modelled as a linear growth (judged reasonable from plots) with a slope parameter depending on the combination of treatment factors and possibly depth.
For all outcomes where the main analysis was stratified by depth, we also present results obtained after summarizing data across all sampling depth in order to be able to discuss the overall effect of the treatment. Residual plots were visually inspected to check that models assumptions were met. Data on root production pattern were transformed by square root prior to statistical analysis to obtain homogeneity of variances.
Random Effects
Random effects were included in the models to account for dependence structures related to the hierarchical structure of the experimental design with sample units organized in plots, within octagons within blocks. For all analyses that were not stratified on depth (or time), we included only a random effect of octagon as inclusion of a block effect did not improve the model fit. For analysis stratified on depth (or time), we included only a random effect of sampling unit (plot). The only exception was the analysis of root net production from ingrowth cores, where inclusion of nested random effect of octagon and plot improved the model fit.
The spatial dependence between observations within the same sampling unit (plot) was modelled by an unrestricted covariance allowing for arbitrary correlation and a different variance at each depth. For the analysis of repeated measures over time for root length (and root production), a spatial exponential (and Gaussian) correlation structure over time turned out to be reasonable. To account for heterogeneity of variance, the variance was modelled as a power function of sampling time. When considering repeated measures of root length, over time allowing for correlations between samples at different depths did not appear to improve the fit of the model. The choice of the random part of the models was mainly based on the Akaike information criterion (AIC), and if relevant confirmed by inspection of semi-variograms for different correlation structures.
Presentation and Interpretation of Results
Specifically for the analysis of root net production from ingrowth cores, we did a likelihood ratio test to confirm that there were no differences between species (Calluna and Deschampsia). Consequently, results are presented for the data obtained by pooling the dry weight of roots for the two species.
The effects of the climate manipulations were quantified as differences of least squares means. For an easy overview, the direction and the P value for relevant contrasts are presented in Table 1. We have excluded the effect of the interaction between all three factors (D × T × CO2) as this was not statistically significant for any outcome considered. Instead the table contains interactions between pairs of treatment factors and main effects. Further, for each pairwise interaction we present contrast to the reference level (ambient) aggregated over the two levels of the third factor. For further explanation, we refer to the legend of Table 1.
For interpretation of the results in Table 1, we emphasize that differences of lsmeans for main effects should only be considered in case of non-significant interaction terms.
The statistical analyses were carried out using R [ref: R: A Language and Environment for Statistical Computing] (R Development Core Team 2011) and SAS 9.4 (SAS Institute Inc 2003). Data are presented as means ± 1 SE and N = 6.
Results
Root Standing Biomass and Root Production: Insight from Soil Coring
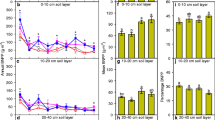
The fine root biomass (<2 mm) sampled in July 2007 after 2 years of treatment was highest in the 0–5 cm depth in all treatments and decreased down the profile (Figure 3A, B). Across treatments, the fine root biomass was distributed with 17 % in 0-horizon, 36 % in 0–5 cm, 19% in 5–10 cm, 17% in 10–30 and 11% in 30–70 cm. The total fine root biomass from the O-horizon to 70 cm depth did not show any treatment effects. In the organic horizon, there was a negative interaction of warming and drought (P = 0.0063), where the combination of the two cancelled out increased fine root biomass. Warming decreased the fine root biomass in 0–5-cm soil depth (P = 0.0461). In the deepest soil layer (30–70 cm), elevated CO2 increased the root biomass by 57 % (P = 0.0485).
A Total fine root biomass (g m−2 ±1 SE of the total, N = 6) from 2007, divided into different depth classes. The treatments are elevated CO2 (CO2), warming (T) and drought (D), and their interactions (T × D, T × CO2, D × CO2, T × D × CO2). B Visualization of the root biomass distribution in the control treatment (A).
The ingrowth cores, in situ from 3.5 to 5 years of treatment, were collected shortly after the drought treatment ended in June 2010, after 5 years of climate manipulations. Both warming and elevated CO2 alone increased the net fine root production across depths (P < 0.0001, see Figure 4), and the effects were additive, that is, there was no significant interaction between the two treatments. When looking at the separate soil depths, warming increased the production in O-horizon and in 0–5 cm (P = 0.0116 and P < 0.0001), whereas elevated CO2 increased the net root production significantly in 0–5 cm (P < 0.0001).
Mean net fine root production (g m−2 ±1 SE of the total; N = 6). The root production was measured in ingrowth bags covering 1.5 year (July 2008–January 2010) and is divided into an organic horizon (3–4 cm thick) and 0–5-cm soil depth. The total production was increased under elevated CO2 (P < 0.0001) and in warming (P < 0.0001).
Root Production: Insight from Minirhizotrons
Similar to the production data from ingrowth cores, the minirhizotron fine root production data measured 27 times showed increased production under elevated CO2 (Figure 5), whereas in contrast no responses to warming were observed. There were, however, tendencies (0.05 < P < 0.1) for drought effects in the period from April to July 2009 (in measurements 13–14, 15–16, 17–18 and 18–19). The fine root production across all treatments followed a seasonal pattern in 2009 with peaks during the summer time. Elevated CO2 significantly increased the fine root production from May to August 2009 (in the intervals between measurement 15–16: P = 0.0108, 16–17: P = 0.0035, 18–19: P = 0.0194, 19–20: P = 0.0059). No treatment effects on the fine root production were observed in 2010 (January to June 2010, data not shown).
Average fine root length production (mm cm−2 day−1, N = 6) in 8–50-cm soil depth and standard errors (±1 SE) are shown for non-CO2 plots (A) and elevated CO2 plots (B), measured as mm growth of root length per day divided by the area of the image. Though measures of production were associated with a large sampling error, significantly larger increments were observed for CO2 enriched plots at various sampling points throughout the summer of 2009, marked with *.
Root Length, Number of Roots and Relative Turnover: Insights from Minirhizotrons
The total standing root length increased in all treatments during the two study years, especially during the first year (Figure 6), and we therefore assume that the last year of measurement is the most representative in terms of treatment effects.
Total standing fine root length (mm/tube image area) of fine roots <1 mm from July 2008 through July 2010 quantified with minirhizotrons in 8–50 cm depth (the organic horizon and the upper 0–5 cm soil was excluded). The analysed tube image area = 78 cm2, which corresponds to c. 10% of the total tube area.
During the full year of 2009 (that is, across 12 measurement campaigns), the main treatment effect on root length was driven by elevated CO2, which increased the total root length and root length in soil depths 15–25 (+47 %) and 25–50 cm (103%) (Table 1). There was also a significant interaction between drought and elevated CO2, mainly driven by a higher root length in the treatment DCO2 in 8–15- and 15–25-cm soil depth, and total.
At the last measurement in July 2010 (measurement 27), after 5 years of treatment, elevated CO2 had increased the root length throughout the whole soil profile (+44%, P = 0.0055, Figure 7A, B). The stimulation of root length by elevated CO2 was observed in 15–25 cm (+57%, P = 0.007) and 25–50 cm (+ 102%, P = 0.0074) (Table 1). In the upper soil layer in 8–15-cm soil depth, warming significantly reduced the root length compared to the non-warmed treatments (P = 0.0081, Figure 8A, B).
A displays the average root length (mm per analysed tube area) in different parts of the soil in ambient CO2 (8–15-, 15–25- and 25–50-cm soil depth). B The average fine root length under elevated CO2 (8–15-, 15–25- and 25–50-cm soil depth). The total shaded area shows the growth of the average root length for the whole soil profile (8–50-cm soil depth). Different shading patterns partition the total root length into the contribution from each soil layer. The height of each shaded area reflects the average root length from that particular soil depth. At the end of the study significantly longer root lengths in elevated CO2 plots were observed for the whole soil profile (P = 0.00055) and in the deep soil layers (P = 0.007/0.0074 for medium/lower layer).
A Displays the average root length (mm per analysed tube area) in different parts of the soil in ambient temperature (8–15-, 15–25- and 25–50-cm soil depth). B The average root length in warming (8–15-, 15–25- and 25–50-cm soil depth). The total shaded area shows the growth of the average root length for the whole soil profile (8–50-cm soil depth). Different shading patterns partition the total root length into the contribution from each soil layer. The height of each shaded area reflects the average root length from that particular soil depth. Root length in 8–15 cm depth was significantly decreased (P = 0.0081) in plots with warming at the end of the study period.
Elevated CO2 increased the total number of roots (P = 0.039) in all depths combined at the end of the study by 39 % (Table 1). When looking at the three depths increments, there was a decrease in the number of roots in the upper most soil layer (8–15 cm, P = 0.0104) in response to warming. Elevated CO2, however, increased the number of roots in 15–25-cm soil depth (P = 0.0571) and in 25–50 cm (P = 0.0155).
Table 2 shows the number of roots for the second year of observation across the whole soil profile. The majority of the roots were below 0.40 mm in diameter (not shown). However, due to low image quality and pixel size, the very fine roots (<0.2 mm) may be underestimated or missed, especially the very fine Calluna roots (Valenzuela-Estrada and others 2008). Over the entire soil profile, the root diameter was similar in all treatments across the 2 years (mean = 0.34, median = 0.32 max = 1.07 and min = 0.16). In 25–50 cm depth layer, warming increased the root diameter (P = 0.045) during the last year of the study (Table 2). Warming also caused increased root length of individual roots (P = 0.025), although this effect was reduced in combination with elevated CO2 (T × CO2, P = 0.007). However, due to the above mentioned biases, we only look at relative differences of root diameter and root length across treatments. The relative root turnover calculated across the whole soil profile was 30 % higher in warmed plots during the last year of the study, that is, from July 2009 to July 2010 (T: turnover = 0.08 y−1, P = 0.050, Table 2).
Discussion
We found that the responses on fine root length, number and production were mainly driven by elevated CO2, with smaller effects of warming and drought. There have not been observed any long-lasting effects on the aboveground biomass in neither Calluna nor Deschampsia, in any of the treatments. Several different types of root research methods overall gave similar responses to the climate treatments.
CO2 Response of Fine Roots
Total root biomass pool measured by soil coring and total standing root length measured by minirhizotrons were strongly stimulated by elevated CO2, which is contrasting to the moderate effects of elevated CO2 on aboveground plant biomass, where only reproductive structures showed a significant increase (Kongstad and others 2012). This suggests that the extra carbon assimilated by photosynthesis (Albert and others 2011a; Albert and others 2011b) was allocated primarily belowground in response to elevated CO2 in this heathland ecosystem. A similar allocation response was found, for example, by Norby and others (2004) in a sweet gum forest.
Elevated CO2 increased both the number and length of roots by the end of our study, and the greater standing root lengths were related to greater number of roots under elevated CO2 rather than longer individual roots, as also reported by Milchunas and others (2005). The increase in root length by 44 % measured in minirhizotrons corresponds to other studies in grasslands of +37% increase (Volder and others 2007; LeCain and others 2006), in steppe +52 % (Milchunas and others 2005), and forest +23% (Pritchard and others 2008).
Although the review from Iversen (2010) mainly focused on forested ecosystems, where deeper rooting is a common response to elevated CO2, they also stated that rooting depth tends to be shallower in grasslands under elevated CO2. However, we do see a change in rooting depth similar to forests and suggest that this change is also taking place in, mainly nutrient limited, grasslands. Arnone and others (2000) reviewed several grassland studies and showed divergent effects of elevated CO2, but most of the studies only focused on shallow depth or was only short term.
Elevated CO2 increased the total standing root length, especially deeper in the soil profile. Root length is often a better indicator of plant nutrient status, and better reflects root activity, than root biomass (Wilson 2014). Hence, the main reason for deeper root distribution and increased root length was probably increased resource demands and increased C allocation to roots. Increased net root production at the site did result in lower N concentrations in roots (Arndal and others 2014), and higher C:N ratio in leaves (Albert and others 2011b), but the total root nitrogen pool was increased under elevated CO2 (Arndal and others 2013). Hence, there were no strong signs of nitrogen limitation after 5 years of climate treatments, which could be explained by the deeper root distribution.
Belowground C allocation through roots may also stimulate microbial community and enhance the rates of soil organic matter decomposition in the rhizosphere (Kuzyakov and others 2007), a process referred to as the ‘priming effect’. This was seen in a grassland study, where labile soil C increased under elevated CO2 and set off the loss of older mineral-associated organic matter (Gill and others 2002). Carbon flux measurements at our site showed increased C efflux under elevated CO2 (Selsted and others 2012). If an ecosystem should act as a C sink in the long run, the extra sequestered C needs to be incorporated in a carbon pool with slow turnover, such as soil organic matter (SOM) or wood (Adair and others 2009). If priming occurs, this could result in old soil organic matter being lost from the ecosystem while young organic matter is being build up. However, it has to be noted that the root and gas flux measurements are not simultaneous and gas fluxes were representing an early response after 2 years of treatment.
Several studies suggest that root litter is an important source of carbon to the soil (Crow and others 2009; Kramer and others 2010; Tefs and Gleixner 2012, Chen and others 2016). Whether a higher fraction of the extra root mass will enter the recalcitrant pool of soil organic matter or whether root stimulated changes in turnover rates of soil organic matter will counterbalance the input, might have great implications for the C balance. Longer-term studies are needed to resolve these questions and see if the changes in root distribution are persistent. As the aboveground plant community at CLIMAITE did not show any significant responses, and were relatively resistant to the climate change on a short time scale, it is suggested that the increased carbon assimilation was allocated belowground and resulted in the observed changes in root length and production.
Temperature Response
From the ingrowth cores, we observed that the net root production increased in response to warming in the top 8 cm of the soil. We hypothesized that warming would increase root biomass and length, but the opposite effect was seen in the minirhizotrons in the upper part of the soil (8–15 cm) where warming reduced the root length. The elevated temperature induced an earlier onset of growing season at the site (Albert and others 2011a), hence increased the water consumption and reduced the water availability, net photosynthesis and growth (Albert and others 2011a). This could potentially reduce the absolute amount of C allocated belowground and result in lower root length in the warming treatment, as we observed in the minirhizotrons in 8–15 cm. Increased mineralization rates at our experimental site have been observed in response to warming alone (Andresen and others 2010a), as well as increased rates of denitrification and potential nitrification but with no changes in belowground pools of N (Larsen and others 2011). Still, the generally higher N turnover in response to warming may explain the lower total root lengths we observed in the warming treatment in the upper soil layers studied by minirhizotrons. As the soil nutrient availability increases, the demand for C allocation to roots decreases. In different tree species and in an alpine meadow, warming and associated increased mineralization resulted in decreased fine root biomass (Bai and others 2010) and increased fine root turnover (Wan and others 2004; Wu and others 2014; Zhou and others 2011).
Warming had a positive effect on the fine root diameter and fine root length of individual roots in the deeper part of the soil (Table 2). This could be due to an increasing dominance of Calluna roots relatively to Deschampsia roots, as the latter has no secondary growth once they have been formed and therefore will not increase in diameter. It could also be due to a change in the relative proportions of first- and second-order roots compared to third- and fourth-order roots (that is, the morphometric system, see Fitter 1982), using root diameter as a proxy for root order (Pilon and others 2013). This would suggest an increase in longer-living roots that act more as conduits. However, as we only looked at small subsamples of the root system, we cannot verify if a shift in root orders took place.
A strategy for roots to cope with low soil moisture is to explore deeper parts of the soil profile (Reid and Renquist 1997), and as the warming treatment decreases the soil water content throughout the whole year (Figure 1C), this may result in drought stress in the upper soil layer. This is contrary to the drought treatment which decreases the SWC only when the drought treatment is active.
Drought Response
The effects of drought manipulations are often very complex and depend on the severity of the drought, and hence plants subjected to moderate drought are often similar in size to control plants (Poorter and others 2012). In our study, the drought treatment did not come out as a significant main effect, probably as the drought was not severe enough. However, the combination of drought and elevated CO2 did increase both the fine root length and production. This suggests that in this dry heathland, elevated CO2 alleviated the drought effect due to enhanced water use efficiency, as also hypothesized. In contrast, the root biomass in the O-horizon from July 2007 decreased when drought and warming were combined. In fact, the lowest soil water content in the summer of 2007 was observed in this treatment combination (data not shown). This is likely because warming stimulated evapotranspiration and therefore decrease the soil water content even further.
Relative Turnover
Nineteen per cent of all observed roots disappeared at our site during the 2 years of study; hence, a longer study period is probably needed to get a solid estimate of root longevity, as also experienced by Milchunas (2009).
The estimated root turnover rate of 0.04–0.08 across treatments suggests that less than 10% of the root biomass is renewed every year. Mommer and others (2015) also found root life spans greater than 3 years in grassland communities, and root length losses were 1–26% per year in all growing seasons. Root life spans are longer in species from low fertility habitats than in species form fertile habitats (Van Der Krift and Berendse 2002). It could be argued that our turnover rate should be divided by 2 and hence be twice as high, as only few roots are being produced during winter in the dormant period, as suggested by (Eissenstat and others 2013), (Turnover = 1/lifespan). As the turnover is not measured from direct measurements of lifespan, the calculation is biased by the lack of disappearance of dead roots and absence of the finest, ephemeral roots due to the image quality. The root longevity includes decomposition time (Pilon and others 2013; Johnson and others 2001), which overestimate the root longevity by the time it takes to decompose a root (Ahrens and others 2014). However, we discuss the turnover results in regard to the relative treatment effects and as such it can provide valuable information on the responses of root dynamics to climate manipulations.
Consistent with Gill and Jackson (2000), who reported exponential increases in root turnover with mean annual temperature for grassland fine roots at a global scale, we also found that warming increased the root turnover. Temperature has a strong role in determining root turnover, through its controls on root respiration and nitrogen mineralization, and further the onset of growing season is often dependent on soil temperature in spring (Gill and Jackson 2000). The growth potential (Growing Degree Days, GDD) increased significantly in the early spring with a 33% higher accumulated GDD during the period from 1 April to 15 May 2006 in warming treatment (Mikkelsen and others 2008). The longer growing season and hence increased maintenance respiration in warmer soils might explain some of the observed higher turnover in warming.
Conclusions
The results from our climate experiment including individual and combined treatments of the most relevant climate change factors, that is, elevated CO2, warming and changes in precipitation patterns confirmed our hypothesis that the additional carbon capture in the plants caused by elevated CO2 was primarily allocated belowground to increased fine root growth. In the future, strong increases of atmospheric CO2 will lead to increased total standing fine root length, especially in deeper soil layers, irrespective of warming and drought. This change in carbon allocation to fine roots may have great impact on the overall ecosystem carbon balance, and must therefore be considered in modelling of future ecosystem responses to climate change.
As no treatment effects have been observed in aboveground plant biomass at the site, although we observed increased allocation belowground, the ecosystem may be in a transition period, where the greater exploitation of deeper soil layers is primarily a response to limited nutrients and water. The increased ability to take up nutrients caused by the higher fine root exploration of especially deeper soil layers may over longer time scales, than captured in our study, results in larger nutrient allocation to the aboveground biomass and therefore potentially to changes in species composition at a later stage. This highlights the need for long-term experiments in ecology.
There were several interactive effects of elevated CO2 and ecosystem warming and drought on fine roots, potentially mediated by changes in soil water content. Nutrient availability may play a role, but this requires further study. In addition, the effect of elevated CO2 on fine root biomass, activity and turnover may alter below ground C storage, and its interactions with the rhizosphere processes and soil C decomposition rates.
References
Adair EC, Reich PB, Hobbie SE, Knops JM. 2009. Interactive effects of time, CO(2), N, and diversity on total belowground carbon allocation and ecosystem carbon storage in a grassland community. Ecosystems 12:1037–52.
Aerts R, Heil GW. 1993. Heath lands. Patterns and processes in a changing environment. Dordrecht: Kluwer Academic Publishers. Geobotany 20:223.
Ahrens B, Hansson K, Solly EF, Schrumpf M. 2014. Reconcilable differences: a joint calibration of fine-root turnover times with radiocarbon and minirhizotrons. New Phytol 204:932–42.
Albert K, Ro-Poulsen H, Mikkelsen TN, Michelsen A, der Linden Van, Beier C. 2011a. Effects of elevated CO2, warming and drought episodes on plant carbon uptake in a temperate heath ecosystem are controlled by soil water status. Plant, Cell Environ 34:1207–22.
Albert K, Ro-Poulsen H, Mikkelsen TN, Michelsen A, der Linden Van, Beier C. 2011b. Interactive effects of elevated CO2, warming, and drought on photosynthesis of Deschampsia flexuosa in a temperate heath ecosystem. J Exp Bot 62:4253–66.
Albert KR, Mikkelsen TN, Michelsen A, Ro-Poulsen H, Van der Linden L. 2011c. Interactive effects of drought, elevated CO(2) and warming on photosynthetic capacity and photosystem performance in temperate heath plants. J Plant Physiol 168:1550–61.
Anderson LJ, Derner JD, Polley HW, Gordon WS, Eissenstat DM, Jackson RB. 2010. Root responses along a subambient to elevated CO2 gradient in a C-3-C-4 grassland. Glob Change Biol 16:454–68.
Andresen LC, Michelsen A, Ambus P, Beier C. 2010a. Belowground heathland responses after 2 years of combined warming, elevated CO2 and summer drought. Biogeochemistry 101:27–42.
Andresen LC, Michelsen A, Jonasson S, Schmidt IK, Mikkelsen TN, Ambus P, Beier C. 2010b. Plant nutrient mobilization in temperate heathland responds to elevated CO2, temperature and drought. Plant Soil 328:381–96.
Arndal M, Merrild M, Michelsen A, Schmidt I, Mikkelsen T, Beier C. 2013. Net root growth and nutrient acquisition in response to predicted climate change in two contrasting heathland species. Plant Soil 369:615–29.
Arndal M, Schmidt IK, Kongstad J, Beier C, Michelsen A. 2014. Root growth and N dynamics in response to multi-year experimental warming, summer drought and elevated CO2 in a mixed heathland-grass ecosystem. Funct Plant Biol 41:1–10.
Arnone JA, Zaller JG, Spehn EM, Niklaus PA, Wells CE, Korner C. 2000. Dymamics of root systems in native grasslands: effects of elevated atmospheric CO2. New Phytol 147:73–85.
Bai WM, Wan SQ, Niu SL, Liu WX, Chen QS, Wang QB, Zhang WH, Han XG, Li LH. 2010. Increased temperature and precipitation interact to affect root production, mortality, and turnover in a temperate steppe: implications for ecosystem C cycling. Glob Change Biol 16:1306–16.
Beier C, Beierkuhnlein C, Wohlgemuth T, Penuelas J, Emmett B, Korner C, de Boeck H, Christensen JH, Leuzinger S, Janssens IA, Hansen K. 2012. Precipitation manipulation experiments - challenges and recommendations for the future. Ecol Lett 15:899–911.
Beier C, Emmett BA, Tietema A, Schmidt IK, Penuelas J, Lang EK, Duce P, de Angelis P, Gorissen A, Estiarte M, de Dato GD, Sowerby A, Kroel-Dulay G, Lellei-Kovacs E, Kull O, Mand P, Petersen H, Gjelstrup P, Spano D. 2009. Carbon and nitrogen balances for six shrublands across Europe. Glob Biogeochem Cycles 23.
Carrillo Y, Dijkstra FA, LeCain D, Morgan JA, Blumenthal D, Waldron S, Pendall E. 2014. Disentangling root responses to climate change in a semiarid grassland. Oecologia 175:699–711.
Chaves MM, Pereira JS, Maroco J, Rodrigues ML, Ricardo CPP, Osorio ML, Carvalho I, Faria T, Pinheiro C. 2002. How plants cope with water stress in the field. Photosynthesis and growth. Ann Bot 89:907–16.
Chen H, Mommer L, van Ruijven J, de Kroon H, Fischer C, Gessler A, Hildebrandt A, Scherer-Lorenzen M, Wirth C, Weigelt A. 2016. Plant species richness negatively affects root decomposition in grasslands. J Ecol . doi:10.1111/1365-2745.12650.
Crow SE, Lajtha K, Filley TR, Swanston CW, Bowden RD, Caldwell BA. 2009. Sources of plant-derived carbon and stability of organic matter in soil: implications for global change. Glob Change Biol 15:2003–19.
Day FP, Schroeder RE, Stover DB, Brown AL, Butnor JR, Dilustro J, Hungate BA, Dijkstra P, Duval BD, Seiler TJ, Drake BG, Hinkle C. 2013. The effects of 11 yr of CO2 enrichment on roots in a Florida scrub-oak ecosystem. New Phytol 200:778–87.
Dieleman WI, Vicca S, Dijkstra FA, Hagedorn F, Hovenden MJ, Larsen KS, Morgan JA, Volder A, Beier C, Dukes JS, King J, Leuzinger S, Linder S, Luo Y, Oren R, de Angelis P, Tingey D, Hoosbeek MR, Janssens IA. 2012. Simple additive effects are rare: a quantitative review of plant biomass and soil process responses to combined manipulations of CO2 and temperature. Glob Change Biol 18:2681–93.
Edwards EJ, Benham DG, Marland LA, Fitter AH. 2004. Root production is determined by radiation flux in a temperate grassland community. Glob Change Biol 10:209–27.
Eissenstat D M, McCormack M L and Du Q. 2013. Global changes and root lifespan. In: Eshel A, Beeckman T, Eds. Plant roots—the hidden half. London: Taylor & Francis Group. pp. 21-1-27-13.
Ferguson SD, Nowak RS. 2011. Transitory effects of elevated atmospheric CO(2) on fine root dynamics in an arid ecosystem do not increase long-term soil carbon input from fine root litter. New Phytol 190:953–67.
Finzi AC, Norby RJ, Calfapietra C, Gallet-Budynek A, Gielen B, Holmes WE, Hoosbeek MR, Iversen CM, Jackson RB, Kubiske ME, Ledford J, Liberloo M, Oren R, Polle A, Pritchard S, Zak DR, Schlesinger WH, Ceulemans R. 2007. Increases in nitrogen uptake rather than nitrogen-use efficiency support higher rates of temperate forest productivity under elevated CO2. Proc Natl Acad Sci USA 104:14014–19.
Fitter AH. 1982. Morphometric analysis of root systems: application of the technique and influence of soil fertility on root system development in two herbaceous species. Plant Cell Environ 5:313–22.
Fitter AH, Self GK, Brown TK, Bogie DS, Graves JD, Benham D, Ineson P. 1999. Root production and turnover in an upland grassland subjected to artificial soil warming respond to radiation flux and nutrients, not temperature. Oecologia 120:575–81.
Garten CT, Classen AT, Norby RJ. 2009. Soil moisture surpasses elevated CO(2) and temperature as a control on soil carbon dynamics in a multi-factor climate change experiment. Plant Soil 319:85–94.
Gill RA, Jackson RB. 2000. Global patterns of root turnover for terrestrial ecosystems. New Phytol 147:13–31.
Gill RA, Polley HW, Johnson HB, Anderson LJ, Maherali H, Jackson RB. 2002. Nonlinear grassland responses to past and future atmospheric CO2. Nature 417:279–82.
Higgins PAT, Jackson RB, Des Rosiers JM, Field CB. 2002. Root production and demography in a california annual grassland under elevated atmospheric carbon dioxide. Glob Change Biol 8:841–50.
IPCC. 2013. the physical science basis. In: Stocker TF, Plattner GK, and M. Tignor, Eds. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press. pp. 1–1535.
Iversen CM. 2010. Digging deeper: fine-root responses to rising atmospheric CO2 concentration in forested ecosystems. New Phytol 186:346–57. doi:10.1111/j.1469-8137.2009.03122.x.
Iversen CM, Ledford J, Norby RJ. 2008. CO(2) enrichment increases carbon and nitrogen input from fine roots in a deciduous forest. New Phytol 179:837–47.
Iversen CM, Murphy MT, Allen MF, Childs J, Eissenstat DM, Lilleskov EA, Sarjala TM, Sloan VL, Sullivan PF. 2012. Advancing the use of minirhizotrons in wetlands. Plant Soil 352:23–39.
Jackson RB, Mooney HA, Schulze ED. 1997. A global budget for fine root biomass, surface area, and nutrient contents. Proc Natl Acad Sci 94:7362–6.
Jobbagy EG, Jackson RB. 2000. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol Appl 10:423–36.
Johnson MG, Tingey DT, Phillips DL, Storm MJ. 2001. Advancing fine root research with minirhizotrons. Environ Exp Bot 45:263–89.
Johnson MG, Rygiewicz PT, Tingey DT, Phillips DL. 2006. Elevated CO2 and elevated temperature have no effect on Douglas-fir fine-root dynamics in nitrogen-poor soil. New Phytol 170:345–56.
Kongstad J, Schmidt IK, Riis-Nielsen T, Beier C, Arndal MF, Mikkelsen TN. 2012. High resilience in heathland plants to changes in temperature, drought and CO2 in combination: results from the CLIMAITE experiment. Ecosystems 15(2):269–83.
Kramer C, Trumbore S, Froberg M, Dozal LMC, Zhang DC, Xu XM, Santos GM, Hanson PJ. 2010. Recent (<4 year old) leaf litter is not a major source of microbial carbon in a temperate forest mineral soil. Soil Biol Biochem 42:1028–37.
Kuzyakov Y, Hill PW, Jones DL. 2007. Root exudate components change litter decomposition in a simulated rhizosphere depending on temperature. Plant Soil 290:293–305.
Larsen KS, Andresen LC, Beier C, Jonasson S, Albert KR, Ambus P, Arndal MF, Carter MS, Christensen S, Holmstrup M, Ibrom A, Kongstad J, Van der Linden L, Maraldo K, Michelsen A, Mikkelsen TN, Pilegaard K, Prieme A, Ro-Poulsen H, Schmidt IK, Selsted MB, Stevnbak K. 2011. Reduced N cycling in response to elevated CO2, warming, and drought in a Danish heathland: Synthesizing results of the CLIMAITE project after two years of treatments. Glob Change Biol 17:1884–99.
LeCain DR, Morgan JA, Milchunas DG, Mosier AR, Nelson JA, Smith DP. 2006. Root biomass of individual species, and root size characteristics after five years of CO2 enrichment on native shortgrass steppe. Plant Soil 279:219–28.
Leuzinger S, Luo YQ, Beier C, Dieleman W, Vicca S, Korner C. 2011. Do global change experiments overestimate impacts on terrestrial ecosystems? Trends Ecol Evol 26:236–41.
Luo Y, Su B, Currie WS, Dukes JS, Finzi AC, Hartwig U, Hungate B, McMurtrie RE, Oren R, Parton WJ, Pataki DE, Shaw MR, Zak DR, Field CB. 2004. Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience 54:731–9.
Madhu M, Hatfield J. 2013. Dynamics of Plant Root Growth under Increased Atmospheric Carbon Dioxide. Agron J 105:657–69.
Mikkelsen TN, Beier C, Jonasson S, Holmstrup M, Schmidt IK, Ambus P, Pilegaard K, Michelsen A, Albert K, Andresen LC, Arndal MF, Bruun N, Christensen S, Danbaek S, Gundersen P, Jorgensen P, Linden LG, Kongstad J, Maraldo K, Prieme A, Riis-Nielsen T, Ro-Poulsen H, Stevnbak K, Selsted MB, Sorensen P, Larsen KS, Carter MS, Ibrom A, Martinussen T, Miglietta F, Sverdrup H. 2008. Experimental design of multifactor climate change experiments with elevated CO2, warming and drought: the CLIMAITE project. Funct Ecol 22:185–95.
Milchunas DG, Morgan JA, Mosier AR, LeCain DR. 2005. Root dynamics and demography in shortgrass steppe under elevated CO2, and comments on minirhizotron methodology. Glob Change Biol 11:1837–55.
Milchunas DG. 2009. Estimating root production: comparison of 11 methods in shortgrass steppe and review of biases. Ecosystems 12:1381–402.
Mokany K, Raison RJ, Prokushkin AS. 2006. Critical analysis of root: shoot ratios in terrestrial biomes. Glob Change Biol 12:84–96.
Mommer L, Padilla FM, van Ruijven J, de Caluwe H, Smit-Tiekstra A, Berendse F, de Kroon H. 2015. Diversity effects on root length production and loss in an experimental grassland community. Funct Ecol 29(12):1560–68. doi:10.1111/1365-2435.12466.
Naudts K, Van den Berge J, Janssens IA, Nijs I, Ceulemans R. 2013. Combined effects of warming and elevated CO2 on the impact of drought in grassland species. Plant Soil 369:497–507.
Nelson JA, Morgan JA, LeCain DR, Mosier A, Milchunas DG, Parton BA. 2004. Elevated CO2 increases soil moisture and enhances plant water relations in a long-term field study in semi-arid shortgrass steppe of Colorado. Plant Soil 259:169–79.
Newingham BA, Vanier CH, Charlet TN, Ogle K, Smith SD, Nowak RS. 2013. No cumulative effect of 10 years of elevated [CO2] on perennial plant biomass components in the Mojave Desert. Glob Change Biol 19:2168–81.
Nielsen PL, Andresen LC, Michelsen A, Schmidt IK, Kongstad J. 2009. Seasonal variations and effects of nutrient applications on N and P and microbial biomass under two temperate heathland plants. Appl Soil Ecol 42:279–87.
Norby RJ, Ledford J, Reilly CD, Miller NE, O’Neill EG. 2004. Fine-root production dominates response of a deciduous forest to atmospheric CO2 enrichment. Proc Natl Acad Sci USA 101:9689–93.
Ostle NJ, Levy PE, Evans CD, Smith P. 2009. UK land use and soil carbon sequestration. Land Use Policy 26:S274–83.
Pilon R, Picon-Cochard C, Bloor JMG, Revaillot S, Kuhn E, Falcimagne R, Balandier P, Soussana JF. 2013. Grassland root demography responses to multiple climate change drivers depend on root morphology. Plant Soil 364:395–408.
Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L. 2012. Biomass allocation to leaves, stems and roots: meta-analysis of interspecific variation and environmental control. New Phytol 193(1):30–50.
Pritchard SG, Strand AE, McCormack ML, Davis MA, Finzi AC, Jackson RB, Matamala R, Rogers HH, Oren R. 2008. Fine root dynamics in a loblolly pine forest are influenced by free-air-CO(2)-enrichment: a six-year-minirhizotron study. Glob Change Biol 14:588–602.
R Development Core Team. 2011. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. ISBN 3-900051-07-0, http://www.R-project.org/.
Reid JB, Renquist AR. 1997. Enhanced root production as a feed-forward response to soil water deficit in field-grown tomatoes. Aust J Plant Physiol 24:685–92.
Reyes-Fox M, Steltzer H, Trlica MJ, McMaster GS, Andales AA, LeCain DR, Morgan JA. 2014. Elevated CO2 further lengthens growing season under warming conditions. Nature 510:259–62.
Rustad LE, Campbell JL, Marion GM, Norby RJ, Mitchell MJ, Hartley AE, Cornelissen JHC, Gurevitch J. 2001. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126:543–62.
SAS Institute Inc. 2003. SAS user’s guide. Statistical Analysis System. Cary: SAS Institute Inc.
Selsted MB, Linden LG, Ibrom A, Michelsen A, Larsen KS, Kongstad J, Mikkelsen TN, Pilegaard K, Beier C, Ambus P. 2012. Soil respiration is stimulated by elevated CO2 and reduced by summer drought: Three years of measurements in a multifactor ecosystem manipulation experiment in a temperate heathland (CLIMAITE). Glob Change Biol 18:1216–30.
Shaw MR, Zavaleta ES, Chiariello NR, Cleland EE, Mooney HA, Field CB. 2002. Grassland responses to global environmental changes suppressed by elevated CO2. Science 298:1987–90.
Steinaker DF, Wilson SD. 2005. Belowground litter contributions to nitrogen cycling at a northern grassland-forest boundary. Ecology 86:2825–33.
Tefs C, Gleixner G. 2012. Importance of root derived carbon for soil organic matter storage in a temperate old-growth beech forest - Evidence from C, N and C-14 content. For Ecol Manage 263:131–7.
Van Der Krift TAJ, Berendse F. 2002. Root life spans of four grass species from habitats differing in nutrient availability. Funct Ecol 16(2):198–203.
Valenzuela-Estrada LR, Vera-Caraballo V, Ruth LE, Eissenstat DM. 2008. Root anatomy, morphology, and longevity among root orders in Vaccinium corymbosum (Ericaceae). Am J Bot 95:1506–14.
Volder A, Gifford RM, Evans JR. 2007. Effects of elevated atmospheric CO2, cutting frequency, and differential day/night atmospheric warming on root growth and turnover of Phalaris swards. Glob Change Biol 13:1040–52.
Wan SQ, Norby RJ, Pregitzer KS, Ledford J, O’Neill EG. 2004. CO2 enrichment and warming of the atmosphere enhance both productivity and mortality of maple tree fine roots. New Phytol 162:437–46.
Wang Z, Ding L, Wang J, Zuo X, Yao S, Feng J. 2016. Effects of root diameter, branch order, root depth, season and warming on root longevity in an alpine meadow. Ecol Res 31(5):739–47.
Wilson SD. 2014. Below-ground opportunities in vegetation science. J Veg Sci 25:1117–25.
Wu YB, Zhang J, Deng YC, Wu J, Wang SP, Tang YH, Cui XY. 2014. Effects of warming on root diameter, distribution, and longevity in an alpine meadow. Plant Ecol 215:1057–66.
Wu Z, Dijkstra P, Koch GW, Penuelas J, Hungate BA. 2011. Responses of terrestrial ecosystems to temperature and precipitation change: a meta-analysis of experimental manipulation. Glob Change Biol 17:927–42.
Zavalloni C, Gielen B, Lemmens CMHM, De Boeck HJ, Blasi S, Van den Bergh S, Nijs I, Ceulemans R. 2008. Does a warmer climate with frequent mild water shortages protect grassland communities against a prolonged drought? Plant Soil 308:119–30.
Zhou YM, Tang JW, Melillo JM, Butler S, Mohan JE. 2011. Root standing crop and chemistry after six years of soil warming in a temperate forest. Tree Physiol 31:707–17.
Acknowledgements
The authors wish to thank The CLIMAITE project (CLIMate change effects on biological processes In Terrestrial Ecosystems), funded by the Villum Kann Rasmussen foundation and further supported by Air Liquide Denmark A/S, DONG Energy and the participating institutions and the INCREASE network funded by the EC FP7-Infrastructure-2008-1 Grant Agreement 227628. The authors wish to thank Nina Thomsen, Preben Jørgensen and Svend Danbæk for keeping the CLIMAITE facilities running and constantly ready for field work. David Eissenstat is thanked for his guidance and help during image analysis at Penn State University, and Victoria Sloan is thanked for her review of the first draft of this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors contributions CB, IKS, MFA conceived and designed the study, MFA performed research, MFA, KSL and AT analysed the data, MFA wrote the manuscript with input and editorial advice by all other authors.
Rights and permissions
About this article
Cite this article
Arndal, M.F., Tolver, A., Larsen, K.S. et al. Fine Root Growth and Vertical Distribution in Response to Elevated CO2, Warming and Drought in a Mixed Heathland–Grassland. Ecosystems 21, 15–30 (2018). https://doi.org/10.1007/s10021-017-0131-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-017-0131-2