Abstract
Aims
To assess the effects of atmospheric N deposition on the C budget of an alpine meadow ecosystem on the Qinghai–Tibetan Plateau, it is necessary to explore the responses of soil-atmosphere carbon dioxide (CO2) exchange to N addition.
Methods
Based on a multi-form, low-level N addition experiment, soil CO2 effluxes were monitored weekly using the static chamber and gas chromatograph technique. Soil variables and aboveground biomass were measured monthly to examine the key driving factors of soil CO2 efflux.
Results
The results showed that low-level N input tended to decrease soil moisture, whereas medium-level N input maintained soil moisture. Three-year N additions slightly increased soil inorganic N pools, especially the soil NH +4 -N pool. N applications significantly increased aboveground biomass and soil CO2 efflux; moreover, this effect was more significant from NH +4 -N than from NO −3 -N fertilizer. In addition, the soil CO2 efflux was mainly driven by soil temperature, followed by aboveground biomass and NH +4 -N pool.
Conclusions
These results suggest that chronic atmospheric N deposition will stimulate soil CO2 efflux in the alpine meadow on the Qinghai–Tibetan Plateau by increasing available N content and promoting plant growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human activities in the past few decades, including fossil fuel combustion, fertilizer production and cultivation, have led to substantial increases in atmospheric nitrogen (N) deposition that may significantly alter the carbon (C) and N cycles of terrestrial ecosystems (Galloway et al. 2008). Anthropogenic reactive N emissions have increased from 15 Tg yr−1 before 1860 to 187 Tg yr−1 in 2005, and about 60% of the reactive N re-enters into the terrestrial and aquatic ecosystems by wet and dry deposition (Galloway et al. 2004, 2008). Over the same period, the global N deposition has increased from 34 to 105 Tg yr−1 and is expected to double over the next 25 years (Neff et al. 2002; Galloway et al. 2008). In China, the mean of dry and wet N deposition is estimated at 12.9 kg N ha−1 yr−1, this is significantly higher than the global average (Lü and Tian 2007). The increase in atmospheric N deposition can alter rates of C and N cycles of terrestrial ecosystems, and thus affect the structure and function of terrestrial ecosystems. Up to now, the effects of atmospheric N deposition on C sequestration in terrestrial ecosystems are highly variable, including increased C sequestration (Magnani et al. 2007), increased C source (Bragazza et al. 2006) and no changes (de Vries et al. 2008). The diverse experimental results indicate that more observations are required to understand the regulatory mechanism of N to key C processes in terrestrial ecosystems.
Soil CO2 efflux is an important process of C cycling in terrestrial ecosystems, vulnerable to climate warming (Luo et al. 2001), changing precipitation (Huxman et al. 2004), increased CO2 concentrations (Gill et al. 2002), and N deposition (Mo et al. 2008). Since the soil C pool is huge, a small change (0.1%) of this pool will lead to an increase of 1 mg m−3 in atmospheric CO2 concentration, which is about 1.5% of the ambient annual soil CO2 efflux (98 ± 12 Pg C) (Eswaran et al. 1993; Bond-Lamberty and Thomson 2010). Similarly, the responses of soil CO2 effluxes to N deposition vary, including promotion (Magill et al. 1997), inhibition (Mo et al. 2008) and no change (Micks et al. 2004). This inconsistency is related to the following aspects: (1) soil CO2 efflux is composed of root autotrophic respiration, rhizosphere heterotrophic respiration and microbial decomposition of soil organic matter, and they may respond differently to N additions (Mo et al. 2007, 2008) and (2) soil CO2 efflux is affected by soil temperature, soil moisture and available nutrients (Dalal and Allen 2008). For example, N additions can decrease soil moisture through promoting plant transpiration and soil evaporation (Inouye 2006); meanwhile, it can also increase soil moisture because of increased vegetation shade (Ma 2009). Soil moisture changes will further affect soil CO2 efflux. In addition, the relative contribution of NO −3 to soil CO2 efflux differs from NH +4 (Fang et al. 2010). To the best of our knowledge, few studies have been conducted to examine the effects of forms and levels of added N on soil CO2 efflux, especially in monitored field experiments.
Up to now, N addition experiments in China have mainly been conducted in forests (Mo et al. 2008), wetlands (Song et al. 2006), and temperate grassland ecosystems (Niu et al. 2010). In contrast, alpine grassland ecosystems have not been investigated as much due to low-level N deposition in these regions. The Qinghai–Tibetan Plateau grassland has a vast area of 51.7 × 104 km2 and a high soil organic C density (9.05 kg m−2) (Yang et al. 2008). Soil C storage at 1 m depth is estimated at 4.68 Pg C, accounting for about 1/20 of total soil C storage in China (Yang et al. 2008). Due to regional economic development and long-range transport of atmospheric reactive N, atmospheric N deposition is very obvious in the eastern Qinghai–Tibetan Plateau, ranging from 8.7 to 13.8 kg N ha−1 yr−1 (Lü and Tian 2007). In contrast to high N deposition, low N input can also significantly change the interaction between plant and soil microbes, and further increase soil N availability and C sequestration in the N-limited alpine meadow ecosystem (Xu et al. 2004). To clarify the mechanism of C turnover in the alpine meadow, it is necessary to deeply explore the responses of soil CO2 efflux to N addition.
This study was designed to: (1) examine the effects of the forms and levels of N addition on the main factors driving soil CO2 efflux, including soil variables (temperature, moisture, inorganic N) and aboveground biomass; (2) clarify the effects of the forms and levels of added N on soil CO2 efflux in the alpine meadow; (3) evaluate the relationships between soil CO2 effluxes and soil variables and aboveground biomass under different levels and forms of N addition.
Materials and methods
Study site
This study was conducted at the Haibei alpine meadow ecosystem research station, Chinese Academy of Sciences (37°37 'N, 101°19' E). The terrain is flat and open with an elevation of 3220 m. The region is characterized by a typical plateau continental climate, with long, cold winters and cool, rainy summers. Mean monthly temperature varies from −14.8°C in January to 9.8°C in July, with a mean annual temperature of −1.7°C. Mean annual precipitation is 580 mm, of which more than 80% appears in May to September (Zhou 2001). The vegetation type is a typical Kobresia humilis meadow. Dominant species are Kobresia humilis, Saussurea superba, Potentilla saundersiana, Leontopodium nanum, Lancea tibetica, Festuca ovina, Festuca rubra, Stipa aliena, Elymus nutans, Helictotrichon tibetica, Koeleria cristata and Poa crymophila. The study area is subject to light grazing with about three sheep ha−1; the vegetation coverage ranges between 90 and 95% (Cao et al. 2004). The soils developed in the Kobresia meadow are Mat–Gryic Cambisol, corresponding to Gelic Cambisol (Zhou 2001).
Experimental design
To simulate deposition of major ions such as NH +4 , NO −3 , Cl−, SO 2−4 , and K+, three N fertilizers, including NH4Cl, (NH4)2SO4 and KNO3, were used in this study. According to the local level of atmospheric N deposition at the Haibei station (8.7–13.8 kg N ha−1 yr−1), three levels referred to as low N (10 kg N ha−1 yr−1), medium N (20 kg N ha−1 yr−1), and high N (40 kg N ha−1 yr−1) were used to simulate a future increase in the atmospheric N deposition by 1-, 2- and 4-fold. A control treatment was designed at each N addition level to calculate the net effect of N addition and to eliminate the interference of micro-topography heterogeneity. Each N treatment was replicated three times, a total of 36 plots. Each plot was 9 m2 in area (3 m × 3 m) and the plots were separated by 2 m wide buffer strips. N fertilizer solutions were sprayed on the plots once a month in 12 equal applications over the entire year, and control plots received 12 equivalent deionized water applications only. All plots are winter pastures, i.e. not grazed in the growing season and grazed in the non-growing season. Chronic N treatments started from May of 2007.
Soil CO2 efflux measurement
At each plot, a square chamber and collar (0.125 m3) was designated to measure CO2 efflux using a static opaque chamber and gas chromatography method (Wang and Wang 2003). The collar was inserted to 10 cm soil depth. A fan with a diameter of 10 cm was installed inside each chamber to make turbulence when the chamber was closed. White insulating material covered the outside of the stainless steel covers to reduce the impact of direct radiative heating during the sampling. The soil CO2 efflux was measured between 9:00 and 11:00 am (China Standard Time, CST) by fitting the chambers to the collars for 30 min (Fang et al. 2010). Four gas samples were taken using 100 ml plastic syringes at intervals of 0, 10, 20 and 30 min. after closing the chambers. All gas samples were analyzed within 24 h following gas collection. Soil CO2 effluxes were calculated based on their rate of concentration change within the chamber, which was estimated as the slope of linear regression between concentration and time (Wang and Wang 2003). All the coefficients of determination (r 2) of the linear regression were greater than 0.95 in our study. Soil CO2 effluxes were measured weekly during the growing season.
Measurements of soil temperature, moisture and aboveground biomass
Soil temperature at the surface, 5 cm, 10 cm and soil moisture at 10 cm below soil surface were monitored at each chamber simultaneously. Soil temperature was measured using portable temperature probes (JM624 digital thermometer, Living–Jinming Ltd., China). Volumetric soil moisture (m3 m−3) was measured using moisture probe meter (MPM160, Meridian Measurement, China). Aboveground biomass was measured by cutting plants at the ground level followed by drying at 60°C to a constant weight. For each vegetation analysis, one 25 cm × 25 cm square area within 1 m from the flux chamber was harvested. During the growing season, soil temperature and moisture were determined weekly, and aboveground biomass was measured monthly.
Soil sampling and mineral N analysis
On the 15th day of each month during the growing season, soil samples were taken nearby the flux chambers from 0–50 cm depth at an interval of 10 cm using an auger (2.5 cm in diameter). Five sub-samples were collected at each site. Soils were immediately passed through a 2 mm sieve to remove roots, gravel and stones. Soil subsamples were extracted in 0.2 M KCl solution (10 g: 100 ml) and shaken for 1 h. The soil suspension was subsequently filtered through Whatman No. 40 filter papers for NH +4 -N and NO −3 -N determination on a continuous-flow autoanalyzer (Bran Luebbe, Germany). Taking the contribution of available N in the entire soil profile into account, soil NH +4 -N and NO −3 -N storages (g m−2) at each plot were calculated based on values of soil bulk density and gravel percentage at each layer.
Statistical analysis
We used three-factors repeated measures multivariate analysis of variance (MANOVA) with N forms, N levels and months as main effects to test differences in soil temperature, soil moisture, aboveground biomass, inorganic N storages and soil CO2 effluxes. Comparisons of the means were conducted using the Tukey’s HSD (Honestly Significant Difference) test. The relationships between soil CO2 efflux, soil temperature, soil moisture content, soil inorganic N pools and aboveground biomass were analyzed using regression analysis. Multiple procedures were used to test the contribution of significant variables to soil CO2 efflux, including 1) the sole contribution of each continuous/categorical variable, 2) nonlinear regression of continuous variables soil temperature and soil moisture based on scatter plots and literature results, 3) overall effects of these independent variables and their co-linearity using a forward stepwise method with an entry significance level of 0.05 and exit significance level of 0.10, which eliminated variable soil moisture from the model. Finally, multiple nonlinear regression models were built for soil temperature, NH +4 -N, aboveground biomass, N level and N form. All statistical analyses were conducted using the SAS software package (version 8.2). Statistical significant differences were set with p values < 0.05 unless otherwise stated.
Results
Effects of N addition on soil temperature and soil moisture content
During the growing season, the surface temperature fluctuated greatly, which correlated with the weather conditions (Fig. 1a–c). Soil temperature at the 5 cm and 10 cm depths changed as a mono-peak curve, i.e. soil temperature at two soil depths was the lowest in early May, gradually reached the maximum value at the end of July, and then decreased (Fig. 1a–c). The soil temperature at the 5 cm and 10 cm depths ranged from 3.2 to 15.9°C, and the difference in average soil temperature between two depths was less than 0.1°C (Fig. 1f). There was no significant difference in soil temperature at the surface, 5 cm and 10 cm depths among various N addition levels (Fig. 1d–f, p > 0.05).
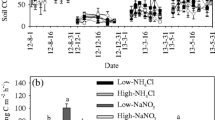
In contrast to soil temperature, soil moisture content showed an overall single parabolic curve. High soil moisture contents occurred in early May and late September, which was attributed to the soil freezing-thawing process. Meanwhile, most of the variations in soil moisture appeared in the early season to the peak growing season, and there was no difference after June or July (Fig. 2a–c, Table 1, p = 0.051). At the same level of N addition, the soil moisture content in different types of N addition showed a similar trend, where low and high levels of N addition tended to decrease soil moisture, while medium N tended to increase soil moisture (Fig. 2d–f). Repeated measures ANOVA results indicated that low N significantly decreased soil moisture content, while medium N significantly increased soil moisture content (Fig. 2f, Table 1, p < 0.0001). In addition, all three levels of KNO3 fertilizer additions altered soil moisture contents, while only low and high levels of (NH4)2SO4 fertilizer significantly decreased soil moisture contents (Fig. 2f). The effects of NO −3 -N additions on soil moisture contents were slightly stronger than for NH +4 -N (Table 1, p = 0.094). Also, there was a significant interaction between the time and N form and the time and N level (Table 1, p < 0.001 and p = 0.006).
Monthly variation of soil moisture and their response to N addition. (a) = Low N, (b) = Medium N, (c) = High N, (d) = 2008, (e) = 2009, (f) = 2008–2009. Different letters above the columns, mean significant differences between N levels. Asterisk (*) means significant difference between N treatments and control
Effects of N addition on soil inorganic N pools
Soil NH +4 -N pool was higher during the growing season than before and after the season, showing obvious seasonal dynamics (Fig. 3a–c, Table 1, p = 0.003). In the control plots, the monthly NH +4 -N pool in the alpine meadow soil ranged from 1.83 to 7.86 g m−2, with an average of 4.01 ± 0.30 g m−2 (Fig. 3a–c). Levels rather than forms of N addition had subtle effects on soil NH +4 -N pool (Table 1, p = 0.065). In 2008, low and medium levels of N addition tended to increase the soil NH +4 -N pool, while high levels of N addition reduced the soil NH +4 -N pool (Fig. 3d). However in 2009, low N reduced, while medium and high N trended to increase the soil NH +4 -N pool (Fig. 3e).
Monthly variation of soil NH +4 -N storage and their response to N addition. (a) = Low N, (b) = Medium N, (c) = High N, (d) = 2008, (e) = 2009, (f) = 2008–2009. Different letters above the columns, mean significant differences between N levels. Asterisk (*) means significant difference between N treatments and control
The monthly variation of the soil NO −3 -N pool was not consistent with that of the soil NH +4 -N pool. Maximum peaks in the soil NO −3 -N pool occurred in June or July, and the minimum was observed in August or September (Fig. 4a–c). In the control plots, the monthly NO −3 -N pool in the alpine meadow soil ranged from 0.59 to 3.14 g m−2, with an average of 1.47 ± 0.12 g m−2, which was lower than the soil NH +4 -N pool (Fig. 3 and Fig. 4). Overall, the levels and forms of N addition tended to increase soil NO −3 -N (Fig. 4d–f); however, the difference between the N addition treatment and the control was not significant (Table 1, p = 0.276 and p = 0.139) while, the differences among various N levels were significant in the same month (Table 1, p = 0.01).
Monthly variation of soil NO −3 -N storage and their response to N addition. (a) = Low N, (b) = Medium N, (c) = High N, (d) = 2008, (e) = 2009, (f) = 2008–2009. Different letters above the columns, mean significant differences between N levels. Asterisk (*) means significant difference between N treatments and control
Effect of N addition on aboveground biomass
Aboveground biomass showed significant seasonal variation, with the lowest in May, gradually increasing and reaching the maximum in August, followed by a decline (Table 1, p < 0.001, Fig. 5a–c). In the control plots, aboveground biomass in the alpine meadow ranged from 147.6 to 581.3 g m−2, with an average of 356.4 ± 9.9 g m−2 (Fig. 5a–c). Overall, N addition significantly increased aboveground biomass (Table 1, p = 0.011). In 2008, medium and high N tended to increase aboveground biomass, while low N decreased aboveground biomass (Fig. 5d). In 2009, N addition consistently increased aboveground biomass (Fig. 5e). However, there was no significant difference between N forms (Fig. 5f, Table 1, p = 0.768).
Monthly variation of aboveground biomass and their response to N addition. (a) = Low N, (b) = Medium N, (c) = High N, (d) = 2008, (e) = 2009, (f) = 2008–2009. Different letters above the columns, mean significant differences between N levels. Asterisk (*) means significant difference between N treatments and control
Effects of N addition on soil CO2 efflux
Similar to soil temperature, soil CO2 efflux showed a single-peak pattern, with the minimum and maximum occurring in May and early August, respectively (Fig. 6a–c). In the control plots, the soil CO2 effluxes ranged from 33.0 to 272.8 mg CO2-C m−2 h−1, with an average of 148.6 ± 10.9 mg CO2-C m−2 h−1 (Fig. 6a–c). In 2008, low N additions decreased soil CO2 effluxes, while medium and high N demonstrated an opposite trend (Fig. 6e). In 2009, N addition tended to promote soil CO2 effluxes and this effect increased with the N addition levels (Fig. 6e). In addition, medium or high levels of (NH4)2SO4 and NH4Cl rather than KNO3 significantly increased soil CO2 effluxes, indicating that the promoting effects of NH +4 -N were more significant than NO −3 -N (Fig. 6d–f, Table 1, p = 0.037).
Monthly variation of soil CO2 effluxes and their response to N addition. (a) = Low N, (b) = Medium N, (c) = High N, (d) = 2008, (e) = 2009, (f) = 2008–2009. Different letters above the columns, mean significant differences between N levels. Asterisk (*) means significant difference between N treatments and control
Relationships between soil CO2 effluxes and environmental variables
Soil CO2 effluxes were positively correlated with soil temperature at the 5 cm depth, while negatively correlated with soil moisture at the 10 cm depth (Fig. 7a–b, Table 2, p < 0.0001). The correlation between soil CO2 effluxes and soil temperature and soil moisture content were demonstrated with exponential equations (Fig. 7a–b). Also, soil CO2 effluxes were positively correlated with the soil NH +4 -N pool rather than the soil NO −3 -N pool (Fig. 7c–d, Table 2, p < 0.0001). Similarly, the soil CO2 effluxes were linearly correlated with aboveground biomass (Fig. 7e, Table 2, p < 0.0001). All significant continuous variables (soil temperature, aboveground biomass, soil NH +4 -N pool) and categorical variables (N level and N form) could as a whole explain more than 90% of the variation in soil CO2 effluxes (Table 2). Regression analysis indicated that soil CO2 efflux was mainly controlled by soil temperature, followed by biomass and the NH +4 -N pool (Table 2). Except soil temperature, the effects of other environmental variables on soil CO2 efflux and the coefficient of categorical variables in the regression equation gradually increased with the increasing N addition level (Table 2). Taking N forms as categorical variables, the contribution of N addition to soil CO2 efflux was higher from NH +4 -N than from NO −3 -N, especially for (NH4)2SO4 (Table 2).
Discussion
N addition changes soil variables and plant growth
Theoretically, the variation of soil moisture content depends on the dynamic balance between the input by precipitation and the loss by soil evaporation and plant transpiration (Yepez et al. 2005). This study showed that low-level N addition tended to decrease soil moisture, while medium level N addition tended to increase soil moisture (Fig. 2). In the study, precipitation was the same among the plots, and thus the differences in soil moisture content among the different N addition plots could be attributed to the differences in soil evaporation and plant transpiration among the plots (Lauenroth and Bradford 2006). Inouye (2006) reported that long-term N addition significantly decreased soil moisture content in sagebrush steppe in SE Idaho, USA. Xia et al. (2009) found that N addition did not affect soil moisture content at the 10 cm depth of the steppe soil in Inner Mongolia, but significantly increased ecosystem C turnover. In contrast, Ma (2009) reported that N addition increased soil moisture content at 10 cm depth by 0.015 m3 m−3 in the Changling meadow. These studies suggest that the responses of soil moisture dynamisms to N addition are ambiguous, and the driving mechanism beyond this pattern needs to be further examined.
The dynamic of soil inorganic N pools is the balance between exogenous N inputs, soil N mineralization, N immobilization, plant uptake, removal by livestock, leaching and nitrogenous gas effluxes in pasture ecosystems (Ammann et al. 2009). Since soil available N and rainfall are low in the alpine meadow ecosystem, soil inorganic N leaching was negligible (Xu et al. 2006). Also, our previous study showed that N addition did not increase soil N2O efflux on the short time scale (Jiang et al. 2010). Consequently, the dynamics of soil NH +4 -N and NO −3 -N pools under N addition was mainly determined by the inorganic N input (N addition and mineralization) and losses by plant uptake and removal by livestock. Low-level N addition could not maintain soil NH +4 -N content, whereas medium and high levels of N addition were conductive to the accumulation of soil NH +4 -N and NO −3 -N pools (Fig. 3 and Fig. 4). Meanwhile, N addition significantly increased aboveground biomass (Fig. 5). These results suggest that, under the low N deposition scenario (≤10 kg N ha−1 yr−1), the loss of soil inorganic N through the accelerated uptake of vegetation and the subsequent removal by livestock exceeded the input of soil inorganic N from N addition and soil organic N mineralization. When exogenous N input was higher than 20 kg N ha−1 yr−1, soil inorganic N began to accumulate. In contrast to the soil NH +4 -N pool, the soil NO −3 -N pool was not responsive to N addition (Fig. 3, Fig. 4 and Table 1), which very likely was correlated with the competition and use of soil inorganic N between plants and soil microorganisms. In the N-limited alpine meadow, the ability of plants to capture soil inorganic N was much stronger than that of soil microorganisms. Moreover, plants prefer to use soil NO −3 -N rather than soil NH +4 -N during the entire growing season (Song et al. 2007). This may be the main reason for the lack of NO −3 -N accumulation in the soil N pool after 3-years of chronic N additions.
Effects of levels of N addition on soil CO2 effluxes
Atmospheric N deposition tends to inhibit soil CO2 efflux and promote ecosystem carbon sequestration (Micks et al. 2004; Magnani et al. 2007; Mo et al. 2008). The inhibiting mechanisms include a decrease in litter decomposition (Franklin et al. 2003; Janssens et al. 2010), a reduction in fine root and soil microbial biomass and their activities (Frey et al. 2004; DeForest et al. 2004; Mo et al. 2008). The study also found that the low level of N addition did not significantly promote soil CO2 efflux, but even showed a significant inhibition in the initial period of N addition (Fig. 6d). The inherent inhibiting mechanisms differed from those in other studies. Based on the combined multiple regression results, soil CO2 efflux was mainly controlled by soil temperature, followed by aboveground biomass and the soil NH +4 -N pool (Table 2). It could be stated that the soil CO2 efflux was not affected by the variations in soil moisture. The decrease in soil CO2 efflux from low N addition was mainly attributed to the decrease of aboveground biomass (Table 2). In the low N plots, most of the applied N is sequestered within the aboveground biomass, and the very conservative N cycling in aboveground plant biomass potentially leads to reduced C allocation to root systems, which in turn could decrease soil CO2 efflux (Burton et al. 2004). To our knowledge, the current study is the first to report this phenomenon, and the different inhibition mechanisms need to be further emphasized.
The significant promotion of soil CO2 effluxes by medium and high levels of N addition in our study confirms findings in previous studies on short-term N addition experiments in N-limited natural ecosystems (Magill et al. 1997; Bowden et al. 2004). The increase in soil CO2 efflux with high N addition was mainly attributed to the increase of aboveground biomass and soil NH +4 -N pool (Table 2). Overall, the dynamics of soil inorganic N dominated the various responses of soil CO2 efflux to N addition. For the N-limited alpine meadow ecosystem, the most energy produced by root respiration was used to absorb soil inorganic N (Bloom et al. 1992). The short-term N addition did not significantly accumulate in the soil inorganic N pools (Fig. 3, Fig. 4 and Table 1), and the alpine meadow ecosystem remains limited in available N. Therefore, plants absorb low amounts of available N and the consumed energy by plant roots increases, which will promote root respiration and surface soil CO2 efflux (Bowden et al. 2004). In addition, some studies propose that N addition can alter the proportion of legume and non-legume plants (Stevens et al. 2004; Clark and Tilman 2008), which further affects soil CO2 efflux by altering the potential productivity of a species and total belowground C allocation (Craine et al. 2001). Generally, the most productive species preferentially utilize the most abundant N form while less productive species use less abundant N forms in N-limited terrestrial ecosystems (McKane et al. 2002). However, our additional data on plant diversity showed that three-year N addition did not change species richness of legume, grass, sedge, and forbs as well as the Shannon–Wiener index (Fig. S1). Therefore, the contribution of plant community composition to the change of soil CO2 efflux caused by N addition is negligible.
Effects of forms from N addition on soil CO2 effluxes
Our studies showed that the promotion of NH +4 -N to soil CO2 efflux was higher than for NO −3 -N fertilizer (Fig. 6 and Table 2). Theoretically, NO −3 -N and NH +4 -N should have contrasting effects on rates of carbon turnover because of their opposite ion charges. First, the less sensitive response to NO −3 amendment is that NO -3 ions are generally very mobile in soil because they are negatively charged (Currey et al. 2010). Consequently, NO −3 ions may not be present in the treated plots as long or at the same concentration as NH +4 ions, which are positively charged and are more strongly adsorbed onto exchange sites in the soil matrix. The accumulation of soil NH +4 -N and NO −3 -N pools with N addition level and time appears to support this deduction (Fig. 3 and Fig. 4). Secondly, the impact on soil acidity caused by NO −3 and NH +4 additions are different, which will further differentiate the impact on soil microbial activity involved in C turnover. In N-limited ecosystems, NO −3 additions generally decreased soil pH (Currey et al. 2010). This decrease was likely enhanced by the fact that NO −3 was added as KNO3 supplying soil with K+ ions and this K+ replaces H+ in soil exchange sites leading to increases in the concentration of H+ in soil solution (Killham 1994). Soil pH values in the NH +4 amended plots remained relatively constant or slightly decreased (Fig. S2). Moreover, although plants prefer to take up soil NO −3 -N (Song et al. 2007), soil NH +4 -N, the most abundant form of N in soil solution, dominates the promotion of added inorganic N to soil CO2 efflux. With increasing soil NO −3 availability, the relationship between soil CO2 efflux and soil NO −3 -N content might become stronger (Fang et al. 2010). Finally, NH +4 -treated soils in the alpine meadow may have the potential to turn over labile sources of C more rapidly than soils with NO −3 amendments. Currey et al. (2010) reported that NH +4 amendments had a stronger effect on the potential usage of labile carbon in an ombrotrophic peatland, with an increase in mineralization of most carbon substrates. The exact cause of this response is uncertain and could result from changes in microbial biomass, community composition physiology (McAndrew and Malhi 1992). The responses of soil microbial activities to N forms should be further investigated.
Conclusions
In this study, the early effects of various N forms and N levels on soil temperature, soil moisture, soil available N, and aboveground biomass and soil CO2 effluxes in an alpine meadow on the Qinghai–Tibetan Plateau were examined to explore the main driving factors of soil CO2 effluxes. The main conclusions are summarized as follows:
-
(1)
N addition does not alter soil temperature, but significantly affects soil moisture content. Low N additions tend to decrease soil moisture, whereas medium N additions are favorable to maintain soil moisture. A certain amount of N addition is needed to significantly increase aboveground biomass, and the effect increases with the time of N addition. Low-level N deposition could not maintain soil NH +4 -N storage, and three-year N addition only slightly accumulates the soil NO −3 -N pool.
-
(2)
The CO2 effluxes from alpine meadow soil are sensitive to exogenous N input. Although medium and high N addition (≥20 kg N ha−1) significantly stimulates soil CO2 effluxes, low N addition (≤10 kg N ha−1) inhibits soil CO2 efflux. Moreover, the stimulation effect on CO2 effluxes is greater from NH +4 than from NO −3 addition. Generally, soil CO2 efflux is mainly determined by soil temperature, followed by vegetation biomass and soil NH +4 -N content. N addition affects soil CO2 effluxes through altering plant growth and soil carbon turnover.
References
Ammann C, Neftel A, Spirig C, Leifeld J, Fuhrer J (2009) Nitrogen balance of hay meadows with and without fertilization. Agrarforschung 16:348–353
Bloom AJ, Sukrapanna SS, Warner RL (1992) Root Respiration Associated with Ammonium and Nitrate Absorption and Assimilation by Barley. Plant Physiol 99:1294–1301
Bond-Lamberty B, Thomson A (2010) Temperature-associated increases in the global soil respiration record. Nature 464:579–583
Bowden RD, Davidson E, Savage K, Arabia C, Steudler P (2004) Chronic nitrogen additions reduce total soil respiration and microbial respiration in temperate forest soils at the Harvard Forest. For Ecol Manag 196:43–56
Bragazza L, Freeman C, Jones T, Rydin H, Limpens J, Fenner N, Ellis T, Gerdol R, Hajek M, Hajek T, Lacumin P, Kutnar L, Tahvanainen T, Toberman H (2006) Atmospheric nitrogen deposition promotes carbon loss from peat bogs. Proc Natl Acad Sci USA 103:19386–19389
Burton AJ, Pregitzer KS, Crawford JN, Zogg GP, Zak DR (2004) Simulated chronic NO −3 deposition reduces soil respiration in northern hardwood forests. Glob Chang Biol 10:1080–1091
Cao GM, Tang YH, Mo WH, Wang YA, Li YN, Zhao XQ (2004) Grazing intensity alters soil respiration in an alpine meadow on the Tibetan plateau. Soil Biol Biochem 36:237–243
Clark CM, Tilman D (2008) Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature 451:712–715
Craine JM, Wedin DA, Reich PB (2001) Grassland species effects on soil CO2 flux track the effects of elevated CO2 and nitrogen. New Phytologist 150:425–434
Currey PM, Johnson D, Sheppard LJ, Leith ID, Toberman H, Van Der W, Dawson LA, Artz RRE (2010) Turnover of labile and recalcitrant soil carbon differ in response to nitrate and ammonium deposition in an ombrotrophic peatland. Glob Chang Biol 16:2307–2321
Dalal RC, Allen DE (2008) Greenhouse gas fluxes from natural ecosystems. Aust J Bot 56:369–407
de Vries W, Solberg S, Dobbertin M, Sterba H, Laubhahn D, Reinds GJ, Nabuurs GJ, Gundersen P, Sutton MA (2008) Ecologically implausible carbon response? Nature 451:E1–E3
DeForest JL, Zak DR, Pregitzer KS, Burton AJ (2004) Atmospheric nitrate deposition and the microbial degradation of cellobiose and vanillin in a northern hardwood forest. Soil Biol Biochem 36:965–971
Eswaran H, Vandenberg E, Reich P (1993) Organic–Carbon in Soils of the World. Soil Sci Soc Am J 57:192–194
Fang HJ, Yu GR, Cheng SL, Zhu TH, Wang YS, Yan JH, Wang M, Cao M, Zhou M (2010) Effects of multiple environmental factors on CO2 emission and CH4 uptake from old-growth forest soils. Biogeosciences 7:395–407
Franklin O, Högberg P, Ekblad A, Ågren GI (2003) Pine forest floor carbon accumulation in response to N and PK additions: bomb 14C modelling and respiration studies. Ecosystems 6:644–658
Frey SD, Knorr M, Parrent JL, Simpson RT (2004) Chronic nitrogen enrichment affects the structure and function of the soil microbial community in temperate hardwood and pine forests. For Ecol Manag 196:159–171
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland CC, Green PA, Holland EA, Karl DM, Michaels AF, Porter JH, Townsend AR, Vorosmarty CJ (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70:153–226
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai ZC, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320:889–892
Gill RA, Polley HW, Johnson HB, Anderson LJ, Maherali H, Jackson RB (2002) Nonlinear grassland responses to past and future atmospheric CO2. Nature 417:279–282
Huxman TE, Smith MD, Fay PA, Knapp AK, Shaw MR, Loik ME, Smith SD, Tissue DT, Zak JC, Weltzin JF, Pockman WT, Sala OE, Haddad BM, Harte J, Koch GW, Schwinning S, Small EE, Williams DG (2004) Convergence across biomes to a common rain-use efficiency. Nature 429:651–654
Inouye R (2006) Effects of shrub removal and nitrogen addition on soil moisture in sagebrush steppe. J Arid Environ 65:604–618
Janssens I, Dieleman W, Luyssaert S, Subke J, Reichstein M, Ceulemans R, Ciais P, Dolman A, Grace J, Matteucci G (2010) Reduction of forest soil respiration in response to nitrogen deposition. Nat Geosci 3:315–322
Jiang C, Yu G, Fang H, Cao G, Li Y (2010) Short-term effect of increasing nitrogen deposition on CO2, CH4 and N2O fluxes in an alpine meadow on the Qinghai–Tibetan Plateau, China. Atmos Environ 44:2920–2926
Killham K (1994) Soil ecology. Cambridge University Press, Cambridge
Lü CQ, Tian HQ (2007) Spatial and temporal patterns of nitrogen deposition in China: Synthesis of observational data. J Geophys Res Atmos 112:D22S05. doi:10.1029/2006jd007990
Lauenroth W, Bradford J (2006) Ecohydrology and the partitioning AET between transpiration and evaporation in a semiarid steppe. Ecosystems 9:756–767
Luo YQ, Wan SQ, Hui DF, Wallace LL (2001) Acclimatization of soil respiration to warming in a tall grass prairie. Nature 413:622–625
Ma LN (2009) Responses of soil organic carbon, nitrogen and phosphorus to simulated warming and nitrogen application in Songnen Grassland. In School of life sciences. Northeast Normal University, Changchun, p29
Magill AH, Aber JD, Hendricks JJ, Bowden RD, Melillo JM, Steudler PA (1997) Biogeochemical response of forest ecosystems to simulated chronic nitrogen deposition. Ecol Appl 7:402–415
Magnani F, Mencuccini M, Borghetti M, Berbigier P, Berninger F, Delzon S, Grelle A, Hari P, Jarvis PG, Kolari P, Kowalski AS, Lankreijer H, Law BE, Lindroth A, Loustau D, Manca G, Moncrieff JB, Rayment M, Tedeschi V, Valentini R, Grace J (2007) The human footprint in the carbon cycle of temperate and boreal forests. Nature 447:848–850
McAndrew D, Malhi S (1992) Long-term N fertilization of a solonetzic soil: effects on chemical and biological properties. Soil Biol Biochem 24:619–623
McKane RB, Johnson LC, Shaver GR, Nadelhoffer KJ, Rastetter EB, Fry B, Giblin AE, Kielland K, Kwiatkowski BL, Laundre JA (2002) Resource-based niches provide a basis for plant species diversity and dominance in arctic tundra. Nature 415:68–71
Micks P, Aber JD, Boone RD, Davidson EA (2004) Short-term soil respiration and nitrogen immobilization response to nitrogen applications in control and nitrogen-enriched temperate forests. For Ecol Manag 196:57–70
Mo J, Zhang W, Zhu W, Gundersen P, Fang Y, Li D, Wang H (2008) Nitrogen addition reduces soil respiration in a mature tropical forest in southern China. Glob Chang Biol 14:403–412
Mo JM, Zhang W, Zhu WX, Fang YT, Li DJ, Zhao P (2007) Response of soil respiration to simulated N deposition in a disturbed and a rehabilitated tropical forest in southern China. Plant Soil 296:125–135
Neff JC, Townsend AR, Gleixner G, Lehman SJ, Turnbull J, Bowman WD (2002) Variable effects of nitrogen additions on the stability and turnover of soil carbon. Nature 419:915–917
Niu SL, Wu MY, Han Y, Xia JY, Zhang Z, Yang HJ, Wan SQ (2010) Nitrogen effects on net ecosystem carbon exchange in a temperate steppe. Glob Chang Biol 16:144–155
Song C, Zhang L, Wang Y, Zhao Z (2006) Annual dynamics of CO2, CH4, N2O emissions from freshwater marshes and affected by nitrogen fertilization. Environ Sci 27:2369–2375
Song M, Xu X, Hu Q, Tian Y, Ouyang H, Zhou C (2007) Interactions of plant species mediated plant competition for inorganic nitrogen with soil microorganisms in an alpine meadow. Plant Soil 297:127–137
Stevens CJ, Dise NB, Mountford JO, Gowing DJ (2004) Impact of nitrogen deposition on the species richness of grasslands. Science 303:1876
Wang YS, Wang YH (2003) Quick measurement of CH4, CO2 and N2O emissions from a short-plant ecosystem. Adv Atmos Sci 20:842–844
Xia JY, Niu SL, Wan SQ (2009) Response of ecosystem carbon exchange to warming and nitrogen addition during two hydrologically contrasting growing seasons in a temperate steppe. Glob Chang Biol 15:1544–1556
Xu XL, Ouyang H, Cao GM, Pei ZY, Zhou CP (2004) Nitrogen deposition and carbon sequestration in alpine meadows. Biogeochemistry 71:353–369
Xu XL, Ouyang H, Kuzyakov Y, Richter A, Wanek W (2006) Significance of organic nitrogen acquisition for dominant plant species in an alpine meadow on the Tibet plateau, China. Plant Soil 285:221–231
Yang YH, Fang JY, Tang YH, Ji CJ, Zheng CY, He JS, Zhu BA (2008) Storage, patterns and controls of soil organic carbon in the Tibetan grasslands. Glob Chang Biol 14:1592–1599
Yepez E, Huxman T, Ignace D, English N, Weltzin J, Castellanos A, Williams D (2005) Dynamics of transpiration and evaporation following a moisture pulse in semiarid grassland: a chamber-based isotope method for partitioning flux components. Agric For Meteorol 132:359–376
Zhou XM (2001) Kobresia humilis meadow in China. Science Press, Beijing
Acknowledgements
We are grateful to Mary–Anne Reeb and Yuanhe Yang for polishing this manuscript. We would also like to thank the two anonymous reviewers and Per Ambus for their helpful comments which helped us to improve the manuscript. This study was funded by the National Key Research and Development Program (2010CB833502 and 2009CB421308), National Natural Science Foundation of China (41071166 and 31070435), Knowledge Innovation Project of the Chinese Academy of Sciences (201003015).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans Lambers.
Rights and permissions
About this article
Cite this article
Fang, H., Cheng, S., Yu, G. et al. Responses of CO2 efflux from an alpine meadow soil on the Qinghai Tibetan Plateau to multi-form and low-level N addition. Plant Soil 351, 177–190 (2012). https://doi.org/10.1007/s11104-011-0942-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-0942-4