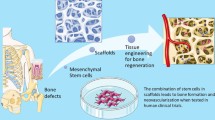
Abstract
Purpose
The current systematic review investigated the results of application of some of the most commonly used scaffolds in conjugation with stem cells and growth factors in animal and clinical studies.
Methods
A comprehensive electronic search was conducted according to the PRISMA guidelines in NCBI PMC and PubMed from January 1970 to December 2015 limited to English language publications with available full texts. In vivo studies in relation to “bone healing,” “bone regeneration,” and at least one of the following items were investigated: allograft, β-tricalcium phosphate, deproteinized bovine bone mineral, hydroxyapetite/tricalcium phosphate, nanohydroxyapatite, and composite scaffolds.
Results
A total of 1252 articles were reviewed, and 46 articles completely fulfilled the inclusion criteria of this study. The highest bone regeneration has been achieved when combination of all three elements, given scaffolds, mesenchymal stem cells, and growth factors, were used. Among studies being reported in this review, bone marrow mesenchymal stem cells are the most studied mesenchymal stem cells, β-tricalcium phosphate is the most frequently used scaffold, and platelet-rich plasma is the most commonly used growth factor.
Conclusion
The current review aimed to inform reconstructive surgeons of how combinations of various mesenchymal stem cells, scaffolds, and growth factors enhance bone regeneration. The highest bone regeneration has been achieved when combination of all three elements, given scaffolds, mesenchymal stem cells, and growth factors, were used.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Treatment of skeletal defects has remained a challenging part of many reconstructive surgeries. Currently, autogenous bone is assumed to be the gold standard for bone grafting [1, 2]. Bone substitute materials are recommended when the quantity of autogenous bone needed is greater than available amounts of autogenous bone [3] and when there is a risk of morbidity at the donor site [4–7]. Bone tissue engineering has been proposed as an alternative therapeutic option by harvesting progenitor cells and engineering graft materials in vitro to be clinically used for reconstruction of skeletal defects in vivo [8]. Over the last few decades, advancements have been achieved in tissue and bone engineering procedures [9–11]. A combination of progenitor/stem cells, growth factors, and scaffolds has been used in bone engineering [12, 13]. Different progenitor/stem cells, including mesenchymal stem cells (MSCs), can be used in bone engineering procedures. The ability of stem cells to self-renew and to differentiate into various cell lineages turns them into a special factor in tissue engineering. Among the different MSCs, bone marrow mesenchymal stem cells (BMMSCs) are most frequently used within the studies [14]. Their osteogenic capability as well as their proliferation have been shown by various studies [12–16]. In vivo studies also have presented higher bone regeneration in treatment with MSCs compared to acellular treatments [16–19]. Osteogenic differentiation of stem cells can be promoted by using cytokines/growth factors, osteoinductive chemical factors, and biomaterials [15]. The addition of various growth factors to cell–scaffold constructs promotes bone regeneration [20, 21]. However, chemical compounds tend to be less unstable and therefore have a longer active half-life in comparison to protein-based cytokines and growth factors. Moreover, they have proven to better promote osteogenic differentiation of stem cells in vitro [15]. An ideal scaffold, grafting material, for bone engineering provides adequate environment for progenitor/stem cells to differentiate and proliferate in an osteoconductive setting. In addition, scaffold permits migration of cells to the site of defect by creating a suitable extracellular matrix for proper vascularization, attachment, infiltration, and cell growth. Scaffold also can be used as a delivery system for biological treatments of bone defects [22]. Growth factors and cells responses to each others, as well as treatment results, depend on physical properties of scaffolds such as porosity, pore size, interconnectivity of the pores, and surface texture. Also, scaffold chemical compositions can make an impression on extracellular matrix production by releasing different ions at different times [23–25]. Numerous scaffolds are commercially available causing confusion for clinicians. Hence, the current systematic review investigated the results of application of some of the most commonly used scaffolds in conjugation with stem cells in animal and clinical studies [1, 13]. The following scaffolds were selected in this study: β-tricalcium phosphate (β-TCP), bone allograft, deproteinized bovine bone mineral (DBBM), hydroxyapetite/tricalcium phosphate (HA/TCP), nano-HA, composite scaffolds, and natural bovine bone mineral (NBBM) [26–30]. This review will help clinicians to determine the suitable scaffolds for bone reconstruction in conjunction with proper stem cells for bone tissue engineering. This review will help clinicians to compare the result of given scaffolds in conjunction with various stem cells and growth factors in bone reconstruction.
Materials and methods
Eligibility criteria
Applied inclusion criteria of this study were as follows:
-
Studies in relation to “bone healing” and “bone regeneration”
-
Studies using at least one of these items: HA/TCP, β-TCP, NBBM, DBBM, composite scaffolds, nano-HA, and allografts
-
Studies using scaffolds on animals and clinical trials
Applied exclusion criteria for this study were as follows:
-
Studies on other tissues, not involving bone tissue or bone healing
-
Studies that did not use our selected scaffolds and items previously explained in inclusion criteria
-
Studies that were performed only in vitro
Information sources and search
An electronic search was conducted in NCBI PMC and PubMed database from January 1970 to December 2015 limited to English language publications with available full texts. Published papers on selected scaffolds were found using the following keywords: ((nano AND [hydroxyapatite OR ha)) OR ((polycaprolactone OR PCL OR PLA or polylactic acid or PLGA or poly(lactic-co-glycolic acid)) AND (“tricalcium phosphate” OR tcp or hydroxyapatite OR HA)) OR osteoporos OR tutogen OR “particulate mineralized bone” OR (dbm AND “calcium sulphate”) OR osteoset OR “natural bone mineral” OR “bio oss” OR “bio-oss” OR “tricalcium phosphate” OR tcp OR ((hydroxyapatite OR ha) AND (“tricalcium phosphate” OR tcp)) OR katsios OR “ha/tcp” OR (biphasic AND (“bone substitutes” OR scaffold)) OR “bone allograft”) AND “stem cell”(all). Figure 1 demonstrated the flow chart diagram of the present study selection according to PRISMA guidelines [31].
Study selection
Primary selection of titles and abstracts were based on the inclusion criteria. Full texts of all eligible studies were obtained and reviewed. Among different reports of one experiment, only the latest report demonstrating the most relevant information with respect to the measurements of this review was included.
Population selection
Studies of animals and human beings in which evaluated newly bone formation in specific bony defect sites.
Results
Study selection
A total of 1252 articles were reviewed. Sixty-seven articles were included as relevant for the purpose of this systematic literature review. Certain cases, showing the exclusion criteria as defined in this study, were excluded. Following the initial screening of titles and abstracts and the final screening of full texts, 46 articles completely fulfilled the inclusion criteria of this study. The qualifying data were extracted from the remaining cases. Since different types of study designs with a wide variation regarding the number of cases were included in this review, the results were reported based on both the number of cases and articles.
Study characteristics
Allograft
Thirty articles that have used bone allograft were evaluated, and five met the requirements for inclusion in this study [16, 28, 32–34]. Allograft was evaluated in one clinical study and two kinds of animal models, rabbit and sheep. Four of the studies used BMMSCs [16, 28, 32, 33] and only one study used stromal stem cells (SCCs) [34]. Computed tomography [35], histological evaluation (HLE), histomorphometrical analysis (HMMA), and radiographical evaluation (RGE) were used to assess the results. Follow-ups varied between 4 and 24 weeks (Table 1).
Human
Combinations of human BMMSCs and autogenous demineralized bone mineral with calcium sulfate were used to repair alveolar cleft in two patients. After 4 months, the amount of bone regeneration was 35.4% in one patient and the second patient showed 25.6% regenerated bone, based on CT evaluation [16].
Sheep
Lucarelli et al. treated 3-cm defects in six sheep with platelet-rich plasma-based scaffold loaded with sSCCs. HMMA demonstrated 42.8 to 54.1% new bone formation (NBF) after a 4-month follow-ups [34].
Rabbit
Three studies used a rabbit model [28, 32, 33]. Autogenous BMMSCs combined with allograft were used in all of these studies. However, different methods/scoring systems were used for assessment of NBF. Lee et al. used allograft wrapped by Gelfoam containing BMMSCs for treatment of femoral defects, and they demonstrated an average Teira score of 14.4 after 12-week follow-ups in treatment groups [33]. A study by Nather et al. treating tibia bone defects, evaluated the NBF with HLE and using NBF index. They showed that the combination of allograft and autogenous BMMSCs presented a significantly higher amount of NBF (NBF index 6.14 ± 2.07) compared to the control (allograft only). In HLE, they also observed a higher percentage of osteocyte formation compared to autograft bone treatment [32]. Khojasteh et al. used particulate bone allograft with fibrin glue in combination with BMMSCs for vertical augmentation of bone around dental implants. HMMA of the results showed the height of NBF was 2.09 mm, and the amount of new supracrestal trabecular bone formation was 28.5–64.5% [28].
NBBM
Thirty-two articles using NBBM were evaluated, and eight articles met the requirements for inclusion in this study [17, 18, 37–42]. NBBM (Bio-Oss) were evaluated in one clinical study and three kinds of animal models, mouse, rat, and dog. MSCs that have been used in these studies were BMMSC, periodontal ligament stem cells (PDLSCs), and dental pulp stem cells (DPSCs). The results were evaluated using HMMA and CT scan analysis. The follow-ups varied between 6 and 15 weeks (Table 2).
Mouse
Park et al. conducted an experiment to compare the regeneration potential of HA, coated with different ratios of TCP, and Bio-Oss as a carrier for human ABMSCs (hABMSCs). HMMA showed that bone regeneration was greater in the hABMSC-loaded HA/TCP groups. No significant difference was observed between HA/TCP groups [41].
Human
Rickert et al. assessed differences in NBF after maxillary sinus floor elevation using bovine bone mineral (Bio-Oss) in combination with either autogenous BMMSCs harvested from the posterior iliac crest (study group) or autogenous bone harvested from the retromolar area (control group). HMMA showed significantly higher NBF in the study group (17.7 ± 7.3%) compared to the control group (12.0 ± 6.6%) [37].
Dog
Three studies used dogs as their animal model [17, 39, 40]. In the study carried out by Jafarian et al., the amount of NBF was assessed by using two types of scaffold: biphasic bone substitutes (HA/TCP) and NBBM (Bio-Oss). A canine full-thickness alveolar defect was used as the model, and MSCs were isolated from dog BMMSC. The results indicated a higher NBF in the HA/TCP + dBMMSCs group (65.78 ± 4.94%) compared to the Bio-Oss + dBMMSCs group (50.31 ± 6.97%) [17]. In the study by Khorsand et al., the effects of DPSCs on regeneration of an experimentally created defect in the periodontium of a canine model were investigated. After 8 weeks post-operation, the regeneration of the periodontal defects, including bone, periodontal ligament (PDL), and cement formation, were evaluated using HMMA. The amount of NBF, new cementum, and PDL formation were 3.60 ± 61.06, 3.83 ± 61.32, and 3.30 ± 61.12 mm, respectively, in study group (DPSCs + Bio-Oss) which were significantly higher than the regeneration level in control groups (Bio-Oss only 3.10 ± 60.82, 2.42 ± 1.40, and 1.77 ± 1.27 mm) [40]. Yu et al. also used Bio-Oss seeded with either PDLSCs or BMMSCs, and they have shown enhanced bone formation and mineralization with the maximum volume of the maxillary sinus augmentation in PDLSCs + Bio-Oss group with only 26.68 ± 2.23 mm3 of bone resorption compared to the BMMSCs + Bio-Oss group (27.34 ± 2.91 mm3) [42].
Rat
Khojaste et al. compared the effect of two different bone substitutes (Bio-Oss and β-TCP) on the rate of bone regeneration in combination with platelet-rich plasma (PRP) or rat BBMSCs. After 6 weeks, HMMA showed the highest bone regeneration in β-TCP + BMMSCs which was 2.53 mm and lowest bone regeneration was observed in the β-TCP + PRP group (1.21 mm) [18]. In the study by Yu et al., the osteogenic effects of PDLSCs and BMMSCs in combination with Bio-Oss scaffolds on 4-mm wide calvarial defects were compared. The results indicated more NBF in the defect treated with Bio-Oss and PDLSCs (28%) [39]. Raposo-Amaral et al. used NBBM loaded rat Orbicularis Oris Muscle MSCs to treat 5-mm alveolar defects in rats. The NBF was 38.35 ± 19.59% in 8 weeks after the surgery [38].
β-TCP
Forty articles using β-TCP were evaluated, and ten articles met the requirements for inclusion in this study [18, 43–51]. Two of them were done in a rabbit model, two in humans, one in a dog model, four in a rat, and one in a pig model. Growth factors such as bone morphogenetic protein-2 (BMP-2) and PRP were used in these studies. BMMSCs, adipose-derived stem cells (ASCs), and urine stem cells (USCs) were used as MSCs. The follow-up periods varied between 1 day and 12 months (Table 3).
Human
Two studies were conducted on humans. In a study by Thesleff et al., the results were compared using CT Hounsfield units (HUs). They evaluated the effect of ASCs loaded on β-TCP in reconstruction of large cranial defects in humans. The mean HU in 1 to 3 days was 791.5, in 3 months was 1025, and in 1 year was 1031 [45]. In the second study by Uede et al., the mean increase in tissue height of 7.3 ± 4.6 mm was evident when β-TCP and hBMMSCs were used in combination with PRP for maxillary sinus floor augmentation [44].
Rabbit
Wang et al. evaluated the bone regeneration capability of alginate microencapsulated rabbit BMMSCs (rBMMSCs) with β-TCP/calcium phosphate cement (CPC). Evaluation of bone substitute degradation and NBF using cone beam computed tomography (CBCT) showed more new bone, bone marrow, and native cells in the group treated with rBMMSCs/β-TCP/CPC than those of control group (β-TCP/CPC) [47]. In a study by Zhou et al., two types of MSCs including BMMSCs and BMMSCs + endothelial cell (EC)-derived MSCs were used on β-TCP scaffold. The amount of NBF on 1.5-cm ulnar defect was assessed. HLE after a 16-week follow-up indicated more NBF in β-TCP + rBMMSCs + EC-derived MSCs group (23.31 ± 1.41%) than β-TCP + rBMMSCs (7.68 ± 0.84%) [46].
Dog
In a study by Li et al., the combination of n β-TCP with ASCs genetically modified by BMP-2 (transduced ADSCs) was used to treat a critical-sized canine ulnar bone defect. After 16 weeks, HMMA indicated that n β-TCP with transduced ASCs showed a significant increase of NBF. Newly formed bone surface area/total surface area of bone defect was 39.95 ± 8.55% [49].
Rat
Three studies evaluated the NBF in rat femoral defects. In a study by Feng et al., combination of β-TCP, rat ADSCs, and vitamin D (VD) were used. The results showed a significant amount of bone volume when both cells and VD were used (64.96 ± 5.17%) [43]. Guan et al. evaluated bone regeneration potential of β-TCP loaded with USCs. Micro-CT analysis showed higher bone volume in β-TCP loaded with USCs (16.3 ± 4.2%) compared to β-TCP alone (8.2 ± 3.7%) [50] Eftekhari et al. compared the regeneration potential of TCP and HA in combination with human BMMSCs, and they showed better bone formation in TCP group [51].
Khojasteh et al. carried out a study to compare BMMSCs and PRP loaded to Bio-Oss and β-TCP for rat calvarial bone repair. The defects were treated either with Bio-Oss plus PRP or Bio-Oss loaded with BMMSCs. The results revealed higher percentage of bone formation in the group treated with BMMSCs groups compared with the PRP group [18].
Pig
Damlar et al. compared three types of commercially viable β-TCP including Cerasorb®, Kasios®, and Poresorb®. No stem cells were used in combination with these bone substitute materials. For the control group, autogenous bone and blood clot were used. HMMA indicated that no significant difference was observed between the three types of β-TCP and the control groups [48].
Nano-HA
Thirteen studies using nano-HA were reviewed, and only five studies were relevant according to inclusion criteria of this study [9, 52–55]. Nano-HA was evaluated in two kinds of animal models, rabbit and goat. Three studies used BMMSCs and two studies used DPSCs. Three of the studies used nano-HA particles in combination with collagen in their studies [52–54]. The results were reported using HLE, RGE, and HMMA. The follow-ups varied between 4 and 12 weeks (Table 4).
Goat
Liu et al. treated 25-mm tibia defect with nanoHA/collagen/poly (L-lactic acid) (PLLA)/chitin fibers (nHACP/CF) loaded with goat BMMSCs. After 8 week follow-up, HMMA demonstrated the highest bone regeneration in treatment groups (16.56 ± 6.5%) compared to other treatments (scaffold, autogenous bone, and control without any treatment) [54].
Rabbit
Four studies used a rabbit model. Three studies used HMMA and one study used synchrotron radiography (SRGE) for result analysis. A study by Liu et al. indicated that the combination of nano-HA + PLA with DPSCs and rhBMP-2 result in bone mineral apposition (BMA) of 1.77 ± 0.11%, whereas in the group without rhBMP-2, BMA was 2.52 ± 0.33% [53]. In another study that evaluated NBF using SRGE after 6, 8, and 12 weeks, the imagology grading rates of nano-HA group were 4.2, 6.6, and 9.6, respectively, which was significantly higher than rates in control groups [52]. Behnia et al. studied the effect nanocrystalline HA silica gel matrix in combination with BMMSCs and PRGF on bone regeneration of parietal bone defects. HMMA after 12 months showed that the amount of NBF was significantly higher in treatment groups with both autogenous BMMSCs and PRGF [9]. Ling et al. suggested higher bone formation in DPSCs loaded on β-TCP group (15 ± 1.7%) compared to DPSCs loaded on nHAC/PLA group (11.15 ± 1%) [55].
HA/TCP
Among 31 articles using HA/TCP scaffold, seven studies were included according to our inclusion criteria [17, 19, 20, 35, 56–58]. Two of the studies compared NBF of HA/TCP with Bio-Oss. The studies were conducted on human and dog models. All of the studies used BMMSCs, and only in one study were BMMSCs and PDLSCs used [57]. The results were reported using HEL, HMMA, RGE, and CBCT. The follow-ups varied between 1 and 12 months (Table 5).
Human
In these studies, HA/TCP loaded with BMMSCs were used. One of the studies conducted the bone augmentation on the maxillary sinus floor, and the other study was performed in the anterior cleft of the maxilla. PRGF was used as growth factor in the second study. The control group was not reported in following studies, and results were based on bone formation mean. Combination of HA/TCP, BMMSCs, and PRGF showed 51.3% mean bone formation after 3-month follow-ups. Interestingly, the combination of HA/TCP and BMMSCs showed similar bone formation after 12 months 41.34% [19, 20].
Dog
Five studies used a dog model. Three studies were conducted on mandibular defects and two were performed on femur defects. Studies carried out by Jafarian et al. and Vahabi et al. were the same in terms of defect size, cells, scaffold, and follow-up periods In both studies after 6-month follow-ups with HMMA, the highest bone formations were observed in the groups treated with bone formation HA/TCP + BMMSCs [17, 56]. A study by Kim et al. showed enhanced bone formation in the treatment with BMMSCs compared to PDLSCs [57]. In two studies on femur bone defects, BMMSCs using the same follow-up period were conducted. In both studies, similar amounts of NBF were reported using both histological and radiological analysis [35, 58].
Composite
Among 22 articles that used composite scaffolds, ten studies were included according to our inclusion criteria [27, 28, 59–66]. Composite was evaluated in five types of animal models: dogs, pigs, rabbits, rats, and mice. Different composite scaffolds including PLGA/CPC, PEG/PCL/HA, PLGA/β-TCP/COL I, PCL/TCP, and PLGA/HA were evaluated. Eight of the studies used autogenous BMMSCs and ASCs. Two other studies used human BMMSCs and ASCs in animal models [62, 63]. The results were mostly reported by using HMMA. In three studies, μ-CT were used. The follow-ups varied between 2 and 36 weeks (Table 6).
Dog
Two studies used dogs as their animal model. Kim et al. investigated the combination of PCL/TCP, auto-fibrin glue (aFG), rhBMP-2, PCL membrane, and BMMSCs for bone regeneration. The results showed the highest NBF in the treatment group [66]. Khojasteh et al. used the combination of TCP/PCL and BMMSCs in the dog mandible. NBF was approximately 48%, and the amounts of remaining scaffold were minor in the cell-seeded group after 2 months [28].
Pig
Liao et al. treated temporal bone defects in two pigs with PEG/PCL/HA scaffold containing BMMSCs. HMMA after 6 months demonstrated a higher NBF (64%) in the PEG/PCL/HA + BMMSCs group than the PEG/PCL + BMMSCs group (22%) [60]. Konopnicki et al. showed higher bone surface area in PCL/TCP + pBMMSCs (22.11 ± 22.45%) than PCL/TCP (1.87 ± 3.66%) [64].
Rabbit
Four studies used rabbit as their animal model. Two of the studies used the same composite scaffold PLGA/β-TCP/COL I and the same experimental defect, 15 mm on radial bone. Two of them used autogenous BMMSCs, and the other used autogenous ASCs. In the study by Pang et al., using BMMSCs, HLE showed 97.27 ± 2.65% bone formation after 36-week follow-ups [61]. The results of the other study by Hao et al., using ASCs, showed similar bone formation (96.4 ± 2.3%) after 24-week follow-ups [27]. He et al. used PRP in combination with PLGA/CPC and BMMSCs. Application of PRP indicated better bone formation 12 weeks after operation [59].
Rat
Rai et al. treated femur defects in rats with PCL/TCP scaffold containing BMMSCs. μ-CT analysis demonstrated more new bone volume (1.0 ± 0.7 mm3) in BMMSC-seeded PCL/TCP group compared to treatment of the defect with scaffold only [63].
Mice
James et al. treated calvarial defects in mice with PLGA/HA scaffold containing human ASCs. In this study, the effect of adding fresh stem cells and frozen stem cells to scaffolds were compared. μ-CT results indicated the highest NBF in the fresh ASCs group (80%) [62].
Discussion
Bone tissue engineering mainly includes the triad of an artificial extracellular matrix/scaffold, stem cells, and growth factors [1, 2]. The efficacy of this combination has been studied in various animal and human studies. A perfect scaffold for bone tissue engineering should have the following features: (1) biocompatibility; (2) convenience for application in defect site, osteoconductivity, and osteoinductivity; (3) biodegradation rate close to natural bone formation rate; (4) structure with proper porosities providing a dynamic extracellular matrix for cell proliferation and differentiation; (5) mechanical properties near to natural bone; and (6) proper structure for vascularization [13]. However, an ideal scaffold having all of these features has not been found for bone tissue engineering. Several studies have attempted to select the most qualified source of stem cells for bone tissue engineering [39, 42, 46, 57]. Expression of special surface markers and capability of differentiation into different cell lineages are the main criteria for detecting stem cells [67, 68]. However, these criteria might not guarantee the efficacy of cells for tissue engineering [47]. Finally, application of exogenous growth factors and osteoinductive cytokines, as the third side of bone tissue engineering, have been shown as important factors in promoting cell adhesion, proliferation, and differentiation in many studies [18, 34, 40, 49].
Combinations of various scaffolds, stem cells, and exogenous factors have been used in tissue engineering, causing mush confusion. The current systematic review aimed to help reconstructive surgeons to find proper combination which are more efficient for bone regeneration. In this study, the most commonly used scaffolds including allograft, NBBM (Bio-Oss), β-TCP, nano-HA, HA/TCP, and composite scaffolds were assessed. Scaffolds were categorized according to the types of scaffolds and animal models. In each study, the application of growth factor, types of stem cells, sample size, follow-up periods, and assessment test also were reported. Although not all the criteria assessed in these studies were the same, they all indicated higher bone formation at follow-up sessions. Several major technical advances have been achieved in the field of bone tissue engineering during the past decade. Particularly, the increased understanding of bone healing at the cellular and molecular levels allowed the conduction of numerous animal and pilot clinical studies using tissue-engineered constructs for local regeneration.
Scaffolds
In general, most of the papers included in this study, according to inclusion criteria, were categorized as composite scaffolds.
Bone allografts are commonly used for reconstruction and repair of skeletal defects due to their appropriate mechanical strength and osteoconductive properties [69]. Although allografts contain protein factors that enhance bone formation, failures due to fracture and infection have been reported in many cases [70–72]. Allograft has been extensively used in combination with BMMSCs [16, 28, 32–34]. Several studies attempted to enhance bone regeneration capability of allograft-BMMSC constructs by addition of various factors. Studies by Khojasteh et al. and Lucarelli et al. demonstrated that the addition of PRP [34] and fibrin glue [28] to allograft bone can increase the amount of bone formation in animal models. Similarly, a study by Lee et al. showed that the amounts of NBF were greater when Gelfoam was added to cell-scaffold constructs [33].
NBBM (Bio-Oss) has been well-established in reconstructive surgeries due to its biocompatibility and osteoconductive properties [73]. Various MSC types have been reported to be used in combination with Bio-Oss. In rat models, the combination of MSCs and NBBM indicated more NBF in alveolar defects compared to calvarial defects [38, 39]. However, in those studies, different types of MSCs were used. Also, different methods were used for assessment of NBF. Khojasteh et al. demonstrated that application of PRP instead of rat BMMSCs can decrease the amount of bone formation [18].
β-TCP is a bioactive bone substitute ceramic which has high biocompatibility and better stability than polymeric bone substitutes [74]. Most of the studies used β-TCP scaffolds alone or in combination with other scaffolds. Since the measurement techniques and follow-up periods to assess NBF were different, it is hard to decide which combinations would be the most suitable. In studies by Hao et al. and Pang et al., β-TCP was used in combination with PLGA(PLGA 70%/β-TCP 30%). The amounts of NBF according to HLE, after 24-month follow-ups, were nearly the same, although different types of stem cells had been used in these studies [27, 61]. Similar amounts of NBF were observed in the studies by Vahabi et al., Jafarian et al., and Kim et al. after 6, 8, and 16 weeks [17, 56, 57]. In these studies, dogs were used as their animal model and HA/TCP loaded with autogenous BMMSCs was used for bone regeneration. Kim et al. also compared BMMSCs with PDLSCs, and they observed higher bone regeneration with BMMSCs. In the studies by Vahabi et al. and Jafarian et al., the bone regeneration capability of HA/TCP were compared with Bio-Oss; they reported better results in HA/TCP loaded with cells. The study by Vahabi et al. was the only study that included negative control in their study design. Although the NBF was highest in HA/TCP + BMMSCs, they observed significantly higher NBF compared to HA/TCP or Bio-Oss-alone groups. In addition, they showed that mean woven bone was significantly higher in the “empty cavity” group (60.80%) than other groups (40.60–46.38%) [56]. Moreover, Eftekhari et al. compared the bone regeneration potential of TCP and HA. The result indicated that combination of hBMMCs and β-TCP for treatment of rat femoral defects showed increased quantity of newly formed lamellar bone compared to HA with hBMMCs in 45 days [51].
The structure of scaffold materials in nanoscale to microscale can resemble the natural structure of cancellous bone [75]. Nanocrystalline HAs were incorporated with silica gel matrix, collagen, chitin fibers, and poly (L-lactide) [9, 52–54]. Behnia et al. performed a study in a rabbit model using a combination of nano-HA, silica gel matrix, autologous PRGF, and BMMSCs. They showed that nano-HA and autologous PRGF group had more bone formation than nano-HA and autogenous BMMSCs. Moreover, application of both autogenous PRGF and BMMSCs showed the best results among the experimental groups However, Ling et al. indicated significantly higher percentage of mature bone formation area in rat DPSCs loaded on β-TCP compared with nHAC + PLA [55]. Liu et al. indicated that application of hBMP-2 could significantly increase protein content, alkaline phosphatase activity and NBF in a rabbit model [53].
Collagen was the most common natural polymer used in combination with composite and nano-HA scaffolds [53–55]. Composition of a biodegradable material, integrating bioactive particles, might improve structural and biological properties of the scaffolds [76–79]. However, there are controversies within the literature regarding advantages and disadvantages of incorporation of collagen in composite scaffolds. Many studies showed better compressive modulus, strength, proliferation, and expression of osteoconductive factors [27, 60, 80]. Others demonstrated that its incorporation in the ceramic phase might cause inadequate mechanical properties [81, 82].
Osteoprogenitor/stem cells
Osteoprogenitor/stem cells are one of the most important parts in bone tissue engineering, and their capabilities in proliferation and osteogenic differentiation have been proven in many studies. In this review, it is also confirmed that the addition of MSCs to different types of scaffolds enhances NBF [16, 28, 34, 45, 52], although in some studies, no statistically significant difference was seen in the amount of NBF while using MSCs [9, 49, 52]. Using enzymatic digestion, MSCs can be isolated from buccal fat pad [83], muscles [84], embryonic tissues [85], and dental tissues [14, 68, 85, 86]. Although in this review the majority of papers used BMMSCs, much evidence indicated other source of MSCs with the same or greater results in NBF. BMMSCs are derived from bone marrow aspirates and are defined as clonogenic cells with the capability of easily culturing and multilineage differentiation [35].
In two different studies on rat, the results demonstrated improvement of bone formation after 28 days and also 12 weeks with different analysis (histological and radiographic evaluation) due to addition of stem cells [43, 50]. In one of them, adipose-derived stem cells were used and in another one human urine stem cells. As a matter of fact, Guan et al. also present a novel idea and suggested that human urine stem cells can be act as a reliable cell source for bone tissue engineering [50].
In the study carried out by Thesleft et al., the combination of β-TCP and ASCs were used in patients following cranioplasty. One-year follow-ups, using CT analysis, revealed successful bone regeneration. Also, satisfactory results were reached in cranium contour regeneration and healing of the wound [45]. Likewise, in three other studies, the application of human BMMSCs in treatment of maxillary sinus floors and clefts indicated acceptable results in bone regeneration [19, 20, 37]. Lucarelli et al. reported higher rates of NBF and new vascularization using the combination of SSCs and PRP with allograft [34]. In the study by Kim et al., two types of stem cells, dog BMMSCS and dog PDLSCs, were used separately with HA/TCP scaffolds. Eight- and 16-week follow-ups showed better outcomes in the dog BMMSCs group although the differences between the two groups were not outstanding [57]. Unlike the previous study, Yu et al. indicates higher bone formation in treatment with Bio-Oss and autogenous PDLSCs compared to Bio-Oss and autogenous BMMSCs in rat and dog models [39, 42]. In studies by Arinzeh et al. and Bruder et al., dog femur defects were treated with HA/TCP loaded with dog allogenic BMMSCs. Although they used allogenic BMMSCs, without any immunosuppressive therapy, no adverse immune reaction was seen [35, 58].
Growth factors
A wide range of exogenous growth factors are currently being used in bone tissue engineering: transforming growth factor beta (TGF-b1), fibroblast growth factor (FGF), insulin growth factor (IGF), vascular endothelial growth factor (VEGF), PDGF, and bone morphogenic proteins (BMPs) [19, 54]. Moreover, rhBMP-2 (INFUSE®, Medtronic, Minneapolis, MN) for alveolar ridge augmentation and sinus lift surgeries and rhBMP-7 (OP-1™, Stryker Biotech, Hopkinton, MA) have received Food and Drug Administration (FDA) approval for long bone nonunion and spinal fusion [87]. BMP-2 is one of the most frequently used growth factor within the studies. PRP and PDGF are two of the most commonly used growth factors in this review. However, due to their differences, a reasonable comparison was not possible. An autologous source of growth factors would be ideal due to low risk of infection transmission and immunological responses. In fact, PRP act as this autologous source and contains not only various growth factors such as TGF-b1, FGF, VEGF, and PDGF, but also adhesion molecules like fibrin, fibronectin, and vitronectin [88–90]. Application of PRP is well established in the treatment of skeletal defects [89, 90]. In this review, three articles also confirmed the beneficial effect of PRP in bone regeneration [34, 44]. PDGF has different types of activity that seem to be beneficial in bone regeneration. PDGF has been shown to have a chemotaxis effect for MSCs. Also, it promotes IGF-1 signaling and VEGF and BMP antagonist expressions [91]. The application of PDGF in the combination of HA/TCP and human BMMSCs in the anterior maxillary cleft among humans showed increased bone formation [20]. In 2013, Behnia et al. showed more NBF (up to 18%) when PRGF was added to nanocrystalline hydroxyapatite silica gel matrix (nHASGM) loaded with rabbit BMMSC [9].
Another biologic adhesive molecule, widely used in reconstructive surgeries, is fibrin glue. Fibrin glues activate fibrinogen and imitate the last part of coagulation, resulting in formation of a fibrin clot with adhesive properties. This element enhances initial stability of scaffolds [16, 17]; it promotes angiogenesis, cell attachment, and proliferation [18].
Almost all studies indicated higher bone formation when growth factor was added, except the study carried out by Liu et al., in which the addition of rhBMP2 to nHA/PLA scaffold had adverse effects on bone regeneration in alveolar defects [53].
Animal models
Animal models within in vivo studies make transitional research between in vitro and clinical trials [92]. Animal models including dogs, rabbits, mice, sheep, pigs, and rats have been frequently used to assess bone regeneration. Each animal model has its own advantages over others, and selection of the best animal model for a specific research study depends on several factors. These factors include cost, availability, life span, animal size, and similarities in physiological and pathological conditions to human beings [93–96]. Dogs, sheep, and pigs have more similarities to human [97]. In this review study, the most common animal model used was the dog. Rabbits, rats, pigs, sheep, and goats were also used. Studies using the same animal model with the same assessed criteria were in agreement and accordance in results. Other studies, differing in their animal models, had no significant differences in NBF. However, factors such as type and size of the defects have great impact on bone regeneration. In this review, defects were placed in various anatomical sites: temporal, parietal, mandibular, maxillary, radial, ulnar, metatarsal, scapula, femoral, and tibial bone. Among them, femoral and mandibular defects were most frequently seen as defect sites.
Human
In general, six studies were done on humans. All the investigations were conducted in the oral cavity except one study that was performed on cranial defects [45]. All studies used BMMSCs, except the study by Thesleft et al., which applied ASCs for the treatment of cranial defects [45].
Limitations
The following variations were observed between included studies which resulted in heterogeneity in reported data and made a reasonable comparison between studies difficult: difference in cell source, scaffold type, animal model, and evaluation method and sacrifice time. Also, several studies did not have the control groups which made in feasible to draw a conclusion. Moreover, not all the studies included growth factors, and some of them which utilized growth factors did not determine dose-response curve.
Conclusion
Tissue engineering is a fast-growing area. Various treatment strategies are already being used clinically. The current review aimed to inform reconstructive surgeons of how combinations of various MSCs, scaffolds, and growth factors enhance bone regeneration. The great success has been achieved using HA/TCP, β-TCP, NBBM, DBBM, scaffolds, nano-HA, and allografts scaffolds in combination with various MSCs and growth factors. The highest bone regeneration has been achieved when combination of all three elements, given scaffolds, MSCs, and growth factors, were used. Among studies being reported in this review, BMMSCs are the most studied MSCs, B-TCP is the most frequently used scaffold, and PRP is the most commonly used growth factor.
References
Behnia H, Khojasteh A, Esmaeelinejad M, Naghdi N (2012) Growth factor carriers in bone formation: a systematic review. Journal of Islamic Dental Association of Iran 24:150–167
Hassani A, Khojasteh A, Alikhasi M (2008) Anterior palate of the maxilla as a donor site for oral and maxillofacial reconstructive procedures. Asian Journal of Oral and Maxillofacial Surgery 20:135–138
Hassani A, Khojasteh A, Alikhasi M, Vaziri H (2009) Measurement of volume changes of sinus floor augmentation covered with buccal fat pad: a case series study. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 107:369–374
Hassani A, Khojasteh A, Shamsabad AN (2005) The anterior palate as a donor site in maxillofacial bone grafting: a quantitative anatomic study. J Oral Maxillofac Surg 63:1196–1200
Khojasteh A, Behnia H, Dashti SG, Stevens M (2012) Current trends in mesenchymal stem cell application in bone augmentation: a review of the literature. J Oral Maxillofac Surg 70:972–982
Khojasteh A, Behnia H, Shayesteh YS, Morad G, Alikhasi M (2011) Localized bone augmentation with cortical bone blocks tented over different particulate bone substitutes: a retrospective study. Int J Oral Maxillofac Implants 27:1481–1493
Morad G, Kheiri L, Khojasteh A (2013) Dental pulp stem cells for in vivo bone regeneration: a systematic review of literature. Arch Oral Biol 58:1818–1827
Amini AR, Laurencin CT, Nukavarapu SP (2012) Bone tissue engineering: recent advances and challenges. Critical Reviews™ in. Biomed Eng 40
Behnia H, Khojasteh A, Kiani MT, Khoshzaban A, Abbas FM, Bashtar M et al (2013) Bone regeneration with a combination of nanocrystalline hydroxyapatite silica gel, platelet-rich growth factor, and mesenchymal stem cells: a histologic study in rabbit calvaria. Oral surgery, oral medicine, oral pathology and oral radiology 115:e7–e15
Khojasteh A, Soheilifar S, Mohajerani H, Nowzari H (2012) The effectiveness of barrier membranes on bone regeneration in localized bony defects: a systematic review. Int J Oral Maxillofac Implants 28:1076–1089
Mortazavi SH, Khojasteh A, Vaziri H, Khoshzaban A, Roudsari MV, Razavi SHE (2009) The effect of fluoxetine on bone regeneration in rat calvarial bone defects. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 108:22–27
Phinney DG, Prockop DJ (2007) Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells 25:2896–2902
Motamedian FSTSR, Khosraviani FGK, Khojasteh A (2012) Craniomaxillofacial bone engineering by scaffolds loaded with stem cells: a systematic review. J Dent Sch 116
Huang G-J, Gronthos S, Shi S (2009) Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res 88:792–806
Zhang Z (2011) Bone regeneration by stem cell and tissue engineering in oral and maxillofacial region. Frontiers of medicine 5:401–413
Behnia H, Khojasteh A, Soleimani M, Tehranchi A, Khoshzaban A, Keshel SH et al (2009) Secondary repair of alveolar clefts using human mesenchymal stem cells. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 108:e1–e6
Jafarian M, Eslaminejad MB, Khojasteh A, Abbas FM, Dehghan MM, Hassanizadeh R et al (2008) Marrow-derived mesenchymal stem cells-directed bone regeneration in the dog mandible: a comparison between biphasic calcium phosphate and natural bone mineral. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 105:e14–e24
Khojasteh A, Eslaminejad MB, Nazarian H (2008) Mesenchymal stem cells enhance bone regeneration in rat calvarial critical size defects more than platelete-rich plasma. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 106:356–362
Shayesteh YS, Khojasteh A, Soleimani M, Alikhasi M, Khoshzaban A, Ahmadbeigi N (2008) Sinus augmentation using human mesenchymal stem cells loaded into a β-tricalcium phosphate/hydroxyapatite scaffold. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 106:203–209
Behnia H, Khojasteh A, Soleimani M, Tehranchi A, Atashi A (2012) Repair of alveolar cleft defect with mesenchymal stem cells and platelet derived growth factors: a preliminary report. J Cranio-Maxillofac Surg 40:2–7
Houshmand B, Behnia H, Khoshzaban A, Morad G, Behrouzi G, Dashti SG et al (2012) Osteoblastic differentiation of human stem cells derived from bone marrow and periodontal ligament under the effect of enamel matrix derivative and transforming growth factor-beta. Int J Oral Maxillofac Implants 28:e440–e450
Takahashi Y, Yamamoto M, Tabata Y (2005) Osteogenic differentiation of mesenchymal stem cells in biodegradable sponges composed of gelatin and β-tricalcium phosphate. Biomaterials 26:3587–3596
Bowers KT, Keller JC, Randolph BA, Wick DG, Michaels CM (1991) Optimization of surface micromorphology for enhanced osteoblast responses in vitro. Int J Oral Maxillofac Implants 7:302–310
Eggli P, Moller W, Schenk R (1988) Porous hydroxyapatite and tricalcium phosphate cylinders with two different pore size ranges implanted in the cancellous bone of rabbits: a comparative histomorphometric and histologic study of bony ingrowth and implant substitution. Clin Orthop Relat Res 232:127–138
Matsuda T, Davies J (1987) The in vitro response of osteoblasts to bioactive glass. Biomaterials 8:275–284
Eslaminejad MB, Jafarian M, Khojasteh A, Mashhadi Abbas F, Dehghan MM, Houshmand B (2007) Enhancing ectopic bone formation in canine masseter muscle by loading mesenchymal stem cells onto natural bovine bone minerals. Iranian J Vet Surg 2:25–35
Hao W, Pang L, Jiang M, Lv R, Xiong Z, Hu YY (2010) Skeletal repair in rabbits using a novel biomimetic composite based on adipose-derived stem cells encapsulated in collagen I gel with PLGA-β-TCP scaffold. J Orthop Res 28:252–257
Khojasteh A, Eslaminejad MB, Nazarian H, Morad G, Dashti SG, Behnia H et al (2013) Vertical bone augmentation with simultaneous implant placement using particulate mineralized bone and mesenchymal stem cells: a preliminary study in rabbit. Journal of Oral Implantology 39:3–13
Liu G, Sun J, Li Y, Zhou H, Cui L, Liu W et al (2008) Evaluation of partially demineralized osteoporotic cancellous bone matrix combined with human bone marrow stromal cells for tissue engineering: an in vitro and in vivo study. Calcif Tissue Int 83:176–185
Neamat A, Gawish A, Gamal-Eldeen AM (2009) β-Tricalcium phosphate promotes cell proliferation, osteogenesis and bone regeneration in intrabony defects in dogs. Arch Oral Biol 54:1083–1090
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151:264–269
Nather A, David V, Teng JW, Lee CW, Pereira BP (2010) Effect of autologous mesenchymal stem cells on biological healing of allografts in critical-sized tibial defects simulated in adult rabbits. Ann Acad Med Singap 39:599
Lee J-Y, Choi M-H, Shin E-Y, Kang Y-K (2011) Autologous mesenchymal stem cells loaded in Gelfoam® for structural bone allograft healing in rabbits. Cell Tissue Bank 12:299–309
Lucarelli E, Fini M, Beccheroni A, Giavaresi G, Di Bella C, Aldini NN et al (2005) Stromal stem cells and platelet-rich plasma improve bone allograft integration. Clin Orthop Relat Res 435:62–68
Bruder SP, Kraus KH, Goldberg VM, Kadiyala S (1998) The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects*. The Journal of Bone & Joint Surgery 80:985–996
Taira H, Moreno J, Ripalda P, Forriol F (2004) Radiological and histological analysis of cortical allografts: an experimental study in sheep femora. Arch Orthop Trauma Surg 124:320–325
Rickert D, Sauerbier S, Nagursky H, Menne D, Vissink A, Raghoebar G (2011) Maxillary sinus floor elevation with bovine bone mineral combined with either autogenous bone or autogenous stem cells: a prospective randomized clinical trial. Clin Oral Implants Res 22:251–258
Raposo-Amaral CE, Bueno DF, Almeida AB, Jorgetti V, Costa CC, Gouveia CH et al (2014) Is bone transplantation the gold standard for repair of alveolar bone defects? Journal of tissue engineering 5:2041731413519352
Yu B-H, Zhou Q, Wang Z-L (2014) Periodontal ligament versus bone marrow mesenchymal stem cells in combination with Bio-Oss scaffolds for ectopic and in situ bone formation: a comparative study in the rat. J Biomater Appl 29:243–253
Khorsand A, Eslaminejad MB, Arabsolghar M, Paknejad M, Ghaedi B, Rokn AR et al (2013) Autologous dental pulp stem cells in regeneration of defect created in canine periodontal tissue. Journal of oral implantology 39:433–443
Park J-C, Oh S-Y, Lee J-S, Park S-Y, Choi E-Y, Cho K-S, Kim C-S (2016) In vivo bone formation by humanalveolar-bone-derived mesenchymal stem cells obtained during implant osteotomy using biphasic calcium phosphate ceramics or Bio-Oss as carriers. J Biomed Mater Res Part B 104B:515–524
Yu B-H, Zhou Q, Wang Z-L (2014) Comparison of tissue-engineered bone from different stem cell sources for maxillary sinus floor augmentation: a study in a canine model. J Oral Maxillofac Surg 72:1084–1092
Feng W, Lv S, Cui J, Han X, Du J, Sun J et al (2015) Histochemical examination of adipose derived stem cells combined with β-TCP for bone defects restoration under systemic administration of 1α, 25 (OH) 2 D 3. Mater Sci Eng C 54:133–141
Ueda M, Yamada Y, Ozawa R, Okazaki Y (2005) Clinical case reports of injectable tissue-engineered bone for alveolar augmentation with simultaneous implant placement. J Prosthet Dent 94:560
Thesleff T, Lehtimäki K, Niskakangas T, Mannerström B, Miettinen S, Suuronen R et al (2011) Cranioplasty with adipose-derived stem cells and biomaterial: a novel method for cranial reconstruction. Neurosurgery 68:1535–1540
Zhou J, Lin H, Fang T, Li X, Dai W, Uemura T et al (2010) The repair of large segmental bone defects in the rabbit with vascularized tissue engineered bone. Biomaterials 31:1171–1179
Wang J, Qiao P, Dong L, Li F, Xu T, Xie Q (2013) Microencapsulated rBMMSCs/calcium phosphate cement for bone formation in vivo. Biomed Mater Eng 24:835–843
Damlar I, Erdoğan Ö, Tatli U, Arpağ OF, Görmez U, Üstün Y (2015) Comparison of osteoconductive properties of three different β-tricalcium phosphate graft materials: a pilot histomorphometric study in a pig model. J Cranio-Maxillofac Surg 43:175–180
Li H, Dai K, Tang T, Zhang X, Yan M, Lou J (2007) Bone regeneration by implantation of adipose-derived stromal cells expressing BMP-2. Biochem Biophys Res Commun 356:836–842
Guan J, Zhang J, Li H, Zhu Z, Guo S, Niu X et al. (2015) Human urine derived stem cells in combination with β-TCP can be applied for bone regeneration
Eftekhari H, Farahpour M, Rabiee S (2014) Histopathological evaluation of potential impact of β-tricalcium phosphate (HA+ β-TCP) granules on healing of segmental femur bone defect. Bratislavske lekarske listy 116:30–34
Sun W, Li Z-R, Yang Y-R, Shi Z-C, Wang B, Liu B et al (2011) Experimental study on phase-contrast imaging with synchrotron hard X-ray for repairing osteonecrosis of the femoral head. Orthopedics 34:699
Liu H-C, Wang D-S, Su F, Wu X, Shi Z-P, Lv Y et al (2011) Reconstruction of alveolar bone defects using bone morphogenetic protein 2 mediated rabbit dental pulp stem cells seeded on nano-hydroxyapatite/collagen/poly (L-lactide). Tissue Eng A 17:2417–2433
Liu X, Li X, Fan Y, Zhang G, Li D, Dong W et al (2010) Repairing goat tibia segmental bone defect using scaffold cultured with mesenchymal stem cells. J Biomed Mater Res B Appl Biomater 94:44–52
Ling LE, Feng L, Liu HC, Wang DS, Shi ZP, Wang JC et al (2015) The effect of calcium phosphate composite scaffolds on the osteogenic differentiation of rabbit dental pulp stem cells. J Biomed Mater Res A 103:1732–1745
Vahabi S, Amirizadeh N, Shokrgozar M, Mofeed R, Mashhadi A, Aghaloo M et al (2011) A comparison between the efficacy of Bio-Oss, hydroxyapatite tricalcium phosphate and combination of mesenchymal stem cells in inducing bone regeneration. Chang Gung Med J 35:28–37
Kim S-H, Kim K-H, Seo B-M, Koo K-T, Kim T-I, Seol Y-J et al (2009) Alveolar bone regeneration by transplantation of periodontal ligament stem cells and bone marrow stem cells in a canine peri-implant defect model: a pilot study. J Periodontol 80:1815–1823
Arinzeh TL, Peter SJ, Archambault MP, Van Den Bos C, Gordon S, Kraus K et al (2003) Allogeneic mesenchymal stem cells regenerate bone in a critical-sized canine segmental defect. The Journal of Bone & Joint Surgery 85:1927–1935
He F, Chen Y, Li J, Lin B, Ouyang Y, Yu B et al (2015) Improving bone repair of femoral and radial defects in rabbit by incorporating PRP into PLGA/CPC composite scaffold with unidirectional pore structure. J Biomed Mater Res A 103:1312–1324
Liao H-T, Chen Y-Y, Lai Y-T, Hsieh M-F, Jiang C-P (2014) The osteogenesis of bone marrow stem cells on mPEG-PCL-mPEG/hydroxyapatite composite scaffold via solid freeform fabrication. Biomed Res Int 2014
Pang L, Hao W, Jiang M, Huang J, Yan Y, Hu Y (2013) Bony defect repair in rabbit using hybrid rapid prototyping polylactic-co-glycolic acid/β-tricalciumphosphate collagen I/apatite scaffold and bone marrow mesenchymal stem cells. Indian journal of orthopaedics 47:388
James AW, Levi B, Nelson ER, Peng M, Commons GW, Lee M et al (2010) Deleterious effects of freezing on osteogenic differentiation of human adipose-derived stromal cells in vitro and in vivo. Stem Cells Dev 20:427–439
Rai B, Lin JL, Lim ZX, Guldberg RE, Hutmacher DW, Cool SM (2010) Differences between in vitro viability and differentiation and in vivo bone-forming efficacy of human mesenchymal stem cells cultured on PCL–TCP scaffolds. Biomaterials 31:7960–7970
Konopnicki S, Sharaf B, Resnick C, Patenaude A, Pogal-Sussman T, Hwang K-G et al (2015) Tissue-engineered bone with 3-dimensionally printed β-tricalcium phosphate and polycaprolactone scaffolds and early implantation: an in vivo pilot study in a porcine mandible model. J Oral Maxillofac Surg 73:1016. e1011–1016. e1011
Prosecká E, Rampichová M, Litvinec A, Tonar Z, Králíčková M, Vojtová L et al (2015) Collagen/hydroxyapatite scaffold enriched with polycaprolactone nanofibers, thrombocyte-rich solution and mesenchymal stem cells promotes regeneration in large bone defect in vivo. J Biomed Mater Res A 103:671–682
Kim S-J, Kim M-R, Oh J-S, Han I, Shin S-W (2009) Effects of polycaprolactone-tricalcium phosphate, recombinant human bone morphogenetic protein-2 and dog mesenchymal stem cells on bone formation: pilot study in dogs. Yonsei Med J 50:825–831
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D et al (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317
Rad MR, Liu D, He H, Brooks H, Xiao M, Wise GE et al (2015) The role of dentin matrix protein 1 (DMP1) in regulation of osteogenic differentiation of rat dental follicle stem cells (DFSCs). Arch Oral Biol 60:546–556
Guo MZ, Xia ZS, Lin LB (1991) The mechanical and biological properties of demineralised cortical bone allografts in animals. Journal of Bone & Joint Surgery, British Volume 73:791–794
Urist MR, Mikulski A, Lietze A (1979) Solubilized and insolubilized bone morphogenetic protein. Proc Natl Acad Sci 76:1828–1832
Enneking WF, Campanacci DA (2001) Retrieved human allografts. The Journal of Bone & Joint Surgery 83:971–986
Enneking W, Mindell E (1991) Observations on massive retrieved human allografts. The Journal of Bone & Joint Surgery 73:1123–1142
Jensen SS, Broggini N, Hjørting-Hansen E, Schenk R, Buser D (2006) Bone healing and graft resorption of autograft, anorganic bovine bone and β-tricalcium phosphate. A histologic and histomorphometric study in the mandibles of minipigs. Clin Oral Implants Res 17:237–243
Suzuki O, Imaizumi H, Kamakura S, Katagiri T (2008) Bone regeneration by synthetic octacalcium phosphate and its role in biological mineralization. Curr Med Chem 15:305–313
Liao S, Cui F, Zhang W, Feng Q (2004) Hierarchically biomimetic bone scaffold materials: nano-HA/collagen/PLA composite. J Biomed Mater Res B Appl Biomater 69:158–165
Guarino V, Causa F, Ambrosio L (2007) Bioactive scaffolds for bone and ligament tissue. Expert review of medical devices 4:405–418
Coombes A, Rizzi S, Williamson M, Barralet J, Downes S, Wallace W (2004) Precipitation casting of polycaprolactone for applications in tissue engineering and drug delivery. Biomaterials 25:315–325
Shikinami Y, Okazaki K, Saito M, Okuno M, Hasegawa S, Tamura J et al (2006) Bioactive and bioresorbable cellular cubic-composite scaffolds for use in bone reconstruction. J R Soc Interface 3:805–821
Mondrinos MJ, Dembzynski R, Lu L, Byrapogu VK, Wootton DM, Lelkes PI et al (2006) Porogen-based solid freeform fabrication of polycaprolactone–calcium phosphate scaffolds for tissue engineering. Biomaterials 27:4399–4408
Zhou Y, Hutmacher DW, Varawan SL, Lim TM (2007) In vitro bone engineering based on polycaprolactone and polycaprolactone–tricalcium phosphate composites. Polym Int 56:333–342
Liao HT, Lee MY, Tsai WW, Wang HC, Lu WC (2016) Osteogenesis of adipose-derived stem cells on polycaprolactone–β-tricalcium phosphate scaffold fabricated via selective laser sintering and surface coating with collagen type I. J Tissue Eng Regen Med 10(10):E337–E353
Azevedo M, Reis R, Claase M, Grijpma D, Feijen J (2003) Development and properties of polycaprolactone/hydroxyapatite composite biomaterials. J Mater Sci Mater Med 14:103–107
Farré-Guasch E, Martí-Pagès C, Hernández-Alfaro F, Klein-Nulend J, Casals N (2010) Buccal fat pad, an oral access source of human adipose stem cells with potential for osteochondral tissue engineering: an in vitro study. Tissue Engineering Part C: Methods 16:1083–1094
Huard J, Cao B, Qu-Petersen Z (2003) Muscle-derived stem cells: potential for muscle regeneration. Birth Defects Research Part C: Embryo Today: Reviews 69:230–237
Chaudhry G, Yao D, Smith A, Hussain A (2004) Osteogenic cells derived from embryonic stem cells produced bone nodules in three-dimensional scaffolds. Biomed Res Int 2004:203–210
Lin NH, Gronthos S, Mark Bartold P (2009) Stem cells and future periodontal regeneration. Periodontol 51:239–251
McKay WF, Peckham SM, Badura JM (2007) A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE® Bone Graft). Int Orthop 31:729–734
Weibrich G, Kleis WK, Hafner G, Hitzler WE (2002) Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Cranio-Maxillofac Surg 30:97–102
Marx RE (2004) Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg 62:489–496
Kanno T, Takahashi T, Tsujisawa T, Ariyoshi W, Nishihara T (2005) Platelet-rich plasma enhances human osteoblast-like cell proliferation and differentiation. J Oral Maxillofac Surg 63:362–369
Hollinger JO, Hart CE, Hirsch SN, Lynch S, Friedlaender GE (2008) Recombinant human platelet-derived growth factor: biology and clinical applications. The Journal of Bone & Joint Surgery 90:48–54
Tare R, Kanczler J, Aarvold A, Jones A, Dunlop D, Oreffo R (2010) Skeletal stem cells and bone regeneration: translational strategies from bench to clinic. Proc Inst Mech Eng H J Eng Med 224:1455–1470
Schimandle JH, Boden SD (1994) The use of animal models to study spinal fusion. Spine-Hagerstown 19:1988–1991
Liebschner MA (2004) Biomechanical considerations of animal models used in tissue engineering of bone. Biomaterials 25:1697–1714
Hazzard DG, Bronson RT, McClearn GE, Strong R (1992) Selection of an appropriate animal model to study aging processes with special emphasis on the use of rat strains. J Gerontol 47:B63–B64
Egermann M, Goldhahn J, Schneider E (2005) Animal models for fracture treatment in osteoporosis. Osteoporos Int 16:S129–S138
Pearce A, Richards R, Milz S, Schneider E, Pearce S (2007) Animal models for implant biomaterial research in bone: a review. Eur Cell Mater 13:1–10
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hosseinpour, S., Ghazizadeh Ahsaie, M., Rezai Rad, M. et al. Application of selected scaffolds for bone tissue engineering: a systematic review. Oral Maxillofac Surg 21, 109–129 (2017). https://doi.org/10.1007/s10006-017-0608-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10006-017-0608-3