Abstract
Despite the importance of future carbon (C) pools for policy and land management decisions under various climate change scenarios, predictions of these pools under altered climate vary considerably. Chronic warming will likely impact both ecosystem C fluxes and the abundance and distribution of plant functional types (PFTs) within systems, potentially interacting to create novel patterns of C exchange. Here, we report results from a 3-year warming experiment using open top chambers (OTC) on the Tibetan Plateau meadow grassland. Warming significantly increased C uptake through gross primary productivity (GPP) but not ecosystem respiration (ER), resulting in a 31.0% reduction in net ecosystem exchange (NEE) in warmed plots. The OTC-induced changes in ecosystem C fluxes were not fully explained by the corresponding changes in soil temperature and moisture. Warming treatments significantly increased the biomass of graminoids and legumes by 12.9 and 27.6%. These functional shifts were correlated with enhanced local GPP, but not ER, resulting in more negative NEE in plots with larger increases in graminoid and legume biomass. This may be due to a link between greater legume abundance and higher levels of total inorganic nitrogen, which can potentially drive higher GPP, but not higher ER. Overall, our results indicate that C-climate feedbacks might be closely mediated by climate-induced changes in PFTs. This highlights the need to consider the impacts of changes in PFTs when predicting future responses of C pools under altered climate scenarios.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to increasing atmospheric CO2 concentrations, global temperatures are likely to increase during the rest of the twenty-first century (Stocker 2014). This will likely have substantial impacts on a wide variety of ecosystem processes and services globally with the states of carbon (C) pools being particularly important. Substantial uncertainties surround predictions of ecosystem C fluxes under projected climate warming. These uncertainties stem from variances among field observations and modeling studies (Heimann and Reichstein 2008; Ballantyne and others 2012; Brient and Bony 2013; Friedlingstein and others 2014). Many current Earth system models (ESMs) project positive feedbacks between ecosystem C fluxes and climate warming due to the higher temperature sensitivity of ecosystem respiration (ER) versus photosynthesis; this is especially evident in temperature-sensitive regions (Davidson and Janssens 2006; Friedlingstein and others 2014; Chen and others 2015a). In contrast, projections combining terrestrial biosphere models with long-term ground observations of phenology and ecosystem plant productivity have shown a net C uptake for a warmer environment (Ballantyne and others 2012; Keenan and others 2014). These types of inconsistencies reflect the urgent need to further develop our understanding of the potential mechanisms that regulate ecosystem C fluxes and storage.
Warming studies have primarily focused on the direct effects of soil temperature and soil moisture on C fluxes. This is likely because of evidence showing that biogeochemical processes were strongly impacted by these factors (Lloyd and Taylor 1994; Davidson and others 1998). However, warming is also likely to alter the composition of plant functional types (PFTs) within ecosystems over time (Smith and others 2009; Wang and others 2012), which can lead to significant impacts on the sensitivity of ecosystem functioning under altered environmental conditions (Niu and others 2013; Ponce-Campos and others 2013; Poyatos and others 2014; Dieleman and others 2015; Su and others 2015; Wilcox and others 2015), and affect ecosystem C pools (Niu and others 2013; Ward and others 2013; Xu and others 2015). An example of the impact of community shifts on C cycling was uncovered in a field experiment that combined warming with manipulations of vegetation in peatlands (Ward and others 2013). That study showed that CO2 uptake was greatest in communities in which shrubs were present although the largest CO2 releases were from communities dominated by graminoids. Another study, conducted in a US tallgrass prairie with a mixture of C4 and C3 grasses, showed that warming-induced changes in ecosystem C fluxes were more correlated with corresponding changes in C4 grass abundance, than with soil temperature or moisture (Niu and others 2013). However, the mechanisms associated with changes in PFTs and ecosystem C fluxes are largely unclear, and constitute a major source of uncertainty when attempting to extend predictions of C fluxes across a wide range of ecosystems under warming.
Studies of ecological responses to climate change from potentially vulnerable regions are valuable, as climate change impacts on these ecosystems may be severe. The ecosystem functions on the Tibetan Plateau is linked with many ecosystem services that are susceptible to climate change, such as ecosystem C pools, plant community composition and plant productivity (Schimel 1995; Liu and Chen 2000; Chen and others 2016b). Moreover, the Tibetan Plateau has experienced rapid climate warming (Thompson and others 2000), and will likely continue to warm at a faster rate than the global average (Liu and Chen 2000). Plant growth on the Tibetan Plateau is primarily limited by low temperatures, and with warming these limitations will likely decrease. Therefore, warming may have a substantial effect on the abundance and distribution of PFTs (Wang and others 2012; Zhang and others 2014). Hence, this temperature- and vegetation-sensitive region provides an ideal model system for examining the effects of warming on PFTs and ecosystem C fluxes.
Previous studies on the Tibetan Plateau have shown that 4 years of experimental warming led to rapid changes in PFTs and species composition (Klein and others 2004; Wang and others 2012). However, it remains unclear whether these changes in PFTs will lead to corresponding changes in ecosystem C fluxes, and what the underlying mechanisms might be. We conducted a warming experiment using open top chambers (OTC) in meadow grassland on the Tibetan Plateau to examine the effects of warming on PFTs and their impacts on ecosystem C fluxes. In light of previous field observations and modeling studies, we tested the following hypotheses: (1) warming would alter the abundance and biomass of PFTs due to the warming-induced changes in microclimate, especially for the plant growth on the Tibetan Plateau which have long been constrained by low temperatures, (2) warming would stimulate ecosystem C fluxes, likely due to the higher temperature sensitivity of C stored on the Tibetan Plateau, and (3) OTC-induced changes in PFTs would affect ecosystem C fluxes, possibly as a result of shifting in PFTs influencing soil nutrient availability and plant productivity.
Materials and Methods
Study Site
The field study site was located at Haibei Grassland Ecological Monitoring Station, which is maintained by China Meteorological Administration in Haibei Prefecture, Qinghai province, China (100°51′E, 36°57′N). The site has a typical plateau continental climate with a short, cool summer and a long, cold winter. The altitude of the site is 3,140 m, mean annual precipitation is 408.45 mm, and the mean annual air temperature is 1.34°C. The soil texture is a sandy loam and is classified as mountain brown according to the Chinese soil classification (cambisols in US Food and Agriculture Organization soil classification) (Chen and others 2015b). The vegetation is a typical meadow grassland dominated by Stipa sareptana var. krylovii, Koeleria cristata, Poa crymophila, Kobresia humilis, Artemisia scoparia, Heteropappus altaicus, Medicago ruthenica, and so on (Table S1). More detailed descriptions about the study site can be found by Chen and others (2015b) and Chen and others (2016b).
Experimental Design
The OTCs were used as passive warming devices. This type of warming method has been shown to be effective in a large number of experiments (Dorrepaal and others 2009; De Frenne and others 2010; Ward and others 2013; Suzuki 2014). The OTCs used in the present study have been described in detail by De Frenne and others (2010) and Väisänen and others (2014). The OTCs were made of 6-mm-thick solar transmitting materials with high light transmittance (>90%) and low infrared transmittance (<5%). They were conical in shape, had a height of 40 cm, and a basal diameter of 160 cm. The OTCs used in the current study closely followed the methods used in the International Tundra Experiment (Marion and others 1997), which have been shown to have minimal effects on photosynthetically active radiation, especially for high latitude regions (Sun and others 2013).
We fenced six replicate study blocks (55 ×180 m) in 2008 to exclude ungulate grazers; before fencing, the study area was used as a low density free-range winter grazing pasture (0.5 yak and 2.5 sheep per hectare). Within each block, we laid out two sub-blocks, one for control and the other one for warming. There were 10 m wide buffer zones along the four sides of each block. Within each block in August 2010, OTCs were randomly assigned to one of the sub-blocks, and the other was designated as the control.
Measurements of Ecosystem Carbon Fluxes
Net ecosystem exchange (NEE) and ER were measured twice each month at 3-h intervals during the growing season. To provide a flat base for the measurements, aluminum frames (0.5 m for each side) were permanently inserted 2–3 cm into the soil in both the control and warming sub-blocks. For the NEE measurements, a transparent cubic glass chamber (0.5 m for each side) was placed and sealed on the aluminum frame. Two small fans (800–1000 rotations per min) continuously mixed the air inside the chamber during the measurements. The C exchange inside the chamber was measured with an infrared gas analyzer (LI-8100, LI-COR, Inc., Lincoln, NE, USA). Consecutive recordings of CO2 concentrations were taken during a 120 s period after the steady-state conditions were achieved. After NEE measurements, the chamber was vented, and then covered with an opaque cloth. The CO2 fluxes measured when light was eliminated were operationally defined as ER (Niu and others 2013; Väisänen and others 2014). Negative NEE represents net C uptake, and positive ER represents net C release. Gross primary productivity (GPP) was calculated as the difference between ER and NEE (GPP = ER–NEE). This static-chamber method has been successfully used to evaluate ecosystem C fluxes in grassland ecosystems, and more detailed information regarding its applications may be found in Niu and others (2013), Väisänen and others (2014), Chen and others (2015b) and Chen and others (2016a).
Plant Functional Biomass
A non-destructive method (Klein and others 2007; Wang and others 2012) was adopted to estimate PFT biomass inside the aluminum frame for both the control and warming treatments. In August of each year, we selected three 0.5 × 0.5 m plots adjacent to the aluminum frame in each control sub-block. Before clipping, the coverage and height of each PFT was recorded using a 0.5 × 0.5 m quadrat with 100 squares. We then clipped the selected plots, sorted to PFT (graminoids, legumes, and forbs), and oven-dried plants at 65°C for 72 h before weighing. These data sets (54 in total) were used to construct the equations to estimate PFT biomass and aboveground biomass (AGB) from coverage and height measurements made in August of each year within the aluminum frames (Table S2). More detailed information regarding these methods can be found in Klein and others (2007) and Wang and others (2012).
Inorganic N and Net N Mineralization Measurements
Soil samples (0–10 cm) were collected from all 12 sub-blocks each month from 2011 to 2013 to measure soil total inorganic nitrogen (TIN). For net N mineralization rates, an in situ soil incubation method (Hook and Burke 1995) was conducted during the 2013 growing season. In each sub-block, two PVC tubes (5 cm in diameter and 12 cm in length) were inserted into the soil to a depth of 10 cm after the plants and litter were removed. One PVC core was sealed with a Parafilm membrane to prevent water penetration while still allowing gas exchange, and then incubated in the field for about 30 days. The other core was taken back to the laboratory and immediately stored at 4°C prior to analysis. Net N mineralization was determined by the equation:
where R min indicates the net N mineralization, A min represents the difference of total inorganic N before and after the incubation, and \( \Delta t \) stands for the length of incubation period (Hook and Burke 1995; Shan and others 2011; Shibata and others 2013; Hu and others 2015). Total inorganic N is the sum of ammonium N and nitrate N.
All fresh soil samples were processed and analyzed within 2 weeks after field sampling. To measure total inorganic N, each sample was first sieved through 2 mm mesh, and then one 10 g sub-sample was extracted with 50 ml of 2 mol L−1 KCl. Concentrations of ammonium and nitrate were determined using a flow injection auto analyzer (FIAstar 5000 Analyzer, Foss Tecator, Denmark). The extractable soil ammonium and nitrate contents were converted to a dry soil mass basis [mg (kg dry soil)−1]. Another 10 g of fresh soil sample was weighed, oven-dried at 105°C for 48 h, and then re-weighed to measure soil moisture.
Soil Temperature and Moisture
Soil temperature was measured using a thermocouple probe. Soil moisture content was measured using gypsum cast around two concentric stainless-steel electrodes, both at a soil depth of 10 cm. 5-min average soil temperature and soil moisture values were recorded by HOBO data loggers (Onset Computer Company, Pocasset, MA, USA) for the duration of the experiment.
Data Analysis
Measurements were averaged over replicate sub-blocks, and monthly mean values were calculated from daily mean values. Two-way repeated measures ANOVAs were used to examine warming, year, and their interactive effects on soil temperature, soil moisture, NEE, ER, GPP, TIN, AGB and biomass for each PFT. Significant differences were evaluated at α = 0.05. Pearson linear regression was adopted to evaluate OTC-induced changes in both biotic and abiotic factors and OTC-induced changes in ecosystem C fluxes. All statistical comparisons were performed in SPSS 13.0 for Microsoft Windows™.
Results
Microclimate
Higher precipitation occurred during the peak of the growing season than during the edges of growing season across all years (Figure S1a). In individual years, growing season precipitation was 427, 447 and 431 mm, and annual precipitation was 447, 471 and 454 mm in 2011, 2012 and 2013, respectively (Figure S1a). Mean growing season air temperature was 8.79, 9.04 and 9.67°C, and mean annual soil temperature was 1.47, 1.42 and 2.02°C in 2011, 2012, and 2013, respectively (Figure S1b).
The OTCs significantly increased soil temperature by an average of 1.03°C during the growing season from 2011 to 2013 (Figure 1). By year, soil temperature during the growing season was increased by 0.99°C in 2011, 1.06°C in 2012 and 1.03°C in 2013. We found significant year effects on soil temperature, but no interactive effects of warming and year (Table 1). The OTCs decreased average soil moisture by 1.1% across the 3 years, with decreases of 1.2, 0.7 and 1.2% in 2011, 2012 and 2013, respectively (Figure 1). We found significant year effects for soil moisture but no significant interactions between warming and year.
Plant Functional Types
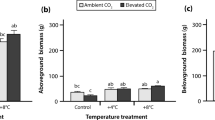
Relative to the control plots, experimental warming significantly increased graminoid, legume, and aboveground biomass by 14.6, 27.6 and 17.4% over the 3 years, but had no effect on forb biomass (Table 1). Increases in AGB were 12.2, 20.3 and 19.1% in 2011, 2012 and 2013, respectively (Figure 2). Warming significantly enhanced graminoid biomass by 10.1, 17.0 and 16.2%, and legume biomass by 20.5, 30.6 and 29.6% in 2011, 2012 and 2013, respectively. Significant year effects were found for AGB, graminoid and legume biomass. No significant effects of warming, year or their interaction were found for forb biomass (Table 1).
Soil Inorganic Nitrogen
Warming significantly enhanced soil TIN by 9.9% across the 3 years (Figure 3a). Specifically, there was an increase in TIN by 11.9% in 2012, and 11.8% in 2013, while warming had no effect on soil TIN in 2011. Soil TIN varied across years, but no significant interaction between warming and year was found (Table 1). Although warming had no effect on soil net N mineralization (Figure 3b), we found a significant positive relationship between OTC-induced increases in legume biomass and soil TIN (Figure 3c).
Annual average values of soil total inorganic nitrogen (TIN, A) from 2011 to 2013. Monthly variations of net nitrogen mineralization in 2013 (B), and relationship between OTC-induced changes in soil TIN and legume biomass (C). Values are mean ± standard errors for six replicates. Asterisk indicates significant difference at p < 0.05.
Ecosystem C Fluxes
Warming resulted in significantly more negative NEE (31.0%) over the 3-year study (Table 1). By year, warming significantly promoted NEE by 20.9, 32.0 and 40.0% in 2011, 2012 and 2013, respectively (Figures 4 and S2). There was no significant year or interactive effects of warming and year on NEE. For ER, there was an effect of year, but there was no effect of warming and interactive effect of warming and year (Table 2).
Seasonal variations of gross primary productivity (GPP), ecosystem respiration (ER) and net ecosystem exchange (NEE) both in control and warming sub-blocks. Values are mean ± standard errors for six replicates. Negative values of NEE represent net C uptake from the ecosystem. Insets indicate the means of C fluxes across the seasons for control (CK) and warming (W) plots, Asterisk indicates significant warming effect at p < 0.05.
Warming significantly enhanced GPP by an average of 18.7% (more negative GPP) for the 3 years of the study (Table 1). There were significant year effects for GPP but no significant interaction between warming and year (Table 1). When the differences for each year were tested separately by comparisons with the controls, the OTC-induced increases in GPP were 13.1% for 2011, 23.4% for 2012 and 18.9% for 2013 (Figures 4a and S2; Table 2).
Factors Affecting Ecosystem C Fluxes
Higher soil temperature and soil moisture were accompanied with relative larger GPP, NEE and ER (more negative values for GPP and NEE) during individual sampling periods (Figure S3), but this did not translate to significant correlations between OTC-induced changes in soil temperature, moisture and the corresponding changes in NEE, ER, GPP (Figure S4). In annual scale, OTC-induced increases in soil temperature were also not correlated with the corresponding changes in ecosystem C fluxes. Despite OTC-induced reductions in annual average soil moisture suppressed ER and GPP, we found no correlation between changes in NEE and corresponding changes in soil moisture (Figure S4).
The OTC-induced changes in PFTs were closely correlated with the corresponding changes in ecosystem C fluxes (Figure. 5). In detail, OTC-induced increases in graminoid and legume biomass explained 36.1 and 55.6% of the variation in NEE, and 39.7 and 29.4% of the variation in GPP, respectively. We did not detect significant relationships between OTC-induced changes in forb biomass and corresponding changes in NEE and GPP. In addition, no significant relationship was found between OTC-induced PFT changes and ER (Figure 5b).
Relationships between OTC-induced changes in the proportion of graminoid, legume, forb biomass and warming-induced changes in gross primary productivity (GPP, A), ecosystem respiration (ER, B), net ecosystem exchange (NEE, C). Negative values of ΔNEE indicate that warming enhanced C uptake, negative values of ΔGPP indicate that warming suppressed C uptake, and negative values of ΔER indicate that warming decreased C respiration.
Discussion
Ecosystem C Fluxes
Experimental warming significantly promoted GPP, but had no effect on ER, resulting in enhanced NEE (Table 1). The OTC-induced changes in ecosystem C fluxes were not fully explained by the corresponding changes in soil temperature and moisture (Figure S4), but they were closely correlated with the corresponding changes in PFTs (Figure 5). Increases in graminoid and legume biomass enhanced GPP, but not ER, resulting in more negative NEE. Our results suggest that warming effects on ecosystem C fluxes were modulated by PFTs.
The lack of ER response to warming resulted from the differential responses of its components, but with different underlying mechanisms. Warming significantly enhanced autotrophic respiration but repressed heterotrophic respiration, resulting in a non-significant response of ER. Increased autotrophic respiration was closely associated with OTC-induced increases in AGB, while decreased heterotrophic respiration was mainly related to the OTC-induced reductions in microbial biomass C (Chen and others 2016b).
Although seasonal patterns of C fluxes were related to seasonal patterns of soil temperature and moisture (Figures 1 and 4) (Lloyd and Taylor 1994; Davidson and others 1998), changes in C fluxes due to experimental warming were not well explained by average changes in soil moisture and temperature caused by OTC. Further analysis revealed that warming had more pronounced effects on NEE and GPP during the start and peak of the growing season, while warming significantly stimulated ER during the end of growing season (Table S3). These asymmetrical responses were not accompanied by OTC-induced changes in soil temperature and moisture (Figure S5). Alternatively, it should be noted that there might be certain periods that are highly sensitive to even small changes in soil temperature and moisture as reported by our previous studies (Chen and others 2015b; Chen and others 2016c), yet the underlying mechanisms are still unclear. Recent evidence reported that seasonal responses of ecosystem C fluxes to experimental warming might also be regulated by many other indirect processes, such as microbial activity (Čapek and others 2015; Creamer and others 2015; Zhou and others 2015), allocation of non-structural C (Cardon and Gage 2006; Zhang and others 2011) and phenology (Richardson and others 2010; Lempereur and others 2015), which deserve further examination.
Our results suggest that indirect processes, induced by OTC, may play more important roles in affecting ecosystem C fluxes than the direct changes in soil temperature and moisture (Wan and others 2005; Luo and others 2011; Wood and others 2012; Giardina and others 2014). Shifts in PFTs may contribute to changes in ecosystem C fluxes under climate change (Figure 5) (Niu and others 2013). One possible mechanism discussed in Niu and others (2013) is that C3 species may suffer from high temperature stress due to having lower optimal temperatures than C4 species. Therefore, warming-induced changes in ecosystem C fluxes may be closely associated with the variations in C4 species biomass. However, different mechanisms are likely responsible in our study, due to the overall lower temperatures and the lower warming magnitudes we imposed than those reported in Niu and others (2013) (Figures 1 and S5).
Plant Functional Types
Experimental warming significantly increased the abundance and biomass of graminoids and legumes, but had no effect on forbs (Figures. 2 and Table S1). This corresponded with results from meta-analyses concerning the effects of warming on PFTs in cooler regions (Arft and others 1999; Walker and others 2006). Plants in cool climates are generally adapted to and constrained by low temperatures (Xu and others 2008). Therefore, a rapid shift in PFTs may occur in these regions due to alterations in OTC-induced resource use efficiency and enhanced substrate availability (Smith and others 2009; Kardol and others 2010), concurring with field observations near our study site showing 4 years of warming led to rapid increases in graminoid and legume biomass (Klein and others 2004; Wang and others 2012). Forb biomass was unaffected by warming (Figure 2). This could be partly due to shallower rooting distribution of forbs on the Tibetan Plateau (Klein and others 2007; Dorji and others 2013), making them more sensitive to reductions in soil moisture (Wang and others 2012) and offsetting any benefit via warming.
Plant Functional Types and AGB
Warming significantly increased AGB, which was primarily driven by OTC-induced biomass accumulation of graminoids and legumes (Figure. 2 and S6). These findings coincide with recent meta-analyses (Lin and others 2010; Way and Oren 2010) and other warming studies on the Tibetan Plateau (Wang and others 2012; Ganjurjav and others 2015). Increased soil TIN is one possible mechanism for greater AGB under experimental warming (Figure S6). Even small changes in N inputs can have substantial impacts on ecosystem plant productivity and flux (Chen and others 2016a), especially on the N-limited Tibetan Plateau (Jiang and others 2010; Fang and others 2012; Field and others 2014). Although increased N mineralization under warming, shown in both observational studies and warming experiments (Bai and others 2013), may result in higher soil TIN, we did not find evidence for this mechanism (Figure 3b). Instead, we found increases in soil TIN might have resulted from an increase in the proportion of legume biomass occurring along with our warming treatment (Figure 3c) (Fornara and Tilman 2008), coinciding with previous findings from a North American prairie (Whittington and others 2013). Due to PFT-AGB relationships, our results also suggest that OTC-induced changes in ecosystem C fluxes may result from corresponding changes in PFTs.
Regulation of Ecosystem C Fluxes by PFTs
The OTC-induced increases in the proportion of graminoid and legume biomass and AGB were linearly correlated with increases in GPP (Figures 5 and S7). One possible explanation might be that increases in legume biomass enhanced soil and leaf N concentrations. These increases led to positive responses of ecosystem photosynthesis and primary production (Figure S6) (Lee and others 2003; Menge and others 2015; Shi and others 2015). Another explanation may be related to the functional traits of legumes and graminoids. Legumes in our study site have relatively larger leaf areas, and graminoids are relatively taller compared to other species (Wang and others 2012). Larger leaf area and increased height are advantageous to plant species when competing for light, potentially leading to higher potentials for GPP and biomass accrual.
The OTC-induced changes in PFTs and AGB did not correspond with changes in ER (Figures 5 and S7), so we suggest PFT effects on GPP may be different from those of ER in this system, resulting in net C uptake under a warming climate (Figures 5 and S2). At large spatial scales, these impacts have implications concerning offsets of the current projected growth rate of atmospheric CO2.
Understanding the relationship between PFTs and ecosystem C fluxes is a critical area of research in ecological sciences (Atkin and others 2015; Dieleman and others 2015; Verheijen and others 2015), and this is especially important in the context of reports of warming-induced shifts in PFTs globally (Arft and others 1999; Klein and others 2004; Walker and others 2006; Xu and others 2015). Our findings are especially relevant to many grassland systems experiencing similar PFTs dynamics (Wang and others 2012; Yang and others 2014; Ganjurjav and others 2016). Overall, we have shown evidence that C-climate feedbacks can be closely modulated by concurrent shifts in PFTs. This highlights the need to incorporate OTC-induced changes in PFTs into ESMs when projecting responses of ecosystem C fluxes under future changes in climate.
Conclusions
The Tibetan Plateau is a temperature- and vegetation-sensitive region likely to be particularly sensitive to future global climate changes. We found that experimental warming significantly increased graminoid and legume abundance, AGB, and promoted GPP and NEE. Annual changes in soil temperature and soil moisture did not fully account for the corresponding changes in GPP, ER and NEE. Increased biomass of graminoids and legumes was closely associated with enhanced GPP, but had no impacts on ER, resulting in an enhancement in NEE. From this, we suggest that changes in community structure may buffer C loss in this system under a warmer climate.
References
Arft AM, Walker MD, Gurevitch J, Alatalo JM, Bret-Harte MS, Dale M, Diemer M, Gugerli F, Henry GHR, Jones MH, Hollister RD, Jónsdóttir IS, Laine K, Lévesque E, Marion GM, Molau U, Mølgaard P, Nordenhäll U, Raszhivin V, Robinson CH, Starr G, Stenström A, Stenström M, Totland Ø, Turner PL, Walker LJ, Webber PJ, Welker JM, Wookey PA. 1999. Responses of tundra plants to experimental warming: meta-analysis of the international tundra experiment. Ecol Monogr 69:491–511.
Atkin OK, Bloomfield KJ, Reich PB, Tjoelker MG, Asner GP, Bonal D, Bönisch G, Bradford MG, Cernusak LA, Cosio EG. 2015. Global variability in leaf respiration in relation to climate, plant functional types and leaf traits. New Phytol 206:614–36.
Bai E, Li S, Xu W, Li W, Dai W, Jiang P. 2013. A meta-analysis of experimental warming effects on terrestrial nitrogen pools and dynamics. New Phytol 199:441–51.
Ballantyne A, Alden C, Miller J, Tans P, White J. 2012. Increase in observed net carbon dioxide uptake by land and oceans during the past 50 years. Nature 488:70–2.
Brient F, Bony S. 2013. Interpretation of the positive low-cloud feedback predicted by a climate model under global warming. Clim Dyn 40:2415–31.
Čapek P, Diáková K, Dickopp J-E, Bárta J, Wild B, Schnecker J, Alves RJE, Aiglsdorfer S, Guggenberger G, Gentsch N. 2015. The effect of warming on the vulnerability of subducted organic carbon in arctic soils. Soil Biol Biochem 90:19–29.
Cardon ZG, Gage DJ. 2006. Resource exchange in the rhizosphere: molecular tools and the microbial perspective. Annu Rev Ecol Evol Syst 37:459–88.
Chen J, Luo Y, Li J, Zhou X, Cao J, Wang RW, Wang Y, Shelton S, Jin Z, Walker LM. 2016a. Costimulation of soil glycosidase activity and soil respiration by nitrogen addition. Glob Chang Biol. doi:10.1111/gcb.13402.
Chen J, Luo Y, Xia J, Jiang L, Zhou X, Lu M, Liang J, Shi Z, Shelton S, Cao J. 2015a. Stronger warming effects on microbial abundances in colder regions. Sci Rep. doi:10.1038/srep18032.
Chen J, Luo Y, Xia J, Shi Z, Jiang L, Niu S, Zhou X, Cao J. 2016b. Differential responses of ecosystem respiration components to experimental warming in a meadow grassland on the Tibetan Plateau. Agric For Meteorol 220:21–9.
Chen J, Shi W, Cao J. 2015b. Effects of grazing on ecosystem CO2 exchange in a meadow grassland on the tibetan plateau during the growing season. Environ Manag 55:347–59.
Chen J, Zhou X, Wang J, Hruska T, Shi W, Cao J, Zhang B, Xu G, Chen Y, Luo Y. 2016c. Grazing exclusion reduced soil respiration but increased its temperature sensitivity in a Meadow Grassland on the Tibetan Plateau. Ecol Evol 6:675–87.
Creamer CA, de Menezes AB, Krull ES, Sanderman J, Newton-Walters R, Farrell M. 2015. Microbial community structure mediates response of soil C decomposition to litter addition and warming. Soil Biol Biochem 80:175–88.
Davidson E, Belk E, Boone RD. 1998. Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Glob Chang Biol 4:217–27.
Davidson EA, Janssens IA. 2006. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440:165–73.
De Frenne P, De Schrijver A, Graae BJ, Gruwez R, Tack W, Vandelook F, Hermy M, Verheyen K. 2010. The use of open-top chambers in forests for evaluating warming effects on herbaceous understorey plants. Ecol Res 25:163–71.
Dieleman CM, Branfireun BA, McLaughlin JW, Lindo Z. 2015. Climate change drives a shift in peatland ecosystem plant community: implications for ecosystem function and stability. Glob Chang Biol 21:388–95.
Dorji T, Totland Ø, Moe SR, Hopping KA, Pan J, Klein JA. 2013. Plant functional traits mediate reproductive phenology and success in response to experimental warming and snow addition in Tibet. Glob Chang Biol 19:459–72.
Dorrepaal E, Toet S, van Logtestijn RS, Swart E, van de Weg MJ, Callaghan TV, Aerts R. 2009. Carbon respiration from subsurface peat accelerated by climate warming in the subarctic. Nature 460:616–19.
Fang H, Cheng S, Yu G, Zheng J, Zhang P, Xu M, Li Y, Yang X. 2012. Responses of CO2 efflux from an alpine meadow soil on the Qinghai Tibetan Plateau to multi-form and low-level N addition. Plant Soil 351:177–90.
Field C, Dise N, Payne R, Britton A, Emmett B, Helliwell R, Hughes S, Jones L, Lees S, Leake J, Leith I, Phoenix G, Power S, Sheppard L, Southon G, Stevens C, Caporn SM. 2014. The role of nitrogen deposition in widespread plant community change across semi-natural habitats. Ecosystems 17:864–77.
Fornara D, Tilman D. 2008. Plant functional composition influences rates of soil carbon and nitrogen accumulation. J Ecol 96:314–22.
Friedlingstein P, Meinshausen M, Arora VK, Jones CD, Anav A, Liddicoat SK, Knutti R. 2014. Uncertainties in CMIP5 climate projections due to carbon cycle feedbacks. J Clim 27:511–26.
Ganjurjav H, Gao Q, Gornish ES, Schwartz MW, Liang Y, Cao X, Zhang W, Zhang Y, Li W, Wan Y. 2016. Differential response of alpine steppe and alpine meadow to climate warming in the central Qinghai-Tibetan Plateau. Agric For Meteorol 223:233–40.
Ganjurjav H, Gao Q, Zhang W, Liang Y, Li Y, Cao X, Wan Y, Li Y, Danjiu L. 2015. Effects of warming on Co2 fluxes in an alpine Meadow ecosystem on the Central Qinghai-Tibetan Plateau. Plos One 10:e0132044.
Giardina CP, Litton CM, Crow SE, Asner GP. 2014. Warming-related increases in soil CO2 efflux are explained by increased below-ground carbon flux. Nat Clim Chang 4:822–7.
Heimann M, Reichstein M. 2008. Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 451:289–92.
Hook P, Burke I. 1995. Evaluation of methods for estimating net nitrogen mineralization in a semiarid grassland. Soil Sci Soc Am J 59:831–7.
Hu R, X-p Wang, Y-x Pan, Y-f Zhang, Zhang H, Chen N. 2015. Seasonal variation of net N mineralization under different biological soil crusts in Tengger Desert, North China. Catena 127:9–16.
Jiang C, Yu G, Fang H, Cao G, Li Y. 2010. Short-term effect of increasing nitrogen deposition on CO 2, CH 4 and N 2 O fluxes in an alpine meadow on the Qinghai-Tibetan Plateau, China. Atmos Environ 44:2920–6.
Kardol P, Campany CE, Souza L, Norby RJ, Weltzin JF, Classen AT. 2010. Climate change effects on plant biomass alter dominance patterns and community evenness in an experimental old-field ecosystem. Glob Chang Biol 16:2676–87.
Keenan TF, Gray J, Friedl MA, Toomey M, Bohrer G, Hollinger DY, Munger JW, O’Keefe J, Schmid HP, Wing IS. 2014. Net carbon uptake has increased through warming-induced changes in temperate forest phenology. Nat Clim Chang 4:598–604.
Klein JA, Harte J, Zhao X-Q. 2004. Experimental warming causes large and rapid species loss, dampened by simulated grazing, on the Tibetan Plateau. Ecol Lett 7:1170–9.
Klein JA, Harte J, Zhao X-Q. 2007. Experimental warming, not grazing, decreases rangeland quality on the Tibetan Plateau. Ecol Appl 17:541–57.
Lee TD, Reich PB, Tjoelker MG. 2003. Legume presence increases photosynthesis and N concentrations of co-occurring non-fixers but does not modulate their responsiveness to carbon dioxide enrichment. Oecologia 137:22–31.
Lempereur M, Martin-StPaul NK, Damesin C, Joffre R, Ourcival JM, Rocheteau A, Rambal S. 2015. Growth duration is a better predictor of stem increment than carbon supply in a Mediterranean oak forest: implications for assessing forest productivity under climate change. New Phytol 207:579–90.
Lin D, Xia J, Wan S. 2010. Climate warming and biomass accumulation of terrestrial plants: a meta-analysis. New Phytol 188:187–98.
Liu X, Chen B. 2000. Climatic warming in the Tibetan Plateau during recent decades. Int J Climatol 20:1729–42.
Lloyd J, Taylor J. 1994. On the temperature dependence of soil respiration. Funct Ecol 8:315–23.
Luo Y, Melillo J, Niu S, Beier C, Clark JS, Classen AT, Davidson E, Dukes JS, Evans R, Field CB. 2011. Coordinated approaches to quantify long-term ecosystem dynamics in response to global change. Glob Chang Biol 17:843–54.
Marion G, Henry G, Freckman D, Johnstone J, Jones G, Jones M, Levesque E, Molau U, Mølgaard P, Parsons A. 1997. Open-top designs for manipulating field temperature in high-latitude ecosystems. Glob Chang Biol 3:20–32.
Menge DN, Wolf AA, Funk JL. 2015. Diversity of nitrogen fixation strategies in Mediterranean legumes. Nat Plant 1:15064. doi:10.1038/nplants.2015.64.
Niu S, Sherry RA, Zhou X, Luo Y. 2013. Ecosystem carbon fluxes in response to warming and clipping in a tallgrass prairie. Ecosystems 16:948–61.
Ponce-Campos GE, Moran MS, Huete A, Zhang Y, Bresloff C, Huxman TE, Eamus D, Bosch DD, Buda AR, Gunter SA. 2013. Ecosystem resilience despite large-scale altered hydroclimatic conditions. Nature 494:349–52.
Poyatos R, Heinemeyer A, Ineson P, Evans JG, Ward HC, Huntley B, Baxter R. 2014. Environmental and vegetation drivers of seasonal CO2 fluxes in a Sub-arctic Forest-Mire Ecotone. Ecosystems 17:377–93.
Richardson AD, Black TA, Ciais P, Delbart N, Friedl MA, Gobron N, Hollinger DY, Kutsch WL, Longdoz B, Luyssaert S. 2010. Influence of spring and autumn phenological transitions on forest ecosystem productivity. Philoso Trans R Soc Lond B Biol Sci 365:3227–46.
Schimel DS. 1995. Terrestrial ecosystems and the carbon-cycle. Glob Chang Biol 1:77–91.
Shan Y, Chen D, Guan X, Zheng S, Chen H, Wang M, Bai Y. 2011. Seasonally dependent impacts of grazing on soil nitrogen mineralization and linkages to ecosystem functioning in Inner Mongolia grassland. Soil Biol Biochem 43:1943–54.
Shi C, Silva LC, Zhang H, Zheng Q, Xiao B, Wu N, Sun G. 2015. Climate warming alters nitrogen dynamics and total non-structural carbohydrate accumulations of perennial herbs of distinctive functional groups during the plant senescence in autumn in an alpine meadow of the Tibetan Plateau, China. Agric For Meteorol 200:21–9.
Shibata H, Hasegawa Y, Watanabe T, Fukuzawa K. 2013. Impact of snowpack decrease on net nitrogen mineralization and nitrification in forest soil of northern Japan. Biogeochemistry 116:69–82.
Smith MD, Knapp AK, Collins SL. 2009. A framework for assessing ecosystem dynamics in response to chronic resource alterations induced by global change. Ecology 90:3279–89.
Stocker TF. 2014. Climate change 2013: the physical science basis: working Group I contribution to the Fifth assessment report of the Intergovernmental Panel on Climate Change: Cambridge University Press.
Su H, Feng J, Axmacher JC, Sang W. 2015. Asymmetric warming significantly affects net primary production, but not ecosystem carbon balances of forest and grassland ecosystems in northern China. Sci Rep 5:9115. doi:10.1038/srep09115.
Sun S, Peng L, Wang G, Wu Y, Zhou J, Bing H, Yu D, Luo J. 2013. An improved open-top chamber warming system for global change research. Silva Fenn 47:960. doi:10.14214/sf.960.
Suzuki RO. 2014. Combined effects of warming, snowmelt timing, and soil disturbance on vegetative development in a grassland community. Plant Ecol 215:1399–408.
Thompson LG, Yao T, Mosley-Thompson E, Davis M, Henderson K, Lin P-N. 2000. A high-resolution millennial record of the South Asian monsoon from Himalayan ice cores. Science 289:1916–19.
Väisänen M, Ylänne H, Kaarlejärvi E, Sjögersten S, Olofsson J, Crout N, Stark S. 2014. Consequences of warming on tundra carbon balance determined by reindeer grazing history. Nat Clim Chang 4:384–8.
Verheijen LM, Aerts R, Brovkin V, Cavender-Bares J, Cornelissen JH, Kattge J, Bodegom PM. 2015. Inclusion of ecologically based trait variation in plant functional types reduces the projected land carbon sink in an earth system model. Glob Chang Biol 21:3074–86.
Walker MD, Wahren CH, Hollister RD, Henry GH, Ahlquist LE, Alatalo JM, Bret-Harte MS, Calef MP, Callaghan TV, Carroll AB. 2006. Plant community responses to experimental warming across the tundra biome. Proc Natl Acad Sci U S A 103:1342–6.
Wan S, Hui D, Wallace L, Luo Y. 2005. Direct and indirect effects of experimental warming on ecosystem carbon processes in a tallgrass prairie. Glob Biogeochem Cycles. doi:10.1029/2004GB002315.
Wang S, Duan J, Xu G, Wang Y, Zhang Z, Rui Y, Luo C, Xu B, Zhu X, Chang X. 2012. Effects of warming and grazing on soil N availability, species composition, and ANPP in an alpine meadow. Ecology 93:2365–76.
Ward SE, Ostle NJ, Oakley S, Quirk H, Henrys PA, Bardgett RD. 2013. Warming effects on greenhouse gas fluxes in peatlands are modulated by vegetation composition. Ecol Lett 16:1285–93.
Way DA, Oren R. 2010. Differential responses to changes in growth temperature between trees from different functional groups and biomes: a review and synthesis of data. Tree Physiol 30:669–88.
Whittington HR, Tilman D, Powers JS. 2013. Consequences of elevated temperatures on legume biomass and nitrogen cycling in a field warming and biodiversity experiment in a North American prairie. Funct Plant Biol 40:1147–58.
Wilcox KR, Fischer JC, Muscha JM, Petersen MK, Knapp AK. 2015. Contrasting above-and belowground sensitivity of three Great Plains grasslands to altered rainfall regimes. Glob Chang Biol 21:335–44.
Wood TE, Cavaleri MA, Reed SC. 2012. Tropical forest carbon balance in a warmer world: a critical review spanning microbial-to ecosystem-scale processes. Biol Rev 87:912–27.
Xu X, Shi Z, Li D, Zhou X, Sherry RA, Luo Y. 2015. Plant community structure regulates responses of prairie soil respiration to decadal experimental warming. Glob Chang Biol 21:3846–53.
Xu Z, Gong T, Li J. 2008. Decadal trend of climate in the Tibetan Plateau—regional temperature and precipitation. Hydrol Process 22:3056–65.
Yang Y, Wang G, Klanderud K, Wang J, Liu G. 2014. Plant community responses to five years of simulated climate warming in an alpine fen of the Qinghai-Tibetan Plateau. Plant Ecol Divers 8:211–18.
Zhang N, Xia J, Yu X, Ma K, Wan S. 2011. Soil microbial community changes and their linkages with ecosystem carbon exchange under asymmetrically diurnal warming. Soil Biol Biochem 43:2053–9.
Zhang SY, Li XY, Ma YJ, Zhao GQ, Li L, Chen J, Jiang ZY, Huang YM. 2014. Interannual and seasonal variability in evapotranspiration and energy partitioning over the alpine riparian shrub Myricaria squamosa Desv. on Qinghai-Tibet Plateau. Cold Reg Sci Technol 102:8–20.
Zhou L, Yin S, An S, Yang W, Deng Q, Xie D, Ji H, Ouyang Y, Cheng X. 2015. Spartina alterniflora invasion alters carbon exchange and soil organic carbon in eastern salt marsh of China. Clean Soil Air Water 43:569–76.
Acknowledgments
This study was supported by the Ministry of Science & Technology (2012BAH31B03), and the State Key Laboratory of Loess and Quaternary Geology, Institute of Earth Environment of Chinese Academy of Sciences (SKLLQG1303). Contributions from Dr. Luo’s Eco-lab to this study was financially supported by US Department of Energy, Terrestrial Ecosystem Sciences Grant DE SC0008270 and US National Science Foundation (NSF) Grant DBI 0850290, EPS0919466, DEB 0743778, DEB 0840964, and EF 1137293. The authors gratefully acknowledge financial support from China Scholarship Council (award for a 1-year study abroad at the University of Oklahoma).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Author contributions
Ji Chen, Junji Cao, Xuhui Zhou, and Rujin Huang conceived and designed the study. Ji Chen, Feng Wu, Tafeng Hu, Junyi Liang, and Zheng Shi performed research. Jianyang Xia, Lifen Jiang, Shuli Niu, Kevin R. Wilcox, and Jianfen Guo analyzed the data. Jianyang Xia, Shuli Niu, and Rui-Wu Wang contributed new methods or models.
Ji Chen, Kevin R. Wilcox, and Katerina Y. Estera wrote the paper.
Our original data set can be found at https://www.researchgate.net/publication/305437349_Warming_effects_on_ecosystem_carbon_fluxes_are_modulated_by_plant_functional_types.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, J., Luo, Y., Xia, J. et al. Warming Effects on Ecosystem Carbon Fluxes Are Modulated by Plant Functional Types. Ecosystems 20, 515–526 (2017). https://doi.org/10.1007/s10021-016-0035-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-016-0035-6