Abstract
A total of 210 Streptomyces were isolated from the soil samples of Tawang, India where temperature varied from 5 °C during daytime to −2 °C during the night. Based on antifungal activity, a total of 33 strains, putatively Streptomyces spp., were selected. Optimal growth temperature for the 33 strains was 16 °C, with growth occurring down to 6 °C but not above 30 °C. Phylogenetic analysis based on 16S rDNA sequences revealed the taxonomic affiliation of the 33 strains as species of Streptomyces. To examine the relatedness of the chitinase genes from six strong antifungal Streptomyces strains, a phylogenetic tree was constructed using the catalytic domain nucleotide sequences and resulted in seven distinct monophyletic groups. A quantitative PCR study for chitinase expressing ability revealed that of the six antifungal strains tested, the strain Streptomyces roseochromogenus TSR12 was the most active producer of family 18 chitinase genes. Streptomyces strains with enhanced inhibitory potential usually encode a family 19 chitinase gene; however, our present study did not show expression of this family in the six strains tested.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With more than 80 % of the biosphere experiencing temperature below 5 °C, nature has posed challenges in the survival of life forms in varied cryoenvironments. The majority of cold environments are represented by the deep sea (nearly 71 % of the Earth is covered by oceans and 90 % of the ocean volume is below 5 °C), followed by snow (35 % of land surface), cryotic soil (24 % of land surface), sea ice (13 % of the Earth’s surface) and glaciers (10 % of land surface) (Margesin and Miteva 2011). Other cold environments are lakes, soils (especially subsoils), deserts, and caves. In order to thrive in cold environments an organism either has to nullify its indirect influence on water, or through its direct influence on its own organic molecules thereby overcoming extremely low rates of nutrient and metabolite transfer, high osmotic potential, low water activity, and potentially high background radiation (Steven et al. 2006). Psychrophilic/psychrotolerant microbes in this regard remodel proteins, nucleic acids, and membrane structure, which allow them to maintain their fluidity, flexibility and associated activity at low temperatures, as well as other adaptations including cryoprotectant production, and highly efficient genetic responses to thermal shifts (Ayala-del-Rio et al. 2010; Bakermans 2008; Deming 2002).
The Tawang district in Arunachal Pradesh, India is situated in the eastern Himalayan global biodiversity hotspot region and experiences large temperature fluctuations, a high number of frost and ice days, regular freeze–thaw events and high precipitation. A varying seasonal fluctuation in such Alpine discontinuous permafrost soils has been shown to result in seasonally different microbial communities (Lipson 2007). Considerable abundance and diversity of microorganisms, including Bacteria, Archaea, phototrophic cyanobacteria and green algae, fungi and protozoa, are present in alpine soils. Studies have shown that high altitude soils (3,000–5,400 m) in the Anapurna Mountains, Nepal, contained the most extreme xerophytic psychrophilic fungi belonging to ascomycetous genera Eurotium and Aspergillus due to a dry climate (Petrovic et al. 2000). Another example of alpine soil bacterial xerophiles is novel species from the genus Deinococcus, which are also radiation resistant.
Most studies relating microbial diversity in cold environment have focused generally on Bacteria and Achaea, with little focus on Streptomyces species, which are the soundest candidate for the exploration of important biological compounds (Margesin and Miteva 2011). Streptomyces are high GC rich, Gram-positive filamentous actinobacteria belonging to the family Streptomycetaceae and are well-known for their ability to produce secondary metabolites, hydrolytic enzymes, anti-tumor agents and enzyme inhibitors many of which are active against pathogenic microorganisms (Harald et al. 2008). Chitinases from Streptomyces species have been implicated in protecting the roots of plants from a variety of plant pathogenic fungi (Stackebrandt and Goebel 1994) by degrading the cell wall.
During the past few years, the activity of several psychrophilic enzymes has been reported (Luo et al. 2006; Yaish et al. 2006) and the three-dimensional structure of some of them has been determined (Van Petegem et al. 2003; Violot et al. 2005). However, few psychrophilic chitinases have been isolated from bacteria (Bendt et al. 2001; Orikoshi et al. 2003) and fungi (Fenice et al. 1998).
The extracellular location of secreted chitinases implies that they must be adapted to function under the physicochemical conditions present in the surrounding environment (LeCleir et al. 2004). Therefore, harsh environmental conditions such as extreme temperatures, high salinity, pH, etc. may impose selective pressure for evolution of proteins with unique catalytic sequences and biochemical properties. Therefore, the diversity of cold adapted rhizospheric Streptomyces in the unexplored niche of high altitude alpine soils of Tawang, and the distribution of their chitinase genes was the focus of our current study.
Materials and methods
Sampling site and sample collection
The soil and water samples were collected from Tawang district (27°30’N, 91°51’E) in Western Arunachal Pradesh, India at an altitude of 4,175 m above the sea level. Samples were collected during December from various geographic locations where the daytime temperature was 5 °C and nighttime temperature was −2 °C. The average humidity was 35 %. Prior to collection, 1 cm of the surface soil was removed with a sterile spatula, and using another sterile spatula, the soil was collected, transferred into sterile polythene bags, transported to the laboratory under sterile conditions in HiDispo BagTM-12 (HiMedia, Mumbai, India) and stored at −10 °C until use. Every sample is a mixture of soils from 5 to 10 holes at a depth of 0–20 cm.
Isolation media and culture condition
Isolation of Streptomyces was carried out following suspension in physiological 25 % w/v NaCl solution, thoroughly homogenized by stirring and plating the serial dilutions (up to 10−5) on standard selection media viz; ISP-4, oatmeal agar, MYM agar, CSPY and Starch-casein (SC), and supplemented with amphotericin B (25 μg/ml) and rifampicin (40 μg/ml). Distinct morphotypic colonies observed after 10–12 days incubation at 6–30 °C were selected and purified by repeated streaking onto CSPY agar slants (g/l) (K2HPO4, 0.5 g; casein, 3.0 g; maize starch, 10.0 g; peptone, 1.0 g; malt extract, 10.0 g; yeast extract, 1.0 g; Agar, 15 g; pH 7.5) and maintained at 4 °C.
Phenotypic characterization
Streptomyces strains were observed under light microscope for acid-fastness and Gram-staining properties. Morphological characters were observed on CSPY agar plate. Physiological criteria such as the ability to degrade casein and tyrosine as substrates by various Streptomyces strains were used for genus confirmation. The utilization of different carbon sources, production of melanin pigment and utilization of urea were also studied (Korn-Wendisch and Kutzner 1991; Sarma et al. 2012).
Screening for antimicrobial activity and chitinolytic system
A total of 210 isolated Streptomyces (TSR1–TSR210) were screened for their antifungal activity against test fungal pathogens, Fusarium oxysporum f. sp. ciceri (NCIM 128), Fusarium moniliforme (NCIM 1099) and Rhizoctonia solani (MTCC 4633). The test pathogens were obtained from Microbial Type Culture Collection (MTCC) and Gene Bank, Institute of Microbial Technology (IMTECH), Chandigarh, India. For agar well diffusion assays, the pure isolates were cultured in 500 ml Erlenmeyer flasks containing 100 ml of CSPY liquid medium for 4–5 days at 16 ± 2 °C at 200 rpm. For the bioassays, 100 μl of crude culture filtrate was centrifuged at 10,000×g for 5 min and the supernatant was used. For the zone of inhibition assays, cleared zones were recorded after 96 h and only strains with positive bioactivity were included for further study. Extracellular chitinase production by the isolates was carried out by spot inoculation on colloidal chitin amended agar media (colloidal chitin 0.2 %, KCl 0.05 %, K2HPO4 0.1 %, (NH4)2SO4 0.2 %, MgSO4·7H2O 0.05 % and FeSO4 0.001 %, pH 7.2). The screening of the chitinase-producing Streptomyces sp. was carried out as described earlier (Li and Liu 2006). Colloidal chitin was incorporated at 1 % w/v in buffer agar media.
To investigate the stability of the antifungal factor in the culture filtrate, exponential culture filtrates were either treated with 0.1 mg/ml of proteinase K at 37 °C for 60 min or boiled for 45 min. The inhibitory effects of the treated filtrates were again determined as described above. Allosamidin (Midwest Biochemical Ltd., US) dissolved in 0.1 M acetic acid was sterilized by passing through a 0.25 μm filter and added to chitin medium at the concentration of 1 μM. Strains TSR23, TSR22, TSR18, TSR24, TSR12 and TSR28 were inoculated and incubated for 5–6 days at 16 °C and 200 rpm on a rotary shaker (Suzuki et al. 2008). The culture filtrate was centrifuged at 10,000×g and the supernatant used for antifungal assay as previously.
PCR amplification of 16S rRNA gene and chitinase gene
The genomic DNA was isolated by GeneiPure DNA extraction kit (Bangalore Genei, India). The small subunit ribosomal RNA gene was amplified using the primer set fD1 (5′-AGA GTT TGA TCC TGG CTC AG-3′) and rP2 (5′-ACG GCT ACC TTG TTA CGA CTT-3′) as done previously (Weisburg et al. 1991). The amplicons were gel separated and then purified using the PureLink® Quick Gel Extraction Kit (Invitrogen). The purified ~1.5 kb fragments were then sequenced.
Antifungal Streptomyces with chitinase-producing ability were PCR screened with family 18 and family 19 chitinase specific primer pairs as described previously (Watanabe et al. 2004). PCR cocktails for 50-μl reaction mixtures contained 1× PCR buffer, 2.5 mM MgCl2, 100 μM each deoxynucleoside, 300 nM each oligonucleotide primer (Eurofins mwg Operon), Platinum Taq polymerase 0.08 U (Invitrogen) and 50–100 ng purified DNA sample, with H2O making up the remaining volume. The 380 bp amplicons from the six strains showing amplification of only a family 18 group A, using the degenerate primer set GA1F (5′-CGT CGA CAT CGA CTG GGA RTD BCC-3′) and GA1R (5′-ACG CCG GTC CAG CCN CKN CCR TA-3′), were purified and sequenced.
Sequencing of 16S rRNA and chitinase gene amplicons
The purified PCR products were sequenced using the same primers for ribosomal RNA gene and using GA1F and GA1R primer for chitinase gene. DNA sequence was determined by fluorescent terminators (Big Dye, Applied Biosystems) and run in an Applied Biosystems ABI prism automated DNA sequencer (3130 × l).
Chimeric artifacts were sorted out using Mallard Software Program version 1.02 (http://www.bioinformatics-toolkit.org/index.html) and Pintail version 1.0. The 16S rRNA partial sequences of the Streptomyces and the partial chitinase gene sequences after analysis and annotation were deposited in NCBI Gene Bank.
GenBank accession number for the 33 Streptomyces strains are JF707861–JF707870, JF755879–JF755888, JF825427–JF825436, JF932302, JF338142, JF338857 and accession number for the 6 partial chitinase sequence genes are JQ321838, JQ320491–JQ320495 (protein_id: AFA36632, AFH01027–AFH01031).
Phylogenetic analysis
The curated sequences were subjected to BLAST sequence similarity search and EzTaxon (Chun et al. 2007) to identify the nearest taxa. Clustal W multiple sequence alignment was done with nucleotide sequences, belonging to the nearest taxa, downloaded from the database (http://www.ncbi.nlm.nih.gov). Pairwise evolutionary distances were computed using the DNADIST programme with the Kimura 2-parameter model. Phylogenetic and molecular evolutionary analyses were conducted using distance-based Neighbor Joining (NJ) and by sequence-based Maximum parsimony option of MEGA version 5 software package (Tamura et al. 2011). Bootstrapping with 1,000 replicates assessed the stability among the clades recovered in the phylogenetic tree.
For chitinase relatedness, the protein sequences were aligned using the Clustal Omega program after protein–protein blast results and Neighbor Joining tree generated by distance matrix method.
Total RNA preparation, reverse transcription and real-time qPCR amplification
Total RNA from strains TSR23, TSR22, TSR18, TSR24, TSR12 and TSR28 was purified using RaFlex™ Total RNA isolation kit (Bangalore Genei, India) according to manufacturer’s protocol, during exponential phase of growth. The Streptomyces were grown in colloidal chitin and F. oxysporum f. sp. ciceri cell walls (0.5 % w/v) supplemented media as inducer for 72 h at 15 °C. Integrity of RNA was confirmed on a 2 % agarose gel. Total RNA was quantified using Qubit® 2.0 fluorometer (Invitrogen).
High capacity cDNA Reverse Transcription kit (Applied Biosystems, USA) was used to reverse transcribe total RNA into cDNA. Furthermore, genomic DNA contamination in RNA samples was detected by performing a ‘no-reverse transcriptase’ control in the cDNA synthesis procedure. Relative quantification was achieved using the comparative C t method taking 16S rRNA gene as an endogenous control. A primer concentration of 200/200 nM (forward/reverse) was chosen for all genes based on optimization of each primer set using standard curves. Expression levels of chitinase were quantified with a real-time q-PCR assay performed in a 96 well StepOnePlus™ system (Applied Biosystems, USA) using Power SyBr Green PCR master mix (Applied Biosystems, USA). No template control (NTC) reactions replacing the cDNA template contained sterile water. Each reaction was replicated in triplicates for each gene in separate tubes. Each 20 μl reaction mixture consisted of 10 μl master mix (2.0×), forward and reverse primer 2.0 μl each (10 pmol/μl), sample cDNA (10×) 2.0 μl, H2O 6.0 μl. The PCR conditions followed the manufacturer’s instruction. The expression level calculated by the formula 2−ΔΔCt represents the x-fold difference from the calibrator.
Results
Isolation, phenotypic characterization and antifungal activity of Streptomyces strains
A total of 210 cold tolerant putative Streptomyces species were isolated from the soil samples of Tawang based on colony morphology. Temperature requirement ranged from 6 to 30 °C with optimal growth for most of the strains to be around 16 °C. Currently the functional low temperature limits of psychrophiles are −12 °C for reproduction and −20 °C for metabolism (Bakermans 2008). A variation in the antagonism of the Streptomyces strains against the pathogens was observed (Table 1). Among the 210 isolates, in vitro analysis for bioactive potential revealed 33 strains putatively Streptomyces, which were active against phytopathogenic fungus.
Most of these strains showed typical morphology of Streptomyces with branched and non-fragmented substrate mycelia, abundant aerial hyphae and short or long spore chains with or without pigmentation. All the colonies of the strains were slow growing, aerobic, glabrous or chalky, heaped with substrate mycelia of colors and possessed an earthy smell. The strains were acid-fast negative and gram positive, degraded the substrates casein, however, degradation of tyrosine was variable according to each isolate (supplementary material, Table S1). Microscopically, it was revealed that the spore chain morphology differed depending on the species, showing straight and flexuous forms, hooks, open loops and coils.
Based on the aerial mycelium color, the strains could be grouped into grey and white. Different colors of mycelia were also observed with light brown and greyish being the predominant. Few had substrate mycelia of a violet, purple or red-violet color. Utilization of several carbohydrates varied according to each strain. Eleven strains had capacity to utilize l-arabinose, while eight utilized sucrose, d-mannitol, raffinose and l-rhamnose. Two strains were able to use d-fructose as a carbon source. A majority of the strains (22 of 33) could degrade urea, and 13 could produce diffusible pigments in the surrounding medium.
Phylogenetic analysis of the antimicrobial Streptomyces
The 16S rRNA gene sequence analysis was carried out to elucidate the within species variation among the Streptomyces strains we isolated. Sequences (1,148–1,466 bp) for each of the 33 strains were compared with each other. Our results showed that 26 of the strains had a 99 % similarity with various Streptomyces spp., while the remaining seven had a 98 % similarity to different species of Streptomyces (supplementary material, Table S2). Ten distinct phyletic clusters were formed from our analysis corresponding to different bootstrap values using Micromonospora aurantiaca as an out-group. A phylogenetic tree based on 16S rDNA sequences was constructed by distance as well as sequence-based method both of which were concordant (Fig. 1a, b).
a Maximum-parsimonious phylogenetic tree, derived from 16S rDNA partial sequence data, showing the affiliation of the 33 Streptomyces strains (black dot) with their nearest phylogenetic relations. The 16S rDNA sequence of Micromonospora aurantiaca W2b was chosen arbitrarily as the out group sequence for the trees. Bootstrap resampling values >70 % are included at the internal nodes. b Neighbor joining tree (1,000 bootstraps) resulting from the alignment of nearly complete (~1,500 bp) 16S rRNA nucleotide sequence of the Streptomyces strains and the database hits, out grouping was done with Micromonospora aurantiaca W2b as out group. Alignment gaps and missing data were completely deleted in pairwise sequence comparison. Scale bar indicates 0.01 substitutions per nucleotide position
Phylogenetic analysis for intraspecies variation showed a stable tree explaining sequence conservation among the Streptomyces genes. Our 16S rDNA-based similarity scores conferred with the rpoB DNA sequences based phylogenetic tree for the genera of Streptomyces and Kitaspora described by Kim and co-workers (2004). TSR50 and TSR23 formed a distinct monophyletic line with Streptomyces shaanxiensis CCNWHQ and Streptomyces griseoplanus NRRLISP 5009 clone 1 whereas TSR41 and TSR43 showed 93 and 95 % relatedness to Streptomyces rishiriensis NRRLB-3239 and Streptomyces humidus NBRC 13254, respectively. Streptomyces kanamyceticus TSR1 at 99 % bootstrap value formed a cluster with NBRC13414 (AB184388.1). In the clad VII Streptomyces purpeofuscus TSR11, TSR6, TSR46 clustered together with S. purpeofuscus NBRC 12905(T) at approximately 90 % bootstrap value. Streptomyces xanthocidicus TSR4 was more related to Streptomyces tsukiyonensis NBRC 14353 than with S. xanthocidicus IFO13469.
However, polyphasic taxonomy for modern prokaryotic taxonomy is important in the genera of Streptomyces due to strain heterogeneity among species used to produce bioactive materials like antibiotics.
PCR detection of chitinolytic system
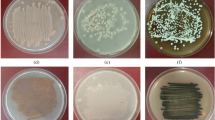
Chitin utilization was observed for the antifungal positive strains, which showed clear halos on spot inoculated colloidal chitin media. In the qualitative spot assay, 17 of 33 Streptomyces strains were able to utilize chitin. PCR screening for the detection of chitinase genes in the Streptomyces strains covered the range of chitinase types found in bacterial community including both family 18 and 19. Most of the strains showed multiplicity and duplicity of the chitinase gene. Since 17 strains inferred the presence of chitinolytic system in the spot assay, they were PCR screened. Of these, nine strains showed the presence of a family 19 chitinase (Table 2) while eight strains (TSR12, TSR24, TSR18, TSR28, TSR22, TSR23, TSR7 and TSR38) showed the presence of a family 18 chitinase (bacterial or Streptomyces specific). Two strains (TSR43 and TSR46) showed no detection with family 18 bacterial, group A or B, Streptomyces chitinase but with only Streptomyces family 19 chitinase. Six of eight strains showed the presence of only bacterial but not Streptomyces specific family 18 chitinase. Coincidentally, they are the same Streptomyces strains that exhibited strong antifungal activity (inhibition zone ≥22 mm) against all the fungal pathogens tested previously. Light micrographs of the six strains are captured by coverslip method (Fig. 2) (Williams and Cross 1971).
Chitinolytic nature of the antifungal active fraction
In order to corroborate whether the antimicrobial activity present in the crude extract is due to a combination of secondary antifungal metabolite(s) and extracellular hydrolytic enzymes or extracellular secondary metabolite(s) alone, bioassays were carried out using heat-treated and proteinase K-treated crude extract taken from the exponential phase of the six potent antifungal strains. Our results showed decreased inhibitory activity by many fold in the treated exponential culture filtrate as compared to untreated exponential culture filtrate, indicating the role of heat labile proteins in the antifungal activity (Fig. 3a–c). On the contrary, the stationary phase culture filtrate under equivalent treatment with heat and proteinase K as well as non-treatment showed a lack of activity against the fungal pathogens. The results of this study suggest that the inhibitory activity of the extracellular culture filtrate against fungal pathogens is largely due to the extracellular exponential phase antifungal enzymes such as chitinases.
Effect of heat and Proteinase K on the antifungal activity of culture filtrates a F. oxysporum f. sp. ciceri, b F. monoliforme, and c R. solani. Exponential culture filtrates from the six potent antifungal strains TSR22, TSR23, TSR18, TSR24, TSR12 and TSR28 were treated with Proteinase K at 37 °C for 60 min or heat treated at 100 °C for 15 min. The treated filtrates were then used for antifungal radial growth inhibition assay against the phytopathogenic fungus (n = 3, n = number of replicates). Fungal growth was assessed after 3–4 days of incubation and the percentage of growth inhibition was calculated. Stationary phase culture filtrates did not show any bioactivity and hence were not included in the study
Allosamidin can inhibit all family 18 chitinases, but does not inhibit family 19 chitinases (Sakuda et al. 1993). Strains were cultured in the chitin medium with and without allosamidin, and chitinase activity in each culture filtrate was measured. The results showed that antifungal activity in the culture filtrates was much reduced by addition of allosamidin as compared to untreated control (Fig. 4a, b). TSR18, which has significant antifungal activity against F. oxysporum f. sp. ciceri and F. moniliforme, showed a total inhibition of activity against the same fungus on allosamidin treatment at 1 μM. Total inhibition of antifungal activity against F. moniliforme and R. solani was shown by TSR12 as compared to untreated extracts. All the other treated extracts showed significantly reduced or minimum inhibition as compared to untreated controls but not total activity inhibition, which may be due to the concentration of allosamidin used. This result suggests that a family 18 chitinase is the active component for fungal inhibition produced extracellularly by the six Streptomyces strains (supplementary material, Fig. S3).
Effect of allosamidin on the activity of crude extracts from TSR23, TSR18, TSR28, TSR22, TSR12 and TSR24. Allosamidin was added at 1 μM concentration in chitin media. Zone of inhibition in mm (n = 3, n = number of replicates) was recorded for inhibitor treated (a) and untreated extracts (b). The values of relative antifungal activity were calibrated based on the activity of the control without allosamidin and mean ± SE were shown (N = 3)
Chitinase gene expression analysis
Saito et al. (2000) reported that family 18 and 19 chitinase gene clusters were transcriptionally co-regulated under the influence of colloidal chitin in Streptomyces coelicolor. The six highly antifungal strains (TSR23, TSR18, TSR22, TSR28, TSR12, TSR24) of the genera under study were subjected to comparative ΔΔCt quantitative PCR for their chitinase expressing ability keeping the 16S rRNA gene as an endogenous control. A single product-specific melt curve was obtained for primers of chi 18 and 16S rRNA gene, respectively, specifying the primers optimal efficiency and efficacy at targeting and amplifying only the genes of interest (data not shown). Our results showed that both the genes (chi and 16S rRNA) were expressed constitutively. Comparison of the respective family 18 chitinase genes in the six potent antifungal strains showed TSR12 to be the most active producer under similar conditions of experimental treatment with 0.5 % w/v colloidal chitin and 0.5 % w/v F. oxysporum f. sp. ciceri cell walls (CWF) taking TSR28 as the calibrator (Fig. 5a, b). Colloidal chitin and CWF’s induced seven- to eightfold increased transcript level than TSR28 which formed similar clearance zone in the in vitro antifungal assay. This incongruity between variations in induced transcript accumulation to bioassay result might be attributed to low translation or export mechanism. TSR24 showed 50 % decreased expression when compared to TSR12. TSR18 and TSR22 have similar expression profile in colloidal chitin amended media and were intermediate producers among the six. In the fungal cell wall-treated growth media, TSR18 and TSR23 showed similar mRNA profile for chitinase induction. The mRNA profile alteration is also observed within colloidal chitin and CWF derived chitin-treated experimental condition. TSR23, TSR22, TSR24 displayed a shift in expression pattern when cultivated in CWF (0.5 % w/v) preparations as against colloidal chitin at (0.5 % w/v). This dissimilarity is mostly due to the complex nature of fungal cell walls containing chitosan and varying degree of chitin oligomers that act as signaling molecule in the chi gene regulation process.
Quantitative comparative gene expression of family 18 groups A chitinase genes. cDNA prepared from strains TSR23, TSR22, TSR18, TSR24, TSR12 and TSR28 after growth in a colloidal chitin (0.5 % w/v) and b Fusarium oxysporum f. sp. ciceri cell wall (0.5 % w/v) amended media were used for regulation of chi gene expression using real-time RT-PCR. The expression level is defined by the formula 2−ΔΔCt. Y-axis represents the x-fold difference from the calibrator. Error bars indicate SD
Family 18 chitinase diversity among the antifungal Streptomyces strains
To observe the relatedness among the chitinase genes of strong antifungal Streptomyces strains (TSR23, TSR18, TSR28, TSR22, TSR12 and TSR24), the amino acid sequences of the proposed catalytic domain were aligned and used for phylogenetic tree construction. Six distinct monophyletic groups were clustered separately (Fig. 6a, b). The partial coding sequence from the TSR23 chitinase (JQ320491, AFH01027) showed a close relationship with the chitinase from Streptomyces roseosporus NRRL15998 (ZP 06582696), while TSR18 (JQ320492, AFH01028) was related to Streptomyces globisporus C-1027 (ZP 11377855). The catalytic domain of the chitinases from TSR12 (AFH01029) formed a cluster with chitinase C from Streptomyces sp. Mg1 (ZP 04997691) whereas a similar strain TSR24 (AFH01030) formed a distinct monophyletic cluster. TSR22 chitinase (JQ321838, AFA36632) belonged to the same cluster as TSR2 but as a sub-clad with chitinase C from Streptomyces sp. C. chitinase (ZP 07289301). TSR28 (AFH01031) did not show any relatedness and showed early divergence forming a unique cluster. The chitinases in our study showed significant variation within the catalytic domain as observed by the phylogram.
a Deduced amino acid sequence alignment of catalytic chitin binding domains of TSR28, TSR22, TSR12, TSR24, TSR18, TSR23 and several other glycosyl hydrolase family 18 chitinase. An EF-hand calcium binding motif- D43GSNGGKLDaaDY55 (“Prosite signature” for glycosyl hydrolase family 18) detected in all six sequences are essential for chitinase activity; b Neighbor-joining tree showing phylogenetic relationships between glycosyl hydrolase family 18, group I chitinase amino acid sequences (catalytic domain) from the six antifungal strains against the GenBank matched entries related to the particular family
Discussion
Owing to its rich biodiversity, North-Eastern India is prioritized for investment by the leading conservation agencies of the world. The conservation international scaled the eastern Himalaya as Indo Burma hotspot (Myers et al. 2000) including all the eight states of North-East India; the WWF has identified the entire Eastern Himalaya as a priority Global 200 Eco-region. Here, we reported the findings of the first study in which psychrotolerant Streptomyces isolates from different areas of Tawang have been investigated for their antimicrobial activity. Comprehensive variation in Streptomyces spp. was found within a limited area. Gangwar et al. (2009) showed that diversity of psychrophilic bacterial communities in high altitude cold soils of the Himalayan Mountains decreased with increasing altitude. We obtained six antifungal strains of Streptomyces (TSR23, TSR18, TSR22, TSR28, TSR12 and TSR24) with enhanced chitinase activity depicting their ability to function effectively without losing significant activity at low temperature (<20 °C). In the present study, 16S rDNA sequence homology revealed the taxonomic affiliation of 98–99 % of cold adapted strains with different species of Streptomyces. Although the Streptomyces genus is known to produce an arsenal of antibiotics and secondary metabolites which largely mediates the inhibitory activity against pathogen role of PR proteins like chitinases, β-glucanase cannot be ruled out. In addition, heat-treated and proteinase K-treated crude extract during the exponential phase from the six potent strains showed decreased inhibitory activity by many fold suggesting the role of heat labile proteins in the antifungal activity.
The conserved nature of chitinolytic system of Streptomyces was also detected at the molecular level as PCR amplicons using primer sets for bacterial chitinase family 18 group A, Streptomyces group A and group B chitinases and family 19 chitinase (Table 2). The screening of the antifungal chitin utilizing Streptomyces accorded with previous reports showing distribution and duplicity of the chitinase gene in this particular genera (Miyashita et al. 2003). Although family 19 chitinases which share high level of similarity to plant class IV chitinases are more prevalent in the genera of Streptomyces (Watanabe et al. 2004), six of the potent antifungal strains in the current study showed no detection with family 19 specific primers but showed amplification with bacterial and Streptomyces specific family 18 group A, group B chitinase. The absence of family 19 chi genes in these actively overproducing antifungal strains gives us an interesting finding (Table 2).
A second approach using allosamidin was adopted to endorse family 18 chitinase liable for the bioactivity and not just conclude the absence of family 19 genes from failed PCR approach using primers for family 18 and 19 genes. Complete to partial inhibition of bioactivity was achieved with allosamidin at 1 μM concentration, although minimum dosage of activity inhibition was not studied. TSR18 showed complete loss of antifungal activity against F. oxysporum f. sp. ciceri. It was shown previously that variation and correlation of nonessential genes such as chitinase and glucanase with that of rRNA phylogeny differ greatly from that of essential genes such as nifH, nirK, nirS, nosZ, pmoA, mxaA basically involved in specific biogeochemical processes due to events of lateral gene transfer, gene duplication and different rates of evolution (Cottrell et al. 2000). Antifungal chitinases in our study followed a similar trend and showed that the phylogeny of chitinase gene contrasted from 16S rRNA phylogeny. In contrast to the 16S rRNA genes, which are located in the conserved core part of the Streptomyces chromosome, genes coding for morphological and physiological characters, such as pigments and production of extracellular enzymes, are often located in the chromosome arms, which can undergo remarkable rearrangements (Bentley et al. 2002). Therefore, the 16S rDNA sequence diversity does not necessarily reflect the diversity of other properties, and depending on application, other genes could be used viz., gyrB, rpoB, rpoD, recA.
Chitinases from glacial microorganisms appear to have adaptations required to function well in cold environments which has been demonstrated for ChiA and ChiB from an Arthrobacter strain isolated from Antarctic sediment (Lonhienne et al. 2001). The increased heat lability of these chitinases is believed to be an effect of structural changes that give the enzymes greater flexibility at lower temperatures (Gerday et al. 1997). Cold-active chitinase is considered to be a useful biocatalyst for the production of pure GlcNAc monomer or oligomers during cold-condition processing in industrial biotechnology. At present, the only example used in biotechnological application for food and health products is an endochitinase from an Arthrobacter strain isolated in Antarctic marine environment (Cavicchioli et al. 2002). One of the interesting finding in plants states that chitinase genes responsive to cold encode antifreeze proteins in plants like Secale cereale (Yeh et al. 2000). These AFP’s are similar to pathogenesis-related (PR) proteins that are normally secreted in response to infection by pathogens as part of the mechanism for disease resistance (Stinzi et al. 1993). Cold-induced accumulation of PR proteins like chitinases is also reported in barley, potatoes, bermudagrass, and carrots (Tronsmo et al. 1993; Zhu et al. 1993; Ergon et al. 1998). These findings provide similar avenues to study the adaptive interaction between a microbe and its environment and the molecular events involved in the evolution of proteins as they acquire new functions.
Lastly, real-time q-PCR data showed regulated expression for the chi genes, where Streptomyces roseochromogenus TSR12 showed very active transcriptional potential. In F. oxysporum f. sp. ciceri cell wall-treated induction media TSR12 showed highest level of gene expression, this result suggested that some soluble component from the cell wall may act as inducer for chi expression. A difference in induction potential between the naturally occurring polymer in fungal cell wall and purified colloidal chitin used in our assay was observed. TSR22 and TSR24 showed up-regulation for the gene with colloidal chitin but were repressed with fungal cell wall treatment. Such alteration in expression data needs more study as recently Price et al. (2013) proposed the widespread occurrence of indirect and suboptimal control of gene expression in bacteria inferring that gene regulation in bacteria is not usually an adaptive response to environmental change. TSR12 and TSR18, however, were not affected in both the treatments.
With cryogenic surfaces imposing incessant challenges to microbial life, frequent evolution of a number of adaptive mechanisms with regard to reproduction, metabolic activities and protection strategies is undoubtedly increasing the window for microbial ecological exploration and also a repertoire of bioactive molecules and factors with diverse functionality.
References
Ayala-del-Rio HL, Chain PS, Grzymski JJ, Ponder MA, Ivanova N, Bergholz PW, Di Bartolo G, Hauser L, Land M, Bakermans C, Rodrigues D, Klappenbach J, Zarka D, Larimer F, Richardson P, Murray A, Thomashow M, Tiedje JM (2010) The genome sequence of Psychrobacter arcticus 273–4, a psychroactive Siberian permafrost bacterium, reveals mechanisms for adaptation to low-temperature growth. Appl Environ Microbiol 76:2304–2312
Bakermans C (2008) Limits for microbial life at sub-zero temperatures. In: Margesin R, Schinner F, Marx J-C, Gerday C (eds) Psychrophiles: from biodiversity to biotechnology. Springer, Berlin, pp 17–28
Bendt A, Huller H, Kammel U, Helmke E, Schweder T (2001) Cloning, expression and characterization of a chitinase gene from the Antarctic psychrotolerant bacterium Vibrio sp. strain Fi: 7. Extremophiles 5:119–126
Bentley SD, Chater KF, Cerdeño-Tárraga AM, Challis GL, Thomson NR, James KD et al (2002) Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141–147
Cavicchioli R, Siddiqui KS, Andrews D, Sowers KR (2002) Low-temperature extremophiles and their applications. Curr Opin Biotechnol 13:253–261
Chun J, Lee JH, Jung Y, Kim M, Kim S, Kim BK, Lim YW (2007) EzTaxon: a web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int J Syst Evol Microbiol 57:2259–2261
Cottrell MT, Wood DN, Yu L, Kirchman DL (2000) Selected chitinase genes in cultured and uncultured marine bacteria in the α- and β-subclasses of the proteobacteria. Appl Environ Microbiol 66:1195–1201
Deming JW (2002) Psychrophiles and polar regions. Curr Opin Microbiol 5:301–309
Ergon Å, Klemsdal SS, Tronsmo AM (1998) Interactions between cold hardening and Microdochium nivale infection on expression of pathogenesis-related genes in winter wheat. Physiol Mol Plant Pathol 53:301–310
Fenice M, Selbmann L, Di Giambattista R, Federici F (1998) Chitinolytic activity at low temperature of an Antarctic strain A3 of Verticillium lecanii. Res Microbiol 149:289–300
Gangwar P, Alam SI, Bansod S, Singh L (2009) Bacterial diversity of soil samples from the western Himalayas, India. Can J Microbiol 55:564–577
Gerday C, Aittaleb M, Arpigny JL, Baise E, Chessa JP, Garsoux G, Petrescu I, Feller G (1997) Psychrophilic enzymes: a thermodynamic challenge. Biochim Biophys Acta 1342:119–131
Harald B, Espen F, Geir Sergey BZ (2008) Actinomycetes from sediments in the Trondheim Fjord, Norway: diversity and biological activity. Mar Drugs 6:12–24
Kim BJ, Kim CJ, Chun J, Koh YH, Lee SH, Hyun JW, Cha CY, Kook YH (2004) Phylogenetic analysis of the genera Streptomyces and Kitasatospora based on partial RNA polymerase β-subunit gene (rpoB) sequences. Int J Syst Evol Microbiol 54:593–598
Korn-Wendisch F, Kutzner HJ (1991) The family Streptomycetaceae. The Prokaryotes. A handbook on the biology of bacteria: ecophysiology, isolation, identification, applications, Chap. 41. Springer, Berlin, pp 965–968
LeCleir GR, Buchan A, Hollibaugh JT (2004) Chitinase gene sequences retrieved from diverse aquatic habitats reveals environment-specific distribution. Appl Environ Microbiol 70:6977–6983
Li ZY, Liu Y (2006) Marine sponge Craniella austrialiensis-associated bacterial diversity revelation based on 16S rDNA library and biologically active actinomycetes screening phylogenetic analysis. Lett Appl Microbiol 43:410–416
Lipson DA (2007) Relationships between temperature responses and bacterial community structure along seasonal and altitudinal gradients. FEMS Microbiol Ecol 59:418–427
Lonhienne T, Mavromatis K, Vorgias CE, Buchon L, Gerday C, Bouriotis V (2001) Cloning, sequences, and characterization of two chitinase genes from the Antarctic Arthrobacter sp. strain TAD20: isolation and partial characterization of the enzymes. J Bacteriol 183:1773–1779
Luo Y, Zheng Y, Jiang Z, Ma Y, Wei D (2006) A novel psychrophilic lipase from Pseudomonas fluorescens with unique property in chiral resolution and biodiesel production via transesterification. Appl Microbiol Biotechnol 73:349–355
Margesin R, Miteva V (2011) Diversity and ecology of psychrophilic microorganisms. Res Microbiol 162:346–361
Miyashita K, Saito A, Fujii T (2003) Distribution and evolution of chitinase genes in Streptomyces species: involvement of gene-duplication and domain-deletion. Antonie Van Leeuwenhoek 84:7–16
Myers N, Mittermeier RA, Mittermeier CG, Gustavo AB da Fonseca, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Orikoshi H, Baba N, Nakayama S, Kashu H, Miyamoto K, Yasuda M, Inamori Y, Tsujibo H (2003) Molecular analysis of the gene encoding a novel cold-adapted chitinase ChiB from a marine bacterium, Alteromonas sp. strain O-7. J Bacteriol 185:1153–1160
Petrovic U, Gunde-Cimerman N, Zaler P (2000) Xerotolerant mycobiota from high altitude Anapurna soils, Nepal. FEMS Microbiol Lett 182:339–342
Price MN, Deutschbauer AM, Skerker JM, Wetmore KM, Ruths T, Mar JS, Kuehl JV, Shao W, Arkin AP (2013) Indirect and suboptimal control of gene expression is widespread in bacteria. Mol Syst Biol 9:660
Saito A, Ishizaka M, Francisco PB Jr, Fujii T, Miyashita K (2000) Transcriptional co-regulation of five chitinase genes scattered on the Streptomyces coelicolor A3(2) chromosome. Microbiology 146:2937–2946
Sakuda S, Isogai A, Suzuki A, Yamada Y (1993) Chemistry and biochemistry of the chitinase inhibitors, allosamidins. Actinomycetologica 7:50–57
Sarma RK, Debnath R, Saikia R, Handique PJ, Bora TC (2012) Phylogenetic analysis of alkaline protease producing fluorescent pseudomonads associated with green gram (Vigna radiata L.) rhizosphere. Folia Microbiol 57:129–137
Stackebrandt E, Goebel BM (1994) Taxonomic note: a place for DNA DNA reassociation and 16S rDNA sequence analysis in the present species definition system, Actinobacteria Classis nov. Int J Syst Bacteriol 47:479–491
Steven B, Leveille R, Pollard WH, Whyte LG (2006) Microbial ecology and biodiversity in permafrost. Extremophiles 10:259–267
Stinzi A, Heitz T, Prasad V, Wiedemann-Merdinoglu S, Kauffmann S, Geoffroy P, Legrand M, Fritig B (1993) Plant pathogenesis-related proteins and their role in defense against pathogens. Biochimie 75:687–706
Suzuki S, Nakanishi E, Furihata K, Miyamoto K, Tsujibo H, Watanabe T, Ohnishi Y, Horinouchi S, Nagasawa H, Sakuda S (2008) Chitinase inhibitor allosamidin promotes chitinase production of Streptomyces generally. Int J Biomac 43:13–19
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tronsmo AM, Gregersen P, Hjeljord L, Sandal T, Bryngelsson T, Collinge DB (1993) Cold-induced disease resistance. In: Fritig B, Legrand M (eds) Mechanism of plant defense responses. Kluwer Academic Publishers, Dordrecht, p 369
Van Petegem F, Collins T, Meuwis MA, Gerday C, Feller G, Van Beeumen J (2003) The structure of a cold-adapted family 8 xylanase at 1.3 A resolution. Structural adaptations to cold and investigation of the active site. J Biol Chem 278:7531–7539
Violot S, Aghajari N, Czjzek M, Feller G, Sonan GK, Gouet P, Gerday C, Haser R, Receveur-Bre′chot V (2005) Structure of a full length psychrophilic cellulase from Pseudoalteromonas haloplanktis revealed by X-ray diffraction and small angle X-ray scattering. J Mol Biol 348:1211–1224
Watanabe T, Kawase T, Saito A, Sato T, Kanai R, Fujii T, Nikaidou N, Miyashita K (2004) Distribution and phylogenetic analysis of Family 19 chitinases in Actinobacteria. Appl Environ Microbiol 70:1135–1144
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Williams ST, Cross T (1971) Actinomycetes isolation from soil. In: Booth C (ed) Methods in Microbio. Academic Press, London, pp 295–334
Yaish MW, Doxey AC, McConkey BJ, Moffatt BA, Griffith M (2006) Cold-active winter rye glucanases with ice-binding capacity. Plant Physiol 141:1459–1472
Yeh S, Moffatt BA, Griffith M, Xiong F, Yang DSC, Wiseman SB, Sarhan F, Danyluk J, Xue YQ, Hew CL, Doherty-Kirby A, Lajoie G (2000) Chitinase genes responsive to cold encode antifreeze proteins in winter cereals. Plant Physiol 124:1251–1263
Zhu B, Chen THH, Li PH (1993) Expression of an ABA responsive osmotin-like gene during the induction of freezing tolerance in Solanum commersonii. Plant Mol Biol 21:729–735
Acknowledgments
This work was supported by grants from the DBT sponsored project (No. BT/PR3679/NDB/39/214/2011) and from the ICAR-AMAAS project. The authors are thankful to the funding agencies. The authors are also thankful to the Director, CSIR-NEIST for providing necessary facilities to carry out the work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Albers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Debnath, R., Saikia, R., Sarma, R.K. et al. Psychrotolerant antifungal Streptomyces isolated from Tawang, India and the shift in chitinase gene family. Extremophiles 17, 1045–1059 (2013). https://doi.org/10.1007/s00792-013-0587-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-013-0587-8