Abstract
Aims
Drought is a major growth limiting factor in the majority of terrestrial ecosystems and is expected to become more frequent in the future. Therefore, resolving the drought response of plants under changing climate conditions is crucial to our understanding of future ecosystem functioning. This study responds to the need for experimental research on the combined effects of warming, elevated CO2 and drought, and aims to determine whether the response to drought is altered under future climate conditions.
Methods
Two grassland species, Lolium perenne L. and Plantago lanceolata L., were grown in sunlit climate-controlled chambers. Four climates were simulated: (1) current climate, (2) current climate with drought, (3) a warmer climate with drought, and (4) a climate with combined warming, elevated CO2 and drought.
Results
Warming did not alter the drought response, neither directly through photosynthesis nor indirectly through changes in water consumption. Also for combined warming and elevated CO2 there were no effects on the plant response to drought for any of the measured parameters. However, simultaneous warming and elevated CO2 mitigated the biomass response to drought through a positive pre-drought effect on photosynthesis and biomass response.
Conclusions
Our results indicate that a positive pre-drought effect of combined warming and elevated CO2 has the potential to compensate for drought-induced biomass losses under future climate conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The impact of single climatic changes like warming, elevated CO2 and extreme drought events, on plant growth have been the subject of numerous studies and have previously been synthesized (e.g. Chaves et al. 2002; Hudson et al. 2011; Long et al. 2004; Rustad et al. 2001). However, experimental research on plant responses to simultaneously occurring climate change factors is still rare, despite some recent efforts (Bloor et al. 2010; Mikkelsen et al. 2008). Yet, these studies are crucial for our understanding of future ecosystem functioning since most multifactor experiments to date have shown interactive and often unpredicted effects of multiple climate change factors (Albert et al. 2011a; Larsen et al. 2010; Shaw et al. 2002).
Precipitation is the major controlling driver of above-ground biomass production in grasslands (Sala et al. 1988) and is expected to exhibit prolonged summer droughts and a higher frequency of extremes in the future (IPCC 2007). Plant responses to drought stress in the field have been intensively studied (Chaves et al. 2002). Low soil water contents limit above-ground biomass production mainly through decreases in stomatal conductance, down-regulation of the photosynthetic machinery and/or increased allocation to the roots (Chaves et al. 2002). Moreover, drought can impair nutrient uptake (Hsiao 1973; Viets 1972) and suppress nitrogen (N) cycling through changes in the balance between immobilization and mineralization of N (Andresen et al. 2010).
In the absence of photosynthetic acclimation, elevated temperature increases rates of photosynthesis as long as the plant’s optimal temperature is not exceeded (Berry and Björkman 1980). In general, biomass production is increased by warming if water is not limiting (Dukes et al. 2005; Penuelas et al. 2007; Rustad et al. 2001; Wu et al. 2011). As the transpiration of a stand is approximately proportional to its green mass, this warming-induced biomass increment could increase water consumption (Larcher 2003). Moreover, this biomass-dependent enhanced water consumption can be further exacerbated by increased evapotranspiration through a direct effect of warming (Allen et al. 2003). Therefore, warming is expected to deteriorate drought stress through enhanced soil water depletion.
Elevated CO2 often decreases plant water consumption due to reduced stomatal conductance, which leads to increased water use efficiency (Ainsworth and Long 2005) and reduced soil water depletion (Leuzinger and Korner 2007; Morison and Gifford 1984; Robredo et al. 2007). This can enable maintained plant carbon uptake during drought periods in elevated CO2 (Leuzinger and Korner 2007; Morison and Gifford 1984; Robredo et al. 2007), but may be insufficient to prevent harmful carbon starvation during intense, frequent or long drought periods (McDowell et al. 2008). Moreover, elevated CO2 causes allocation of carbon to root growth, which can enhance the plants’ capacity to acquire water (De Luis et al. 1999). Hence, under future climate, elevated CO2 could compensate for the possible detrimental effect of warming during extreme drought periods through a stimulation of photosynthesis or through indirect effects on water consumption and uptake. Besides effects on the plant water status, elevated CO2 and warming have been found to alter the availability of N through complex changes in the release of nutrients from soil organic matter turnover and mineralization (Andresen et al. 2010).
To assess the impact of drought under future climate conditions, monocultures and mixtures of two grassland species were grown in four simulated climates, i.e. (i) current climate, (ii) current climate with a drought period, (iii) a warmer climate with a drought period and (iv) a climate with combined warming and elevated CO2, and a drought period. In an earlier experiment on grassland communities we found no effect of combined warming and elevated CO2 on the biomass response to drought (Naudts et al. 2011). However, in that experiment we only studied the combined effect of warming and elevated CO2, whereas the additive experimental design of the present study provides insight on the effects of the single factors and allows comparison between current climate conditions without drought and future climate conditions, including drought, warming and elevated CO2. We hypothesize that: (1) warming deteriorates the negative impact of drought on plant growth through enhanced water consumption, and (2) combined warming and elevated CO2 mitigates the detrimental drought impact through stimulated photosynthesis, reduced water consumption, carbon allocation to the roots and/or enhanced nutrient availability.
Material and methods
Experimental set-up
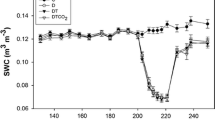
The study was performed at the Drie Eiken Campus, University of Antwerp, Wilrijk, Belgium (51 ° 09′ N, 04 ° 24′ E), where average annual precipitation is 776 mm (evenly distributed throughout the year) with an average annual air temperature of 10.8 °C. The experimental set-up consisted of 16 sunlit, south facing climate-controlled chambers. The distances between the chambers were maximised to avoid mutual shading. The interior surface area was 1.5 × 1.5 m, the height at the north side was 1.5 m and at the south side 1.2 m. The top of the chambers consisted of a colourless polycarbonate plate (4 mm thick), whereas the sides were made of polyethylene film (200 μm thick), both UV transparent. In the chambers four climate treatments (four chambers per climate) were simulated in an additive design: (1) a climate treatment mimicking current temperature and atmospheric [CO2] (current climate, C); (2) a climate also mimicking current temperature and atmospheric [CO2], but including a drought period (D);(3) a climate mimicking future temperature and current atmospheric [CO2], including a drought period (DT); and (4) a climate treatment mimicking future temperature and atmospheric [CO2], including a drought period (DTCO2). The chambers simulating current temperatures (C and D) followed fluctuating air temperatures mimicking an average daily air temperature course, calculated for the period 1996–2005. The chambers with future temperature simulated a 3 °C warming compared to the simulated current climate. The climate treatment with elevated CO2 had a target CO2 concentration of 620 ppm. The CO2 concentration was measured and regulated with a CO2 control group with an infrared analyser (WMA-4, PPSystems, Hitchin, UK). In C, D and DT chambers the CO2 concentration was 392 ± 42 ppm (SD), while it was 615 ± 81 ppm (SD) in DTCO2. Relative humidity and air temperature (Tair) were monitored with a humidity-temperature sensor (Siemens, type QFA66, Germany) and photosynthetically active radiation (PAR) was measured with a quantum sensor (SDEC, type JYP1000, France). All microclimate parameters were automatically logged every 30 min. Monthly average air temperature in C and D chambers was 12.33, 16.60, 18.80, 14.69 and 15.53 °C in May, June, July, August and September, respectively. For DT and DTCO2, chambers were on average 3.02 ± 0.82 °C (SD) warmer than chambers with current temperature. Average daily PAR sum inside the chambers was 23.1, 25.3, 34.6, 42.1, 39.7 mol m−2d−1 in May, June, July, August and September, respectively, and differed little between climate treatments (maximum delta of 2.4 ± 0.5 mol m−2d−1). Average vapour pressure deficit was 0.35 ± 0.02 and 0.46 ± 0.02 (SD) in the climate treatments with ambient and warmed air temperature, respectively. The experimental drought period was applied by withholding water for 20 days (DOY 197–217).
Inside the chambers, plant communities with two common and co-occurring grassland species, Lolium perenne L. and Plantago lanceolata L. were assembled. Both species have a C3 photosynthetic pathway. The plants were sown at the end of March 2010 and the seedlings were transplanted at the end of April (day of year, DOY 116–118) into PVC containers (19 cm inner diameter, 40 cm height), filled with sandy soil (93.2 % sand, 4.6 % silt, 2.2 % clay; field capacity 0.13 m3 m−3; pH 7.6; total Kjeldahl-N 0.42 g kg−1; 1 % C in humus). Each community (and thus each container) contained six individuals planted in a hexagonal grid with a 5 cm interspace and one individual positioned at the centre of the grid. In each chamber 24 communities with four different plant compositions (further referred to as composition) were assembled: (1) six monocultures of Lolium perenne L.; (2) six monocultures of Plantago lanceolata L.; (3) six mixtures of both species with Lolium perenne L. as central plant; and (4) six mixtures of both species with Plantago lanceolata L. as central plant. All communities were fertilised with 10 g m−2 NH4NO3, 5 g m−2 P2O5, 10 g m−2 K2O and micro-elements (Fe, Mn, Zn, Cu, B, Mo). The fertiliser was given dissolved in water in two equal amounts at DOY 140 and 180. Irrigation was calculated from the monthly rainfall over the period 1995–2005 and corrected for differences in evapotranspiration (ET) inside and outside the chambers. To this end, De Boeck et al. (2006) calculated ET inside the chambers from changes in soil water content (SWC) and the amount of administered water. The outside ET was calculated with Hamon’s equation (Haith and Shoemaker 1987) based on day length, vapour pressure and air temperature. The containers were watered every 2 days according to the 10 year average of 14 to 15 rain days per month during the growing season. Total monthly irrigation matched 61.5, 64.4, 85.1, 80.2 and 80.9 mm in May, June, July, August and September, respectively. During the experimental drought period irrigation was interrupted in D, DT and DTCO2. Water could freely drain from the containers while capillary rise of ground water towards the containers was prevented by a drainage system placed below the chambers. In each chamber, four containers (one in each composition) contained a profile probe tube for the PR2 soil moisture sensor (Delta-T Devices Ltd., UK). Soil water content (SWC) was measured once a week before the drought treatment (DOY 130–193) and twice a week during the experimental drought period.
Biomass harvest and resin bags
In each chamber above-ground biomass (shoot above 3.5 cm and stubble) of two communities per composition (eight communities per climate treatment) was harvested before drought, after drought and at the end of the growing season (DOY 197, 217 and 307). At the same time root biomass was also determined in one community per chamber (four replicates per climate treatment). Root samples were washed until they were free of soil.
Total community biomass included the sum of above-ground and root biomass and root/shoot ratio was calculated by dividing root biomass with above-ground biomass. Relative growth rate (RGR) during and after the drought period represents the increase in total biomass during the respective period, relative to the initial total biomass. All plant material was dried at 70 °C for 48 h and then weighed. Total leaf area was determined on four communities per climate treatment (one per chamber per composition) with a portable area meter (LI-3000A, Li-COR, NE, USA) before and after the drought period. Leaf area index (LAI) was calculated as the ratio of total leaf area (of the community) to the ground area of a container. Ion exchange resin bags were used to estimate N availability in the presence of functioning plant roots (Binkley and Matson 1983). One resin bag was buried 5 cm below the soil surface in one community per composition in each chamber (four replicates per climate treatment) and collected during each harvest. Ammonium (NH4) and nitrate (NO3) were extracted from the resin bags in 50 mL 2 M HCl and measured colorimetrically. Resin adsorption quantity (RAQ) was calculated as cV/MA, where c is the concentration of nutrient in the HCl extract, V is the volume of the extract, M is the molar mass of the nutrient, and A is the capsule surface (11.4 cm2).
Leaf gas exchange
Leaf stomatal conductance (gs) was measured before and after the drought period (DOY 197 and 217), on the most recently matured leaf of the central plant, with an automatic porometer (AP4, Delta-T Devices Ltd., UK), taking into account abaxial and adaxial sides of the leaves. Also light-saturated net CO2 assimilation rate (Asat) was determined on the most recently matured leaf of the central plant with a portable gas exchange system (LI-6400, Li-COR, NE, USA) before and after drought (DOY 197 and 217). Leaf chamber conditions were controlled at 380 ppm CO2 and 23.5 °C (block temperature) at saturating PAR (1,500 μmol m−2 s−1) and ambient relative humidity for the current climate treatment. In DT the block temperature was 26.5 °C and for DTCO2 the CO2 concentration was controlled at 610 ppm. Measurements were performed on two communities per composition in each chamber, yielding eight measurements per composition and climate combination.
Data analysis
Analyses of variance (ANOVA) were performed on above-ground, root and total community biomass, Asat, gs, LAI, RAQ and RGR with climate and composition as fixed factors. For Asat and gs also species was included in the model. Soil water content was analysed with repeated measures ANOVA with DOY, composition and climate as fixed factors. Chamber was always included as a random factor nested within climate. Non-significant factors were excluded from the model. In case of significant effects, the means were compared a posteriori with Tukey corrections for multiple comparisons. Effects of drought were examined by comparing C and D, effects of warming on drought by comparing D and DT and effects of combined warming and elevated CO2 on drought by comparing D and DTCO2. Analyses were performed in SAS (version 9.1, SAS Institute Inc., Cary, NC) using the mixed procedure (Littell et al. 1996).
Parameters were analysed at three moments in the experiment: ‘before drought’ (DOY 197), ‘(immediately) after drought’ (DOY 217) and at the end of the growing season ‘after recovery’ (DOY 307). The study focuses on the effects of warming and elevated CO2 on the drought response and to clearly represent these results the intrinsic differences between plant compositions were not extensively discussed in the results, but briefly summarized here. Overall differences between monocultures and mixtures were not significant, however, there was an intrinsic difference between monocultures of Lolium perenne L. and Plantago lanceolata L. (the latter had a slightly lower biomass production). Differences in the drought response between plant compositions (climate x composition) were not significant for any of the parameters in none of the climate treatments (P > 0.1 in all cases) and therefore the climate x composition interaction could be excluded from the model. For reasons of clarity, and because the drought response did not differ between compositions for any of the climate treatments, we only show the results of the mixtures in the figures.
Results
Drought response under current climate conditions
The drought response under current climate conditions was determined by comparing plant communities in C and D. Soil water content decreased during the imposed drought from DOY 203 onwards and remained lower after re-watering until DOY 235 (Fig. 1; Table 1). Drought reduced above-ground, root and total biomass production respectively with 15.3 %, 21.5 % and 22.1 %. Also gs, LAI, Asat and RGR were lower in D than in C (data not shown, Figs. 2 and 3, respectively; Table 1). Except for root biomass production, plant communities were fully recovered from the drought-induced growth reduction by the end of the growing season (Fig. 3; Table 1). Root/shoot ratio was not altered by drought, neither immediately after drought nor at the end of the season (data not shown; Table 1). Also N availability in the plant communities was not changed by drought (Fig. 4; Table 1).
Time course of soil water content (SWC) in communities with mixtures of Lolium perenne L. and Plantago lanceolata L. in current climate conditions (white square), current climate conditions with a drought period (white circle), warmer climate conditions with a drought period (white triangle) and future climate conditions with combined warming, elevated CO2 and a drought period (white diamond). The drought period was initiated at day of year (DOY) 197 and re-watering started at DOY 217. Means±SE are indicated
Light-saturated net assimilation rate (Asat) of Lolium perenne (Lp) and Plantago lanceolata (Pl) at the end of a drought period in communities with mixtures of these species. Measurements were performed on the most recently matured leaf of the central individual in the plant community. Plants were grown in current climate conditions (white bars), current climate conditions with drought (light grey bars), warmer climate conditions with drought (dark grey bars) or future climate conditions (warming and elevated CO2) with drought (black bars). In climate conditions with a drought period, irrigation was stopped for 20 days (DOY 197–217 in 2010). Means±SE are indicated. Letters indicate differences for a posterior comparisons between climate treatments, separately tested for three moments in the experiment: ‘before drought’ (DOY 197), immediately ‘after drought’ (DOY 217) and ‘at the end of the growing season’ (DOY 307)
Total (top panel), above-ground (middle panel) and root biomass (bottom panel) before drought, after drought and at the end of the growing season in communities with mixtures of Lolium perenne L. and Plantago lanceolata L. Plants were grown in current climate conditions (white bars), current climate conditions with drought (light grey bars), warmer climate conditions with drought (dark grey bars) and future climate conditions with combined warming, elevated CO2 and drought (black bars). In climate treatments with a drought period irrigation was stopped for 20 days (DOY 197–217). Means±SE are indicated. Letters indicate differences for a posterior comparisons between climate treatments, separately tested for three moments in the experiment: ‘before drought’ (DOY 197), immediately ‘after drought’ (DOY 217) and ‘at the end of the growing season’ (DOY 307)
Resin absorption quantity (RAQ) in communities with mixtures of Lolium perenne L. and Plantago lanceolata L. before and after a drought (D) period and at the end of the growing season. Communities were grown in current climate conditions (white bars), current climate conditions with drought (light grey bars), warmer climate conditions with drought (dark grey bars) and future climate conditions with combined warming, elevated CO2 and drought (black bars). In climate treatments with a drought period irrigation was interrupted for 20 days (DOY 197–217). Means±SE are indicated. Letters indicate differences for a posterior comparisons between climate treatments, separately tested for three moments in the experiment: ‘before drought’ (DOY 197), immediately ‘after drought’ (DOY 217) and ‘at the end of the growing season’ (DOY 307).
Effect of warming on the drought response
The effect of warming on the drought response was determined by comparing D and DT. Warming did not enhance soil water depletion compared to the drought period under current climate conditions (Fig. 1; Table 1). Moreover, except for LAI, which was slightly lower in DT than D during drought, warming did not alter any of the observed responses to drought, neither immediately after drought nor at the end of the growing season (Figs. 1, 2, 3, 4; Table 1). Because the overall absence of a warming effect on the drought response could originate from a possible beneficial effect of warming before the drought, we also considered the pre-drought period. However, also before the drought, warming did not affect SWC, gs, Asat, LAI, N availability, RGR and biomass production (Figs. 1, 2, 3, 4; Table 1).
Combined effect of warming and elevated CO2 on the drought response
The combined effect of warming and elevated CO2 on the drought response was assessed by comparing D and DTCO2. Immediately after drought, above-ground and total biomass production was significantly higher in DTCO2 than in D, suggesting that the drought response was alleviated by combined warming and elevated CO2 (Fig. 2; Table 1). However, this mitigating effect on the drought response was not supported by any of the other parameters: LAI, gs, RGR, Asat, N availability did not differ between D and DTCO2 (data not shown, Figs. 2 and 4, respectively; Table 1). This can be explained because the mitigating effect of combined warming and elevated CO2 originated in a positive pre-drought effect of combined warming and elevated CO2: stimulation of Asat resulted in a higher above-ground and total biomass production before drought (Fig. 3; Table 1).
Discussion
Drought inhibited photosynthesis and biomass production, but did not alter root/shoot ratio or N availability. Further, LAI and gs, which are the main plant traits affecting evapotranspiration, where decreased. We did not observe any changes in root/shoot ratio or N availability, which could be explained by the short duration of the experiment. Decreased photosynthesis, biomass production, gs and LAI are common plant responses to drought (Chaves et al. 2002), but the effects of changing climate on these responses have rarely been examined (Albert et al. 2011a; Bloor et al. 2010; Kongstad et al. 2012).
Warming did not affect the biomass or photosynthetic response to drought, i.e. biomass production and photosynthesis were equally restrained by drought under current and warmer climate conditions. Combined warming and elevated CO2 alleviated the drought response, or in other words, biomass production and photosynthesis were higher in DTCO2 than in D immediately after drought. However, relative growth rate during drought did not differ between D and DTCO2, indicating that the mitigating effect of combined warming and elevated CO2 mediated through a pre-drought effect rather than through the expected changes during drought stress. Indeed, combined warming and elevated CO2 enhanced photosynthesis and biomass production before drought, resulting in more biomass in DTCO2 than in D immediately after the drought period. In a study that examined interactive effects between warming and precipitation change, overall above-ground biomass in drought stressed plots decreased along a warming gradient (Hoeppner and Dukes 2012). However, the biomass response to drought under current climate conditions was very small in that experiment, whereas in our study biomass was substantially decreased by single drought and did not decrease further by additional warming. Regarding the pre-drought mitigating effect of combined warming and elevated CO2, our results are in agreement with an earlier study on grassland communities (Naudts et al. 2011). At the end of the growing season the overall effects of changed climate conditions on biomass production were small to absent, confirming the high resilience to climate changes previously found in heath land plants (Kongstad et al. 2012).
Warming and/or elevated CO2 can alter the biomass response to drought through direct changes on photosynthesis or indirect changes in evapotranspiration, root/shoot ratio and/or nutrient availability (Newman et al. 2011). The reduction in stomatal conductance during drought was not affected by warming and/or elevated CO2 in our study. Similarly, gs of temperate heath plants did not differ between D, DT and DTCO2 (Albert et al. 2011b). Also the drought-induced inhibition of photosynthesis was not altered by single warming, however, combined warming and elevated CO2 mitigated this photosynthetic inhibition. This is in contrast to an earlier study on grassland communities, in which stronger stomatal closure was found to cause a larger drought-induced reduction in Asat in combined warming and elevated CO2 (Naudts et al. 2011). The drought-induced decrease in LAI in our study was not altered by warming/or elevated CO2. Effects of warming and elevated CO2 on LAI have been found to be closely related to soil water availability: enhanced soil drying due to warming resulted in reduced LAI, while elevated CO2 counteracted this effect by mitigating soil water depletion during drought (Dermody et al. 2007). Likewise, also in other studies, warming has been found to enhance soil drying in grassland communities (Zavalloni et al. 2008), while elevated CO2 counteracted this effect (Naudts et al. 2011). The unaltered drought response of soil water availability and LAI under combined warming and elevated CO2 in our study, confirm these results. However, the absence of a warming-induced enhanced soil drying was unexpected because evapotranspiration was anticipated to increase in a warmer climate due to a higher atmospheric demand. As additional warming did not further decrease gs or LAI during drought, other warming-induced responses, like changes in phenology (e.g. accelerated senescence) might have contributed to the unaltered soil water availability under warmer environmental conditions (Zavaleta et al. 2003).
Single warming and warming combined with elevated CO2 did not affect the root/shoot ratio from drought-stressed plants in this study. In literature, root/shoot ratio has generally been found to be increased by elevated CO2 and/or drought (De Luis et al. 1999; Luo et al. 2006), but also unaltered root/shoot ratio has been reported in response to elevated CO2, warming and/or drought (Lilley et al. 2001).
Hence, the drought response of photosynthesis, gs, LAI, soil water availability and root/shoot ratio was not affected by changed climate conditions. Also N availability was not influenced by warming and/or elevated CO2 during drought and across the entire growing season. Warming has been found to stimulate N mineralization, however, this effect can be counteracted when combined with elevated CO2 and/or drought (Andresen et al. 2010; Hovenden et al. 2008). The lack of an effect of climate on the impact of drought on photosynthesis, water consumption, root/shoot ratio and N availability during drought confirmed the absence of an effect of warming and/or elevated CO2 on the drought response at the biomass level.
Complementarity in the acquisition of water due to a combination of deep-rooted and shallow-rooted species can lead to more efficient water use in multi-species communities compared to monocultures (Berendse 1982; De Boeck et al. 2006). Therefore, one might expect an increased resistance against drought in mixtures. Nevertheless, our results show no difference in the biomass response to drought between monocultures and mixtures for any of the climate treatments. This is in agreement with several studies that show no effect of species number on the resistance of communities to abiotic stress (De Boeck et al. 2008; van Ruijven and Berendse 2010). However, these studies also report an increased pre-disturbance biomass and/or improved recovery in species rich communities (De Boeck et al. 2008; van Ruijven and Berendse 2010). The absence of such effects in our study can be explained by the low number of species and the rather short duration of the experiment. A study on the combined effects of species richness, drought, warming and elevated CO2 has, to our knowledge, never been conducted. Although a much wider range of species numbers need to be tested and long-term experiments are required, our results suggest that also under future climate conditions, more diverse communities are not necessarily more resistant against drought.
To date, the main comparison of concern to semi-arid regions is the comparison between C and DT. Our results showed reduced stomatal conductance and, consequently, inhibited photosynthesis under dry, warm climate conditions. The decreased photosynthetic rate resulted in a lower total biomass production at the end of the growing season, which was mainly explained by impaired root biomass production. The latter is in agreement with the warming-induced root death found in temperate grasslands (Edwards et al. 2004). As drought is predicted to become more frequent in the future (Meehl et al. 2000), the key comparison regarding the response of grasslands to future climate conditions, is the comparison between C and DTCO2. Our results clearly indicate that biomass production did not differ between these climate conditions, because of a beneficial effect of combined warming and elevated CO2 early in the season. These findings are in agreement with the second year of an experiment on an upland grassland in the French Central Massif, which also had an additive design, yielding T, DT and DTCO2 treatments (Bloor et al. 2010). We cannot compare our findings on the effect of climate on the drought response with that study, because it did not include a single drought treatment. Yet, we can compare the results on the comparison between C and DTCO2: similar to our findings, a positive effect on above-ground biomass in DTCO2 during spring resulted in the lack of an effect between C and DTCO2 later in the growing season. However, the beneficial pre-drought effect under future climate conditions was due to warming, whereas in our study it was mainly due to elevated CO2. The larger responsiveness to warming in the upland grassland study can be explained by lower ambient temperatures than in our experiment, while the lack of an effect of elevated CO2 can be explained by nutrient limitation. Our results contrasted with those of the third year of the experiment on upland grasslands (Bloor et al. 2010). In that third year, above-ground biomass in DTCO2 was lower than in C, despite a positive effect of future climate conditions in spring. Further research on the long-term response of grasslands should clarify this inter-annual difference and determine whether the mitigating pre-drought effect of combined warming and elevated CO2 will be sufficient to prevent drought-induced biomass loss in the future.
We conclude that, in contrast to our first hypothesis, single warming did not deteriorate the drought-induced inhibition of biomass production, neither directly on the photosynthetic level nor indirectly through changes in water consumption. In agreement with our second hypothesis, combined warming and elevated CO2 alleviated the drought response of above-ground and total biomass production. However, this mitigation mediated through a positive pre-drought effect and not through changes during drought, as was expected. Most importantly, our results indicate that the beneficial pre-drought effect of combined warming and elevated CO2 has the potential to compensate for drought-induced biomass losses under future climate conditions.
Abbreviations
- C:
-
Climate treatment with current air temperature and current atmospheric [CO2]
- D:
-
Climate treatment with current air temperature, current atmospheric [CO2] and a drought period
- DOY:
-
Day of year
- DT:
-
Climate treatment with future temperature, current atmospheric [CO2] and a drought period
- DTCO2 :
-
Climate treatment with future temperature, future atmospheric [CO2] and a drought period
- Tair :
-
Air temperature
- PAR:
-
Photosynthetically active radiation
- ET:
-
Evapotranspiration
- SWC:
-
Soil water content
- RGR:
-
Relative growth rate
- LAI:
-
Leaf area index
- RAQ:
-
Resin adsorption quantity
- gs :
-
Stomatal conductance
- Asat :
-
Light-saturated net CO2 assimilation rate
- ANOVA:
-
Analysis of variance
- SD:
-
Standard deviation
- SE:
-
Standard error
References
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy. New Phytol 165:351–371
Albert KR, Mikkelsen TN, Michelsen A, Ro-Poulsen H, van der Linden L (2011a) Interactive effects of drought, elevated CO2 and warming on photosynthetic capacity and photosystem performance in temperate heath plants. J Plant Physiol 168:1550–1561
Albert KR, Ro-Poulsen H, Mikkelsen TN, Michelsen A, Van der Linden L, Beier C (2011b) Effects of elevated CO2, warming and drought episodes on plant carbon uptake in a temperate heath ecosystem are controlled by soil water status. Plant Cell Environ 34:1207–1222
Allen LH, Pan DY, Boote KJ, Pickering NB, Jones JW (2003) Carbon dioxide and temperature effects on evapotranspiration and water use efficiency of soybean. Agron J 95:1071–1081
Andresen LC, Michelsen A, Jonasson S, Schmidt IK, Mikkelsen TN, Ambus P, Beier C (2010) Plant nutrient mobilization in temperate heathland responds to elevated CO2, temperature and drought. Plant Soil 328:381–396
Berendse F (1982) Competition between plant-populations with different rooting depths. 3. Field experiments. Oecologia 53:50–55
Berry J, Björkman O (1980) Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol Plant Mol Biol 31:491–543
Binkley D, Matson P (1983) Ion-exchange resin bag method for assessing forest soil-nitrogen availability. Soil Sci Soc Am J 47:1050–1052
Bloor JMG, Pichon P, Falcimagne R, Leadley P, Soussana JF (2010) Effects of warming, summer drought, and CO2 enrichment on aboveground biomass production, flowering phenology, and community structure in an upland grassland ecosystem. Ecosystems 13:888–900
Chaves MM, Pereira JS, Maroco J, Rodrigues ML, Ricardo CPP, Osorio ML, Carvalho I, Faria T, Pinheiro C (2002) How plants cope with water stress in the field. Photosynthesis and growth. Ann Bot 89:907–916
De Boeck HJ, Lemmens C, Bossuyt H, Malchair S, Carnol M, Merckx R, Nijs I, Ceulemans R (2006) How do climate warming and plant species richness affect water use in experimental grasslands? Plant Soil 288:249–261
De Boeck HJ, Lemmens C, Zavalloni C, Gielen B, Malchair S, Carnol M, Merckx R, Van den Berge J, Ceulemans R, Nijs I (2008) Biomass production in experimental grasslands of different species richness during three years of climate warming. Biogeosciences 5:585–594
De Luis I, Irigoyen JJ, Sanchez-Diaz M (1999) Elevated CO2 enhances plant growth in droughted N-2-fixing alfalfa without improving water status. Physiol Plant 107:84–89
Dermody O, Weltzin JF, Engel EC, Allen P, Norby RJ (2007) How do elevated [CO2], warming, and reduced precipitation interact to affect soil moisture and LAI in an old field ecosystem? Plant Soil 301:255–266
Dukes JS, Chiariello NR, Cleland EE, Moore LA, Shaw MR, Thayer S, Tobeck T, Mooney HA, Field CB (2005) Responses of grassland production to single and multiple global environmental changes. PLoS Biol 3:1829–1837
Edwards EJ, Benham DG, Marland LA, Fitter AH (2004) Root production is determined by radiation flux in a temperate grassland community. Global Change Biol 10:209–227
Haith DA, Shoemaker LL (1987) Generalized watershed loading functions for stream-nutrients. Water Resour Bull 23:471–478
Hoeppner SS, Dukes JS (2012) Interactive responses of old-field plant growth and composition to warming and precipitation. Global Change Biol 18:1754–1768
Hovenden MJ, Newton PCD, Carran RA, Theobald P, Wills KE, Vander Schoor JK, Williams AL, Osanai Y (2008) Warming prevents the elevated CO2-induced reduction in available soil nitrogen in a temperate, perennial grassland. Global Change Biol 14:1018–1024
Hsiao TC (1973) Plant responses to water stress. Annu Rev Plant Physiol Plant Mol Biol 24:519–570
Hudson JMG, Henry GHR, Cornwell WK (2011) Taller and larger: shifts in Arctic tundra leaf traits after 16 years of experimental warming. Global Change Biol 17:1013–1021
IPCC 2007 Climate Change (2007) The physical science basis. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Contribution of Working Group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
Kongstad J, Schmidt IK, Riis-Nielsen T, Arndal MF, Mikkelsen TN, Beier C (2012) High resilience in heathland plants to changes in temperature, drought, and CO2 in combination: results from the CLIMAITE experiment. Ecosystems 15:269–283
Larcher W (2003) Physiological plant ecology: ecophysiology and stress physiology of functonal groups, 4th edn. Springer, Berlin
Larsen KS, Andresen LC, Beier C, Jonasson S, Albert KR, Ambus P, Arndal MF, Carter MS, Christensen S, Holmstrup M, Ibrom A, Kongstad J, van der Linden L, Maraldo K, Michelsen A, Mikkelsen TN, Pilegaard K, Prieme A, Ro-Poulsen H, Schmidt IK, Selsted MB, Stevnbak K (2010) Reduced N cycling in response to elevated CO(2), warming, and drought in a Danish heathland: synthesizing results of the CLIMAITE project after two years of treatments. Global Change Biol 17:1884–1899
Leuzinger S, Korner C (2007) Water savings in mature deciduous forest trees under elevated CO2. Global Change Biol 13:2498–2508
Lilley JM, Bolger TP, Gifford RM (2001) Productivity of Trifolium subterraneum and Phalaris aquatica under warmer, high CO2 conditions. New Phytol 150:371–383
Littell RC, Milliken GA, Stroup WW, Wolfinger RD (1996) SAS System for mixed models. SAS Institute Inc., Cary
Long SP, Ainsworth EA, Rogers A, Ort DR (2004) Rising atmospheric carbon dioxide: plants face the future. Annu Rev Plant Biol 55:591–628
Luo YQ, Hui DF, Zhang DQ (2006) Elevated CO2 stimulates net accumulations of carbon and nitrogen in land ecosystems: a meta-analysis. Ecology 87:53–63
McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, Yepez EA (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178:719–739
Meehl GA, Zwiers F, Evans J, Knutson T, Mearns L, Whetton P (2000) Trends in extreme weather and climate events: issues related to modeling extremes in projections of future climate change. Bull Am Meteorol Soc 81:427–436
Mikkelsen TN, Beier C, Jonasson S, Holmstrup M, Schmidt IK, Ambus P, Pilegaard K, Michelsen A, Albert K, Andresen LC, Arndal MF, Bruun N, Christensen S, Danbaek S, Gundersen P, Jorgensen P, Linden LG, Kongstad J, Maraldo K, Prieme A, Riis-Nielsen T, Ro-Poulsen H, Stevnbak K, Selsted MB, Sorensen P, Larsen KS, Carter MS, Ibrom A, Martinussen T, Miglietta F, Sverdrup H (2008) Experimental design of multifactor climate change experiments with elevated CO2, warming and drought: the CLIMAITE project. Funct Ecol 22:185–195
Morison JIL, Gifford RM (1984) Plant-growth and water-use with limited water-supply in high CO2 concentrations. 1. Leaf-area, water-use and transpiration. Aust J Plant Physiol 11:361–374
Naudts K, Van den Berge J, Janssens IA, Nijs I, Ceulemans R (2011) Does an extreme drought event alter the response of grassland communities to a changing climate? Environ Exp Bot 70:151–157
Newman JA, Anand M, Henry HAL, Hunt S, Gedalof Z (2011) Climate change biology. CABI, Oxfordshire
Penuelas J, Prieto P, Beier C, Cesaraccio C, de Angelis P, de Dato G, Emmett BA, Estiarte M, Garadnai J, Gorissen A, Lang EK, Kroel-Dulay G, Llorens L, Pellizzaro G, Riis-Nielsen T, Schmidt IK, Sirca C, Sowerby A, Spano D, Tietema A (2007) Response of plant species richness and primary productivity in shrublands along a north–south gradient in Europe to seven years of experimental warming and drought: reductions in primary productivity in the heat and drought year of 2003. Global Change Biol 13:2563–2581
Robredo A, Perez-Lopez U, de la Maza HS, Gonzalez-Moro B, Lacuesta M, Mena-Petite A, Munoz-Rueda A (2007) Elevated CO2 alleviates the impact of drought on barley improving water status by lowering stomatal conductance and delaying its effects on photosynthesis. Environ Exp Bot 59:252–263
Rustad LE, Campbell JL, Marion GM, Norby RJ, Mitchell MJ, Hartley AE, Cornelissen JHC, Gurevitch J (2001) A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126:543–562
Sala OE, Parton WJ, Joyce LA, Lauenroth WK (1988) Primary production of the central grassland region of the United States. Ecology 69:40–45
Shaw MR, Zavaleta ES, Chiariello NR, Cleland EE, Mooney HA, Field CB (2002) Grassland responses to global environmental changes suppressed by elevated CO2. Science 298:1987–1990
van Ruijven J, Berendse F (2010) Diversity enhances community recovery, but not resistance, after drought. J Ecol 98:81–86
Viets JF (1972) Water deficits and nutrient availability. In: KT (ed) Water deficits and plant growth. Academic Press, New York
Wu ZT, Dijkstra P, Koch GW, Penuelas J, Hungate BA (2011) Responses of terrestrial ecosystems to temperature and precipitation change: a meta-analysis of experimental manipulation. Global Change Biol 17:927–942
Zavaleta ES, Thomas BD, Chiariello NR, Asner GP, Shaw MR, Field CB (2003) Plants reverse warming effect on ecosystem water balance. Proc Natl Acad Sci USA 100:9892–9893
Zavalloni C, Gielen B, Lemmens CMHM, De Boeck HJ, Blasi S, Van den Bergh S, Nijs I, Ceulemans R (2008) Does a warmer climate with frequent mild water shortages protect grassland communities against a prolonged drought? Plant Soil 308:119–130
Acknowledgements
This research was funded by the University of Antwerp as concerted research project “Changes in the stress sensitivity of plants and ecosystems under climate change conditions” (GOA-BOF-UA-2007). K. Naudts holds a grant from the Institute for the Promotion of Innovation by Science and Technology in Flanders (IWT). J. Van den Berge is a Research Assistant of the Fund for Scientific Research-Flanders (FWO). We thank N. Calluy and M. Wellens for technical assistance and H. Van De Velde for field assistance. We thank three reviewers for their valuable comments and highly appreciated suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Rafael S. Oliveira.
Rights and permissions
About this article
Cite this article
Naudts, K., Van den Berge, J., Janssens, I.A. et al. Combined effects of warming and elevated CO2 on the impact of drought in grassland species. Plant Soil 369, 497–507 (2013). https://doi.org/10.1007/s11104-013-1595-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1595-2