Abstract
Using ectoine-excreting strain Halomonas salina DSM 5928T, we developed a new process for high-efficiency production of ectoine, which involved a combined process of batch fermentation by growing cells and production by resting cells. In the first stage, batch fermentation was carried out using growing cells under optimal fermentation conditions. The second stage was the production phase, in which ectoine was synthesized and excreted by phosphate-limited resting cells. Optimal conditions for synthesis and excretion of ectoine during batch fermentation in a 10 l fermentor were 0.5 mol l−1 NaCl and an initial monosodium glutamate concentration of 80 g l−1 respectively. The pH was adjusted to 7.0 and the temperature was maintained at 33°C. In phosphate-limited resting cells medium, monosodium glutamate and NaCl concentration was 200 g l−1 and 0.5 mol l−1, respectively, as well as pH was 7.0. The total concentration of ectoine produced was 14.86 g l−1, the productivity and yield of ectoine was 7.75 g l−1 day−1 and 0.14 g g−1, respectively, and the percentage of ectoine excreted was 79%. These levels of ectoine production and excretion are the highest reported to date.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ectoine (1,4,5,6-tetrahydro-2-methyl-4-pyrimidinecarboxylic acid) is the cyclic amino acid derivative of aspartate (Galinski et al. 1985) and appears to be the nearly universal organic compatible solute in bacteria. Ectoine was originally discovered in anoxygenic phototrophs of the Ectothiorhodospira-Halospira group, and ectoine was recently identified as a major organic compatible solute in halophilic methanotrophs and methylotrophs. The biosynthesis of ectoine has been documented in a wide variety of halophilic and halotolerant species, and especially in species with simple growth demands. In the domain Bactiera, ectoine synthesis is far more abundant than the synthesis of the compatible solute glycine betaine (Oren 2008). Ectoine is currently produced for commercial usages such as cosmetic additives, which proved to have extensive applications in biological and enzyme preparations, pharmaceutical industry, and other fields (Ventosa and Nieto 1995; Ventosa et al. 1998; Kanapathipillai et al. 2005; Zhang et al. 2006; Lentzen and Schwarz 2006). There is therefore great interest in the technology that leads to efficient production of ectoine.
To date, reported processes for ectoine production have the following common features. First, high cell density cultivation has been adopted to improve ectoine production. Non-ectoine excreting strains can accumulate ectoine in the cells, but cannot excrete ectoine into the medium unless they are exposed to hypoosmotic shock. Therefore in these strains, the total concentration of ectoine is equal to the intracellular ectoine concentration. For this reason, high cell density cultivation of these cells was adopted in order to obtain as much ectoine as possible (Sauer and Galinski 1998; Onraedt et al. 2005). Secondly, there was essentially linear relationship between ectoine content of the cells and the salinity of the medium over a wide range of osmotic growth conditions (Kuhlmann and Bremer 2002), therefore the bacteria must be grown in media with a relatively high NaCl concentration in order to enhance the accumulation of ectoine. It has been reported that, NaCl concentrations of 2.6 and 1 mol l−1 yielded maximum ectoine concentrations when using Halomonas elongata DSM 2581T (Sauer and Galinski 1998) and Brevibacterium epidermis (Onraedt et al. 2005), respectively. Growth medium with high NaCl concentration aggravates corrosion of the equipment and reduces the growth rates. This means that the fermentation period for ectoine synthesis is usually extended due to fed-batch fermentation of substrate. In high cell density fermentations of H. elongata DSM 2581T and B. epidermis, the fermentation period was as long as 120 and 100 h, respectively, which resulted in lower volumetric productivity. (3) During high cell density cultivation of non-ectoine excreting strains the dissolved oxygen limitation makes it difficult to operate the fermentor and increases the cost of production.
Zhang et al. (2009) reported an ectoine-excreting strain Halomonas salina DSM 5928T, formerly known as Deleya salina (Dobson and Franzmann 1996), excreted ectoine into the medium and accumulated ectoine in the medium when extracellular osmolarity was constant. The total concentration of both intracellular and excreted ectoine was not affected by NaCl concentration in the medium. However, the excreted ectoine concentration is negatively correlated with NaCl concentration in the medium. Therefore, using ecoine-excreting strain, a new production process could be developed for high-efficiency production of ectoine under low NaCl concentration to avoid the problems encountered when using non-ectoine excreting strains in the above-mentioned processes.
The resting cells (also called as non-growing cells) refer to the group of non-proliferating and still metabolically active cells (Cotter et al. 2009; Achkar and Ferrandez 2010), which could be controlled to either not grow or to grow at low specific growth rates by the limitation of essential nutrients such as inorganic phosphate in the medium (Ramana and Karanth 1989). Resting cells have a number of advantages over growing cells, including selective product transformation, a higher bioconversion ratio, cells stability and lower consumption of oxygen, and so on (Cotter et al. 2009; Achkar and Ferrandez 2010; Pareilleux and Vinas 1984; Tanaka et al. 2002) and therefore, resting cells have been widely used for the synthesis and transformation of some products, for example, candicidin (Martin and McDaniel 1976), alkaloid (Pareilleux and Vinas 1984), ethanol and acetate (Cotter et al. 2009), mannitol (Khan et al. 2009), hydroxytyrosol (Achkar and Ferrandez 2010), glycolipid (Ramana and Karanth 1989), etc. However, to date, there has been no report of ectoine production using resting cells. In order to improve the productivity of ectoine and to overcome the limitation of dissolved oxygen during high cell density fermentation, we tried to efficiently synthesize ectoine using resting cells of the ectoine-excreting strain H. salina DSM 5928T.
This paper reports the development of a new process for high-efficiency production of ectoine by ectoine-excreting H. salina DSM 5928T. The process is performed in a combined process of batch fermentation using growing cells and production using resting cells. In the first stage, batch fermentation was carried out using growing cells under optimal fermentation conditions such as NaCl and initial monosodium glutamate concentration, pH and temperature. The second stage is the production phase in which phosphate-limited cells were used for ectoine synthesis and excretion of ectoine to the medium.
Materials and methods
Strain and media
H. salina DSM 5928T was purchased from DSMZ (German Collection of Microorganisms and Cell Cultures). The activation medium (g l−1): monosodium glutamate 30, KH2PO4 3, K2HPO4 9, MgSO4·7H2O 0.4, and MnSO4·4H2O 0.01, yeast extract 1, NaCl 30, pH 7.0, which was sterilized at 121°C for 20 min. The fermentation medium (g l−1): monosodium glutamate and NaCl concentrations, as well as pH were set according to experimental condition; KH2PO4 3, K2HPO4 9, MgSO4·7H2O 0.4, and MnSO4·4H2O 0.01. The medium was sterilized at 121°C for 20 min.
Batch fermentations
H. salina DSM 5928T was cultivated in the activation medium at 30°C by 120 rpm for 24 h. Batch fermentations were performed in a fermentor with a working volume of 10 l. The fermentor was filled with 6 l of fermentation medium. The fermentor was inoculated with shake flask cultures of H. salina DSM 5928T (300 ml). The pH and the temperature were set according to the experimental conditions shown in Table 1. The maximum aeration and stirrer speed were 10 l min−1 and 1,000 rpm, respectively. The dissolved oxygen level was measured by a polarization pO2 electrode. The antifoam agent Paodi (Lushun Chemical Plant, China), 0.015%, was added to the fermentation medium to avoid foaming.
Ectoine synthesis using resting cells
The cells were grown in batch fermentation medium until the end of exponential phase of growth, and then the resting cells medium was added. Culture system of resting cells was limited by phosphate, and the ingredient in resting cells medium was the same as that in batch fermentation medium, but the concentrations of some ingredients were different. The concentrate of resting cells medium contained monosodium glutamate (200 g l−1), KH2PO4 (3.0 g l−1), K2HPO4 (9.0 g l−1), MgSO4·7H2O (0.4 g l−1), MnSO4·4H2O (0.01 g l−1) and NaCl (0.5 mol l−1), which was sterilized at 121°C for 20 min (pH 7.0). After 18 h of incubation, resting cells medium was added to the fermentor at a rate of 1.12 ml min−1, and the total volume adding was 1.21 l.
Cell dry weight
Fermentation broth was centrifuged by 15,000×g at 4°C for 15 min and then pellets were washed by a 100 mmol l−1 phosphate buffer with same concentrations of NaCl and pH values as the fermentation broth. After centrifugation, the pellets were dried at 90°C and weighed until no further change in weight occurred.
Analyses of ectoine and monosodium glutamate
Concentration of intracellular ectoine (g l−1)
1 ml broth was centrifuged by 15,000×g at 4°C for 15 min, and then 80% ethanol was added to the pellets and kept overnight after suspension. After centrifugation by 15,000×g at 4°C for 15 min, the supernatant was applied for the measurement (Nagata et al. 2008).
The concentration of the excreted ectoine (g l−1)
1 ml broth was centrifuged by 15,000×g at 4°C for 15 min and the supernatant was applied for the measurement.
Total concentration of ectoine (g l−1) was the sum-up of the concentrations of intracellular and excreted ectoine.
HPLC determination
The concentration of ectoine was measured by HPLC with a TSK-GEL reversed-phase column (Tosoh, Japan) with 50 mmol l−1 phosphate buffer as mobile phase at 35°C. The flow rate was 1 ml min−1 and UV detection at 210 nm was adopted (Nagata et al. 2008). The retention time of ectoine was determined using commercially available authentic ectoine ((S)-β-2-methyl-1,4,5,6-tetrahydro-pyrimidine-4-carboxylic acid, purity >97%, Biomol, Hamburg, Germany).
Monosodium glutamate concentration was measured with HPLC using the same column and conditions as that of ectoine. The preserving time was determined by chromatographic grade of authentic monosodium glutamate.
Extraction and purification of ectoine
Ultrafiltration with hollow fiber
Polysulfone hollow fiber ultrafiltrate cylinder (CLW-003, Beijing Xubang Film Equipment Co., Ltd) was used, and the molecular weight cut-off of the hollow fiber membranes is approximately 10,000. The flux of pure water was 60 l h−1 at 25°C under 0.06 MPa.
Sedimentation with ethanol
Ethanol (95%, v/v) was added into the pre-purified fluid, and the proportion of the pre-purified fluid against ethanol was 1:9 (v/v). After 30 min at 4°C, filtration was performed.
Separation by ion exchange
Cation exchange resin of highly acidic (732-type, Dandong Dong Fan Rsin Plant, China) was used. The separation was carried out according to the method described in Sauer and Galinski (1998).
Results
Optimization of fermentation conditions for ectoine excretion
Batch fermentations using ectoine-excreting strain H. salina DSM 5928T were performed in a 10 l fermentor. The effects of NaCl and initial monosodium glutamate concentration, pH and temperature in fermentation broth were investigated on the excretion and synthesis of ectoine (Table 1).
Effect of NaCl concentration on the excretion and synthesis of ectoine
To examine the effect of NaCl concentration on the excretion and synthesis of ectoine, NaCl concentrations were set at 0.25, 0.5, 0.75 or 1 mol l−1. The initial monosodium glutamate concentration was 60 g l−1. Batch fermentation (pH 7.0) was performed at 30°C. As shown in Table 1, excretion and synthesis of ectoine from H. salina DSM 5928T are intimately related to NaCl concentration. Some of these relationships are summarized as follows: (1) the total concentration of ectoine was the sum-up of the concentrations of intracellular and excreted ectoine. The NaCl concentration has little influence on the total concentration of ectoine, which remains at an almost constant level regardless of the NaCl concentration in the medium. When NaCl concentration was 0.5, 0.75 and 1 mol l−1, total concentration of ectoine was 6.91, 6.87 and 6.85 g l−1, respectively, which was not significantly different. (2) NaCl concentration has a decisive effect on the concentration of intracellular ectoine, which is positively correlated with the osmotic pressure of the medium, i.e., it increased with the increase of NaCl concentration in the medium. (3) The distributions of ectoine inside and outside the cells are determined by the NaCl concentration. The excreted ectoine concentration is negatively correlated with the osmotic pressure of the medium. The lower the NaCl concentration, the higher the concentration of excreted ectoine. The excreted ectoine concentration was maximal (4.25 g l−1) at 0.5 mol l−1 NaCl. Cells could not grow better with 0.25 mol l−1 NaCl (12 h, OD value was 1.2) compared with others. In this paper, the optimal NaCl concentration for ectoine excretion and synthesis from H. salina DSM 5928T was 0.5 mol l−1.
Effect of initial monosodium glutamate concentration on the excretion and synthesis of ectoine
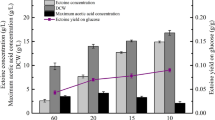
The initial monosodium glutamate concentration was set at 40, 60, 80 or 100 g l−1 in fermentation medium. NaCl concentration was 0.5 mol l−1. As shown in Table 1, the excreted and total concentrations of ectoine reached maximum when the initial monosodium glutamate concentration was 80 g l−1. When the initial monosodium glutamate concentration was 100 g l−1, the residual glutamate in the medium remained at a relatively high concentration of 30.84 g l−1.
Effect of pH on the excretion and synthesis of ectoine
When monosodium glutamate was used as the source of carbon and nitrogen in the medium for the fermentation with H. salina DSM 5928T, the pH was significantly increased because of the production of ammonia through monosodium glutamate metabolism, from pH 7.0 to 8.9 (Zhang et al. 2009). Because high pH inhibits growth of H. salina DSM 5928T, the maintenance of an optimal pH is essential during fermentation. The pH value of the fermentation medium was adjusted to 6.5, 7.0 or 7.5. NaCl and the initial monosodium glutamate concentrations were 0.5 mol l−1 and 80 g l−1, respectively. As shown in Table 1, the excreted and total concentrations of ectoine were the highest when the pH value was 7.0.
Effect of temperature on the excretion and synthesis of ectoine
The temperature of the batch fermentation was set at 30, 33 or 36°C. NaCl and initial monosodium glutamate concentrations were 0.5 mol l−1 and 80 g l−1, respectively. Excreted and total concentrations of ectoine were the highest at 33°C. Although 30°C was the most appropriate temperature for cell growth, a higher temperature (33°C) enhanced the permeability of the cell membrane, which was beneficial for ectoine excretion.
To conclude, the optimal condition of ectoine excretion during batch fermentation was that NaCl and initial monosodium glutamate concentrations were 0.5 mol l−1 and 80 g l−1, respectively, the pH and temperature were maintained at 7.0 and 33°C respectively, which were also the conditions to obtain the highest total concentration of ectoine.
Time course of ectoine batch fermentation under optimal conditions
Using the optimal conditions mentioned above, ectoine batch fermentation was monitored over time. As shown in Fig. 1, after a lag phase of 5–7 h, H. salina DSM 5928T entered the exponential phase of growth, and the stationary phase was observed from 19 to 26 h of incubation. Ectoine was synthesized and excreted during both the exponential phase of growth and the stationary phase. Both the excreted and the total concentrations of ectoine reached their maximum of 6.84 and 8.21 g l−1, respectively, at the end of the stationary phase of growth. These concentrations were significantly higher than those achieved at the exponential phase of growth.
Combined process for ectoine production
The combined process included two stages, namely, batch fermentation by growing cells and ectoine production by resting cells. Batch fermentation was carried out using growing cells and the optimal fermentation conditions as shown in Table 1. Fermentation was then followed by ectoine synthesis using resting cells. The initial monosodium glutamate concentration in batch fermentation medium was 80 g l−1. After 18 h of fermentation, the resting cells medium (monosodium glutamate concentration was 200 g l−1) was added. The actual concentration of monosodium glutamate in the fermentor is shown in Fig. 2. There was a transition phase which began at the time of feeding of the phosphate-limited resting cells medium (18 h) and extent to the time when cells stopped growing (20 h). During this period, cell dry weight increased by 5.83 g l−1. Following consumption of a specific level of phosphate, cell growth stopped and the cells entered the resting state.
Combined process of ectoine production using both growing and resting cells. Fermentation condition: NaCl 0.5 mol l−1, pH 7.0, initial monosodium glutamate 80 g l−1, temperature 33°C. Legends: intracellular ectoine (black bar), excreted ectoine (white bar), cell dry weight (open circles), monosodium glutamate (open triangles). After 18 h (of fermentation) the resting cells medium was added (↓)
During batch fermentation using growing cells (0–20 h, this phase includes growth phase (0–18 h) and transition phase (18–20 h)), cell dry weight was 42.25 g l−1, the excreted and total concentrations of ectoine were 2.51 and 4.72 g l−1, respectively, the productivity was 0.24 g l−1 day−1.
The maximum and minimum cell dry weight of resting cells during production of ectoine (20–46 h), was 42.68 and 41.81 g l−1, respectively. Thus, cell dry weight and viable cell numbers were almost maintained at a constant level. By the end of this stage, the total concentration of ectoine had increased from 4.72 g l−1 (20 h) to 14.86 g l−1 (46 h), of which, the excreted ectoine concentration had increased from 2.51 g l−1 (20 h) to 11.75 g l−1 (46 h), while the intracellular ectoine concentration was maintained at 2.90 ± 0.21 g l−1. Ectoine productivity was 9.36 g l−1 day−1 using resting cells.
Using the combined process, the total concentration of ectoine was 14.86 g l−1, while excreted and intracellular concentrations of ectoine were 11.75 and 3.11 g l−1, respectively. The productivity and yield of ectoine were 7.75 g l−1 day−1 and 0.14 g g−1, respectively. Compared with batch fermentation under optimal conditions shown in Table 1 (0.5 mol l−1 NaCl, initial monosodium glutamate 80 g l−1, pH 7.0, 33°C), the excreted and total concentrations of ectoine as well as yield of ectoine increased by 71.83, 80.98 and 34.95%, respectively.
Purification of excreted ectoine
When the production phase was finished, the main ingredients in the medium were ectoine (11.75 g l−1), cells (dry weight 42.68 g l−1), monosodium glutamate (4.5 g l−1), NaCl (29.2 g l−1) and proteins (1.33 g l−1). Ectoine extraction and purification done by hollow fiber ultrafiltration, ethanol precipitation and ion-exchange chromatography.
-
1.
The fermentation broth was centrifuged to remove cells when the production phase was finished. Then cells, proteins and other substances in the supernatant were first filtered using hollow fiber ultrafiltration. The removal rates of cells and proteins were 100 and 96%, respectively. And ectoine cannot be cut off.
-
2.
The resultant fluid after hollow fiber ultrafiltration was concentrated by depression vaporization to reduce the volume to about one fifth of the original volume. Next, ethanol (95%, v/v) was added to yield an ethanol/concentrated liquid ratio of 9:1 (v/v) in order to precipitate contaminating substances such as monosodium glutamate, NaCl and proteins, etc. The removal rates of monosodium glutamate, NaCl, protein were ≥97, 71 and 98%, respectively. The rate of recovery of ectoine was ≥79%.
-
3.
The supernatant after ethanol precipitation was concentrated and crystallized by depression vaporization. Subsequently, deuterium oxide was added to dissolve the crystal. Then ectoine was purified by strongly acidic cation exchange chromatography according to the method described by Sauer and Galinski (1998). The rate of recovery of ectoine was ≥86%.
The liquid recovered by ion exchange was concentrated and crystallized by depression vaporization. The purity of ectoine was 97.6% (mass) and the recovery rate was ≥70%. For intracellular ectoine, purification could be performed according to Onraedt et al. (2005).
Discussion
In batch fermentation with 10 l fermentor, optimal conditions for synthesis and excretion of ectoine by H. salina DSM 5928T were as follows: NaCl and initial monosodium glutamate concentrations in the medium were 0.5 mol l−1 and 80 g l−1, respectively. The pH and the temperature were maintained at 7.0 and 33°C, respectively. The combined process of batch fermentation using growing cells and production using resting cells was performed. The use of this combined process significantly increased the total concentration of ectoine that was synthesized as well as excreted. Moreover, 79% of the total concentration of synthesized ectoine was excreted into the medium, which could simplify the process of extraction and purification of ectoine.
Grammann et al. (2002) discovered a specific ectoine transporter TeaABC in Halomonas elongata DSM 2581T. Transporter TeaABC takes up ectoine that is excreted from the cell to the periplasm, as well as exogenous ectoine that is supplied with the medium. Through a putative export-uptake cycle, it is proposed that ectoine synthesis could be regulated to reach and maintain an optimal concentration of ectoine in the cell. Therefore, ectoine was not accumulated in the medium. However, TeaABC-deficient mutants of H. elongata DSM 2581T could excrete ectoine to the medium, i.e, the concentration of excreted ectoine resulted in 370 μmol l−1 (Grammann et al. 2002). The percentage of ectoine excreted from the ectoine-excreting wild-type strain of H. salina DSM 5928T was much greater than that from TeaABC-deficient mutants of H. elongata DSM 2581T, which have also been reported to increase ectoine production.
A biotechnological process called as ‘‘bacterial milking’’ has been established in order to improve the productivity of ectoine synthesis (Sauer and Galinski 1998). By this process, ectoine is synthesized by H. elongata DSM 2581T under higher NaCl concentration. When transferred to a low-salinity medium (osmotic downshock), H. elongata DSM 142 cells rapidly release ectoine to achieve osmotic equilibrium and the procedure can be repeated, where the productivity of ectoine is about 3.3 g l−1 day−1. Osmotic downshock is essential for ectoine excretion in this process because the H. elongata DSM 142 a non-ectoine excreting strain is used. However, if the ectoine-excreting strain H. salina DSM 5928T is used for ectoine production, as shown in our study ectoine would be excreted into the medium when the intracellular ectoine concentration reached an optimal concentration. Thus, osmotic downshock is not necessary in our process, which could therefore simplify the equipment and processes used for industrialized production of ectoine.
Onraedt et al. (2005) reported the results of fed-batch fermentation with high cell density, which indicated that an increase in ectoine concentration depends on an increase in cell density. After fermentation of 100 h in the medium with 1 mol l−1 NaCl, the maximal cell density was 49 g l−1 and ectoine concentration was about 8 g l−1. Thus, the productivity and yield of ectoine were 1.92 g l−1 day−1 and yield was 0.05 g g−1, respectively. The ectoine concentration that they achieved was the highest so far reported using batch fermentation and a non-excreting halophile. In their process, when cell growth stopped, ectoine concentration leveled off and the dissolved oxygen level was maintained above 50% saturation during the fermentation. In contrast, the results of the present paper, using a combined process of batch fermentation by growing cells and production by resting cells, show high ectoine productivity, involving both ectoine excretion and synthesis, using resting cells. The reasons for the highly efficient ectoine synthesis were as follows: (1) cells that synthesized ectoine with high efficiency were obtained through batch fermentations, and these cells, which possessed an ectoine synthase system, were then used as the source of resting cells for further ectoine production. (2) The synthesized ectoine was partly excreted to the medium at low NaCl concentrations, since the H. salina DSM 5928T used is an ectoine-excreting strain. Therefore, ectoine was continuously synthesized during the production phase using resting cells. Tanaka et al. (2002) measured the oxygen consumption of growing and resting cells of Pseudomonas putida. Compared with the growing cells, resting cells showed lower oxygen consumption. The concentration of ectoine that was synthesized was limited by aeration during high cell density fermentation. However, oxygen consumption was significantly reduced when resting cells were used for synthesis of ectoine. In the present paper, the concentration of ectoine produced was significantly improved by adopting a combined process of batch fermentation by growing cells and production by resting cells. The total concentration, productivity and yield of ectoine, were 14.86 g l−1, 7.75 g l−1 day−1 and 0.14 g g−1, respectively.
Achkar and Ferrandez (2010) reported a combined process of tyrosol tranformation to hydroxytyrosol by E. coli TOP10/pD1. The process consisted of three different phases: growth phase; transition phase, where cultivation conditions could be modified so that the growth rate of the microorganism decreased until a resting cells mode was reached; production phase using resting cells. The three phases were performed in the same fermentor. However, in their process, when growth entered the transition phase, the cells had to be separated by centrifugation or other methods, which might be inconvenient for industrialized production (Achkar and Ferrandez 2010). In contrast, in the present paper, the media components of the two phases were the same, although their concentrations were different. Therefore, the transition from the growth phase to production phase using resting cells could be completed by means of nutrition consumption in the growth medium and feeding after the growth phase. This result means that the step of harvesting of the cells may be omitted, and that fermentation with two phases can be performed in the same vessel. Thus, the preparation process is simplified and the cost of medium can be reduced. Therefore, the present research might provide a foundation for the development of methods for the continuous production of ectoine and for ectoine synthesis using immobilized resting cells.
References
Achkar J, Ferrandez A (2010) Method for preparing hydroxytyrosol. US patent US2010047887A1
Cotter JL, Chinn MS, Grunden AM (2009) Ethanol and acetate production by Clostridium ljungdahlii and Clostridium autoethanogenum using resting cells. Bioprocess Biosyst Eng 32:369–380
Dobson SJ, Franzmann PD (1996) Unification of the genera Deleya (Baumann et al. 1983), Halomonas (Vreeland et al. 1980), and Halovibrio (Fendrich 1988) and the species Paracoccus halodenitrificans (Robinson and Gibbons 1952) into a single genus, Halomonas, and placement of the genus Zymobacter in the family Halomonadaceae. Int J Syst Evol Microbiol 46:550–558
Galinski EA, Pfeiffer HP, Trüper HG (1985) 1, 4, 5, 6-tetrahydro-2-methyl-4-pyrimidinecarboxylic acid. A novel cyclic amino acid from halophilic phototrophic bacteria of the genus Ectothiorhodospira. Eur J Biochem 149:135–139
Grammann K, Volke A, Kunte HJ (2002) New type of osmoregulated solute transporter identified in halophilic members of the bacteria domain: TRAP-transporter TeaABC mediates the uptake of ectoine and hydroxyectoine in Halomonas elongata DSM 2581T. J Bacteriol 184:3078–3085
Kanapathipillai M, Lentzen G, Sierks M, Park CB (2005) Ectoine and hydroxyectoine inhibit aggregation and neurotoxicity of Alzheimer’s β-amyloid. FEBS Lett 579:4775–4780
Khan A, Bhide A, Gadre R (2009) Mannitol production from glycerol by resting cells of Candida magnoliae. Bioresource Technol 100:4911–4913
Kuhlmann AU, Bremer E (2002) Osmotically regulated synthesis of the compatible solute ectoine in Bacillus pasteurii and related Bacillus spp. Appl Environ Microb 68:772–783
Lentzen G, Schwarz T (2006) Extremolytes: natural compounds from extremophiles for versatile applications. Appl Microbiol Biotechnol 72:623–634
Martin JF, McDaniel LE (1976) Biosynthesis of Candicidin by phosphate-limited resting cells of Streptomyces griseus. Eur J Appl Microbiol 3:135–144
Nagata S, Wang YQ, Oshima A, Zhang LH, Miyake H, Sasaki H, Ishida A (2008) Efficient cyclic system to yield ectoine using Brevibacterium sp. JCM 6894 subjected to osmotic downshock. Biotechnol Bioeng 99:941–948
Onraedt AE, Walcarius BA, Soetaert WK, Vandamme EJ (2005) Optimization of ectoine synthesis through fed-batch fermentation of Brevibacterium epidermis. Biotechnol Prog 21:1206–1212
Oren A (2008) Microbial life at high salt concentrations: phylogenetic and metabolic diversity. Saline Syst 4:2
Pareilleux A, Vinas R (1984) A study on the alkaloid production by resting cell suspensions of Catharanthus roseus in a continuous flow reactor. Appl Microbiol Biotechnol 19:316–320
Ramana KV, Karanth NG (1989) Production of biosurfactants by the resting cells of Pseudomonas aeruginosa CFTR-6. Biotechnol Lett 11:437–442
Sauer T, Galinski EA (1998) Bacterial milking: a novel bioprocess for production of compatible solutes. Biotechnol Bioeng 57:306–313
Tanaka T, Xing XH, Matsumoto K, Unno H (2002) Preparation and characteristics of resting cells of bioluminescent Pseudomonas putida BLU. Biochem Eng J 12:29–36
Ventosa A, Nieto JJ (1995) Biotechnological applications and potentialities of halophilic microorganisms. World J Microbiol Biotechnol 11:85–94
Ventosa A, Nieto JJ, Oren A (1998) Biology of moderately halophilic aerobic bacteria. Microbiol Mol Biol Rev 62:504–544
Zhang LH, Wang Y, Zhang CY, Wang YJ, Zhu DC, Wang CX, Nagata S (2006) Supplementation effect of ectoine on thermostability of phytase. J Biosci Bioeng 102:560–563
Zhang LH, Lang YJ, Nagata S (2009) Efficient production of ectoine using ectoine-excreting strain. Extremophiles 13:717–724
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 20776021).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Santos.
Rights and permissions
About this article
Cite this article
Lang, Yj., Bai, L., Ren, Yn. et al. Production of ectoine through a combined process that uses both growing and resting cells of Halomonas salina DSM 5928T . Extremophiles 15, 303–310 (2011). https://doi.org/10.1007/s00792-011-0360-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-011-0360-9