Abstract
Objectives
We previously showed that accelerated degradation of collagen membranes (CMs) in diabetic rats is associated with increased infiltration of macrophages and blood vessels. Since pre-implantation immersion of CMs in cross-linked high molecular weight hyaluronic acid (CLHA) delays membrane degradation, we evaluated here its effect on the number of macrophages and endothelial cells (ECs) within the CM as a possible mechanism for inhibition of CM resorption.
Materials and methods
Diabetes was induced with streptozotocin in 16 rats, while 16 healthy rats served as control. CM discs were labeled with biotin, soaked in CLHA or PBS, and implanted under the scalp. Fourteen days later, CMs were embedded in paraffin and the number of macrophages and ECs within the CMs was determined using antibodies against CD68 and transglutaminase II, respectively.
Results
Diabetes increased the number of macrophages and ECs within the CMs (∼2.5-fold and fourfold, respectively). Immersion of CMs in CLHA statistically significantly reduced the number of macrophages (p < 0.0001) in diabetic rats, but not that of ECs. In the healthy group, CLHA had no significant effect on the number of either cells. Higher residual collagen area and membrane thickness in CLHA-treated CMs in diabetic animals were significantly correlated with reduced number of macrophages but not ECs.
Conclusions
Immersion of CM in CLHA inhibits macrophage infiltration and reduces CM degradation in diabetic animals.
Clinical relevance
The combination of CLHA and CM may represent a valuable approach when guided tissue regeneration or guided bone regeneration procedures are performed in diabetic patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The regeneration of tissues lost due to disease is the goal of periodontal treatment [1]. Collagen-based barrier membranes (collagen membranes; CMs) are routinely applied in guided tissue regeneration (GTR) and guided bone regeneration (GBR) procedures. Membranes create and maintain a space over a bony defect and act as a protective barrier against ingrowth of rapidly proliferating epithelial and connective tissue cells into the defect [2]. Resorption of the CM occurs via a biodegradation process. This process starts when cells within the surgical site release matrix metalloproteinases (MMPs) to the wound area during healing, and continues with infiltration and colonization by fibroblasts and capillaries, leading to collagen scaffold remodeling, followed by its replacement with new extracellular matrix [3,4,5,6].
It has been established that CM longevity plays a crucial role in the success of regenerative procedures [7,8,9,10]. Furthermore, premature exposure of the CM to the oral cavity or its early degradation may jeopardize the success of GTR or GBR procedures [11,12,13]. The degradation rate may be influenced by the composition, structure, and dimension of the CM [1, 3, 14] as well as by systemic diseases, such as uncontrolled diabetes. Many studies have demonstrated that diabetes severely exacerbates the onset, progression, and severity of periodontitis [15,16,17,18] due to dysregulation of the inflammatory process and also impairs periodontal regeneration by interfering with proper wound healing and diminishing bone formation [19,20,21].
In this regard, several studies have demonstrated faster CM resorption in rats with uncontrolled diabetes [22,23,24], showing that the inflammatory infiltrate inside the CMs in diabetic rats was more marked, compared with normoglycemic rats, and that accelerated degradation of CM was associated with increased inflammatory components like macrophages and capillaries [24]. Reducing the inflammatory process within the CM plays an important factor in enhancing the longevity and integrity of the CM in diabetic conditions.
Hyaluronic acid (HA) is a natural glycosaminoglycan. It is an essential component of connective tissues and plays an important role during wound healing [25]. Moreover, HA may also be introduced as a hydrogel with inherent absorption properties that are desirable in the wound healing environment [25,26,27]. The properties of HA have been investigated since 1934. It was found that HA possesses biocompatible, biodegradable, bacteriostatic, antioxidant, anti-edematous, and anti-inflammatory properties [28]. There are 2 main forms of HA: high molecular weight HAs (HMW) and low molecular weight HAs (LMW). It has been shown that LMW HAs (100–500 kDa), but not the HMW HA molecules (~ 4,000 kDa), stimulate inflammatory cells creating an inflammatory environment [29]. In medicine and dentistry, the use of HMW HAs is preferable for several reasons. HMW HAs exhibit higher viscosity, longer residence time, and higher biocompatibility when compared to LMW HAs [30]. HMW HAs also show anti-inflammatory effects by decreasing interleukin (IL) − 1β, IL-6, tumor necrosis factor-α (TNF-α), and prostaglandin E2 (PGE2) production [31,32,33], whereas LMW HAs have been reported to stimulate angiogenesis [34] and induce an inflammatory response [35]. On the other hand, HMW HAs predominate in healthy tissues and typically inhibit inflammation. Due to their desirable properties, HMW HAs have been used in various medical [36] and dental [37, 38] therapies. In medicine, HMW HAs are routinely applied in the treatment of diabetic ulcers since they increase the rate of wound healing [38, 39]. Recently, it has been also demonstrated that HA enhances the proliferative, migratory, and wound healing properties of several cell types involved in soft tissue wound healing without impairing the healing process by prolonging inflammation or causing excessive MMP expression. Interestingly, recent data [39] also revealed that HA has the potential to induce the growth of osteoprogenitors by maintaining their stemness, which may exert a possible effect on the balance between self-renewal and differentiation during bone healing/regeneration. Recent systematic reviews and meta-analysis in dentistry have shown favorable results using HA as an adjunct to periodontal surgical procedures [40].
Immersion of CM in HA is a currently new area of investigation. Recently, several in vitro and in vivo animal studies have demonstrated some advantages using cross-linked HMW HA [41]. In a recent animal study, it was concluded that HMW HA does not interfere with tissue integration and structural degradation of Bio-Guide® (Geistlich Pharma AG, Wolhusen, Switzerland) or OsseoGuard® (Zimmer Biomet, Warsaw, IN, USA) [41], two CMs which are frequently used for periodontal and bone regeneration.
In another recent publication, we showed histologically that in rats with type-1-like diabetes, the immersion of CMs in HMW cross-linked HA delayed membrane degradation compared with non-immersed CMs [42]. As a continuation of that study, which looked only at the influence of hyperglycemia and HA on the amount of residual collagen, the current study aimed to add some insights into the mechanisms responsible for increased membrane resorption in diabetic animals on one hand and the mitigating effect of HA immersion on the other hand. For this purpose, we chose to assess the inflammatory response within CMs, as evidenced by the number of macrophages and endothelial cells that have penetrated the implanted CMs and test whether it is correlated to the degree of membrane resorption. Different interventions that slow down CM resorption may have distinct mechanisms. For instance, immersion of CMs in tetracycline reduces their resorption [22] possibly due to its anti-collagenolytic properties. Therefore, it is important to elucidate the way in which HA protects CMs from excessive resorption in diabetic animals.
We hypothesize that HMW HA, due to its anti-inflammatory properties, protects CM from accelerated resorption by reducing the number of M1 macrophages inside the implanted CMs.
Materials and methods
Details of the animal procedures of this study appear in [42]. Thirty-two 12-week-old male Wistar rats weighing 300–350 g were randomly divided into 2 groups. The institution Animal Care and Use Committee of Tel Aviv University, Tel Aviv, Israel, approved the study (TAU 1–16-031). Diabetes was induced in 16 rats by a single intraperitoneal injection of 65 mg/kg streptozotocin (Sigma Chemical Co. MO, USA). The remaining animals, which served as normoglycemic controls, were given a similar volume of citrate buffer. All animals were fed a regular diet. Pericardial CMs (Smartbrane, Regedent AG, Zürich, Switzerland) were cut with a disposable biopsy punch (Miltex, Lake Success, NY) to 8-mm-diameter discs. Membrane labeling with biotin has been described previously [22].
One week after diabetes induction, animal surgeries were performed by the same experienced operator (CN). Animals were anesthetized by an intramuscular injection of 0.1 mL/100 g 10% ketamine hydrochloride (Rhone Merieux, Lion, France) and 0.1 mL/100 g 2% xylazine hydrochloride (Vitamed, Binyamina, Israel). The dorsal part of the skin covering the scalp was shaved and aseptically prepared for surgery. A 15-mm incision was made on the mid-sagittal line and a subperiosteal pouch over the calvaria was created using a Kirkland periodontal knife (Hu-Friedy, Chicago, IL). One disc, immersed in a 20 mg/mL CLHA (Hyadent BG; Regedent AG) solution or in PBS, was implanted in each animal underneath the periosteum. Periosteum and skin were repositioned, covering the implanted membrane, and the skin was sutured with resorbable sutures (Vicryl Rapide, Ethicon).
Fourteen days later, animals were euthanized with an overdose of ketamine and xylazine, followed by asphyxiation with carbon dioxide (CO2). The discs and overlying skin were retrieved and fixed in 4% paraformaldehyde, decalcified for 10 weeks in a 10% ethylenediaminetetraacetic acid (EDTA, pH 7.3) solution, dehydrated in ethanol and xylene, and embedded in paraffin. Sagittal 5-μm sections were made, and those that included the central area of the membrane were selected for comparative analysis of CM cell infiltration and degradation. Sections were stained with hematoxylin and eosin (H&E), and adjacent sections were stained with horseradish peroxidase (HRP)‐conjugated streptavidin (ZytoMed, Berlin, Germany) according to the protocol of the manufacturer, to detect biotinylated collagen [22, 42].
Two neighboring sagittal sections were used for immunohistochemistry. Endogenous peroxidase activity was blocked with 3% H2O2 for 10 min. Antigen retrieval was performed with 0.1% proteinase K in PBS for 15 min at 37 °C (for CD68) or by heating under pressure of the sections in a citrate buffer, pH = 6, for a total of 12 min (for TGII). Nonspecific binding sites were blocked by incubation with Background Buster (Innovex, Richmond, CA, USA) for 40 min. Primary antibodies used were a mouse anti-rat CD68 monoclonal antibody (Millipore Corporation, Billerica, MA, USA) (at a 1:100 dilution) for macrophage identification and a mouse monoclonal anti-transglutaminase II (TGII) antibody (Thermo Fisher Scientific, Waltham, MA, USA) (at a 1:150 dilution) for the identification of endothelial cells [43]. Both antibodies were diluted in an antibody diluent (Zytomed) and were incubated with the sections for 1 h at room temperature. Bound primary antibodies were detected with a goat anti-mouse HRP-conjugated antibody (Zytomed), incubated for 30 min at room temperature, and followed by a detection with DAB substrate kit (ScyTek, Logan, UT, USA) and hematoxylin counterstain. Negative controls were performed by omitting the primary antibody. All slides were mounted with an aqueous solution of glycerol vinyl alcohol (Zytomed).
Histological evaluation was performed by the same experienced investigator (DB). Stained sections (one from each animal for collagen content/membrane thickness and 2 for immunohistology) were photographed with a digital camera (AxioCam MRC, Carl Zeiss) mounted on a light microscope (AxioImager M2, Carl Zeiss) with a × 20 objective.
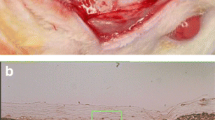
Measurements of CM thickness and collagen content in streptavidin-stained sections (reported previously ([42]) were made by superimposing three rectangular regions of interest (ROIs) per disc, one anterior, one middle, and one posterior. Each ROI had dimensions of 0.625 mm × 0.4 mm (= 0.25 mm2). A grid containing 40 μm × 40 μm cells was superimposed on the ROIs (Fig. 1) and the number of cell intersections in the grids that hit collagen within the ROI was registered. Mean residual collagen content (% of baseline) and CM thickness (mm) were determined for each disc. H&E stained sections were used to better identify the surrounding tissues (Fig. 2). For macrophage and endothelial cell counts, identical ROIs were used and all positive cells were counted within each ROI and the mean per disc was calculated.
Photomicrograph showing the 40-μm-cell grid superimposed on the ROI (stained green) overlying the disc, which is stained in red/brown with an Avidin‐biotin‐HRP reaction. The horizontal borders of the ROI are set to include the total width of the membrane disc. Methods used to measure collagen content and immunostained cells are listed in the “Methods” section
Data were analyzed for statistical significance using the non-parametric Kruskal–Wallis test (p values < 0.05 were considered significant), where CM treatment with CLHA/PBS was the within-subject variable and health status (normo- vs hyper-glycemia) was the between-subject variable. The Dunn’s multiple comparison test was used to test differences between groups. The Spearman’s correlation procedure was used to test correlations between collagen area or thickness and the number of endothelial cells or macrophages. All statistical calculations were made using the GraphPad Prism 7.0 software (GraphPad, La Jolla, CA, USA).
Results
Hyperglycemia was successfully induced by STZ in all animals in the diabetic groups (mean blood glucose level was 291.1 ± 5.5 mg/dL vs 108.2 ± 6.6 mg/dL in the control groups). Diabetes increased ~ 2.5-fold the number of CD-68 positive macrophages, which infiltrated the PBS-immersed membrane discs, compared to the healthy group (from 44.3 ± 35.1 per mm2 to 109.2 ± 41.7) (Figs. 3 and 4). Numerous CD68 + cells could also be seen at the CM borders. Immersion of the collagen discs placed in diabetic animals in CLHA statistically significantly reduced (~ 3.5-fold) the number of CD-68 positive macrophages compared to the PBS-immersed discs (from 109.2 ± 41.7 per mm2 to 30.9 ± 14.7). In CM placed in control animals, immersion in CLHA had no significant effect on the number of macrophages. Therefore, CLHA immersion reduced macrophage number only in the diabetic animals.
Photomicrographs of CD-68 positive cells within the CM placed in a normoglycemic (c − /c +) or a diabetic (d − /d +) rat, showing fewer macrophages in CLHA-immersed CM in the diabetic animal (d + vs d −). C − = control group, no CLHA. C + = control group, with CLHA. d − = diabetic group, no CLHA. d + = diabetic group with CLHA. Green rectangles represent the ROI
Since we have previously shown that immersion of CMs in CLHA statistically significantly increased residual collagen content and membrane thickness in the diabetic but not in the normoglycemic animals, we tested whether this effect correlated with the number of macrophages or endothelial cells in this group. Figures 5 and 6 show a significant negative linear correlation between the number of macrophages and membrane thickness (r = − 0.9021, p = 0.0002) or residual collagen (r = − 0.7802, p = 0.0025) in the diabetic animals. In both figures, all the data from the D − group (no HA) were concentrated in the upper left area of the scatter diagrams (more macrophages/lower residual membrane) and all data from the D + group were localized in the lower right area of the diagrams (fewer macrophages/higher residual membrane). Thus, reduced membrane resorption in diabetic rats seems to be associated with a reduction in macrophage infiltration.
Diabetes statistically significantly increased (by more than fourfold) the number of TGII-positive endothelial cells within the PBS-immersed membrane discs compared to the healthy group (from 26.8 ± 13.4 per mm2 to 115.9 ± 32.6, Figs. 7 and 8). Unlike macrophages, immersion of the discs in CLHA had no effect on the growth of TGII-positive endothelial cells into the collagen discs in both the control and diabetic animals.
In agreement, Figs. 9 and 10 show no correlation between membrane thickness or residual collagen and the number of endothelial cells, positive for TGII, within the CM in diabetic animals. Thus, the protective effect of CLHA against CM resorption was not correlated to changes in the infiltration of blood vessels into the membrane.
Discussion
Several studies, including our recent publication, demonstrated that CM degradation is markedly accelerated in uncontrolled diabetes compared with a healthy situation [22, 25, 42]. Given these data, our aim has been to advance the understanding of how hyperglycemia results in faster degradation of implanted devices such as collagen membranes and what measures can be devised to counteract its deleterious effect. For this purpose, we extended the mere measurements of residual collagen performed in the previous study [42] into an assessment of the inflammatory response within CMs, as evidenced by the number of macrophages and endothelial cells within the implanted CMs. All our previous studies with this model showed that the destructive effect of diabetes on membrane survival in rats is evident 2–4 weeks after implantation [22, 24, 42, 75]. Much shorter periods (a few days) may not be enough for hyperglycemia to affect the physio-pathology of the implantation site and significantly longer periods may result in complete loss of the membrane in diabetic animals (rats in this case). Thus, a post-implantation of 2 weeks was chosen for the present study.
Earlier studies showed distinctly more inflammatory cells penetrating the CMs that were implanted in diabetic rats, compared with healthy rats. This observation, together with a finding of a fourfold increase in the number of macrophages inside the CM in diabetic rats [24], suggested an association between faster CM degradation in diabetic rats and the presence of inflammation in and around the CM [22, 24, 42]. The current study, showing that diabetes increased 2.5-fold the number of macrophages inside the CM discs (Figs. 3, 4), is in agreement with these studies. In support of a hyperglycemia-induced local inflammation, a recent study using the same model reported that the expression and abundance of several inflammatory molecules (IL-6, TNFα, MMP-9, MIF, MIP-1α, and MIP-2α) in the tissues around and within CMs implanted in diabetic rats were markedly elevated, compared with those in normoglycemic rats [44].
In the current study, we found a significant negative correlation between the number of macrophages and the amount of residual collagen in the implanted CMs (Figs. 5, 6). Macrophages are known to participate in the host defense by their production of inflammatory cytokines, nitric oxide, and toxic oxygen metabolites contributing to collagen degradation [45]. These cells also have the capacity to secrete several members of the matrix metalloproteinase (MMP) family, including collagenase-1 (MMP-1) [46], stromelysin (MMP-3) [47], gelatinase B (MMP-9) [48], and macrophage elastase (MMP-12) [49]. While the activity of these proteinases likely contributes to both the host defense function of macrophages and to normal tissue remodeling and repair [45], various reports have shown that the levels of tissue MMPs in diabetes are elevated and those of the tissue inhibitors of matrix metalloproteinases (TIMPs) are lower [50, 51]. Excessive MMP-driven proteolysis has been associated with several pathological conditions including arthritis, cancer growth and metastasis, chronic obstructive pulmonary disease [52,53,54], and diabetes [50, 51]. Since CM degradation depends on collagenolytic activity of the host cells, specifically on MMPs [55, 56], it is understandable why CM degradation is accelerated in diabetic rats.
Macrophages are divided into several arbitrary functional classes, mainly M1 (“classical activation”), which are pro-inflammatory, and M2 (“alternative activation”), which are anti-inflammatory and pro-healing [57]. Accordingly, they differ significantly in the array of cytokines and growth factors they produce (TNFα, IL-1β, IL-6, etc. vs IL-10, TGFβ, etc., respectively). While the former have significant roles in mounting inflammation upon injury and combatting infection, the latter are vital during the formative and remodeling phases of wound healing and in tissue regeneration [57].
It is well known that diabetes induces accumulation of M1 macrophages in many organs and these cells contribute greatly to the respective diabetic complications [58,59,60,61]. Our findings of a dramatic increase in CD-68 + cells (putative M1 macrophages) within CM implanted in diabetic rats [24 and Figs. 3 and 4, current study] are in agreement with this notion. Macrophage accumulation in diabetes is attributed to increased non-enzymatic glycation of proteins (formation of advanced glycation end products (AGEs) and oxidative stress (OxS)) [58]. AGEs may affect many cell types by binding the receptor for AGEs (RAGE) and inducing the local formation of molecules that attract and retain macrophages such as monocyte chemoattractant protein-1 (MCP-1) and macrophage migration inhibitory factor (MIF) [58]. These activated macrophages, in turn, secrete a variety of pro-inflammatory mediators such as IL-1 and TNFα and contribute to diabetic tissue injury by producing reactive oxygen species (ROS) and MMPs [58].
The first part of the current experiment [42] showed the delaying effect of HMW CLHA on the degradation of the CM. CMs that were immersed in CLHA showed slower degradation in diabetic rats than those immersed in PBS. In contrast, such an effect of CLHA was not observed in the healthy rats. The fact that HMW CLHA had a significant effect only in the animals with uncontrolled diabetes is in agreement with other studies that showed an effect of HMW HA only in inflamed conditions (like uncontrolled diabetes) [62]. Thus, HMW HA can be beneficial towards the healing of chronically inflamed wounds, including most difficult-to-treat diabetic foot ulcers [62].
Although we previously showed that HA reduced the resorption of CMs in diabetic animals [42], we were seeking in the present study some mechanisms that would explain this beneficial effect of HA. One of the explanations for better outcomes with HMW CLHA in a chronic inflammatory state could be its effect on macrophages. Chronic inflammation is often associated with persistently activated macrophages, leading to most detrimental effects [63]. Therefore, macrophage reduction may help to reduce collagen degradation. This study examined the influence of CLHA on the number of macrophages present in the CM implanted in rats with uncontrolled diabetes. Our results clearly showed the effect of HMW CLHA on the degradation of the CM in uncontrolled diabetic conditions through the reduction of M1 macrophages. High-MW HA has been shown to promote M1-to-M2 phenotype switch, which results in dampening of the production of pro-inflammatory molecules (including MMPs) and stimulation of anti-inflammatory molecules [64,65,66]. Also, HMW HA represses the production of MIF in some cells [67] and the production of inflammatory cytokines/MMPs in other cells (e.g., chondrocytes) [68,69,70]. These observations could explain our finding of reduced M1 macrophages within HA-immersed CM discs, either by direct effects of HA on macrophages or through neighboring, host cells. Our conclusion that reduced CM degradation is strongly associated with reduction in the number of infiltrating macrophages is supported by the strong, negative linear correlations between these two variables, depicted in Figs. 5 and 6. Since HA may promote post-inflammatory healing by shifting macrophages towards an M2 phenotype, future studies may examine the possible promotive effect of HA on M2 macrophages in our model, as reported in other systems [64, 65].
The current study also aimed at evaluating the effects of CLHA on endothelial cells invading the CMs. It is well known that aberrant angiogenesis in different tissues can play a role in the pathogenesis of many complications of diabetes (e.g., retinopathy, nephropathy) [71, 72]. The enhancing effect of diabetes on blood vessel invasion into CM was found in several of our studies, where the number of endothelial cells was significantly (two- to three-fold) higher in the diabetic group compared to the healthy group [22, 24]. The data of the current study (a 2.5-fold increase, Figs. 7, 8) support these observations. The current study also found that adding CLHA to the CM did not reduce the number of endothelial cells inside the CM in the diabetic group. Consequently, we found no correlation between the number of endothelial cells and the residual collagen content and thickness of the CM in the diabetic group (Figs. 9, 10). This can be explained by studies showing that HA increases angiogenesis in certain circumstances [73, 74]. This study, together with our previous ones [22, 24, 42, 75], clearly shows that excessive CM degradation in hyperglycemic animals can be reduced by several means. While tetracycline probably inhibits collagenolysis by targeting MMPs, HA attenuates the inflammation that develops within the implanted CMs.
Furthermore, a few studies that compared tissue reactions to other ECM-based implantable devices between healthy and diabetic animals showed that hyperglycemia increased signs of inflammation (lymphocytes, macrophages, TNFα, MCP-1) and collagenolysis within these devices [76,77,78]. The results of our study may point to a method of prolonging the longevity of these devices used for medical purposes.
It is important to remember that the results of our study, both in terms of the effects of hyperglycemia and those of HA, are relevant to our T1D (type 1 diabetes)-like model and may be somewhat different in a T2D (type 2 diabetes) model.
Lastly, the fact that CLHA inhibits macrophage infiltration/collagen degradation (this study) and, in the same time, enhances the proliferative and migratory properties of cell types involved in soft tissue wound healing [79] may point to its potential usefulness in recession coverage procedures with or without the use of subepithelial connective tissue grafts (SCTG)[80]. A very recent study in dogs has evaluated the healing of gingival recessions treated with coronally advanced flap (CAF) with or without CLHA both clinically and histologically. The results revealed statistically significant differences in clinical attachment level gain favoring the use of CLHA. Most importantly, the study has for the first time provided histologic evidence for periodontal regeneration of gingival recession defects following the use of CLHA. These preclinical findings are in line with clinical studies that have evaluated the healing of single and multiple recessions treated with either CAF and CLHA [81] or modified coronally advanced tunnel (MCAT) in conjunction with CLHA and SCTG [82, 83], revealing excellent outcomes in terms of mean and complete root coverage.
Conclusions
Accelerated degradation of implanted CMs in rats with uncontrolled diabetes is strongly associated with increased infiltration of macrophages. Pre-implantation immersion of the CMs in cross-linked HMW HA slowed down their degradation most probably by reducing the number of infiltrating macrophages. Therefore, immersion of CM, to be applied in diabetic conditions, in CLHA can lead to a longer maintenance of CM thickness and collagen density, better preserving the barrier functions of CM. From a clinician’s perspective, the present findings suggest that the combination of CLHA and CM may represent a valuable approach when guided tissue regeneration or guided bone regeneration procedures are performed in diabetic patients.
References
Karring T, Nyman S, Gottlow J (2000) Laurell L (1993) Development of the biological concept of guided tissue regeneration–animal and human studies. Periodontol 1:26–35
Caton JG, DeFuria EL, Polson AM, Nyman S (1987) Periodontal regeneration via selective cell repopulation. J Periodontol 58(8):546–552
Rothamel D, Benner M, Fienitz T, Happe A, Kreppel M, Nickenig HJ, Zoller JE (2014) Biodegradation pattern and tissue integration of native and cross-linked porcine collagen soft tissue augmentation matrices - an experimental study in the rat. Head Face Med 10:10
Rothamel D, Schwarz F, Sager M, Herten M, Sculean A, Becker J (2005) Biodegradation of differently cross-linked collagen membranes: an experimental study in the rat. Clin Oral Implants Res 16(3):369–378
Willershausen I, Barbeck M, Boehm N, Sader R, Willershausen B, Kirkpatrick CJ, Ghanaati S (2014) Non-cross-linked collagen type I/III materials enhance cell proliferation: in vitro and in vivo evidence. J Appl Oral Sci 22(1):29–37
Calciolari E, Ravanetti F, Strange A, Mardas N, Bozec L, Cacchioli A, Kostomitsopoulos N, Donos N (2018) Degradation pattern of a porcine collagen membrane in an in vivo model of guided bone regeneration. J Periodontal Res 53(3):430–439
Tal H, Kozlovsky A, Artzi Z, Nemcovsky CE, Moses O (2008) Cross-linked and non-cross-linked collagen barrier membranes disintegrate following surgical exposure to the oral environment: a histological study in the cat. Clin Oral Implants Res 19(8):760–766
Pfeifer J, Van Swol RL, Ellinger R (1989) Epithelial exclusion and tissue regeneration using a collagen membrane barrier in chronic periodontal defects: a histologic study. Int J Periodontics Restorative Dent 9(4):262–273
Chung KD, Liu WM, Wu YW, Hui YL (1990) Painless labor and instrumentation. Ma Zui Xue Za Zhi 28(3):303–306
Patino MG, Neiders ME, Andreana S, Noble B, Cohen RE (2002) Collagen: an overview. Implant Dent 11(3):280–285
Machtei EE (2001) The effect of membrane exposure on the outcome of regenerative procedures in humans: a meta-analysis. J Periodontol 72(4):512–516
Tal H, Kozlovsky A, Artzi Z, Nemcovsky CE, Moses O (2008) Long-term bio-degradation of cross-linked and non-cross-linked collagen barriers in human guided bone regeneration. Clin Oral Implants Res 19(3):295–302
Zitzmann NU, Scharer P, Marinello CP (2001) Long-term results of implants treated with guided bone regeneration: a 5-year prospective study. Int J Oral Maxillofac Implants 16(3):355–366
Ferreira AM, Gentile P, Chiono V, Ciardelli G (2012) Collagen for bone tissue regeneration. Acta Biomater 8(9):3191–3200
Graves DT, Ding Z (2000) Yang Y (2020) The impact of diabetes on periodontal diseases. Periodontol 82(1):214–224
Polak D, Sanui T, Nishimura F (2000) Shapira L (2020) Diabetes as a risk factor for periodontal disease-plausible mechanisms. Periodontol 83(1):46–58
Preshaw PM, Bissett SM (2013) Periodontitis: oral complication of diabetes. Endocrinol Metab Clin North Am 42(4):849–867
Genco RJ, Graziani F (2000) Hasturk H (2020) Effects of periodontal disease on glycemic control, complications, and incidence of diabetes mellitus. Periodontol 83(1):59–65
Chang PC, Chung MC, Wang YP, Chien LY, Lim JC, Liang K, Chong LY, Kuo YP, Chen CH, Chiang HC (2012) Patterns of diabetic periodontal wound repair: a study using micro-computed tomography and immunohistochemistry. J Periodontol 83(5):644–652
Corrêa MG, Gomes Campos ML, Marques MR, Casati MZ, Nociti FH Jr, Sallum EA (2013) Histometric analysis of the effect of enamel matrix derivative on the healing of periodontal defects in rats with diabetes. J Periodontol 84(9):1309–1318
Retzepi M, Calciolari E, Wall I, Lewis MP, Donos N (2018) The effect of experimental diabetes and glycaemic control on guided bone regeneration: histology and gene expression analyses. Clin Oral Implants Res 29(2):139–154
Eliezer M, Nemcovsky C, Romanos G, Kozlovsky A, Tal H, Kolerman R, Weinreb M, Moses O (2013) Opposing effects of diabetes and tetracycline on the degradation of collagen membranes in rats. J Periodontol 84(4):529–534
Aamar S, Saada A, Rotshenker S (1992) Lesion-induced changes in the production of newly synthesized and secreted apo-E and other molecules are independent of the concomitant recruitment of blood-borne macrophages into injured peripheral nerves. J Neurochem 59(4):1287–1292
Moses O, Eliezer M, Nemcovsky C, Tal H, Weinreb M (2016) Accelerated degradation of collagen membranes in diabetic rats is associated with increased infiltration of macrophages and blood vessels. Clin Oral Investig 20(7):1589–1596
Croce MA, Dyne K, Boraldi F, Quaglino D Jr, Cetta G, Tiozzo R, Pasquali Ronchetti I (2001) Hyaluronan affects protein and collagen synthesis by in vitro human skin fibroblasts. Tissue Cell 33(4):326–331
Alexander SA, Donoff RB (1980) The glycosaminoglycans of open wounds. J Surg Res 29(5):422–429
Alexander SA, Donoff RB (1980) The histochemistry of glycosaminoglycans within hypertrophic scars. J Surg Res 28(2):171–181
Fraser JR, Laurent TC, Laurent UB (1997) Hyaluronan: its nature, distribution, functions and turnover. J Intern Med 242(1):27–33
Eberlein M, Scheibner KA, Black KE, Collins SL, Chan-Li Y, Powell JD, Horton MR (2008) Anti-oxidant inhibition of hyaluronan fragment-induced inflammatory gene expression. J Inflamm (Lond) 5:20
Kato Y, Nakamura S, Nishimura M (2006) Beneficial actions of hyaluronan (HA) on arthritic joints: effects of molecular weight of HA on elasticity of cartilage matrix. Biorheol 43(3,4):347–354
Takahashi K, Goomer RS, Harwood F, Kubo T, Hirasawa Y, Amiel D (1999) The effects of hyaluronan on matrix metalloproteinase-3 (MMP-3), interleukin-1beta(IL-1beta), and tissue inhibitor of metalloproteinase-1 (TIMP-1) gene expression during the development of osteoarthritis. Osteoarthritis Cartilage 7(2):182–190
Feinberg RN, Beebe DC (1983) Hyaluronate in vasculogenesis. Sci 220(4602):1177–1179
Mitsui Y, Gotoh M, Nakama K, Yamada T, Higuchi F, Nagata K (2008) Hyaluronic acid inhibits mRNA expression of proinflammatory cytokines and cyclooxygenase-2/prostaglandin E(2) production via CD44 in interleukin-1-stimulated subacromial synovial fibroblasts from patients with rotator cuff disease. J Orthop Res : Off Pub Orthop Res Soc 26(7):1032–1037
West DC, Hampson IN, Arnold F, Kumar S (1985) Angiogenesis induced by degradation products of hyaluronic acid. Sci 228(4705):1324–1326
Fakhari A, Berkland C (2013) Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler and in osteoarthritis treatment. Acta Biomater 9(7):7081–7092
Robert L (2015) Hyaluronan, a truly “youthful” polysaccharide. Its medical applications Pathol Biol (Paris) 63(1):32–34
Bertl K, Bruckmann C, Isberg PE, Klinge B, Gotfredsen K, Stavropoulos A (2015) Hyaluronan in non-surgical and surgical periodontal therapy: a systematic review. J Clin Periodontol 42(3):236–246
Chen CP, Hung W, Lin SH (2014) Effectiveness of hyaluronic acid for treating diabetic foot: a systematic review and meta-analysis. Dermatol Ther 27(6):331–336
Asparuhova MB, Chappuis V, Stahli A, Buser D, Sculean A (2020) Role of hyaluronan in regulating self-renewal and osteogenic differentiation of mesenchymal stromal cells and pre-osteoblasts. Clin Oral Investig 24(11):3923–3937. https://doi.org/10.1007/s00784-020-03259-8
Eliezer M, Imber JC, Sculean A, Pandis N, Teich S (2019) Hyaluronic acid as adjunctive to non-surgical and surgical periodontal therapy: a systematic review and meta-analysis. Clin Oral Investig 23(9):3423–3435
Silva EC, Omonte SV, Martins AG, de Castro HH, Gomes HE, Zenobio EG, de Oliveira PA, Horta MC, Souza PE (2017) Hyaluronic acid on collagen membranes: An experimental study in rats. Arch Oral Biol 73:214–222
Eliezer M, Sculean A, Miron RJ, Nemcovsky C, Weinberg E, Weinreb M, Zoabi H, Bosshardt DD, Fujioka-Kobayashi M, Moses O (2019) Hyaluronic acid slows down collagen membrane degradation in uncontrolled diabetic rats. J Periodontal Res 54(6):644–652. https://doi.org/10.1111/jre.12665
Schwarz F, Rothamel D, Herten M, Sager M, Becker J (2006) Angiogenesis pattern of native and cross-linked collagen membranes: an immunohistochemical study in the rat. Clin Oral Implants Res 17(4):403–409
Zoabi H, Nemcovsky CE, Bender O, Moses O, Weinreb M (2020) Accelerated degradation of collagen membranes in type 1 diabetic rats is associated with increased expression and production of several inflammatory molecules. J Periodontol 91(10):1348–1356
Murray PJ, Wynn TA (2011) Protective and pathogenic functions of macrophage subsets. In Nat Rev Immunol 11:723–737
Cury JD, Campbell EJ, Lazarus CJ, Albin RJ, Welgus HG (1988) Selective up-regulation of human alveolar macrophage collagenase production by lipopolysaccharide and comparison to collagenase production by fibroblasts. J Immunol 141(12):4306–4312
Campbell EJ, Cury JD, Shapiro SD, Goldberg GI, Welgus HG (1991) Neutral proteinases of human mononuclear phagocytes Cellular differentiation markedly alters cell phenotype for serine proteinases, metalloproteinases, and tissue inhibitor of metalloproteinases. J Immunol 146(4):1286–1293
Welgus HG, Campbell EJ, Cury JD, Eisen AZ, Senior RM, Wilhelm SM, Goldberg GI (1990) Neutral metalloproteinases produced by human mononuclear phagocytes Enzyme profile, regulation, and expression during cellular development. J Clin Invest 86(5):1496–502
Shapiro SD (1994) Elastolytic metalloproteinases produced by human mononuclear phagocytes. Potential roles in destructive lung disease. Am J Respir Crit Care Med 150(6 Pt 2):S160–S164
Yang C, Zhu P, Yan L, Chen L, Meng R, Lao G (2009) Dynamic changes in matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 levels during wound healing in diabetic rats. J Am Podiatr Med Assoc 99(6):489–496
Han SY, Jee YH, Han KH, Kang YS, Kim HK, Han JY, Kim YS, Cha DR (2006) An imbalance between matrix metalloproteinase-2 and tissue inhibitor of matrix metalloproteinase-2 contributes to the development of early diabetic nephropathy. Nephrol Dial Transplant 21(9):2406–2416
McCachren SS (1991) Expression of metalloproteinases and metalloproteinase inhibitor in human arthritic synovium. Arthritis Rheum 34(9):1085–1093
Ohashi K, Nemoto T, Nakamura K, Nemori R (2000) Increased expression of matrix metalloproteinase 7 and 9 and membrane type 1-matrix metalloproteinase in esophageal squamous cell carcinomas. Cancer 88(10):2201–2209
Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD (1997) Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 277(5334):2002–2004
Reynolds JJ, Hembry RM, Meikle MC (1994) Connective tissue degradation in health and periodontal disease and the roles of matrix metalloproteinases and their natural inhibitors. Adv Dent Res 8(2):312–319
Armstrong DG, Jude EB (2002) The role of matrix metalloproteinases in wound healing. J Am Podiatr Med Assoc 92(1):12–18
Yang D, Yang L, Cai J, Hu X, Li X, Zhang X, Zhang X, Chen X, Dong H, Nie H, Li Y (2021) A sweet spot for macrophages: Focusing on polarization. Pharmacol Res 167:105576
Tesch GH (2007) Role of macrophages in complications of type 2 diabetes. Clin Exp Pharmacol Physiol 34(10):1016–1019
Barman P, Koh TJ (2020) Macrophage Dysregulation and Impaired Skin Wound Healing in Diabetes. Front Cell Dev Biol 8:528
Kanter JE, Hsu CC, Bornfeldt KE (2020) Monocytes and Macrophages as Protagonists in Vascular Complications of Diabetes. Front Cardiovasc Med 7:10
Meshkani R, Vakili S (2016) Tissue resident macrophages: Key players in the pathogenesis of type 2 diabetes and its complications. Clin Chim Acta 462:77–89
Voigt J, Driver VR (2012) Hyaluronic acid derivatives and their healing effect on burns, epithelial surgical wounds, and chronic wounds: a systematic review and meta-analysis of randomized controlled trials. Wound Repair Regen 20(3):317–331
Mosser DM (2003) The many faces of macrophage activation. J Leukoc Biol 73(2):209–212
Kim H, Cha J, Jang M, Kim P (2019) Hyaluronic acid-based extracellular matrix triggers spontaneous M2-like polarity of monocyte/macrophage. Biomater Sci 7(6):2264–2271
He H, Zhang S, Tighe S, Son J, Tseng SCG (2013) Immobilized heavy chain-hyaluronic acid polarizes lipopolysaccharide-activated macrophages toward M2 phenotype. J Biol Chem 288(36):25792–25803
Rayahin JE, Buhrman JS, Zhang Y, Koh TJ, Gemeinhart RA (2015) High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomater Sci Eng 1(7):481–493
van der Gaag R, Broersma L, Koornneef L (1987) The influence of high molecular weight sodium hyaluronate (Healon) on the production of migration inhibitory factor. Curr Eye Res 6(12):1433–1440
Bauer C, Niculescu-Morzsa E, Jeyakumar V, Kern D, Späth S, Nehrer S (2016) Chondroprotective effect of high-molecular-weight hyaluronic acid on osteoarthritic chondrocytes in a co-cultivation inflammation model with M1 macrophages. J Inflamm (Lond).13(1):31.
Tarricone E, Mattiuzzo E, Belluzzi E, Elia R, Benetti A, Venerando R, Vindigni V, Ruggieri P, Brun P (2020) Anti-Inflammatory Performance of Lactose-Modified Chitosan and Hyaluronic Acid Mixtures in an In Vitro Macrophage-Mediated Inflammation Osteoarthritis Model. Cells 9(6):1328
Campo GM, Avenoso A, Campo S, D’Ascola A, Nastasi G, Calatroni A (2010) Molecular size hyaluronan differently modulates toll-like receptor-4 in LPS-induced inflammation in mouse chondrocytes. Biochimie 92(2):204–215
Martin A, Komada MR, Sane DC (2003) Abnormal angiogenesis in diabetes mellitus. Med Res Rev 23(2):117–145
Tahergorabi Z, Khazaei M (2012) Imbalance of angiogenesis in diabetic complications: The mechanisms. Int J Prev Med 3:827–838
Ciccone V, Zazzetta M, Morbidelli L (2019) Comparison of the Effect of Two Hyaluronic Acid Preparations on Fibroblast and Endothelial Cell Functions Related to Angiogenesis. Cells 8(12):1479. https://doi.org/10.3390/cells8121479
Cirligeriu L, Cimpean AM, Calniceanu H, Vladau M, Sarb S, Raica M, Nica L (2018) Hyaluronic Acid/Bone Substitute Complex Implanted on Chick Embryo Chorioallantoic Membrane Induces Osteoblastic Differentiation and Angiogenesis, but not Inflammation. Int J Mol Sci 19(12):4119. https://doi.org/10.3390/ijms19124119
Tal H, Weinreb M, Shely A, Nemcovsky C, Moses O (2016) Tetracycline impregnation affects degradation of porcine collagen matrix in healthy and diabetic rats. Clin Oral Investig 20(6):1237–1242. https://doi.org/10.1007/s00784-015-1615-0
Chow JP, Simionescu DT, Warner H, Wang B, Patnaik SS, Liao J, Simionescu A (2013) Mitigation of diabetes-related complications in implanted collagen and elastin scaffolds using matrix-binding polyphenol. Biomaterials 34(3):685–695
Socarrás TO, Vasconcelos AC, Campos PP, Pereira NB, Souza JPC, Andrade SP (2014) Foreign body response to subcutaneous implants in diabetic rats. PLoS One. 9(11):e110945
Oviedo-Socarrás T, Vasconcelos AC, Barbosa IX, Pereira BB, Campos PP, Andrade SP (2014) Diabetes alters inflammation, angiogenesis, and fibrogenesis in intraperitoneal implants in rats. Microvasc Res 93:23–29
Asparuhova MB, Kiryak D, Eliezer M, Mihov D, Sculean A (2019) Activity of two hyaluronan preparations on primary human oral fibroblasts. J Periodontal Res 54(1):33–45
Shirakata Y, Nakamura T, Kawakami Y, Imafuji T, Shinohara Y, Noguchi K, Sculean A (2021) Healing of buccal gingival recessions following treatment with coronally advanced flap alone or combined with a cross linked hyaluronic acid gel An experimental study in dogs. J Clin Periodontol 48(4):570–580
Pilloni A, Schmidlin PR, Sahrmann P, Sculean A, Rojas MA (2019) Effectiveness of adjunctive hyaluronic acid application in coronally advanced flap in Miller class I single gingival recession sites: a randomized controlled clinical trial. Clin Oral Investig 23(3):1133–1141
Guldener K, Lanzrein C, Eliezer M, Katsaros C, Stähli A, Sculean A (2020) Treatment of single mandibular recessions with the modified coronally advanced tunnel or laterally closed tunnel, hyaluronic acid, and subepithelial connective tissue graft: a report of 12 cases. Quintessence Int 51(6):456–463
Lanzrein C, Guldener K, Imber JC, Katsaros C, Stähli A, Sculean A (2020) Treatment of multiple adjacent recessions with the modified coronally advanced tunnel or laterally closed tunnel in conjunction with cross-linked hyaluronic acid and subepithelial connective tissue graft: a report of 15 cases. Quintessence Int 51(9):710–719
Acknowledgements
The authors thank Hana Vered, Dept. of Pathology, Tel Aviv University School of Dental Medicine, and Owusu Silvia, Thuy-Trang Nguyen, and Monika Aeberhard (University of Bern) for technical laboratory assistance.
Funding
This study was supported by a grant from Regedent AG, Zurich, Switzerland.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Meizi Eliezer, Carlos Nemcovsky, Dieter D. Bosshardt, Masako Fujioka-Kobayashi, and Ofer Moses. The first draft of the manuscript was written by Meizi Eliezer and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
The institution Animal Care and Use Committee of Tel Aviv University, Tel Aviv, Israel, approved the study (TAU 1–16-031).
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eliezer, M., Sculean, A., Miron, R.J. et al. Cross-linked hyaluronic acid slows down collagen membrane resorption in diabetic rats through reducing the number of macrophages. Clin Oral Invest 26, 2401–2411 (2022). https://doi.org/10.1007/s00784-021-04206-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-021-04206-x