Abstract
Winter climate change is an important environmental driver that alters the biogeochemical processes of forest soils. The decrease in snowpack amplifies soil freeze–thaw cycles and decreases the snowmelt water supply to soil. This study examined how snow decrease affects nitrogen (N) mineralization and nitrification in forest soil in northern Japan by conducting an in situ experimental snowpack manipulation experiment and a laboratory incubation of soil with different moisture, temperature and freeze–thaw magnitudes. For the incubation studies, surface mineral soil (0–10 cm) was collected from a cool-temperate natural mixed forest and incubated using the resin core method during the winter. In the field, there were two treatments: 50 and 100 % snow removal and control plots. The increase in the soil freeze–thaw cycle increased net N mineralization and marginally decreased the net nitrification in soil. The dissolved organic carbon (DOC) and DOC/DON ratio in soil increased with the decrease in snowpack especially during the snow melt period. These results suggested that the change in substrate quality by the increase in freeze–thaw cycles caused the significant enhancement of microbial ammonium production in soil. The lower soil moisture and higher gross immobilization of inorganic N by soil microbes may be maintaining the slow net nitrification and low nitrate leaching in freeze–thaw cycles with less snowpack. The results indicate that winter climate change would strongly impact N biogeochemistry through the increase in ammonium availability in soil for plants and microbes, whereas it would be unlikely that nitrate loss from surface soil would be enhanced.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen (N) is an essential nutrient for all biota and is often the limiting nutrient in most temperate forest ecosystems (Vitousek et al. 1997). At the same time, anthropogenic excess N often causes serious environmental problems such as air pollution, acidification of soil and water, biodiversity loss, water degradation and eutrophication of aquatic ecosystems (Galloway et al. 2008; Porter et al. 2012). Understanding N biogeochemistry in forest ecosystems is important for assessing ecosystem conditions and to predict future forest ecosystem qualities, functions and services (Vitousek et al. 1997; Compton et al. 2011). The N cycle in forest ecosystems are fluctuated by biotic (e.g. vegetation, microbe and animals) and abiotic (e.g. temperature, moisture and atmospheric deposition) factors and their interactions (Gundersen et al. 2006). Climate is a fundamental driver for biogeochemical processes in forest ecosystems. Seasonal and spatial patterns in water and heat regimes influence dynamic N pools and flows between vegetation, microbes, soil, air and water.
The winter climate in cool-temperate and boreal zones of the northern hemisphere is cold and snowy, providing dormant periods for vegetation and microbes, which are the key biotic players in the N cycle. Recent studies have suggested that the winter climate is not a negligible driver of N biogeochemistry in temperate and boreal forest ecosystems (Kielland et al. 2006; Stottlemyer and Toczydlowski 2006). Although nutrient uptake and evapotranspiration by trees almost stops in winter, it has been recognized that certain soil microbes can survive and maintain microbial N transformation (mineralization, nitrification and denitrification) even in severe cold environments (Groffman et al. 2011). Inorganic N produced by soil microbes in winter would be a potential source of N leaching to streams during snow melt season and might be an important source of N for growth of vegetation and microbes in the early growing season.
Recent modeling studies of global climate change imply that winter climate could be affected by an increase of greenhouse gasses derived from anthropogenic activities (Hosaka et al. 2005). Changes in winter climate will cause alterations in the heat and water regimes of forest ecosystems through a lower snowpack depth that reduces the insulation function of the snowpack on the soil (Hardy et al. 2001; Iwata et al. 2008). This reduction in the heat insulation function enhances soil freeze–thaw cycles that are influenced by rapid fluctuations in surface air temperature, especially in early winter and in the late melting season as these periods have a lesser snowpack but a cold air temperature (Christopher et al. 2008).
An increase in frequency and magnitude of soil freeze–thaw cycles often stimulates soil microbial N transformation in winter (Matzner and Borken 2008; Groffman et al. 2001a; Schimel and Mikan 2005; Gilliam et al. 2010; Groffman et al. 2011) through the increase in N mineralization and nitrification (DeLuca et al. 1992; Groffman et al. 2001b; Christopher et al. 2008; Shibata et al. 2011), nitrate leaching (Mitchell et al. 1996; Fitzhugh et al. 2001; Callesen et al. 2007), labile carbon availability (Hentschel et al. 2008; Haei et al. 2010) and emission of nitrous oxide (Bochove et al. 2000; Teepe et al. 2001). It has been suggested that increased freeze–thaw cycles will stimulate microbial activity, change the microbial composition, and alter the substrate quality and quantity for soil microbes (Morley et al. 1983; Skogland et al. 1988; Allen-Morley and Coleman 1989; Henry 2007; Templer 2012). Brooks et al. (2011) recently summarized the current understanding of carbon and N cycling in snow-covered environments, focusing on soil temperature and water availability under the freeze–thaw cycle (Hobbie and Chapin 1996; Öquist et al. 2009; Harrysson-Drotz et al. 2009), microbial responses in cold environments (Lipson et al. 2002; Schadt et al. 2003), gaseous emission (carbon dioxide, methane and nitrous oxide) (Monson et al. 2006; Brooks et al. 1997) and solute exports (nitrate and dissolved organic carbon) in winter from the watershed (Brooks and Williams1999; Callesen et al. 2007). However, previous studies have indicated that patterns and responses of soil microbial N transformation in winter varied greatly both temporally and spatially and between regions (Matzner and Borken 2008; Brooks et al. 2011; Groffman et al. 2011).
Christopher et al. (2008) examined the impacts of soil freezing on net ammonium and nitrate production in forest surface soil by transplant incubation during the winter climate in northern Hokkaido, Japan. They found that net ammonium production increased with a decreased snowpack, while there were no significant differences in net nitrate production. Shibata et al. (2011) found that transplant incubation from warm to cold climate during the dormant season enhanced the net ammonium production of surface soils in Japanese forests. Although these studies revealed the distinct response to cold climate, the drivers and mechanisms behind these alterations due to winter climate changes are still unclear. Detailed understanding of the patterns, processes and mechanisms of the impacts of winter climate changes on soil N dynamics would enable better quantification of N leaching and soil nutrient fertility in early growing seasons and further understanding of the resilience and vulnerability of forest ecosystem to winter climate changes.
The objectives of this study were to address the following research questions:
-
(i)
How does an increase of frequency and magnitude (i.e. amplitude of temperature range) in soil freeze–thaw cycles, caused by a decrease in snowpack, affect soil N mineralization and nitrification in winter?
-
(ii)
What are the environmental factors and mechanisms that alter the microbial N transformation with the increase in winter soil freeze–thaw cycles?
The working hypotheses are (i) Increase of frequencies and magnitudes of soil freeze–thaw cycles by snowpack decrease would enhance microbial N transformation, and (ii) The altered substrate quality and energy availability for soil microbes by the increase in freeze–thaw cycle are important factors driving the changes in soil microbial activity. To test these hypotheses, experimental manipulation of snowpack in situ and laboratory incubations of forest surface soils in northernmost Hokkaido, northern Japan, were conducted.
Materials and methods
Study site
Forest surface soil was collected from a natural cool-temperate mixed forest (N44° 21′ 21″, E142° 15′ 29″) in the Uryu Experimental Forest of Hokkaido University located in northern Hokkaido, Japan. The dominant vegetation was Quercus crispula, Abies sachalinensis, Betula ermanii, Acer mono and Picea glehnii with a dense understory vegetation of Sasa senenensis. The basal area at breast height of the overstory vegetation was 19.6 m−2 ha−1 (Christopher et al. 2008). The soil was brown forest soil, Cambisols (IUSS Working group 2006) on tertiary andesite. The depth of the A horizon ranged from 6 to 13 cm and soil pH (H2O) in the A horizon ranged from 4.4 to 5.0 (Shibata et al. 2011). The annual precipitation was ~1,390 mm with almost half of the precipitation supplied as snowfall from December to April. The annual mean temperature was 3.1 °C.
The in situ incubation studies were conducted in experimental fields of the Northern Forestry and Development Office, Forest Research Station, Field Science Center for Northern Biosphere of Hokkaido University, northern Hokkaido, Japan (N44° 19′ 49″, E142° 27′ 11″). The distance between sampling and incubation site was ~16 km. We used different sampling and in situ incubation locations because of the difficulties of snow manipulation in the remote forested area due to inaccessibility during winter.
Soil sampling
Surface mineral soil (0–10 cm) was collected in 10 places randomly distributed in 1 ha of the studied forest stands in late October 2009. The collected soil was sieved to 5 mm to remove coarse gravel and roots and well mixed to prepare the one pooled sieved sample for the in situ and laboratory incubations. The total weight of the prepared soil sample was about 30 kg fresh weight. The soil was kept at 4 °C prior to chemical analysis and incubation studies. Soil moisture was measured by drying soil at 110 °C for 48 h.
Snow manipulation
Three experimental plots (control, 50 and 100 % removal of snowpack) were established for the in situ soil incubation during winter in flat, open fields at the Northern Forestry and Development Office. The area of each plot was 48 m2 (4 × 12 m) with a 1 m buffering-strip. Snow was removed to keep the snowpack within 50–60 cm and 0–10 cm in the 50 and 100 % removal plots, respectively. Snowpack was removed once a week by manual shoveling and at additional times in the case of heavy snowfall. Snow was removed from mid-December 2009 to mid-April 2010.
In situ incubation
The in situ soil incubation used the resin core method (Binkley et al. 1986; Shibata et al. 2011) from late October 2009 to late April 2010. A resin core was constructed from a polyvinyl chloride (PVC) cylinder. The core consisted of a column for the soil (150 cm3 volume and 67 mm inner diameter) and two columns for the ion exchange resin (Amberlite MB-1; Organo, Japan) arranged above and below the soil column. The upper resin column retained the external inputs of N into the soil column. The lower resin column retained the leached N (ammonium and nitrate) from the soil column. Thus, using this resin core, the net production of inorganic N as the whole and the components (N accumulation in soil and N leaching from soil), can be quantified. The details of the resin core method are available in Shibata et al. (2011). A 100 g sample of the prepared soil and 40 g of ion exchange resin were packed into the soil and two resin columns, respectively. Twenty cores were incubated in each plot for four sampling times with five replications. The surface soil in the studied forest was carried and packed around each core in each plot to keep the change in heat environments around the soil core the same as the original sites. The resin cores were incubated at 4 cm, with the midpoint of the soil column. The in situ incubation began on 28th October 2009 and the resin cores were collected on 14th December 2009 and 9th February, 30th March and 20th April 2010.
Snowpack depth was measured manually once a week at the time of snow shoveling. The frost tube method (Hardy et al. 2001) with methylene blue dye was used to measure the soil freezing depth. The dye filled PVC tube was installed within PVC casing in the soil and the length of the frozen dye was measured after removal from the casing. Frost depth was measured at the same interval as the snowpack measurements.
Soil temperature at 2 cm was measured using a temperature probe with a data logger (20 min interval, UA-001-64, Onset Co. Ltd., Tokyo) throughout the field incubation period.
Laboratory incubation
Laboratory incubation was conducted with different soil moisture levels at the same temperature. A 100 g sample was packed in the resin core column as in the in situ incubation. The cores were incubated at 5 °C under different soil moisture treatments (control, 5 mm and 10 mm addition) each with five replications. The deionized water was added once a week throughout the incubations. The soil was analyzed before and 1 week, 2 weeks and 3 weeks after incubation initiation.
Laboratory incubation with different freeze–thaw cycles was conducted separately. Incubation temperature was controlled automatically at constant (0 °C), medium changes (0 ± 5 °C) and large changes (0 ± 10 °C) where the temperature was changed every 12 h. A 50 g soil sample was incubated in a glass bottle without moisture control with five replications. The soil with each treatment was incubated for 1 week and the soil inorganic N content before and after the incubation was analyzed.
Chemical analysis
100 mL of potassium chloride (KCl) solution (2 mol L−1) was used to extract of inorganic N (ammonium [NH4 +] and nitrate [NO3 −]) from 10 g soil for both the in situ and laboratory incubations. In the ion exchange resin it was extracted using 100 mL of 1 mol L−1 KCl twice for 4 g resin (Shibata et al. 2011). After 1 h of extraction and paper filtering (Advantec 5B, Advantec Co Ltd., Tokyo), the concentrations of NH4 + and NO3 − in the extract solutions were analyzed using a continuous flow injection analyzer (AACS-4, BL-Tech Co. Ltd., Osaka).
Water extractable dissolved organic carbon and N (DOC and DON, respectively) in soil were analyzed using a TOC analyzer (TOC-5000A, Shimadzu Co Ltd., Kyoto) for DOC and a continuous flow injection analyzer (AACS-4, BL-Tech Co Ltd., Osaka) for dissolved total N after the extraction of soil using 100 mL of ultrapure water for 10 g soil and paper filtering (Advantec 5B, Advantec Co Ltd., Tokyo). DON was calculated from the dissolved total N minus the sum of the water extractable inorganic N (ammonium and nitrate).
Data analysis and statistics
Net production rates of NH4 + and NO3 − using the resin core methods were obtained as the sum of net changes in the soil pool (difference before and after the incubation) and the leaching from soil (net increase in resin below the soil column) for both ions during the incubation. Thus, the obtained rates of net NH4 + and NO3 − using the resin core was evaluated by each component i.e. the net change of N in soil column and the leaching of N from the soil column. The net production of ammonium and nitrate was quantified in different periods; early (Oct–Dec 2009), mid (Dec 2009–Feb 2010), late (Feb–Apr 2010) and whole (Oct 2009–Apr 2010) winter.
Similarly, the net production rates in the laboratory incubation with different temperature changes and freeze–thaw cycles was obtained as the difference of NH4 + and NO3 − contents in soil and resin before and after the incubation. Significant differences in net production rates of NH4 + and NO3 − and DOC and DON contents in soil as well as soil moisture contents between treatments and observed times were tested using two way ANOVAs (treatment, time and the interaction) and Tukey HSD with significance at P < 0.05. Significant differences in net accumulation, leaching and production during the whole winter between treatments was tested using a one way ANOVA and Tukey HSD with significance at P < 0.05. The R software (R version 2.15.2; The R foundation for statistical computing http://www.r-project.org/) was used to perform the statistical analysis.
Results
Snowpack and soil frost
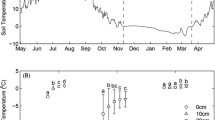
Snowpack accumulation began in early December 2009 and reached maximum (~120 cm) in the control plot in mid-February (Fig. 1). Experimental manipulation of snowpack worked well in the 50 and 100 % removal plots with snowpack depth kept at ~60 and 10 cm, respectively. Snow pack decreased in all plots in early April, and diminished in the control, 50 and 100 % removal plots in late, mid and early April 2010, respectively (Fig. 1).
The first soil frost was observed in early December 2009 in all plots, while the soil in the control plot was not frozen until after late December (Fig. 1). In the 100 % removal plot, the maximum soil frost depth reached ~19 cm in late February to March 2010. The soil frost in the 50 % removal plot increased slightly from mid-February and reached 2 cm in late February.
Soil temperature and moisture
There were no remarkable differences in soil temperature at 2 cm between the control and 50 % removal plots throughout the experiments (Fig. 2). The temperature was almost constant above 0 °C in the control plot from December 2009 to April 2010. In the 50 % removal plot, soil temperature was ~0 °C from December 2009 to mid-April 2010. Soil temperature in the 100 % removal plot fluctuated largely with repeated freeze–thaw cycles from late January and especially from mid-February to early March 2010 (Fig. 2) with a minimum soil temperature of about −5 °C in early March.
Initial soil water content (w/w) was 41.6 %. Soil water contents increased with time gradually during the incubation (Table 1). There were significant differences in soil moisture among treatments and time with a significant interaction (Table 1). Soil moisture in the 100 % removal plot tended to decrease from mid to late winter and was significantly lower than those in the control and 50 % removal plots at late winter (April 20, 2010).
In situ net production of ammonium and nitrate during winter
There was a significant difference in net ammonium production among the treatments and periods with a significant interaction (Table 2). The net production rate of ammonium in soil was significantly higher in the 100 % removal plot than in the control and 50 % removal plots in late winter, while there was no significant difference between the control and 50 % removal plots (Table 2). Although there was no significant difference in net nitrate production rates among treatments by two-way ANOVA, the net production rate of nitrate in the 100 % removal in late winter showed a significantly lower value (P < 0.05; Tukey HSD) compared with the 50 % removal plot. The net N mineralization rate (sum of net ammonium and nitrate production) was not significantly changed by either treatment throughout the experiments (Table 2).
The net increase in ammonium accumulation in soil in late winter was significantly higher in the 100 % removal plot than in the control and 50 % removal plots, while there were no significant differences in ammonium leaching among treatments during any period (Table 3). The net change of nitrate accumulation in soil was quite small in all plots for any period compared with those for ammonium but a large amount of nitrate was leached from soil in all plots. The leaching of nitrate from soil in the 100 % removal plot in late winter was significantly lower than that in the 50 % removal site but had no difference with the control plots (Table 3).
Seasonal changes in dissolved carbon and nitrogen contents in soil during winter
The DOC contents significantly decreased from October 2009 to April 2010 (Table 4). There was also a significant difference in the DOC contents among treatments with a significant interaction with time (Table 4). The DOC content in the 100 % removal plots in March 2010 was significantly higher than in the control plot. There were no significant differences in DON contents among treatments and time (Table 4). The DOC/DON ratio of soil fluctuated greatly with time, and was marginally but insignificantly higher in the 100 % removal plot in March and April than those in the control plot.
Total production of ammonium and nitrate during winter
The net ammonium accumulation and the production (accumulation + leaching) in the 100 % removal plot during the whole winter was significantly the highest among plots (Table 5) and was 141 and 156 % of that in the control and 50 % removal plots, respectively. On the other hand, net nitrate production in the 100 % removal plot was the lowest among plots (although the difference was insignificant) and was 55 and 41 % of that in the control and 50 % removal plots, respectively (Table 5). There was no significant difference in net N mineralization (ammonium + nitrate) during the whole winter among the treatments (Table 5).
Moisture impact on ammonium and nitrate production in the laboratory incubation
There were significant differences in net ammonium production in the laboratory incubation among water addition treatments and the incubation period with a significant interaction between treatments and periods (Table 6). After 3 weeks incubation, net ammonium production in the 10 mm addition was significantly higher than that in the control (Table 6). There were also significant differences in net nitrate production among treatments and periods with a significant interaction between treatments and periods (Table 6). The net nitrate production in the control and the 5 mm addition after 3 weeks incubation was significantly lower than that in the 10 mm addition. After 2 weeks incubation, all treatments indicated a net decrease of nitrate compared with the initial nitrate pool (negative values for net nitrate production). There were also significant differences in the ratio of the ammonium and dissolved inorganic N (DIN = ammonium + nitrate) among treatments and periods with a significant interaction between treatments and periods (Table 6). The ratio of the ammonium and DIN in the control after 3 weeks was significantly higher than that in the 10 mm addition treatment. There were also significant differences in soil moisture among treatments and periods with a significant interaction between treatments and periods (Table 6). The soil moisture (~44−48 %) in both the 5 and 10 mm addition treatments (Table 3) ranged within the soil moisture values in the soil column for in situ incubation from December 2009 to April 2010 (Table 1) although the soil moisture at the 100 % removal plot from mid to late winter was lower than other plots.
Effect of freeze–thaw magnitude on nitrogen mineralization and nitrification in the laboratory incubation
Temperature fluctuations (0 ± 5 and 0 ± 10 °C) significantly increased the net ammonium production in the laboratory incubation experiment (Table 7). Nitrate production was generally smaller than the net production of ammonium in all treatments. The medium changes (0 ± 5 °C) significantly decreased the net nitrate production compared with constant temperature, while there were no significant differences in net nitrate production in the large change (0 ± 10 °C) compared to constant and medium changes (Table 7). Net DOC production was significantly higher in the larger temperature changes (0 ± 10 °C) than those in the constant temperature and the medium temperature changes (0 ± 5 °C) (Table 7).
Discussion
Impacts of soil freeze–thaw cycle on microbial ammonium and nitrate production
The results of the in situ soil incubation with experimental snowpack manipulation clearly indicated increased freeze–thaw cycles with 100 % removal (Figs. 1 and 2) which significantly enhanced net ammonium production in soil during late winter from February to April (Table 2). Freeze–thaw frequencies and magnitude were largest after mid-February (Fig. 2) which coincided with the increase in net ammonium production. The increased ammonium accumulated possibly by absorption to the soil exchangeable sites (Table 3), suggesting that the produced ammonium was retained to be utilized by vegetation and microbes in the following season. The laboratory incubation with temperature fluctuations (Table 7) also supported the hypothesis that net ammonium production was enhanced with an increase in soil freeze–thaw cycles. Significant increases of net ammonium production by soil freezing in cold environments were also reported by Christopher et al. (2008) and Shibata et al. (2011).
There were no significant differences in net nitrate production among treatment while the 100 % removal plot marginally increased compared to the control plot in early winter (Table 2). The occasional below freezing temperature observed in early winter (Fig. 2) might slightly enhance the activity of nitrifiers in early winter. However, the stronger freeze–thaw cycles in the 100 % removal from mid to late winter did not increase net nitrate production, but significantly decreased it in late winter (Table 2). The decrease in net nitrification with soil freeze–thaw was also found in the laboratory incubation studies with medium temperature fluctuations (0 ± 5 °C) (Table 7), suggesting that nitrifiers are more sensitive to freeze–thaw environments than the soil microbes which contribute to ammonium N production under similar environments. These results also suggested that an increase of nitrate leaching by amplified soil freeze–thaw cycles from mid to late winter was unlikely in this region. These are different patterns from the previous studies in northeastern US which indicated the increase of nitrate leaching due to the soil freezing (Mitchell et al. 1996; Fitzhugh et al. 2001).
Although the ammonium and nitrate dynamics were affected by freeze–thaw cycles (Table 2 and 3), total net N mineralization (ammonium + nitrate) did not change even in the 100 % removal plots (Table 5), indicating that freeze–thaw cycles affect soil N dynamics qualitatively, but not quantitatively as a whole. The qualitative alteration towards an ammonium dominated system in soil provides a longer residence time of inorganic nitrogen as ammonium could be easily absorbed in the soil and microbes, while nitrate could be easily leached to groundwater and surface water.
The results also indicated that soil freeze–thaw cycles and microbial N transformations are not linear changes with snowpack removal. The response of most measured parameters in the 50 % removal plots was not half the level of the 100 % removal plot, suggesting a threshold level (<50 cm) exists for abrupt changes in soil heat-water regimes and the related changes of microbial N transformation.
Mechanisms of the alteration in microbial nitrogen dynamics during winter
Dissolved organic matter is an important energy source for microbes, and DON is a substrate for N mineralization and the subsequent nitrification (Groffman et al. 2011). A significant increase in DOC and marginal increase in the DOC/DON ratio in the 100 % removal plot in late winter (Table 4) suggested that more fresh organic matter was dissolved by the increase in soil freeze–thaw cycles. The physical pressures by freezing (<0 °C) and the subsequent thawing with warmer temperatures (>0 °C) would mechanically breakdown the structure of the fresh organic matter (freshly fallen litter, fine roots and microbial detritus), resulting in changes in their solubility. The warmer temperature after the soil freezing might also enhance soil microbial activity (Matzner and Borken 2008). The laboratory incubation with temperature fluctuations (Table 7) also suggested an increase in DOC by the amplification of soil freeze–thaw cycles. These suggested that the increased availability of energy sources and substrate by the amplified soil freeze–thaw cycle are important factors enhancing net ammonium production by soil microbes in late winter and during the whole winter (Tables 2 and 5).
The decrease of snowpack not only enhanced the soil freeze–thaw cycle (Figs. 1 and 2), but also decreased the soil water contents in the 100 % removal plot through a decrease of melt water supply at the snowpack bottom (Table 1). The laboratory incubation with different soil moisture controls indicated that dryer soil tended to decrease the net production of ammonium and nitrate (Table 6). Since soil microbial activities are driven by soil moisture generally, these results suggested that the marginal decrease in net nitrification in late winter (Table 2) might be influenced by the decrease in water contents by soil freezing (Table 1). Below 0 °C, soil water is a mixture of liquid, solid, and vapor with the majority of water at 0 °C being in liquid form due both to matric and osmotic effects on the orientation of water molecules (Stähli and Stadler 1997; Teepe et al. 2001). Although the liquid, solid and vapor in soil water in each treatment was not quantified, it was implied that the 100 % removal treatment had higher vapor losses (as evidenced by loss in gravimetric soil moisture over the winter in Table 1), further reducing the volume of water potentially moving through soil and resins. Because the leaching rates of produced N in soil in a function of concentration on solution (ammonium and nitrate) multiplied by the volume of solution moved, any reduction in transport of water (due to frost or drying) might be expected to reduce leaching N losses from soil even without any microbial processing.
The production of ammonium and nitrate in soil is calculated as their net changes; the balances of gross production and immobilization by soil microbes. Kuroiwa et al. (2011) found that gross immobilizations of ammonium and nitrate are quite high and comparable to their gross production rates in this soil. Thus, it was suggested that the lower values of net nitrate production and the decrease of net nitrification in the 100 % removal plot in late winter (Table 2) might be related to the high rates of gross ammonium (as substrate of nitrification) and nitrate immobilization by soil microbes in winter. The decrease of the net nitrate changes in the laboratory medium temperature changes (0 ± 5 °C) compared to the constant temperature also imply that the net immobilization by soil microbes occurred during the incubation period (Table 7). Groffman et al. (2011) found a positive relationship between DOC and nitrate concentration in soil during winter in northeastern US, suggesting that soil freeze–thaw cycles mobilized available C which may stimulate microbial N immobilization. The increase of DOC (Table 4) in this study might stimulate not only net production of ammonium, but also net nitrate immobilization during the winter although further study is required.
Limitation and future research needs
A limitation of the resin core method is that the incubated condition is a closed system without vegetation uptake of water and nutrients. The closed system enables quantification of microbial processes, but simultaneously creates different conditions from the natural environment. It is well known that vegetation competes with soil microbes for N under N limiting conditions. In early spring, vegetation starts to uptake water and nutrients, suggesting possible influences on the soil microbial processes during these periods. Further studies using lysimeter studies and other techniques are necessary for an in depth understanding of these processes (Fitzhugh et al. 2001). Soil disturbance during column setting is also a fundamental problem of column incubation. The removal of fine roots is necessary to quantify the microbial processes separately from natural soil microbial N transformation and artificial root decay by the soil disturbances. However, the soil disturbance during fine root removal would cause changes in moisture and oxygen contents in the soil matrix, and possibly influencing the soil N transformation (Raison et al. 1987; Abril et al. 2001). In addition, both soil frost tubes and mineralization tubes might interrupt ground heat and latent heat fluxes in soil, promote soil frost and possibly overestimate the extent of freezing. This especially might happen when the snowpack is shallow and tubes are a conduit for heat loss from soil. The frost tubes thus may provide an index of soil frost when used consistently at a location, but further study is required to confirm their ability to compare soil frost between treatments. Getting the fraction of soil water that is frozen correct is critical because it disrupts cellular membranes, increasing labile solutes in soil for microbial metabolism, and also because liquid water is the most reliable predictor of microbial activity (Hentschel et al. 2008; Haei et al. 2010; McMahon et al. 2009). The unfrozen water content of mineral soils (excluding clay soils) is primarily determined by the osmotic potential of the soil solution (Brooks et al. 2011; Harrysson-Drotz et al. 2009), while matric potential is also an important factor in highly organic soils (Harrysson-Drotz et al. 2009). As ice is formed with an increase in the solute concentration of the remaining liquid water in soil, its osmotic potential and freezing point might be lowered (Brooks et al. 2011), implying that freezing depth and duration in the actual unsaturated soil even at soil temperatures below 0 °C (Fig. 2) might be different from those observed in the water within the frost tube (Fig. 1). Therefore, further study is required to understand the fractional changes of liquid, solid and vapor by the freeze–thaw cycle and their effect on N mineralization and nitrification under the frozen, water limited environment.
The net changes of ammonium and nitrate as soil microbial activities were analyzed but the microbial composition which contributed to these N transformations was not. A recent study has reported that soil archaea play important roles in nitrification, especially in acidic soil (Isobe et al. 2011) such as this study soil. The responses of archaea activity to environmental stress might be different from other nitrifiers. Molecular biological studies such as denaturing gradient gel electrophoresis (DGGE), 16S-rRNA and Gene marker methods on soil microbes (Isobe et al. 2011) with functional analysis would be necessary for further understanding. In addition with the analysis of changes in microbial biomass and species composition (e.g. Templer 2012), the study of trace gas emission (i.e. nitrogen oxide) would be helpful to understand the changes in processes and underlying mechanism of the altered N cycle in soil under the freeze–thaw cycle (Bochove et al. 2000; Goldberg et al. 2010).
Conclusions
Based on in situ soil incubation studies with manipulation of snowpack and laboratory incubations with different soil moisture and temperature changes, the findings are as follows;
-
1.
Net ammonium production was significantly increased by the increase in soil freeze–thaw frequencies and magnitude from the snowpack decrease in late winter.
-
2.
The increased availability of energy sources and substrate for soil microbes (high DOC and DOC/DON ratio) was a possible reason for the enhanced microbial ammonium production in winter.
-
3.
The net nitrate production increased slightly in early winter, but marginally decreased in late winter. The decrease of soil moisture due to soil freezing and higher gross N immobilization of soil microbe might decrease nitrifier activity.
-
4.
The decrease of snowpack and the subsequent increased soil freeze–thaw cycles enhanced the ammonium accumulation in soil that would be a possible nutrient source for vegetation and microbes in the following early growing season, but it was unlikely that it enhanced the nitrate loss from soil during the snowmelt season.
References
Abril A, Caucas V, Bucher VEH (2001) Reliability of the in situ incubation methods used to assess nitrogen mineralization: a microbiological perspective. Appl Soil Ecol 17:125–130
Allen-Morley CR, Coleman DC (1989) Resilience of soil biota in various food webs to freezing perturbations. Ecology 70:1127–1141
Binkley D, Aber J, Pastor J, Nadelhoffer K (1986) Nitrogen availability in some Wisconsin forests: comparisons of resin bags and on-site incubations. Biol Fertil Soils 2:77–82
Bochove EV, Prevost D, Pelletier F (2000) Effects of freeze-thaw and soil structure on nitrous oxide produced in a clay soil. Soil Sci Am J 64:1638–1643
Brooks PD, Williams MW (1999) Snowpack controls on nitrogen cycling and export in seasonally snow covered catchments. Hydrol Process 13:2177–2190
Brooks PD, Schmidt SK, Williams MW (1997) Winter production of CO2 and N2O from alpine tundra: environmental controls and relationship to inter-system C and N fluxes. Oecologia 110:403–413
Brooks PD, Grogan P, Templer PH, Groffman P, Oquist MG, Schimel J (2011) Carbon and nitrogen cycling in snow-covered environments. Geogr Compass 5:682–699
Callesen I, Borken W, Kalbitz K, Matzner E (2007) Long-term development of nitrogen fluxes in a coniferous ecosystem: does soil freezing trigger nitrate leaching? J Plant Nutr Soil Sci 170:189–196
Christopher SF, Shibata H, Ozawa M, Nakagawa Y, Mitchell MJ (2008) The effect of soil freezing on N cycling: comparison of two headwater subcatchments with different vegetation and snowpack conditions in the northern Hokkaido Island of Japan. Biogeochemistry 88:15–30
Compton JE, Harrison JA, Dennis RL, Greaver TL, Hill BH, Jordan SJ, Walker H, Campbell HV (2011) Ecosystem services altered by human changes in the nitrogen cycle: a new perspective for US decision making. Ecol Lett 14:804–815
DeLuca TH, Keeney DR, McCarty GW (1992) Effect of freeze–thaw events on mineralization of soil nitrogen. Biol Fertil Soils 14:116–120
Fitzhugh R, Driscoll C, Groffman P, Tierney G, Fahey T, Hardy J (2001) Effects of soil freezing disturbance on soil solution nitrogen, phosphorus, and carbon chemistry in a northern hardwood ecosystem. Biogeochemistry 56:215–238
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320:889–892
Gilliam FS, Cook A, Lyter S (2010) Effects of experimental freezing on soil nitrogen dynamics in soils from a net nitrification gradient in a nitrogen-saturated hardwood forest ecosystem. Can J For Res 40:436–444
Goldberg SD, Borken W, Gebauer G (2010) N2O emission in a Norway spruce forest due to soil frost: concentration and isotope profiles shed a new light on an old story. Biogeochemistry 97:21–30
Groffman PM, Driscoll CT, Fahey TJ, Hardy JP, Fitzhugh RD, Tierney GL (2001a) Colder soils in a warmer world: a snow manipulation study in a northern hardwood forest ecosystem. Biogeochemistry 56:135–150
Groffman PM, Driscoll CT, Fahey TJ, Hardy JP, Fitzhugh RD, Tierney GL (2001b) Effects of mild winter freezing on soil nitrogen and carbon dynamics in a northern hardwood forest. Biogeochemistry 56:191–213
Groffman PM, Hardy JP, Fashu-Kanu S, Driscoll CT, Cleavitt NL, Fahey TJ, Fisk MC (2011) Snow depth, soil freezing and nitrogen cycling in a northern hardwood forest landscape. Biogeochemistry 102:223–238
Gundersen P, Schmidt IK, Raulund-Rasmussen K (2006) Leaching of nitrate from temperate forests—effects of air pollution and forest management. Environ Rev 14:1–57
Haei M, Öquist MG, Buffam I, Ågren A, Blomkvist P, Bishop K, Ottosson-Löfvenius M, Laudon H (2010) Cold winter soils enhance dissolved organic carbon concentrations in soil and stream water. Geophys Res Lett 37:L08501. doi:10.1029/2010GL042821
Hardy JP, Groffman PM, Fitzhugh RD, Henry KS, Welman AT, Demers JD, Fahey TJ, Driscoll CT, Tierney GL, Nolan S (2001) Snow depth manipulation and its influence on soil frost and water dynamics in a northern hardwood forest. Biogeochemistry 56:151–174
Harrysson-Drotz S, Tilston EL, Sparrman T, Schleucher J, Nilsson M, Öquist MG (2009) Contributions of matric and osmotic potentials to the unfrozen water content of frozen soils. Geoderma 148:392–398
Henry HAL (2007) Soil freeze-thaw cycle experiments: trends, methodological weaknesses and suggested improvements. Soil Biol Biochem 39:977–986
Hentschel K, Borken W, Matzner E (2008) Repeated freeze–thaw events affect leaching losses of nitrogen and dissolved organic matter in a forest soil. J Plant Nutr Soil Sci 171:699–706
Hobbie SE, Chapin FS (1996) Winter regulation of tundra litter carbon and nitrogen dynamics. Biogeochemistry 35:327–338
Hosaka M, Nohara D, Kitoh A (2005) Changes in snow cover and snow water equivalent due to global warming simulated by a 20 km mesh global atmospheric model. SOLA 1:93–96
Isobe K, Koba K, Otsuka S, Senoo K (2011) Nitrification and nitrifying microbial communities in forest soils. J For Res 16:351–362
IUSS Working group WRB (2006) World reference base for soil resources 2006. A framework for international classification, correlation and communication. World Soil Resources Reports 103, FAO, Rome
Iwata Y, Hayashi M, Hirota T (2008) Comparison of snowmelt infiltration under different soil-freezing conditions influenced by snow cover. Vadose Zone J 7:79–86
Kielland K, Olson K, Ruess RW, Boone RD (2006) Contribution of winter processes to soil nitrogen flux in taiga forest ecosystems. Biogeochemistry 81:349–360
Kuroiwa M, Koba K, Isobe K, Tateno R, Nakanishi A, Inagaki Y, Toda H, Otsuka S, Senoo K, Suwa Y, Yoh M, Urakawa R, Shibata H (2011) Gross nitrification rates in four Japanese forest soils: heterotrophic versus autotrophic and the regulation factors for the nitrification. J For Res 16:363–373
Lipson DA, Schadt CW, Schmidt SK (2002) Changes in soil microbial community structure and function in an alpine dry meadow following spring snow melt. Microbial Ecol 43:307–314
Matzner E, Borken W (2008) Do freeze-thaw events enhance C and N losses from soils of different ecosystems? a review. Eur J Soil Sci 59:274–284
McMahon SK, Wallenstein MD, Schimel JP (2009) Microbial growth in Arctic tundra soil at −2°C. Environ Microbiol Rep 1:162–166
Mitchell M, Driscoll C, Kahl J, Likens G, Murdoch P, Pardo L (1996) Climatic control of nitrate loss from forested watersheds in the northeast United States. Environ Sci Technol 30:2609–2612
Monson RK, Burns SP, Williams MW, Delany AC, Weintraub M, Lipson DA (2006) The contribution of beneath-snow soil respiration to total ecosystem respiration in a high-elevation, subalpine forest. Global Biogeochem Cycle 20, GB3030, doi:10.1029/2005GB002684
Öquist MG, Sparrman T, Klemedtsson L, Drotz SH, Grip H, Schleucher J, Nilsson M (2009) Water availability controls microbial temperature responses in frozen soil CO2 production. Global Change Biol 15:2715–2722
Porter EM, Bowman WD, Clark CM, Compton JE, Pardo LH, Soong JL (2012) Interactive effects of anthropogenic nitrogen enrichment and climate change on terrestrial and aquatic biodiversity. Biogeochemistry (in press)
Raison RJ, Connell MJ, Khanna PK (1987) Methodology for studying fluxes of soil mineral-N in situ. Soil Biol Biochem 19:521–530
Schadt CW, Martin AP, Lipson DA, Schmidt SK (2003) Seasonal dynamics of previously unknown fungal lineages in tundra soils. Science 301:1359–1361
Schimel JP, Mikan C (2005) Changing microbial substrate use in Arctic tundra soils through a freeze-thaw cycle. Soil Biol Biochem 37:1411–1418
Shibata H, Urakawa R, Toda H, Inagaki Y, Tateno R, Koba K, Nakanishi A, Fukuzawa K, Yamasaki A (2011) Changes in nitrogen transformation in forest soil representing the climate gradient of the Japanese archipelago. J For Res 16:374–385
Skogland T, Lomeland S, Goksøyr J (1988) Respiratory burst after freezing and thawing of soil: experiments with soil bacteria. Soil Biol Biochem 20:851–856
Stähli M, Stadler D (1997) Measurement of water and solute dynamics in freezing soil columns with time domain reflectometry. J Hydrol 195:352–369
Stottlemyer R, Toczydlowski D (2006) Effect of reduced winter precipitation and increased temperature on watershed solute flux, 1988–2002, Northern Michigan. Biogeochemistry 77:409–440
Teepe R, Brumme R, Beese F (2001) Nitrous oxide emissions from soil during freezing and thawing periods. Soil Biol Biochem 33:1269–1275
Templer PH (2012) Changes in winter climate: soil frost, root injury, and fungal communities. Plant Soil 353:15–17
Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman DG (1997) Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7:737–750
Acknowledgments
The authors thank the graduate students and technical staff of the Northern Forestry Research and Development Office and the Uryu Experimental Forest, Forest Research Center, Field Science Center for Northern Biosphere, Hokkaido University for help conducting the snowpack manipulation and field work. Uryu Experimental Forest is a part of North Hokkaido Experimental Forest, a core-site of Japan Long-Term Ecological Research Network (JaLTER). This study is partly supported by research funds (22248016, 25252026) of the Japan Society for the Promotion of Science, and the Environmental research and technology development fund (S-9-3) of the Ministry of the Environment, Japan. This study was conducted as a part of the research project, “ReSIN; regional and comparative soil incubation study on nitrogen dynamics in forest ecosystems”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shibata, H., Hasegawa, Y., Watanabe, T. et al. Impact of snowpack decrease on net nitrogen mineralization and nitrification in forest soil of northern Japan. Biogeochemistry 116, 69–82 (2013). https://doi.org/10.1007/s10533-013-9882-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-013-9882-9