Abstract
A lipase-producing bacterium strain B68 screened from soil samples of China was identified as Pseudomonas fluorescens. With GenomeWalker, the open reading frame of lipase gene lipB68, encoding 476 amino acids, was cloned and expressed in Escherichia coli BL21 (DE3). By affinity chromatography, the recombinant LipB68 protein was purified to the purity of 95%. As a member of lipase subfamily I.3, LipB68 has a unique optimum temperature of 20 °C, which was the lowest in this subfamily. In chiral resolution, LipB68 effectively catalyzed the transesterification of both α-phenylethanol and α-phenylpropanol at 20 °C, achieving E values greater than 100 and 60 after 120 h, respectively. Among all the known catalysts in biodiesel production, LipB68 produced biodiesel with a yield of 92% after 12 h, at the lowest temperature of 20 °C, and is the first one of the I.3 lipase subfamily reported to be capable of catalyzing the transesterification reaction of biodiesel production. Since lipase-mediated biodiesel production is normally carried out at 35–50 °C, the availability of a highly active lipase with a low optimal temperature can provide substantial savings in energy consumption. Thus, this novel psychrophilic lipase (LipB68) may represent a highly competitive energy-saving biocatalyst.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lipases (E.C. 3.1.1.3) catalyze the hydrolysis of acylglycerides and other fatty acid ester bonds. Because of the far ranging of substrates, these enzymes were recognized as valuable biocatalysts.
Pseudomonas genus produce a variety of lipases and are well-known psychrophilic lipase-producing bacteria (Rajmohan et al. 2002). Most of the lipases produced by Pseudomonas fluorescens belong to lipase subfamily I.1, I.2, or I.3. Among this group, lipases from subfamily I.3 have comparably larger molecular masses (50 kDa or above) and do not need additional gene products for correct folding (Rosenau and Jaeger 2000). Although both the biochemical and structural properties of lipases from subfamilies I.1 and I.2 have been well-studied, few lipases from subfamily I.3 have been characterized and found to be applicable in enzymatic industry. Even fewer of these enzymes have been found to be useful for enzymatic reactions at industrial scales (Lee et al. 1993; Amada et al. 2000; Rashid et al. 2001).
Biodiesel, produced through transesterification of fat or vegetable oils, has emerged as an attractive alternative to petroleum fuel, since it is biodegradable, nontoxic, and essentially free of sulfur and aromatics. Current industrial biodiesel production primarily relies on base-catalyzed transesterification of animal fat or vegetable oils, which have the drawbacks of pollution during the removal of catalyst and the requirement of energy-intensive process, because of the need of high temperatures and pressures. Therefore, the use of lipases to catalyze biodiesel production under mild conditions was studied by many researchers (Mohamed and Uwe 2003), and the development of new biocatalysts, particularly those highly active at lower temperatures, is the key point of studies.
In this article, we focused on the characterization of a newly cloned psychrophilic lipase from a lipase-producing P. fluorescens strain B68, investigated the enzymatic properties, including its enantioselectivity to two popular enantiomers, and evaluated the transesterification activity of the enzyme in the production of biodiesel.
Materials and methods
Screening of strains
Soil samples were collected from parts of China, and the microbes inside were accumulated and separated as described previously (Gao et al. 1998). The strains with lipase-producing abilities were identified according to their substrate utilization and biochemical traits at Shanghai Institute of Preventive Medicine (Shanghai, China).
Strains, plasmids, and enzymes
Escherichia coli strains DH5α and BL21 (DE3) were used as host cells, and plasmid pET-28a purchased from Novagen (Madison, WI) was used as the expression vector. DNA polymerase and restriction enzymes were purchased from Takara (Kyoto, Japan). Genomic DNA was isolated using genomic DNA isolation kit (Vitagene, China).
Cloning of full-length lipase gene
Pairs of primers (Table 1) were designed according to the homology of the lipase genes from P. fluorescens. Using genomic DNA extracted from P. fluorescens strain B68, highly conserved core sequences of lipase genes were amplified and determined.
The full-length gene cloning protocol was performed according to the method of GenomeWalker (Morris et al. 1998). Briefly, genomic DNA was cleaved by blunt-end-restriction enzymes (PvuII, EcoRV, ScaI, and DraI), to generate blunt-end DNA fragments. These fragments were then ligated with the universal walking adaptor (Table 1) at both sides. The ligation products were used as templates in subsequent polymerase chain reaction (PCR) amplifications. PCR was performed with three primers, one of them was complementary to the adaptors, and the others were complementary to the 5′ and 3′ end of a conserved segment (AP1 and 68-GSP1, respectively, see Table 1). To increase the PCR specificity, secondary PCR (nested PCR) was conducted with primers AP2 and 68-GSP2 (Table 1). The products of the secondary PCR were then sequenced and analyzed to reconstitute the complete open reading frame of lipase gene.
Sequencing and analysis of the gene lipB68
DNA sequencing of lipB68 was performed by Shenergy Biocolor Biological Science and Technology (Shanghai, China). Amino acid sequence alignments were performed with GENEDOC program. Lipase sequences for comparative study were retrieved from protein and nucleotide databases on the NCBI Entrez server, at http://www.ncbi.nlm.nih.gov/Entrez/.
Accession number of the gene lipB68
The GenBank accession number of gene lipB68 in NCBI is AY694785.
Expression and purification of LipB68 in E. coli
The recombinant protein LipB68 was expressed in E. coli BL21 (DE3) with the expression vector pET-28a. Isopropylthiogalactoside induced expression of LipB68, and LipB68 protein was produced in high quantity in insoluble inclusion bodies. The bacteria were harvested, sonicated, and inclusion bodies were collected by centrifugation. The inclusion bodies were then dissolved in 25 mM Tris–HCl (pH 8.0), containing 50 mM NaCl and 8 M urea. The solution was loaded on a Ni–NTA sepharose column (Amersham Pharmacia Biotech) equilibrated with the same buffer. The enzyme was eluted with a linear gradient of imidazole (from 0 to 1 M) in the same buffer and dialyzed against 5 M, 3 M, and 0 M urea in 25 mM Tris–HCl (pH 8.0), respectively. The purity of the protein was examined on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Immobilization of LipB68
Five grams of cellulose fabric was added to 20 ml of solution containing about 4 mg of purified recombinant LipB68, and the mixture was stirred for 3 h at room temperature. The cellulose fabric containing immobilized lipase was recovered through filtration, dried in a vacuum desiccator, and stored at 4 °C. The activity of immobilized LipB68 was detected to be 110 U/g by the olive oil hydrolyzation method described previously (Sugihara et al. 1991).
Lipase activity assay
Enzyme activities of purified LipB68 solution were assayed by measuring the absorbance at 410 nm of liberated p-nitrophenol. One unit of enzymatic activity was defined as the amount of enzyme needed to release 1 μmol p-nitrophenol per minute.
The reaction mixture (1.6 ml) contained 100 μl of p-nitrophenyl caprate solution (final concentration of 6.25 mM in the solution of isopropanol), 900 μl lipase assay buffer (100 mM Tris–HCl buffer, pH 8.0), 100 μl 80 mM CaCl2 solution, and 500 μl purified enzyme solution (containing 95 μg LipB68 protein). The solutions were incubated at a certain temperature for 10 min, and the reactions were terminated by adding 1 ml of ethanol.
The temperature-dependent activities of LipB68 were determined by adding the enzyme solutions to assay mixtures that had been preheated at the designated temperatures. The data of enzymatic activities were replicated at least three times.
Enantioselectivity of LipB68
R-α-phenylethanol and R-α-phenylpropanol and their ethyl esters were purchased from Sigma as standard samples. Racemic α-phenylethanol (Acros, USA) or α-phenylpropanol (Lancaster, UK; 0.5 mmol) was dissolved in 10 ml toluene, containing 1 mmol of vinyl acetate (Sinopharm, China), and 0.6 g of immobilized enzyme was added. The reactions were performed at 20, 35, or 50 °C, respectively, on a platform shaking at 200 rpm. The samples from reaction mixtures were analyzed by an Agilent 4890N gas chromatograph (Agilent, USA) equipped with HP-Chiral Column (J&W, USA) and flame ionization detection. The oven was initially held at 100 °C for 2 min and was then elevated to 120 °C at 5 °C/min and held 2 min, to 140 °C at 0.5 °C/min, and finally held for 5 min. The results were replicated at least three times and presented by mean value.
Enantiomeric excesses (ee), conversions (c), and enantiomeric ratios (E) were calculated using the corresponding peak areas:
-
ee=100×(A−B)/(A+B), where (A and B) were the peak areas of enantiomers, and A)>B);
-
c=ees/(ees+eep) (Mori and Akao 1980), where s and p represented substrate and product; \(E = {\ln {\left[ {1 - c{\left( {1 + {\text{ee}}_{{\text{p}}} } \right)}} \right]}} \mathord{\left/ {\vphantom {{\ln {\left[ {1 - c{\left( {1 + {\text{ee}}_{{\text{p}}} } \right)}} \right]}} {\ln {\left[ {1 - c{\left( {1 - {\text{ee}}_{{\text{p}}} } \right)}} \right]}}}} \right. \kern-\nulldelimiterspace} {\ln {\left[ {1 - c{\left( {1 - {\text{ee}}_{{\text{p}}} } \right)}} \right]}} = {\ln {\left[ {{\left( {1 - c} \right)}{\left( {1 - {\text{ee}}_{{\text{s}}} } \right)}} \right]}} \mathord{\left/ {\vphantom {{\ln {\left[ {{\left( {1 - c} \right)}{\left( {1 - {\text{ee}}_{{\text{s}}} } \right)}} \right]}} {\ln {\left[ {{\left( {1 - c} \right)}{\left( {1 + {\text{ee}}_{{\text{s}}} } \right)}} \right]}}}} \right. \kern-\nulldelimiterspace} {\ln {\left[ {{\left( {1 - c} \right)}{\left( {1 + {\text{ee}}_{{\text{s}}} } \right)}} \right]}}\) (Mori and Akao 1980).
The transesterification activity of LipB68 in the production of biodiesel
Methyl esters of palmitic, stearic, oleic, linoleic, and linolenic acids were purchased from Sigma as standard samples. Methyl heptadecanoate (Sigma, USA) was used as the internal standard. Furthermore, n-heptane was obtained from Shanghai Chemical Reagents Company.
Two millimoles (about 1.7 ml) of soybean oil (Luhua Group, Shandong) was dissolved in 8.3 ml of n-heptane, to which immobilized LipB68 (1 g) was added as catalyst and a total of 6 mmol of methanol was added in three equal additions of 2 mmol at 2-h intervals. The transesterification reactions were carried out at 20, 30, 40, or 50 °C as indicated, on a shaker table operated at 180 rpm.
Samples (5 μl) taken from the reactions were mixed with 7.5 μl of 40 mM heptadecanoic acid methyl ester and 290 μl of n-heptane, and were analyzed by gas chromatography with a HP-5 column (J&W). The oven was initially held at 180 °C for 1 min and was then elevated to 300 °C at 10 °C/min and held for 2 min. The results were replicated at least three times. The yields of biodiesel were calculated by following equations:
Yield=100×(A+B+C+D+E)/(3×F), where A, B, C, D, E, and F are the peak areas of methyl esters of palmitic, stearic, oleic, linoleic, linolenic, and heptadecanoic acids, respectively.
Results
Gene cloning, expression, and purification of LipB68
The open reading frame of lipB68 consisted of 1,428 nucleotides, encoding a protein of 476 amino acids with a molecular mass of 50,193 Da (Fig. 1). Searches in the Conserved Domain Database at NCBI showed that lipase LipB68 belonged to lipase subfamily I.3. Lipase LipB68 did not contain a secretion signal sequence on its N terminus, while its C-terminal region contained four GGXGXD consensus sequences, an 18 amino-acid residual amphiphilic α-helix, and a negatively charged amino acid followed by four hydrophobic residues (DGVSI). These indicated that its secretion mechanism might follow the ATP-binding cassette transporter system (Andersson 1980; Jaeger et al. 1994; Binet et al. 1997; Ahn et al. 1999; Rosenau and Jaeger 2000). Sequence analysis also revealed a highly reserved amino acid motif (GXSXG) among lipases in lipase LipB68: GHSLG (residues 205–209), therefore the Ser207, have been predicted to be the serine residue that could take part in formation of the catalysis triad Ser-His-Asp, of the lipase (Jaeger et al. 1994).
Multiple-sequence alignment of amino acid sequence of LipB68. The amino acid sequences of LipB68 and two homologous Pseudomonas fluorescens lipases, lipase SIK-W1 and lipase KB700 (GenBank Accession nos.: AAD09856 and BAB64913), were aligned with GENEDOC. The highly conserved amino acid motif (GXSXG) among lipases in LipB68. GHSLG (residues 206–209) is underlined with solid line. Four glycine-rich motifs (GGXGXD) and the sequence composed of a negatively charged amino acid followed by four hydrophobic residuals (DGVSI) is underlined by dotted lines, and the putative amphiphilic α-helix is underlined by a dot-dash line, all of which were the properties of proteins belonging to ABC-transporter secretion system
BLAST at NCBI showed that the amino acid sequence of LipB68 shared high identity with other lipases for which enzyme properties have been highlighted, such as the lipase from thermostable P. fluorescens SIK W1 lipase (Lee et al. 1993), KB700A (Rashid et al. 2001), and Pseudomonas sp. MIS38 (Amada et al. 2000) (GenBank accession nos. are AAD09856, BAB64913, and BAA84997, respectively), with identities being 89, 88, and 71%, respectively.
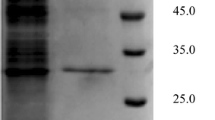
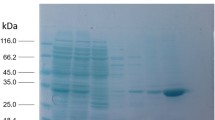
LipB68 can be highly expressed in pET28a at BL21 (DE3), and the recombinant lipase protein can be purified to greater than 95% by SDS-PAGE (Fig. 2).
Enzymatic properties of LipB68
The lipase activities toward various p-nitrophenyl esters (including p-nitrophenyl acetate, p-nitrophenyl butyrate, p-nitrophenyl caprate, p-nitrophenyl laurate, p-nitrophenyl palmitate, and p-nitrophenyl stearate) were examined at 20 °C and pH 8.0 using purified recombinant LipB68. Results showed that p-nitrophenyl caprate (C10 acyl group) was the most favorable substrate, and the specific activity of purified LipB68 toward p-nitrophenyl caprate was 423 U/mg of protein (data not shown).
With p-nitrophenyl caprate as substrate, LipB68 exhibited maximal activity at 20 °C, with decreased activities at higher and lower temperatures showing about 50% of maximal activity at 4 °C. The enzyme was almost totally inactivated at 60 °C (Fig. 3). The results suggest that LipB68 is a psychrophilic enzyme.
The enantioselectivity of LipB68 was investigated in the acylation of α-phenylethanol and its analogue, α-phenylpropanol (Scheme 1), at 20, 35, and 50 °C. Toward both substrates, immobilized LipB68 showed high preference to the R enantiomer (Table 2, Fig. 4). Consistent with the observation that LipB68 had an optimal temperature of 20 °C, the highest E value and conversion for both reactions were also observed at 20 °C. When α-phenylpropanol was used as a substrate, LipB68 yielded a higher conversion but lower E value at 50 °C than at 35 °C, likely due to increased reaction rates at the higher temperature.
The GC graphs of the samples of enantioselective transesterification of α-phenylethanol and α-phenylpropanol by immobilized LipB68. Racemic α-phenylethanol, 0.5 mmol, or α-phenylpropanol was dissolved in 10 ml toluene, containing 1 mmol of vinyl acetate and 0.6 g of immobilized enzyme. The reactions were performed at 20 °C, 200 rpm. Samples were taken after 120 h and analyzed by GC. a the GC graph with α-phenylethanol as substrate; b, the GC graph with α-phenylpropanol as substrate. PeaksA–F stand for AS-α-phenylethanol ethyl ester, BR-α-phenylethanol ethyl ester, CR-α-phenylethanol, DS-α-phenylethanol, ES-α-phenylpropanol ethyl ester, FR-α-phenylpropanol ethyl ester, GR-α-phenylpropanol, and HS-α-phenylpropanol, respectively
The transesterification activity of LipB68 in the production of biodiesel
We investigated the productivity of LipB68 and the influence of temperature in the transesterification of soybean oil. Remarkably, the highest activity of the enzyme proved to be at 20 °C, with a yield of about 92% after 12 h, and the activities decreased with increasing temperatures (Fig. 5). As with other substrates, immobilized LipB68 also exhibited the highest enzymatic activity toward soybean oil at temperatures as low as 20 °C.
The effect of temperature on the immobilized LipB68 catalyzing production of biodiesel (◆20 °C, ◼30 °C, ▲40 °C, ● 0 °C). Methanolysis of soybean oil (2 mmol in n-heptane) was carried out with 1.0 g of immobilized LipB68 and addition of 3 molar equivalents of methanol, using a shaker table operating at 180 rpm
Discussion
P. fluorescens species are spread widely in the environment and utilize lipids as nutrients. Because these bacteria can be isolated easily, they have been the natural source for novel and highly active lipase catalysts. In this report, P. fluorescens lipase LipB68 was cloned and expressed in E. coli, and its enzymatic properties and application in biodiesel production were explored.
Among all the previously described members of the I.3 lipase subfamily, LipB68 showed unique psychrophilic properties. The optimum temperature for hydrolyzing p-nitrophenyl caprate, a commonly used substrate, was 20 °C, which is substantially lower than the optimum hydrolyzing temperatures that have been reported for other homologous lipases. The lipase KB700A cloned from Pseudomonas sp. exhibits an optimum temperature of 35 °C (Rashid et al. 2001), while the optimum temperature of the SIK W1 lipase isolated from P. fluorescens was reported to be in the range of 45–55 °C (Lee et al. 1993). Among all the I.3 lipase subfamily members described so far, the MIS38 lipase isolated from Pseudomonas sp. has the highest optimum temperature of 55 °C (Amada et al. 2000). Since the amino acid sequence of LipB68 is highly homologous to KB700A and SIK W1 (Fig. 1) with 87 and 88% identities respectively, the substantially lower optimum temperature exhibited by LipB68 suggest that the seemingly minor amino acid differences may cause a large change in the optimal temperature of the enzyme.
Furthermore, the enantioselective transesterification activities of LipB68 toward α-phenylethanol and α-phenylpropanol were both considerable and also more pronounced at 20 °C, while the common temperature range of this reaction was 35–60 °C. Another homologue of LipB68, reported to have enantioselectivities toward the same substrates, was P. fluorescens SIK W1 lipase (88% identity with LipB68), which shows high enantioselectivities toward α-phenylethanol but a low selectivity toward α-phenylpropanol (Liu et al. 2001). Considering the broad usage of the R enantiomers of both substrates as chiral auxiliaries or reagents or intermediate in pharmacological applications (Ley et al. 1990), LipB68 again shows a better substrate adaptability than the other homologues, along with the unique psychrophilic catalytic ability.
The differences between gene lipB68 and its two homologues, lipase from Pseudomonas sp. KB700A and the lipase from P. fluorescens SIK W1 (Fig. 1), are worth exploring to investigate why only LipB68 preferred low temperature. Studies on the traits of the structures of psychrophilic proteins indicate that psychrophilic proteins generally adopt more flexible structures to form enzyme–substrate complex at low temperature. Substitution of arginyl residues with lysyl residues results in increased rigidity and stabilization of the protein. For example, Feller et al. (1996) have demonstrated that lower ratios of Arg/(Arg+Lys) correlate with greater protein flexibility. The Arg/(Arg+Lys) ratio in LipB68 is 0.33, which is lower than that of the P. fluorescens SIK W1 lipase (0.4) and the Pseudomonas sp. KB700A lipase (0.43). The lower Arg/(Arg+Lys) ratio of LipB68 may explain to some degree the unique psychrophilic property of LipB68. Determination of the crystal structure of LipB68 in the future may provide valuable insight into the molecular basis of its unique psychrophilic properties.
The present results add to the numerous previous reports on catalysts for the production of biodiesel (Iso et al. 2001; Soumanou and Uwe 2003). In the studies of enzymatic production of biodiesel, the development of new biocatalysts is slow up to now. Most investigations focus on the exploration of new plant oils as substrate or the optimization of the reaction conditions. This article is the first to report the application of lipase subfamily I.3 in the enzymatic production of biodiesel. Furthermore, it is also the first time to report the enzymatic production of biodiesel at temperatures as low as 20 °C. In fact, although the optimal temperature for biodiesel production by LipB68 was 20 °C, the yield at 30 °C was also considerable, which suggested that the reaction would be feasible at room temperature. The excellent productivity of LipB68 at 20–30 °C offers an advantage in industrial applications over other lipases that require temperatures in the range of 40–50 °C (Soumanou and Uwe 2003), and facilitates the substitution of a mild and clean industry procedure for the conventional energy-costing and pollution-inducing chemical one.
Today, when industry development is directed toward green chemistry and energy economization, the psychrophilic property of LipB68, exhibited along with its high enantioselectivity toward α-phenylethanol and α-phenylpropanol, and especially its excellent ability in biodiesel production, makes LipB68 an appealing enzymatic catalyst in the chemical industry.
References
Ahn JH, Pan JG, Rhee JS (1999) Identification of the tliDEF ABC Transporter Specific for Lipase in Pseudomonas fluorescens SIK W1. J Bacteriol 181:1847–1852
Amada K, Haruki M, Imanaka T, Morikawa M, Kanaya S (2000) Overproduction in Escherichia coli, purification and characterization of a family I.3 lipase from Pseudomonas sp. MIS38. Biochim Biophys Acta 1478:201–210
Andersson RE (1980) Microbial lipolysis at low temperatures. Appl Environ Microbiol 39:36–40
Binet R, Letoffe S, Ghigo JM, Delepelaire P, Wandersman C (1997) Protein secretion by Gram-negative bacterial ABC exporters—a review. Gene 192:7–11
Feller G, Narinx E, Arpigny JL, Aittaleb M, Baise E, Genicot S, Gerday C (1996) Enzymes from psychrophilic organisms. FEMS Microbiol Rev 18:189–202
Gao XG, Zhang K Ch, Cao Sh G (1998) Isolation of a lipase-producing pseudomonas strain and optimization of its fermentation conditions. Acta Microbiol Sin 38:313–317
Iso M, Chen B, Eguchi M, T Kudo T, Shresth S (2001) Production of biodiesel fuel from triglycerides and alcohol using immobilized lipase. J Mol Catal B Enzym 16:53–58
Jaeger KE, Ransac S, Dijkstra BW, Colson C, van Heuvel M, Misset O (1994) Bacterial lipases. FEMS Microbiol Rev 15:29–63
Lee YP, Chung GH, Rhee JS (1993) Purification and characterization of Pseudomonas fluorescens SIK W1 lipase expressed in Escherichia coli. Biochim Biophys Acta 1169:156–164
Ley SV, Parra M, Redgrave AJ, Sternfeld F (1990) Microbial oxidation in synthesis: preparation of myoinositol phosphates and related cyclitol derivatives from benzene. Tetrahedron 46:4995–5026
Liu AMF, Somers NA, Kazlauskas RJ, Brush TS, Zocher F, Enzelberger MM, Bornscheuer UT, Horsman GP, Mezzetti A, Schmidt-Dannert C, Schmid RD (2001) Mapping the substrate selectivity of new hydrolases using colorimetric screening: lipases from Bacillus thermocatenulatus and Ophiostoma piliferum, esterases from Pseudomonas fluorescens and Streptomyces diastatochromogenes. Tetrahedron Asymmetry 12:545–556
Mohamed MS, Uwe TB (2003) Improvement in lipase-catalyzed synthesis of fatty acid methyl esters from sunflower oil. Enzyme Microb Technol 33:97–103
Mori K, Akao H (1980) Synthesis of optically active alkenyl alcohols and α-hydroxyl esters by microbial asymmetric hydrolysis by the corresponding acetates. Tetrahedron 36:91–96
Morris DD, Gibbs MD, Chin CW, Koh MH, Wong RW, Allison RW, Nelson PJ, Bergquist PL (1998) Cloning of the xynB gene from Dictyoglomus thermophilum Rt46B.1 and action of the gene product on kraft pulp. Appl Environ Microbiol 64:1759–1765
Rajmohan S, Dodd CER, Waites WM (2002) Enzymes from isolates of Pseudomonas fluorescens involved in food spoilage. J Appl Microbiol 93:205–213
Rashid N, Shimada Y, Ezaki S, Atomi H, Imanaka T (2001) Low-temperature lipase from psychrotrophic Pseudomonas sp. strain KB700A. Appl Environ Microbiol 67:4064–4069
Rosenau F, Jaeger KE (2000) Bacterial lipases from Pseudomonas: regulation of gene expression and mechanisms of secretion. Biochimie 82:1023–1032
Soumanou MM, Uwe TB (2003) Lipase-catalyzed alcoholysis of vegetable oils. Eur J Lipid Sci Technol 105:656–660
Sugihara A, Tani T, Tominaga Y (1991) Purification and characterization of a novel thermostable lipase from Bacillus sp. J Biochem (Tokyo) 109:211–216
Acknowledgements
This work was supported by the grant from the Ministry of Science and Technology, Basic Research project No.: 2002CCA400, and Program for New Century Excellent Talents in University, project No.: NCET-04-0411. Furthermore, we thank Drs. Yusen Liu and Charles V. Smith of the Ohio State University for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, Y., Zheng, Y., Jiang, Z. et al. A novel psychrophilic lipase from Pseudomonas fluorescens with unique property in chiral resolution and biodiesel production via transesterification. Appl Microbiol Biotechnol 73, 349–355 (2006). https://doi.org/10.1007/s00253-006-0478-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-006-0478-3