Abstract
Three novel Gram-positive, endospore-forming bacteria were isolated from a cold and alkaline environment. Phylogenetic analysis showed that the strains were almost identical, and that they were related to Natronobacillus azotifigens 24KS-1T (95.8% identity), Paraliobacillus quinghaiensis YIM-C158T (95.1%), Paraliobacillus ryukyuensis O15-7T (94.5%), and Halolactibacillus miurensis M23-1T (93.9%). The isolates produced amylase, α-galactosidase, β-galactosidase, and β-glucuronidase, and showed optimal growth at pH 10, at 20°C, and at 2–8% (w/v) NaCl. Major fatty acids were C14:0 (10.6–11.6%), anteiso-C15:0 (25.7–32.7%), C16:1 ω11c (12.2–16.0%), and C16:0 (14.0–20.4%). The major polar lipids were diphosphatidylglycerol and phosphatidylglycerol, and meso-diaminopimelic acid was found in the cell-wall peptidoglycan. The G+C content was 38.4%. DNA–DNA hybridization between strain GCM68T and H. miurensis M23-1T was 32.4%, while hybridization to N. azotifigens 24KS-1T, Amphibacillus tropicus Z-7792T, and Paraliobacillus ryukyuensis O15-7T was below 30%. The phylogenetic analysis and G+C content place strain GCM68T in relation to species belonging to Bacillus rRNA group 1, but phylogenetic and physiologic data combined with chemotaxonomic analyses support our proposal for a new genus, Alkalilactibacillus, gen. nov., with the novel species Alkalilactibacillus ikkensis, sp. nov. (type strain is GCM68T = DSM 19937 = LMG 24405).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gram-positive bacteria within the rRNA group 1 in the phyletic assemblage classically defined as the genus Bacillus (Ash et al. 1991) have been described from a number of saline and hypersaline environments, seawater and decomposing, dead marine organisms. For example, bacteria representing the genera Natronobacillus, Amphibacillus, Gracilibacillus, Halolactibacillus, and Paraliobacillus have been isolated from soda solonchak soils and soda lake sediments in the Kulunda Steppe (Altai, Russia), from soda lakes like Lake Magadi, salt lakes in Inner Mongolia, and decaying marine organisms from Japan (Sorokin et al. 2008; Zhilina et al. 2001, 2002; Wainø et al. 1999; Carrasco et al. 2006; Chen et al. 2009; Ishikawa et al. 2002, 2005).
In this paper, we describe the isolation and characterization of three novel alkaliphilic, halotolerant and cold-active bacterial strains related to the genera Natronobacillus, Amphibacillus, Gracilibacillus, Halolactibacillus, and Paraliobacillus. The strains were isolated from an alkaline, low-saline and cold environment, the ikaite columns in Ikka Fjord in Greenland. The Ikka Fjord in South-West Greenland harbors rare ikaite tufa columns, which are cold (4°C), alkaline (pH 10.4) and low salinity (0.9%) environments. The major mineral component in the columns is ikaite (CaCO3·6H2O), which forms a porous centre in the columns with seeping, alkaline spring water floating through the column (Buchardt et al. 1997, 2001). The oxygen tension in the interior of the columns varies from 25% to approximately 50% of atmospheric oxygen levels. Previous studies have shown that the columns harbor a large diversity of bacterial species (Stougaard et al. 2002; Schmidt et al. 2006) with representatives of alpha- and gamma-Proteobacteria as the most abundant bacterial groups. Phylogenetic investigations of cultured as well as uncultured bacteria indicate that approximately one-third of the phylotypes represent new species or new genera (Stougaard et al. 2002; Schmidt et al. 2006). The new bacterial strains were selected due to their ability to produce cold-active enzymes, in particular a novel β-galactosidases with industrial application potential (Schmidt and Stougaard 2010). The strain GCM68T was characterized according to the suggestions put forward by Logan et al. (2009).
Materials and methods
Isolation and cultivation
Ikaite material was collected from ikaite tufa columns from the Ikka Fjord in South-West Greenland (61°11′N; 48°01′W) in 2006 at a depth of approximately 6–10 m. The pH of the water from the interior of the column was measured to pH 10.4 and the temperature was 2–4°C. For isolation of bacteria, ikaite material was drilled out from three different ikaite columns and the material was suspended in 250 ml R2 broth pH 10 (per liter: yeast extract 0.5 g, peptone 0.5 g, casamino acids 0.5 g, glucose 0.5 g, soluble starch 0.5 g, Na-pyruvate 0.3 g, K2HPO4 0.3 g, and MgSO4 × 7 H2O 0.05 g; pH was adjusted with Na2CO3/NaHCO3 buffer) (Schmidt et al. 2006). The R2 broth medium was sterilized by autoclaving and buffers, separately sterilized by autoclaving, were added at a concentration of 0.1 M. The pH of the growth media throughout this study was measured prior to inoculation. R2 broth medium pH 10 was inoculated with ikaite material and incubated aerobically at 5°C for 2 months. After 2 months, the cultures were plated onto R2 agar medium pH 10 (R2 composition as above for R2 broth but solidified with agar, final concentration 1.5%) supplemented with 10 g/l of lactose (instead of glucose), 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG), and 40 mg/l of 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-gal). After 1–2 weeks of incubation at 5°C, blue colonies representing β-galactosidase-producing bacteria were selected for further characterization. Anaerobic growth was attempted on R2 agar plates, in R2 broth and in nitrogen-free soda medium (NF) used by Sorokin et al. (2008). Agar plates and liquid cultures were flushed with N2 gas and incubated in gas-tight jars (Oxoid) under 20% CO2, 80% N2 atmosphere for 4 weeks at 10 and 20°C.
Phylogenetic analysis, DNA–DNA hybridization and G+C content
A total of 22 blue colonies were isolated and subjected to phylogenetic analysis. DNA for phylogenetic analysis and full genome sequencing were extracted using a conventional phenol–chloroform extraction method (Sambrook and Russell 2001). 16S rRNA gene amplification was carried out on all three strains using the primers 27F and 1492R (Lane 1991), and the 16S rRNA gene sequence was established by sequencing the PCR fragment at GATC Biotech (Germany). Furthermore, the full length 16S rRNA gene sequence was identified in the full genome sequence. The full length DNA sequence (1,567 nucleotides) of the 16S rRNA gene from strain GCM68T was submitted to GenBank/EMBL/DDBJ with the accession number EU281853. The 16S rRNA gene sequences of the remaining two isolates GCM74 and GCM75 were similarly submitted with the accession numbers HM016849 and HM016850. Related sequences were retrieved from public databases using BLASTn at the NCBI server (http://www.ncbi.nlm.nih.gov/blast/). The closest related 16S rRNA gene sequences were aligned using the alignment tool in the CLC Main Workbench 5.0 software (CLC bio). DNA–DNA hybridizations were carried out at DSMZ. DNA was isolated using a French pressure cell (Thermo Spectronic) and was purified by chromatography on hydroxyapatite as described by Cashion et al. (1977). DNA–DNA hybridization was carried out as described by De Ley et al. (1970) under consideration of the modifications described by Huß et al. (1983) using a model Cary 100 Bio UV/vis-spectrophotometer equipped with a Peltier-thermostatted 6 × 6 multicell changer and a temperature controller with in situ temperature probe (Varian). DNA base composition (G+C content) was similarly performed at DSMZ. The DNA was hydrolyzed with P1 nuclease and the nucleotides dephosphorylated with bovine alkaline phosphatase (Mesbah et al. 1989). The resulting deoxyribonucleotides were analyzed by HPLC. Full genome sequencing of isolate GCM68T was carried out using a paired-end library with an insert size of 500 bp prepared from 5 μg of high molecular weight DNA according to the Illumina GA II paired-end library preparation protocol (Illumina, San Diego, CA, USA). Sequencing on the Illumina GA II instrument for 2 × 32 cycles using the paired-end settings resulted in 18.2 M paired-end reads. Assembly of the reads was performed using Velvet version 0.7.59 (Zerbino and Birney 2008) with parameters obtained using VelvetOptimizer and were as follows: velveth: 31 -fastq -shortPaired. velvetg: -ins_length 500 -exp_cov auto -min_contig_lgth 500 -cov_cutoff 2.85364760913563. The assembly yielded a total of 201 contigs (longest contig 229,656 nucleotides, n50 = 39,472) indicating a chromosome size of approximately 3.3 Mbp with an average GC content of 34%.
Phenotypic and growth determination
Strain GCM68T was tested for a number of physiological and morphological characteristics using standard procedures such as Gram staining, oxidase test (Microbiology Bactident® Oxidase-strips) and catalase test (H2O2). Cell morphology was analyzed by phase-contrast microscopy and scanning electron microscopy. Temperature- and pH-dependent growth was carried out in shaking flasks in triplicate with 200 ml R2 broth buffered to pH 6, 7 and 8 with NaH2PO4/Na2HPO4, to pH 9 with NaHCO3/HCl, and to pH 10 and 10.7 with NaHCO3/Na2CO3. The pH 6, 7, 8, and 9 cultures were inoculated with stationary cultures grown in R2 broth at pH 9, since it was not possible to establish stationary cultures at pH 6, 7 or 8. Cultures at pH 10 and 10.7 were inoculated with stationary pH 10 and 10.7 cultures, respectively. Cultures were grown at 0, 5, 10, 20, 25, and 30°C. Growth was detected by measuring the optical density at 600 nm, and pH was measured throughout the experiment. Since the pH at high pH values are unstable (Supplementary Fig. 5), growth performance was measured during exponential growth only over a few hours. Salt tolerance of strain GCM68T was tested in DeepWell microtiter plates with seven replicates and one blank control within each salt concentration incubated with shaking at 300 rpm. The medium used was R2 broth pH 10 adjusted to the following concentrations of NaCl: 0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, and 25%. This experiment was carried out at 5, 15, and 25°C to test if there was any correlation between temperature and salt tolerance. Oxidation of different carbon sources was tested using the Biolog GP2 MicroPlate™ System. The recommended medium was adjusted to pH 9.2 using a 1 M sodium carbonate buffer, and the plates were incubated at 15°C for 2 weeks with OD600 measurements every second day. Otherwise, the instructions given by the manufacturer were followed. Enzymatic activities were all tested at pH 10 using an R2 agar-based medium. Extracellular hydrolytic enzymes were detected on media without glucose and soluble starch but supplemented with AZCL-coupled substrates (Megazyme) and un-coupled, native substrates: AZCL-amylose + amylose, AZCL-casein + casein, AZCL-cellulose + CM-cellulose, AZCL-galactomannan + mannan, AZCL-pachyman + β-1,3-glucan, and AZCL-xylan + xylan from birch wood and oat spelt. Activity of β-galactosidase, β-glucosidase, β-glucuronidase and α-galactosidase was tested using 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal), 5-bromo-4-chloro-3-indolyl-β-d-glucopyranoside (X-Glc), 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid (X-GlcA), and 5-bromo-4-chloro-3-indolyl-α-d-galactopyranoside (X-α-gal), respectively, and phosphatase activity was tested using 5-bromo-4-chloro-3-indolyl phosphate disodium salt (BCIP). Screening for enzymatic activity was performed at 5, 15, and 25°C. The following tests were all performed at both neutral pH and at pH 10: Detection of deoxyribonuclease (DNase) activity on a DNase test agar, protease activity (degradation of casein), degradation of starch, degradation of aesculin, degradation of tryptophan (indole test using Kovacs reagent), methyl red and Voges-Proskauer tests, nitrate reduction to NO2, and dihydrogensulfide (H2S) production (on triple sugar iron agar). All media for these tests are originally made for tests at neutral pH, but to accommodate the activity of strain GCM68T, we also made the tests at pH 10, even though the high pH in the media may affect the reaction. The following antibiotics were tested: Ampicillin (25, 50, and 100 μg/ml), chloramphenicol (17 and 34 μg/ml), kanamycin (12.5, 25, 50, and 100 μg/ml), tetracyclin (5 and 10 μg/ml), gentamicin (12.5 and 25 μg/ml), and streptomycin (12.5 and 25 μg/ml).
Chemotaxonomic characterization
The membrane fatty acid composition in bacteria has been shown to be dependent on the growth conditions (Valderrama et al. 1998). Therefore, in order to make correct comparisons between fatty acids of strain GCM68T and of the closest relative, N. azotifigens 24KS-1T , two different media were used, R2 agar and the nitrogen-free soda medium (NF) used by Sorokin et al. (2008). The two isolates, strain GCM68T and N. azotifigens 24KS-1T, were grown on R2 agar and NF medium at pH 10 and 5°C. Strain GCM68T was incubated aerobically at 15°C, while N. azotifigens 24KS-1T was incubated anaerobically at 37°C. Analysis and identification of whole-cell fatty acids were performed using freeze-dried washed bacteria and gas chromatographic analysis (Johansen and Olsson 2005; Mansfeld-Giese et al. 2002). Analysis of respiratory quinones and polar lipid analysis was carried out by the Identification Service of the DSMZ and Dr. Brian Tindall, DSMZ, Braunschweig, Germany. Peptidoglycan composition was carried out by the Identification Service of the DSMZ. Hydrolysates were subjected to TLC on cellulose plates using the solvent system of Rhuland et al. (1955).
Results and discussion
Morphology
Isolate GCM68T formed white to transparent colonies when cultivated on standard R2 agar pH 10. The bacterium was Gram-positive, chemoheterotrophic, aerobic and oxidase and catalase negative (Table 1). Phase-contrast microscopy and scanning electron microscopy showed that the cells were motile rods and that they were 1.5–5 μm long and 0.5 μm wide and endospore-forming. Spore formation was also confirmed by genome sequencing, which showed the presence of 37 genes believed to be involved in sporulation and spore germination. Chains of up to four cells were observed, and they divided by binary fission (Supplementary Fig. 1). No flagella were observed by phase-contrast microscopy or scanning electron microscopy, but a total of 44 genes believed to be involved in flagellar motility were predicted from full genome sequencing of isolate GCM68T (cf. below). Strain GCM68T was Gram-positive, and oxidase and catalase test showed that GCM68T was both oxidase and catalase negative The main morphological and physiological features are summarized in Table 1.
Growth
Growth experiments showed that isolate GCM68T grew from 0 to 25°C and from pH 9 to 10.7 (Supplementary Fig. 2). Measurements of pH in prolonged incubation of cultures at pH 10 and 10.7 showed that the pH dropped over time, whereas pH in cultures at pH 7, 8 and 9 stayed constant (Supplementary Fig. 5). Therefore, measurements of growth characteristics were performed in cultures in exponential growth phase within the first 12 h after inoculation. Analyses of exponentially growing cultures showed that optimal growth was around 20°C and pH 10, where the highest growth rate measured was 0.16 generations per hour (Supplementary Figs. 2 and 3). No exponential growth could be established at pH 6, 7 or 8 or at 30°C or above. Growth at 0°C was slow, but after 25 days the cell density reached the same level as obtained at optimal temperature (data not shown). Isolate GCM68T grew in the range of 0–10% NaCl (w/v) at pH 10. At 5°C, the optimal salt concentration was 8% NaCl, at 15°C it was 8% NaCl, and at 25°C it was 6% NaCl (Supplementary Fig. 4). Attempts to establish growth under anaerobic conditions were unsuccessful. Growth physiology and morphological features are summarized in Table 1, where characteristics of related species are also listed. Oxidation of different carbon sources was tested using the Biolog GP2 MicroPlateTM System. The recommended medium was adjusted to pH 9.2 using a 1 M sodium carbonate buffer, and the plates were incubated at 15°C for 2 weeks with OD600 measurements every second day. Otherwise, the instructions given by the manufacturer were followed. The Biolog assay showed that several compounds were utilized as the sole carbon source when the strain was incubated chemotropically (aerobic/dark), cf. results in description of new species below. Analysis of enzyme activities showed that isolate GCM68T produced α-amylase activity at 5, 15, and 25°C, β-galactosidase, β-glucuronidase, and α-galactosidase activity at 5 and 15°C, and β-1,3-glucanase activity at 15°C. Furthermore, conventional assays at neutral pH and at pH 10 for deoxyribonuclease (DNase) activity, protease activity, degradation of starch, degradation of aesculin, degradation of tryptophan, methyl red and Voges-Proskauer tests, nitrate reduction to NO2, and dihydrogensulfide (H2S) production showed that isolate GCM68T displayed no DNase activity at any of the pH values, protease activity and degradation of starch were negative at both pH values, while degradation of aesculin was detected at pH 10. Degradation of tryptophan was detected at low levels at pH 10 and not at all at neutral pH. The methyl red and Voges-Proskauer tests turned out negatively at both pH values. Nitrate degradation to NO2 was detected at a low level at pH 10, but the bacterium only grew to a low density in this medium. Dihydrogensulfide (H2S) production was positive at pH 10 and negative at neutral pH. Analyses for antibiotic resistance were carried out on R2 agar pH 10 at 15°C. Analysis of antibiotic resistance showed that isolate GCM68T was unable to grow at any of the concentrations tested of ampicillin, chloramphenicol, or streptomycin, while it showed full growth with 5 and 10 μg/ml of tetracyclin and with 12.5 and 25 μg/ml gentamicin. On kanamycin, the isolate showed full growth with 12.5 μg/ml and reduced growth with increasing concentration. Only very weak growth was observed with 100 μg/ml kanamycin.
Phylogeny
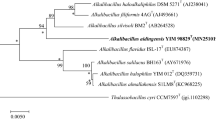
A full-length DNA sequence of the 16S rRNA gene from isolate GCM68T was established and BLASTn analysis showed that the closest relatives were Natronobacillus azotifigens 24KS-1T (95.8% identity; EU143681), Paraliobacillus quinghaiensis YIM-C158T (95.1% identity; EU135728), Paraliobacillus ryukyuensis O15-7T (94.5%; AB087828), Halolactibacillus miurensis M23-1T (93.9% identity; AB362701), and Amphibacillus tropicus Z-7792T (93.9% identity; AF418602). Thus, the distance in 16S rRNA gene sequence similarities between strain GCM68T and the closest relatives was below 97% similarity, which is often used as a preliminary guideline for species separation. A phylogenetic tree using the Neighbor Joining alignment method showed that GCM68T groups with N. azotifigens 24KS-1 and A. tropicus Z-7792T, but that the distances between GCM68T and the two closest related species are rather large (Fig. 1). Supplementary Fig. 6 shows a Neighbor Joining tree with several more related genera in the rRNA group 1 of the genus Bacillus. Supplementary Fig. 6 confirms the result in Fig. 1 that the three new isolates GCM68T, GCM74 and GCM75 constitute a novel lineage separated from the related genera Natronobacillus, Amphibacillus, Halolactibacillus, Streptobacillus, Gracilibacillus, Oceanobacillus, and Virgibacillus. The DNA–DNA hybridization analyses between strain GCM68T and some of the phylogenetically closest related bacteria gave the following results: GCM68T and N. azotifigens 24KS-1: 26.8%, GCM68T and A. tropicus Z-7792T: 22.6%, GCM68T and P. ryukyuensis O15-7T: 13.7%, and GCM68T and H. miurensis M23-1T: 32.4%. The DNA G+C content of strain GCM68T was determined to be 38.4 mol% in conventional HPLC analysis of nuclease P1 digestion and approximately 34% as determined from genome sequencing. This number is fairly similar to that of the closest related genera: The G+C content of H. halophilus M2-2T and H. miurensis M23-1T has been reported to be 38.5–40.7 mol% (Ishikawa et al. 2005), for P. ryukyuensis O15-7T it was 35.6 mol% (Ishikawa et al. 2002), for N. azotifigens 24KS-1T it was 36.1–38.5 mol% (Sorokin et al. 2008), and for G. halotolerans NNT it was reported to be 38 mol% (Wainø et al. 1999).
Chemotaxonomy
The predominant fatty acids of strain GCM68T were iso-C14:0 (12.4% on R2), C14:0 (11.6% on R2 and 10.6% on NF), anteiso-C15:0 (25.7% on R2 and 32.7 on NF), C16:1 ω11c (12.2% on R2 and 16.0% on NF), and C16:0 (20.4% on R2 and 14.0 on NF) (Table 2). No matches were found to GCM68T in the TSBA40 aerobic bacteria database. The fatty acid profiles from the closest related species, N. azotifigens 24KS-1T, were also experimentally established in parallel with isolate GCM68T, while data from other related species were retrieved from literature. Based on these data (Table 2), the fatty acid profile of strain GCM68T was rather different from those of the closest relative, N. azotifigens 24KS-1T. Significant differences were observed in the low content of iso- and anteiso-C13:0, the high content of C14:0, and the high content of C16:1 ω11c of GCM68T compared to the other strains. The fatty acid profile for P. ryukyuensis has, to our knowledge, not been published. However, care should be taken when using the fatty acid content as an indicator in speciation of psychrophilic, psychrotrophic, or alkaliphilic bacteria since the composition of fatty acids may be a response to the surrounding environment (Valderrama et al. 1998). Strain GCM68T was isolated from a low-saline environment, whereas the related species have been isolated from saline to highly saline environments and thus, we cannot exclude that the differences may be, in part, due to different environmental adaptations and growth requirements. No respiratory quinones were detected in strain GCM68T and in N. azotifigens 24KS-1T. The major polar lipids of strain GCM68T were diphosphatidylglycerol and phosphatidylglycerol, and minor to trace amounts of four unknown phospholipids and two glycolipids. N. azotifigens 24KS-1T contained diphosphatidylglycerol, phosphatidylglycerol as well as phosphatidylethanolamine and four unknown phospholipids and one aminophospholipid. The diagnostic diamino acid of the peptidoglycan in both strains was meso-diaminopimelic acid (m-Dpm). However, the amount of m-Dpm in strain GCM68T was remarkably higher than in N. azotifigens 24KS-1T.
Enzymatic activities
Isolate GCM68T was analyzed for enzymatic activities at pH 10 on solid media supplemented with chromogenic enzyme substrates. Isolate GCM68T showed α-amylase activity at 5, 15, and 25°C, β-galactosidase, β-glucuronidase, and α-galactosidase activity at 5 and 15°C, and β-1,3-glucanase activity at 15°C.
In summary, strain GCM68T is phylogenetically related to the rRNA group 1 in the phyletic assemblage classically defined as the genus Bacillus, with species from the genera Natronobacillus, Paraliobacillus, Amphibacillus, Gracilibacillus, and Halolactibacillus as the closest relatives. Chemotaxonomical analysis confirm the relationship of strain GCM68T since the cell-wall peptidoglycan of GCM68T and of isolates from the genera Natronobacillus, Paraliobacillus, Amphibacillus, Gracilibacillus, and Halolactibacillus was m-Dpm. However, the amount of m-Dpm in strain GCM68T was remarkably higher than in N. azotifigens 24KS-1T, the closest relative based on 16S rRNA gene comparison. Strain GCM68T together with N. azotifigens 24KS-1T, A. tropicus Z-7792T and H. halophilus M2-2T contained no respiratory quinones in contrast to P. quinghaiensis YIM-C158T, P. ryukyuensis O15-7T, H. alkaliphilus H-5T, and G. halotolerans NNT, which all produced quinones. Major polar lipids of strain GCM68T were diphosphatidylglycerol and phosphatidylglycerol, whereas N. azotifigens 24KS-1T in addition to diphosphatidylglycerol and phosphatidylglycerol also contained phosphatidylethanolamine. Cellular fatty acid composition also showed differences, since strain GCM68T contained C14:0, anteiso-C15:0, C16:1 ω11c, and C16:0 as the dominant fatty acids, whereas N. azotifigens 24KS-1T had iso-C13:0, iso-C15:0, anteiso-C15:0, C16:0 and anteiso-C17:0 as the dominant fatty acids (Table 2). Finally, DNA–DNA hybridization analyses showed that hybridization between strain GCM68T and the closest relatives was below 30%. Thus, phylogenetical, physiological, and chemotaxonomical analyses support the notion of strain GCM68T being a novel species belonging to a new genus. We propose a new genus Alkalilactibacillus gen. nov. comprising the species Alkalilactibacillus ikkensis sp. nov. Strain GCM68T is type strain for A. ikkensis sp. nov.
Description of Alkalilactibacillus gen. nov
Alkalilactibacillus [Al.ka.li.lacti.ba’cil.lus. N.L. n. alkali (from Arabic article al, the; Arabic n. qaliy, ashes of saltwort), alkali; L. n. lactis milk; L. masc. n. bacillus, a rod; N.L. masc. n. Alkalilactibacillus, milk (lactose)-degrading rod living under alkaline conditions]. Cells are Gram-positive, rod-shaped, motile, oxidase negative and catalase negative. Growth is heterotrophic, aerobic, and chemoheterotrophic. Growth is observed between pH 9 and 10.7 and between 5 and 25°C. NaCl is not required for growth, and growth is observed in the range of 0–10% NaCl. The genus Alkalilactibacillus belongs to the class “Bacilli” and the family “Bacillaceae”. The type species is Alkalilactibacillus ikkensis.
Description of Alkalilactibacillus ikkensis sp. nov
Alkalilactibacillus ikkensis (ik.ken’sis. N.L. masc. adj. ikkensis of or belonging to the Ikka Fjord, referring to the origin of the type strain). Colonies are smooth, circular, and white to transparent. Cells are Gram-positive, rod shaped, 1.5–5 μm in length and 0.5 μm in width, oxidase negative and catalase negative. Growth occurs at temperatures from 0 to 25°C, with an optimum around 20°C. Growth occurs from pH 9 to at least pH 10.7. NaCl is not required for growth, but up to 10% (w/v) NaCl is tolerated. Optimal growth occurs at 2–8% (w/v) NaCl at 5°C, around 6% at 15°C and 2% at 25°C. The strains are able to use a wide spectrum of carbon sources such as d-alanine, l-alanine, l-glutamic acid, 2,3-butanediol, and glycerol and to a lesser degree dextrin, l-arabinose, arbutin, d-fructose, l-fructose, d-galactose, α-d-glucose, d-mannose, palatinose, d-psicose, l-rhamnose, d-ribose, salicin, d-tagatose, turanose, d-xylose, d-fructose-6-phosphate, and d-glucose-6-phosphate. And the strain shows α-amylase (alkaline, extracellular), β-galactosidase (intracellular), β-glucosidase (intracellular), β-glucuronidase (intracellular), α-galactosidase (intracellular), and β1,3 glucanase (alkaline, extracellular) activity. Antibiotic resistance is seen toward gentamycin, tetracyclin, and kanamycin. The predominant fatty acids were iso-C14:0 (12.4% on R2), C14:0 (11.6% on R2 and 10.6% on NF), anteiso-C15:0 (25.7% on R2 and 32.7 on NF), C16:1 ω11c (12.2% on R2 and 16.0% on NF), and C16:0 (20.4% on R2 and 14.0 on NF). DNA G+C content of the type strain is 38.4 mol%. The type strain is GCM68T (=DSM 19937 = LMG 24405).
References
Ash C, Farrow JAE, Wallbanks S, Collins MD (1991) Phylogenetic heterogeneity of the genus Bacillus revealed by comparative analysis of small-subunit-ribosomal RNA sequences. Lett Appl Microbiol 13:202–206
Buchardt B, Seaman P, Stockmann G, Vous M, Wilken U, Düwel L, Kristiansen A, Jenner C, Whitticar MJ, Kristensen RM, Petersen GH, Thorbjørn L (1997) Submarine columns of ikaite tufa. Nature 390:129–130
Buchardt B, Israelson C, Seaman P, Stockmann G (2001) Ikaite tufa towers in Ikka Fjord, Southwest Greenland: their formation by mixing of seawater and alkaline spring water. J Sediment Res 71:176–189
Cao SJ, Qu JH, Yang JS, Sun Q, Yuan HL (2008) Halolactibacillus alkaliphilus sp. nov., a moderately alkaliphilic and halophilic bacterium isolated from a soda lake in Inner Mongolia, China, and emended description of the genus Halolactibacillus. Int J Syst Evol Microbiol 58:2169–2173
Carrasco IJ, Marquez MC, Yanfen X, Ma Y, Cowan DA, Jones BE, Grant WD, Ventosa A (2006) Gracilibacillus orientalis sp. nov., a novel moderately halophilic bacterium isolated from a salt lake in Inner Mongolia, China. Int J Syst Evol Microbiol 56:599–604
Cashion P, Hodler-Franklin MA, McCully J, Franklin M (1977) A rapid method for base ratio determination of bacterial DNA. Anal Biochem 81:461–466
Chen YG, Cui XL, Zhang YQ, Li WJ, Wang YX, Xu LH, Wen ML, Peng Q, Jiang CL (2009) Paraliobacillus quinghaiensis sp. nov., isolated from salt-lake sediment in China. Int J Syst Evol Microbiol 59:28–33
De Ley J, Cattoir H, Reynaerts A (1970) The quantitative measurement of DNA hybridization from renaturation rates. Eur J Biochem 12:133–142
Huβ VAR, Festl H, Schleifer KH (1983) Studies on the spectrophotometric determination of DNA hybridization from renaturation rates. Syst Appl Microbiol 4:184–192
Ishikawa M, Ishizaki S, Yamamoto Y, Yamasato K (2002) Paraliobacillus ryukyuensis gen. nov., sp. nov., a new Gram-positive, slightly halophilic, extremely halotolerant, facultative anaerobe isolated from a decomposing marine alga. J Gen Appl Microbiol 48:269–279
Ishikawa M, Nakajima K, Itamiya Y, Furukawa S, Yamamoto Y, Yamasato K (2005) Halolactibacillus halophilus gen. nov., sp. nov. and Halolactibacillus miurensis sp. nov., halophilic and alkaliphilic marine lactic acid bacteria constituting a phylogenetic lineage in Bacillus rRNA group 1. Int J Syst Evol Microbiol 55:2427–2439
Johansen A, Olsson S (2005) Using phospholipid fatty acid technique to study short-term effects of the biological control agent Pseudomonas fluorescens DR54 on the microbial microbiota in barley rhizosphere. Microb Ecol 49:1–10
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, New York, pp 115–175
Logan NA, Berge O, Bishop AH, Busse HJ, De Vos P, Fritze D, Heyndrickx M, Kämpfer P, Rabinovitch L, Salkinoja-Salonen MS, Seldin L, Ventosa A (2009) Proposed minimal standards for describing new taxa of aerobic, endospore-forming bacteria. Int J Syst Evol Microbiol 59:2114–2121
Mansfeld-Giese K, Larsen J, Bødker L (2002) Bacterial populations associated with mycelium of the arbuscular mycorrhizal fungus Glomus intraradices. FEMS Microbiol Ecol 41:133–140
Mesbah M, Premachandran U, Whitman W (1989) Precise measurement of the G+C content of deoxyribonucleic acid by high performance liquid chromatography. Int J Syst Bact 39:159–167
Rhuland LE, Work E, Denman RF, Hoare DS (1955) The behavior of the isomers of α,ε-diaminopimelic acid on paper chromatograms. J Am Chem Soc 77:4844–4846
Sambrook J, Russell DW (2001) Commonly used techniques in molecular cloning, Molecular cloning: A laboratory manual, vol 3. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Schmidt M, Stougaard P (2010) Identification, cloning and expression of a cold-active β-galactosidase from a novel Arctic bacterium, Alkalilactibacillus ikkense. Environ Technol 31:1107–1114
Schmidt M, Priemé A, Stougaard P (2006) High microbial diversity in permanently cold and alkaline ikaite columns from Greenland. Extremophiles 10:551–562
Sorokin ID, Zadorina EV, Kravchenko IK, Boulygina ES, Tourova TP, Sorokin DY (2008) Natronobacillus azotifigens gen. nov., sp. nov., an anaerobic diazotrophic haloalkaliphile from soda-rich habitats. Extremophiles 12:819–827
Stougaard P, Jørgensen F, Johnsen MG, Hansen OC (2002) Microbial diversity in ikaite tufa columns: an alkaline, cold ecological niche in Greenland. Environ Microbiol 4:487–493
Valderrama MJ, Monteoliva-Sanchez M, Quesada E, Ramos-Cormenzana A (1998) Influence of salt concentration on the cellular fatty acid composition of the moderately halophilic bacterium Halomonas salina. Res Microbiol 149:675–679
Wainø M, Tindall BJ, Schumann P, Ingvorsen K (1999) Gracilibacillus gen. nov., with description of Gracilibacillus halotolerans gen. nov., sp. nov.; transfer of Bacillus dipsosauri to Gracilibacillus dipsosauri comb. nov., and Bacillus salexigens to the genus Salibacillus gen. nov., as Salibacillus salexigens comb. nov. Int J Syst Bacteriol 49:821–831
Zerbino DR, Birney E (2008) Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res 18:821–829
Zhilina TN, Garnova ES, Tourova TP, Kostrikina NA, Zavarzin GA (2001) Amphibacillus fermentum sp. nov., Amphibacillus tropicus sp. nov., new alkaliphilic, facultatively anaerobic, saccharolytic bacilli from Lake Magadi (English translation of Mikrobiologiia). Microbiology 70:711–722
Zhilina TN, Garnova ES, Tourova TP, Kostrikina NA, Zavarzin GA (2002) Amphibacillus fermentum sp. nov. and Amphibacillus tropicus sp. nov. In validation of publication of new names and new combinations previously effectively published outside the IJSEM, List no. 85. Int J Syst Evol Microbiol 52:685–690
Acknowledgments
We acknowledge Michael Hansen for doing the SEM microscopy and Dorte Rasmussen and Karin Vestberg for technical assistance. Professor Jean Euzéby, University of Toulouse, is thanked for guidance in nomenclature. Referring to the Convention on Biological Diversity, we thank the Greenland Home Rule for permission to sample ikaite columns from the Ikka Fjord. This work was partly funded by the Villum Kann Rasmussen Foundation and the The Danish Council for Technology and Innovation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Oren.
The GenBank/EMBL/DDBJ accession number for the 16S rRNA gene sequence of Alkalilactibacillus ikkensis strain GCM68T is EU281853.
Electronic supplementary material
Below is the link to the electronic supplementary material.
792_2012_430_MOESM1_ESM.doc
Supplementary Figure 1. Scanning electron microscopy pictures of strain GCM68T, showing the rod shaped cells of 1.5-5 μm in length and around 0.5 μm in width. Furthermore, cells performing binary fission can be seen as long chains of only partly separated cells. (DOC 322 kb)
792_2012_430_MOESM2_ESM.doc
Supplementary Figure 2. Growth of strain GCM68T at different temperatures and pH. Liquid cultures in shake flasks were inoculated with strain GCM68T and samples were withdrawn for OD600 measurements at the time intervals indicated. Growth was investigated at pH 6, 7, and 8 (not shown) and at pH, 9 (dashed lines, ♦), pH 10 (solid lines, ■) and pH 10.7 (initial pH; dotted lines, ▲). The shake flasks were incubated at 10°C, 20°C, and 25°C. The pH values at the end of the growth experiment were measured to pH 9, 10, and 10.5, respectively. All incubations were in triplicates. (DOC 380 kb)
792_2012_430_MOESM3_ESM.doc
Supplementary Figure 3. Growth rate of strain GCM68T at different temperatures and pH. Regression lines were drawn from the growth curves in Supplementary Fig. 2 in the exponential growth phase. The pH values at the end of the growth experiment were measured to pH 9, 10, and 10.5, respectively. Open bars, 10°C; solid bars, 20°C; hatched bars, 25°C. (DOC 23 kb)
792_2012_430_MOESM4_ESM.doc
Supplementary Figure 4. Growth of strain GCM68T at increasing concentrations of NaCl measured in Deep Well microtiter plates. The concentrations ranged from 0 to 25% NaCl, and growth was investigated at pH 10 at 5, 15, and 25°C. The figure shows that isolate GCM68T grows in the range from 0 to 10% NaCl with optimal growth from 2 to 8% NaCl. (DOC 28 kb)
792_2012_430_MOESM5_ESM.doc
Supplementary Figure 5. Measurement of pH in growing cultures in shake flasks. The growth media were adjusted to pH 6.0 - 8.0 using NaH2PO4/Na2HPO4, to pH 8.5 - 9.0 using NaHCO3/HCl, and to 9.4 - 11.5 using NaHCO3/Na2CO3. Samples were withdrawn for pH measurements at the time intervals indicated. (DOC 163 kb)
792_2012_430_MOESM6_ESM.doc
Supplementary Figure 6. Neighbor Joining phylogenetic tree showing strains GCM68T, GCM74 and GCM75 and their closest relatives within the rRNA group 1 in the phyletic assemblage classically defined as the genus Bacillus. Sequences were retrieved from NCBI database, trimmed in length, and aligned with the CLC Main Workbench 5.0 software (CLC bio). Bootstrap (n=100) values are shown. Bar, 0.022 substitutions per nucleotide position. (DOC 140 kb)
Rights and permissions
About this article
Cite this article
Schmidt, M., Priemé, A., Johansen, A. et al. Alkalilactibacillus ikkensis, gen. nov., sp. nov., a novel enzyme-producing bacterium from a cold and alkaline environment in Greenland. Extremophiles 16, 297–305 (2012). https://doi.org/10.1007/s00792-012-0430-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-012-0430-7