Abstract
Objectives
Root canal sealers are widely used worldwide in endodontics to prevent reinfection and growth of surviving microorganisms. Considering the strong correlation between genetic damage and carcinogenesis, evaluation of genotoxicity induced by endodontic sealers is recommended for elucidating the true health risks to patients and professionals. The purpose of this article was to provide a comprehensive review of studies involving genotoxicity analysis of endodontic sealers and the used methodologies.
Materials and methods
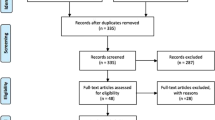
A literature search was made in PubMed using the following combination of words “genotoxicity,” “mutagenicity,” “endodontic sealers,” and “root canal sealers.” A total of 39 articles with genotoxicity studies were selected for the present study.
Results
Sealers have been ranked in decreasing order of their genotoxicity as: ZOE sealers > GIC sealers > S sealers > ER sealers > MR sealers > Novel sealers > CH sealers > CS sealers.
Conclusions
All published data showed some evidence of genotoxicity for most of the commercial root canal sealers; however, contradictory results were found, mainly for AH Plus, the most studied sealer.
Clinical relevance
The information provided would direct the endodontists to use the less genotoxic materials in endodontic treatment in a way to reduce DNA damage promoting oral healthcare.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tooth pulp is protected from injurious elements by enamel, dentin, and periodontium; however, when these barriers are breached, microorganisms and substances may adversely affect the stability, causing inflammation (pulpitis) and even tissue death (pulp necrosis). To overcome this, the recommended procedures are pulpectomy, which involves surgical removal of all the material in the pulp chamber and root canal, or pulpotomy, which refers only to removal of the coronal portion of the pulp [1]. The root canal system is sealed with a filling material to prevent reinfection and growth of surviving microorganisms. The deposition of cementum is considered a desired healing response and a prerequisite for restoring a functional periodontal attachment. The effectiveness of the filling materials is critically dependent on its physical and chemical properties, however being biological safety a prerequisite for its clinical use [2].
Genotoxicity is a critical issue in determining the safety of agents that might contact biological structures and should be considered within a biological risk assessment process [3]. Mutagens or genotoxic substances induce DNA damage directly or indirectly through inactivation of enzymes involved in the maintenance of genome integrity. Mutagen-target interactions may result in different types of DNA damage (DNA adducts, alkali labile sites, strand breaks) that can be pre-mutagenic. Cellular mechanisms to overcome these harmful effects include protective antioxidant activity (mediating elimination/neutralization processes) and the removal of induced lesions by the DNA repair machinery [4]. Nevertheless, insufficient cell-protecting mechanisms and/or high DNA-inflicted damage result in the disruption of the replication and/or transcription processes hindering the cell self-repairing potential leading, ultimately, to cell apoptosis [5].
Several methodologies able of detecting genetic damage and/or mutations have been established and are approved by international regulatory agencies for validation of chemical agents commercially available. The potential health risks are thus elucidated as it has been established that genetic damage is intimately linked to diseases such as cancer [6]. Understanding the impact of exposure scenarios (dose, chronic, acute) on cancer risk is important but remains a scientific challenge. Dental materials persist in the oral cavity for long periods which imply that risk assessment is required to ensure the safety profile of such materials [7].
Due to the current demand of enhanced clinical performance of dental materials, the number of commercial products is continuously increasing. Physical properties, biocompatibility, sealing ability, adhesion, solubility, and antibacterial efficacy results are abundant for root canal sealers, and some reviews on those issues have been written [2, 8,9,10,11,12,13]. However, genotoxic stress as a reaction to endodontic sealers is also an important parameter to be assessed to validate the safety of biomaterials in clinical practice [14]. So far, limited reviews have reported about the genotoxicity of endodontic sealers [15].
The present review intends to provide detailed information on the genotoxicity of root canal sealers (RCS), displaying the reported results considering the sealer’s composition. A comprehensive literature search on “genotoxicity,” “mutagenicity,” “endodontic sealers,” and “root canal sealers” was performed on studies conducted between 1998 and 2020. In brief, a search of PubMed, MEDLINE, Embase, and Google Scholar for a plethora of articles was carried out using the aforementioned keywords. Case reports and articles not written in English were excluded from this review.
Genotoxicity as a biocompatibility requirement
ISO 10993-1 lists two components for biological response evaluation of biomaterials [16]. The first normative component requires a number of aspects, such as physicochemical information, cytotoxicity, sensitization, irritation or intracutaneous reactivity, material-mediated pyrogenicity, toxicity, implantation, hemocompatibility, genotoxicity, carcinogenicity, reproductive/developmental toxicity, and biodegradation. The second one provides suggestions and considerations. These aspects are combined together with the nature of the tissues and contact time to assist in the selection of an appropriate evaluation technique [14]. However, the clinical relevance of these assays is limited because they do not take into account the complexity of a living organism, as well as the clinical presentation of the apical region. Despite that assessment, it is also mandatory to determine the biocompatibility of a material within in vivo setting [16]. Of the above-mentioned aspects, cytotoxicity and genotoxicity are most commonly reported in the literature [10, 11].
Genotoxicity is defined as toxicity that affects DNA structure, i.e., the ability of a substance (genotoxin) to modify the chemical structure of DNA, causing DNA lesions [14]. However, not all genotoxins act directly on the DNA molecule. Some genotoxins interact with DNA repair proteins, increasing mutation rate, or mitotic spindle proteins, leading to chromosomal misaggregation or even with proteins involved in the cell cycle, increasing the proliferation rate [17]. Several assays are addressed to detect DNA damage, i.e., comet assay, sister chromatid exchange, detection of γH2AX or 32P-postlabeling assay, and its transition to mass spectrometry [18]. Once damaged DNA can mispair during replication, an alteration in nucleotide sequence can arise, characterizing the mutation, which can involve a single (point mutation), few base pairs or a whole chromosome. Mutagenicity can be assessed using the Ames test, cytogenetics, or micronucleus (MN) assay [19]. The aim of genotoxicity assays is the identification of probable mutagens.
Regulatory agencies require testing for biomaterials to be available in the market. However, multiple tests are needed to monitor all potential endpoints related to DNA damage or mutations [14]. Registration, Evaluation, Authorisation, and restriction of Chemicals (REACH) is a European Union (EU) regulation adopted to improve protection of human health and the environment from the risks posed by chemicals. REACH Annexures describe the requirements on genotoxicity, specifying the information that must be submitted for purposes of registration and evaluation [20]. A set of specifications for testing chemicals and also medical devices is established by the Organization for Economic Co-operation and Development (OECD) with appropriate model systems, methodologies, reference standards, and recommendations for statistical analysis [21]. For medical devices, ISO10993-33 provides guidance on tests to evaluate the potential genotoxicity, carcinogenicity, or reproductive toxicity [22]. Table 1 summarizes the methodologies used to assess materials genotoxicity according to EU/OECD/ISO10993-33 guidelines.
Results
Commonly used sealers are based on zinc oxide-eugenol (ZOE sealers), silicone (S sealers), glass ionomer cements (GIC sealers), methacrylate resin (MR sealers), epoxy resin (ER sealers), polyvinyl resin (PR sealers), calcium silicate (CS sealers), calcium hydroxide (CH sealers), and novel sealers (Table 2). Detailed information of the commercial sealers with reported results on genotoxicity is presented in the Supplementary material (Table S1).
The present survey addresses the information on the genotoxicity of available root canal sealers reported from 1998 to the present date. A total of 39 articles were included. Figure 1 shows the publication periodicity grouped over a 4-year time span. On average, 1.9 articles were published per year, with two peak periods (2006 to 2009, 9 articles; 2014 to 2017, 11 articles). The two more recent articles were available in 2018 and 2020. Table 3 presents an overview of the included studies in terms of the experimental protocol (cell line/type, exposure time, concentration range, genotoxicity assay) and the relevant results, considering the sealers’ groups. Most of the studies were performed in vitro; the comet assay and the micronucleus test were widely used and broadly applied to assess genotoxic and mutagenic effects, respectively. Relevant results are given below.
Zinc oxide-eugenol-based sealers
Six articles addressed the genotoxicity of eight ZOE sealers (five of them published from 1999 to 2009, and the last one in 2016). One or two studies were performed for each sealer (Table 3). All sealers were considered genotoxic in the tested cell lines through different methods, i.e., comet assay [36, 40], DNA precipitation assay [37], proto-oncogenes expression [38], and micronuclei assay [39]. The Ames test was performed for Tubli-Seal and Endométhasone N, and the results were considered negative [35].
Silicone-based sealers
RoekoSeal demonstrated time-dependent positive genotoxicity results on V79 cells using comet assay [41], and GuttaFlow showed negative results performing chromosomal alteration analysis and comet assay in hPB lymphocytes [40].
Glass-ionomer cement-based sealers
Ketac Endo was not mutagenic on S. typhimurium strains [35]. Vitrebond was tested on CHO cells exhibiting a clear genotoxic effect in the hprt test and umu chromotest [42]. Additionally, the genotoxic compounds, being hydrophilic, leached out rapidly when in contact with saliva [42], and when tested on V79 cells using the MN test, results were also considered positive [43].
Resin-based sealers
The prototype of R sealers was developed as a bis-phenol resin using methenamine for polymerization; however, the product released formaldehyde during setting [74]. Alternatives were sought and variants include phenol-formaldehyde, resorcin-formaldehyde, and methylmethacrylate, which were strongly antibacterial however presenting shrinkage and discoloration and poor biocompatibility during setting. Genotoxicity results for resin-based sealers were considered for methacrylate resin (MR)-, epoxy resin (ER)-, and polyvinyl resin (PR)-based sealers and are summarized in Table 3.
MR sealers were involved in a variety of studies performed from 2009 to 2018, and a higher number revealed absence of genotoxicity. Several studies that compared single MR sealer (EndoRez) with multi-MR sealer (RealSeal) found no toxic effects on FMM1 cells and transfected COS-7 cells [47] and also hGF [44]; EndoRez caused low double-strand break (DSB) formation in PDL cells while RealSeal did not [46]. EndoRez also showed positive results on V79 cells [41, 45] and on PDL cells [46]. Epiphany, using the comet assay, showed negative effects on hPB lymphocytes [40] and hPB leucocytes [49]; however, results were positive with the micronucleus test on V79 cells [48]. Coming to RealSeal, only one study (out of six) reported dose- and time-dependent DNA damage, namely on BHK-21 cells using the comet assay [50], while others reported negative genotoxicity on several cell types [45, 49]. Hybrid Root Seal, a self-etching hydrophilic material as a result of the inclusion of 4-META, showed significant DSB formation in PDL cells [46].
ER-based sealers have also been extensively tested, with published studies from 1998 to 2020, prevailing the results reporting genotoxicity. Results are available for Topseal, AH26, AH Plus/AH Plus Jet, and Acroseal (Table 3). Most concern AH Plus. AH26 and AH Plus showed positive genotoxicity [36, 37, 51] but negative effects on the Ames test [35, 52,53,54]. AH Plus was also genotoxic in V79 cells [41, 48, 55], FMM1 cells [56], UO2S cells [38], hDPS cells [57], and, recently, in hGF cells [58]. However, controversial results were found with the same cell lines, i.e., results were negative with V79 cells [45], FMM1 cells [47], hGF cells [44], and PDL cells [46] perhaps due to different methodologies. Other ER sealers have also been investigated. Topseal showed some DNA damage in OC2 cells using the comet assay [36], and Acroseal increased the production of ROS and MN and delayed the cell cycle in G2 phase on V79 cells [48]; however, it did not alter DSB formation on PDL cells [46].
Diaket, a PR sealer, sets by chelation, but contains polyvinyl chloride as polymer and showed positive genotoxicity on hPB lymphocytes [40].
Calcium silicate-based sealers
CS sealers were the most tested, from 2005 to 2020, and only few studies demonstrated genotoxicity in mammalian cells (Table 3). Portland cement (PC) did not cause genotoxic effects in four cell lines, using the comet assay [59,60,61,62]. ProRoot MTA was also safe in most studies [43, 63, 65, 66], except for one exhibiting some genotoxicity on L929 cells using comet assay [64], perhaps due to the high concentrations used. Endocem MTA [65] and MTA Plus [50] were not genotoxic using the comet assay on CHO cells and BHK-21 cells, respectively. MTA Fillapex was tested in five cell lines being genotoxic in four of them [46, 50, 55, 57], except with hGF cells [58]. MTA Angelus, the most tested CS sealer, showed absence of genotoxicity, with the comet assay, on L5178Y cells [67], CHO-K1 cells [60, 68], hPB lymphocytes [61, 69], and 3T3-L1 cells [62]. MTA Angelus also showed negative results using the MN test with V79 cells [55] and the bone marrow of Swiss mice [39]. Genotoxicity was observed on hDPS cells, evaluated by gene expression analyses [57]. Other CS-based sealers not displaying genotoxicity were a CS cement [63] and TheraCal LC [66], a light-cure single-component material for direct and indirect pulp capping.
Calcium hydroxide-based sealers
CH sealers have a complex and inhomogeneous setting reaction, producing a hard surface with the deeper part retaining a dough-like structure. They perform remarkably well in laboratory leakage tests and also on biological, animal, and human tests [12]. Lack of physical sturdiness, poor clinical and radiographic outcomes when compared with other sealers are causes for concern [75]. CH is also added to other cements such as resins and ZOE sealers [76,77,78,79].
As a group, CH sealers appeared safe, as suggested from the studies performed through 1999 to 2018 (Table 3). CRCS [35], Sealapex [36, 38], Hydro C [43], Calcicur [44], P.A. CH [39], and Apexit [47] showed negative results. Positive genotoxicity was only noted for Calcipex II and Vitapex with CHO-K1 cells, observed by a higher incidence of micronuclei and higher tail moment values when compared with MTA [65].
Other novel sealers
Bioceramics exhibit excellent biocompatibility properties due to compositional similarity with biological hydroxyapatite, producing mineral hydroxyapatite with an osteoconductive effect, leading to bone formation at the interface between material and bone. Even though these advantages have contributed to their rapid spread in the dental field, they are not widely used, and products on the market are not yet known or used by many dentists [9]. Other novel sealers include the use of medicinal plants with studies showing the effect of natural products in pulpal and dentin repair with variable effectiveness such as alkaloids, coumarins, saponins, and flavonoids [80].
Genotoxicity results of novel sealers are summarized in Table 3. Studies were reported through 2008 to 2020. EndoSequence BC and iRoot SP are the same product, a hydrophilic sealer where the moisture inside the tubules initiates setting and shows genotoxicity [46], however lower than ZOE with L929 cells [70] and AH Plus with FMM1 cells [56]. Biodentine, a Ca3SiO5-based material indicated as a dentine substitute, was not mutagenic or genotoxic [66, 71]. However, using the MN test, an increase in the frequency of micronuclei was observed [72] which may have occurred because the high concentrated test solution BioRoot RCS demonstrated negligible DSB formation [46]. Two novel natural resin-based sealers have been recently created, namely Polifill [43, 45] (based on a polymer from R. communis—castor oil) and Bioseal [45] (based on Copaifera multijuga oil-resin—copaiba—with CH and zinc oxide) which tested negative for genotoxicity with V79 cells. Other experimental sealers tested with the comet assay include calcium silicate-hydroxyapatite (CS+HA) which was not genotoxic with hPB lymphocytes [73] and calcium-enriched mixture (CEM) which was genotoxic with L929 cells [64]. Novel sealers with favorable biocompatibility results that require genotoxicity studies include polymer nanocomposite resins [81], PC-based partial stabilized cement with zinc [82], and new resin cement [83].
Discussion
In 1981, Grossman listed requirements for RCS, as they should (1) be tacky when mixed to provide good adhesion to the canal wall when set; (2) make a hermetic seal; (3) be radiopaque to be visualized on radiographs; (4) have fine particles of powder to mix easily with liquid; (5) not shrink upon setting; (6) not discolor tooth structure; (7) be bacteriostatic or at least not encourage bacterial growth; (8) set slowly; (9) be insoluble in tissue fluids; (10) be well tolerated by the periapical tissue; and (11) be soluble in common solvents if it is necessary to remove it [84]. In 2007, de Ingle et al added two more requirements: (1) should not provoke an immune response and (2) should not be mutagenic or carcinogenic [85]. Thus, a RCS should have an acceptable level of biocompatibility and according to regulations, successfully pass a clinical risk assessment before commercialization, fulfilling the technical, biological, handling, and biocompatibility requirements [86].
This review put together the results concerning the studies addressing the genotoxicity of commercially available endodontic sealers and endodontic agents, included in 39 articles published from 1998 to the present data. The results’ outcome was presented considering the chemical composition of the sealers and are summarized in Table 3. Most studies were performed in vitro, with only three studies being conducted in vivo [39, 72, 87]. A great heterogeneity on the experimental protocols was evident, involving a variety of animal and human cell lines, exposure conditions (sealers’ concentration and exposure time) and the genotoxicity assays, hampering the results’ analyses and the characterization of genotoxicity patterns. Nevertheless, some trends were clearly noted. As a whole, all sealers’ groups presented agents with reported genotoxicity, but differences were observed among the groups, and also within each group. Figure 2 gives a quantitative overview of the reported studies, displaying also the genotoxicity outcome. Resin-based sealers (methacrylate- and epoxy-based) and calcium silicate sealers were addressed in a higher number of studies, followed by the zinc oxide-eugenol sealers and calcium hydroxide sealers.
Root canal sealers’ genotoxicity studies presented by composition: methacrylate-based sealers (MR), epoxy resin-based sealers (ER), polyvinyl resin-based sealers (PR), calcium silicate-based sealers (CS), zinc oxide eugenol-based sealers (ZOE), silicone-based sealers (S), glass ionomer cement-based sealers(GIC), calcium hydroxide-based sealers (CH), and other novel sealers
As displayed in Fig. 2, resin-based sealers were tested in a great number of studies. Detailing the methacrylate-based sealers, EndoRez, Epiphany and RealSeal presented different outcomes depending on the cell line and experimental protocol; however, absence of genotoxic effects was reported in most studies. RealSeal was the most tested sealer of this group, and genotoxicity was rarely observed [50]. Otherwise, MetaSeal was only addressed in one study, being genotoxic [46]. On the epoxy resin-based sealers, AH 26 but, particularly, AH Plus, were thoroughly tested. This sealer yielded different outcomes although prevailing an absence of toxicity [44, 46, 47]. TopSeal and Acroseal were less addressed. The same was verified with Diaket, a polyvinyl resin-based sealer [40]. In the R sealers, the monomers and co-monomers appear potentially associated with genotoxicity, and a relationship between the structural and biological activities has been reported [88]. Resin monomers enhance the formation of ROS affecting the expression and levels of protective antioxidant enzymes as superoxide dismutase, glutathione peroxidase, and catalase [89], and the redox imbalance triggers DNA damage and apoptosis [90]. In methacrylate resin-based sealers, leached compounds as BisGMA, BisDMA, HEMA, PEGDMA, TEGDMA, UDMA, and the photoinitiator camphorquinone [91], possibly due to their solubility [92], alter tightly regulated metabolic pathways, by inducing cellular stress responses, including oxidative DNA damage and DSB [93,94,95]. In the oral environment, HEMA increases production of ROS and causes oxidative DNA damage through DSB as evidenced by the presence of micronuclei, cell-cycle delay, and apoptosis [96], as well as the transcription of early inflammatory genes [97]. For epoxy resin-based sealers, identified leachable components include bisphenol-A diglycidyl ether and formaldehyde, which are considered carcinogens and probably responsible for cellular toxicity [98] and apoptotic cell death [91].
Calcium silicate-based sealers are mainly composed of mineral trioxide aggregate (MTA) and were described as the most biocompatible material with most predictable outcomes [99], however with some drawbacks [100]. These sealers were tested in a large variety of studies and genotoxicity was observed only occasionally (Fig. 2). The most tested were Portland cement, ProRoot MTA, MTA Fillapex and, particularly, MTA Angelus. Portland cement (and also MTA Plus, Endocem MTA, CS cement) did not reveal genotoxic effects. ProRoot MTA showed toxic effects in only one study, as well as MTA Angelus. Comparatively, MTA Fillapex displayed higher toxicity. As a whole, MTA-based sealers are associated with low genotoxicity potential [65, 69]. However, calcium-enriched mixtures of MTA were genotoxic [64]. Nevertheless, pure CS-based cements, modified CS-based cements, and three resin-based CS cements showed no genotoxicity in human osteoblast cells [101].
Zinc oxide-eugenol sealers were also involved in a number of studies. Overall, these sealers appeared to be genotoxic. The eight tested sealers presented deleterious effects in several in vitro [51,52,53,54] and in vivo [39] assays. In the other way, calcium hydroxide-based sealers showed low genotoxicity [35, 36, 38, 39, 43, 44, 47]. Eight sealers were analyzed, and only Calcipex II and Vitapex were genotoxic [65]. Sealapex did not exhibit genotoxicity [36, 38].
The other sealers’ groups were less tested, as shown in Fig. 2. Two silicone-based sealers were tested, i.e., RoekoSeal and Gutta Flow presenting, respectively, positive [41] and negative [40] genotoxicity. For the glass ionomer cement sealers, three genotoxicity studies were reported, namely involving Ketac-Endo [35] and Vitrebond [42, 43]. The presence of the resin component in the sealer composition might play a role in the observed deleterious effect [102, 103]. Miscellaneous commercially available sealers were grouped together, and tested sealers included Endosequence BC [56, 70], iRootSP [56, 70], Biodentine [66, 71, 72], BioRoot RCS, Polifil, CEM, and CS+HA. Although only one or two studies were conducted per sealer, overall, genotoxicity potential was low (Fig. 2).
Considering the three groups of sealers involved in a higher number of studies, calcium silicate-based sealers presented the lower genotoxicity potential, followed by methacrylate resin-based sealers, which appeared slightly lower genotoxic than the epoxy resin-based sealers, whereas zinc oxide based sealers presented the higher genotoxic potential. The other groups of sealers were less addressed. Calcium hydroxide-based sealers appeared to present low genotoxic potential, whereas silicone-based and glass ionomer-based sealers showed variable outcomes. Nevertheless, establishing rankings of genotoxicity is a risky exercise. Major drawbacks are the great heterogeneity of the experimental protocols, namely the use of multiple cell lines, some of them not recommended by OECD guidelines, differences in the exposure conditions and in the performed genotoxicity assays. Also, there is insufficient information to draw firm conclusions concerning the sealers’ safety. Gene expression profiles and epigenetic mechanisms for damage response, antioxidant, or DNA repair genes, despite being indirect methods of analysis and not commonly recommended by standard guidelines, could provide relevant information on the involved genotoxicity mechanisms [104].
In a translational view, the reported information on the sealers’ genotoxicity must be placed in a proper context. Results were collected mostly from in vitro studies, using static cell culture models, and exposure conditions that are far from those anticipated in a clinical setting. Following the endodontic treatment, the levels of degradable/leachable compounds eventually observed are expectably low due to the very small contact area of the sealer potentially yielding leachable components. Also, due to the dynamic in vivo conditions, namely the continuous extracellular fluid flow, eventual leachates are continuously being cleared decreasing the local levels. The exposure features also deserve some observations. In vivo, cells of the periapical tissues are embedded in a collagenous extracellular matrix organized in a three-dimensional structure, thus with lower susceptibility to deleterious effects from the surrounding environment. Due to these unique physiological features and exposure conditions, the genotoxicity trends observed in vitro might not translated to the periapical environment with, eventually, less noticeable differences and/or outcomes among sealers [105]. Nonetheless, efforts to regulate the in vitro and in vivo genotoxicity assays is critical, in order to standardize protocols allowing representative information and translational usefulness.
Conclusions
An overview of the collected studies suggests that most sealers present some degree of genotoxicity and DNA damage, however with a trend toward low or high genotoxic potential. As the available information is mostly from in vitro studies, and involving a great heterogeneity on the experimental protocols, there is insufficient information to draw firm conclusions concerning the sealers’ safety.
Abbreviations
- BisDMA:
-
Bisphenol-A dimethacrylate
- BisEMA:
-
Ethoxylated bisphenol-A dimethacrylate
- BisGMA:
-
Bisphenol-A-glycidylmethacrylate
- CH:
-
Calcium hydroxide
- CS:
-
Calcium silicate
- DSB:
-
Double-strand breaks
- GIC:
-
Glass ionomer cements
- ER:
-
Epoxy resin
- hDPF:
-
Human dental pulp fibroblast
- hDPSC:
-
human dental pulp stem cells
- HEMA:
-
Hydroxyethylene methacrylate
- hGF:
-
Human gingival fibroblast
- hPB:
-
Human peripheral blood
- MR:
-
Methacrylate resin
- MN:
-
Micronucleus
- MTA:
-
Mineral trioxide aggregate
- PBMC:
-
Peripheral blood mononuclear cells
- PDL:
-
Periodontal ligament cells
- PEGDMA:
-
Polyethylene glycol dimethacrylate
- PR:
-
Polyvinyl resin
- RCS:
-
Root canal sealer
- ROS:
-
Reactive oxygen species
- S:
-
Silicone
- SHE:
-
Syrian hamster embryo
- TEGDMA:
-
Triethyleneglycoldimethacrylate
- UDMA:
-
Urethanedimethacrylate
- ZOE:
-
Zinc oxide-eugenol
References
Gadallah L, Hamdy M, El Bardissy A, Abou El Yazeed M (2018) Pulpotomy versus pulpectomy in the treatment of vital pulp exposure in primary incisors. A systematic review and meta-analysis. F1000Res 7:1560. https://doi.org/10.12688/f1000research.16142.2
Kaur A, Shah N, Logani A, Mishra N (2015) Biotoxicity of commonly used root canal sealers: a meta-analysis. J Conserv Dent 18:83–88. https://doi.org/10.4103/0972-0707.153054
International Organization for Standardization (2014) Biological evaluation of medical devices. Part 3. Tests for genotoxicity, carcinogenicity and reproductive toxicity. ISO 10993-3:2014:1-34
Chatterjee N, Walker GC (2017) Mechanisms of DNA damage, repair, and mutagenesis. Environ Mol Mutagen 58:235–263. https://doi.org/10.1002/em.22087
Surova O, Zhivotovsky B (2013) Various modes of cell death induced by DNA damage. Oncogene 32:3789–3797. https://doi.org/10.1038/onc.2012.556
Tubbs A, Nussenzweig A (2017) Endogenous DNA damage as a source of genomic instability in cancer. Cell 168:644–656. https://doi.org/10.1016/j.cell.2017.01.002
Anderson JM (2016) Future challenges in the in vitro and in vivo evaluation of biomaterial biocompatibility. Regen Biomater 3:73–77. https://doi.org/10.1093/rb/rbw001
Al-Haddad A, Che Ab Aziz ZA (2016) Bioceramic-based root canal sealers: a review. Int J Biomater 2016:1–10. https://doi.org/10.1155/2016/9753210
Jitaru S, Hodisan I, Timis L, Lucian A, Bud M (2016) The use of bioceramics in endodontics - literature review. Clujul Med 89:470–473. https://doi.org/10.15386/cjmed-612
Munitić MS, Peričić TP, Utrobičić A et al (2019) Antimicrobial efficacy of commercially available endodontic bioceramic root canal sealers: a systematic review. PLoS One 14:e0223575. https://doi.org/10.1371/journal.pone.0223575
Fonseca DA, Paula AB, Marto CM, Coelho A, Paulo S, Martinho JP, Carrilho E, Ferreira MM (2019) Biocompatibility of root canal sealers: a systematic review of in vitro and in vivo studies. Materials (Basel) 12:4113. https://doi.org/10.3390/ma12244113
Desai S, Chandler N (2009) Calcium hydroxide-based root canal sealers: a review. J Endod 35:475–480. https://doi.org/10.1016/j.joen.2008.11.026
Kim YK, Grandini S, Ames JM, Gu LS, Kim SK, Pashley DH, Gutmann JL, Tay FR (2010) Critical review on methacrylate resin-based root canal sealers. J Endod 36:383–399. https://doi.org/10.1016/j.joen.2009.10.023
Cvetković VJ, Takić Miladinov D, Stojanović S (2018) Genotoxicity and mutagenicity testing of biomaterials. In: Biomaterials in clinical practice. Springer International Publishing, Cham, pp 501–527
Ribeiro DA, Yujra VQ, DE Moura CFG et al (2017) Genotoxicity induced by dental materials: a comprehensive review. Anticancer Res 37:4017–4024. https://doi.org/10.21873/anticanres.11786
International Organization for Standardization (2018) Biological evaluation of medical devices. Part 1. Evaluation and testing within a risk management process. ISO 10993-1:2018:1-41
Mateuca R, Lombaert N, Aka PV, Decordier I, Kirsch-Volders M (2006) Chromosomal changes: induction, detection methods and applicability in human biomonitoring. Biochimie 88:1515–1531. https://doi.org/10.1016/j.biochi.2006.07.004
Klaene JJ, Sharma VK, Glick J, Vouros P (2013) The analysis of DNA adducts: The transition from 32P-postlabeling to mass spectrometry. Cancer Lett 334:10–19. https://doi.org/10.1016/j.canlet.2012.08.007
Ranganatha R, Chakravarthy S, Sukumaran S (2016) High-throughput approaches for genotoxicity testing in drug development: recent advances. Int J High Throughput Screen 6:1. https://doi.org/10.2147/IJHTS.S70362
European Commission (2008) Council Regulation (EC) No 440/2008 of 30 May 2008 laying down test methods pursuant to Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). Off J Eur Union L142:1–739
Maibach H, Wilhelm K-P (2007) OECD guidelines for testing of chemicals. In: Dermatotoxicology, seventh edition. Informa Healthcare, p 303–305
International Organization for Standardization (2015) Biological evaluation of medical devices. Part 33. Guidance on tests to evaluate genotoxicity ISO/TR 10993-33:2015:1-47
OECD (1997) Test No. 471: bacterial reverse mutation test. OECD
OECD (2016) Test No. 476: in vitro mammalian cell gene mutation tests using the Hprt and xprt genes. OECD
OECD (2016) Test No. 490: in vitro mammalian cell gene mutation tests using the thymidine kinase gene. OECD
OECD (2016) Test No. 473: in vitro mammalian chromosomal aberration test. OECD
OECD (2016) Test No. 487: in vitro mammalian cell micronucleus test. OECD
OECD (1986) Test No. 482: Genetic toxicology: DNA damage and repair, unscheduled DNA synthesis in mammalian cells in vitro. OECD
OECD (1986) Test No. 479: genetic toxicology: in vitro sister chromatid exchange assay in mammalian cells. OECD
OECD (2016) Test No. 475: mammalian bone marrow chromosomal aberration test. OECD
OECD (2016) Test No. 474: mammalian erythrocyte micronucleus test. OECD
OECD (2013) Test No. 488: transgenic rodent somatic and germ cell gene mutation assays. OECD
OECD (2016) Test No. 489: in vivo mammalian alkaline comet assay. OECD
OECD (1997) Test No. 486: unscheduled DNA synthesis (UDS) test with mammalian liver cells in vivo. OECD
Ersev H, Schmalz G, Bayirli G, Schweikl H (1999) Cytotoxic and mutagenic potencies of various root canal filling materials in eukaryotic and prokaryotic cells in vitro. J Endod 25:359–363. https://doi.org/10.1016/S0099-2399(06)81172-6
Huang T, Hueilee D, Kao C (2001) Evaluation of the genotoxicity of zinc oxide eugenol-based, calcium hydroxide-based, and epoxy resin-based root canal sealers by comet assay. J Endod 27:744–748. https://doi.org/10.1097/00004770-200112000-00008
Tai K, Huang F-M, Huang M, Chang Y (2002) Assessment of the genotoxicity of resin and zinc-oxide eugenol-based root canal sealers using anin vitro mammalian test system. J Biomed Mater Res 59:73–77. https://doi.org/10.1002/jbm.1218
Huang F, Hsieh Y, Tai K et al (2002) Induction of c-fos and c-jun protooncogenes expression by formaldehyde-releasing and epoxy resin-based root-canal sealers in human osteoblastic cells. J Biomed Mater Res 59:460–465. https://doi.org/10.1002/jbm.10022
Santos NCN, Ramos MESP, Ramos AFB, Cerqueira AB, Cerqueira EMM (2016) Evaluation of the genotoxicity and cytotoxicity of filling pastes used for pulp therapy on deciduous teeth using the micronucleus test on bone marrow from mice (Mus musculus). Mutagenesis 31:589–595. https://doi.org/10.1093/mutage/gew026
Brzovic V, Miletic I, Zeljezic D, Mladinic M, Kasuba V, Ramic S, Anic I (2009) In vitro genotoxicity of root canal sealers. Int Endod J 42:253–263. https://doi.org/10.1111/j.1365-2591.2008.01510.x
Camargo CHR, Oliveira TR, Silva GO, Rabelo SB, Valera MC, Cavalcanti BN (2014) Setting time affects in vitro biological properties of root canal sealers. J Endod 40:530–533. https://doi.org/10.1016/j.joen.2013.08.009
Müller BP, Eisenträger A, Jahnen-Dechent W, Dott W, Hollender J (2003) Effect of sample preparation on the in vitro genotoxicity of a light curable glass ionomer cement. Biomaterials 24:611–617. https://doi.org/10.1016/S0142-9612(02)00375-7
Camargo SEA, Camargo CHR, Hiller K-A, Rode SM, Schweikl H, Schmalz G (2009) Cytotoxicity and genotoxicity of pulp capping materials in two cell lines. Int Endod J 42:227–237. https://doi.org/10.1111/j.1365-2591.2008.01506.x
Van Landuyt KL, Geebelen B, Shehata M et al (2012) No evidence for DNA double-strand breaks caused by endodontic sealers. J Endod 38:636–641. https://doi.org/10.1016/j.joen.2011.12.037
Silva GO, Cavalcanti BN, Oliveira TR, Bin CV, Camargo SEA, Camargo CHR (2016) Cytotoxicity and genotoxicity of natural resin-based experimental endodontic sealers. Clin Oral Investig 20:815–819. https://doi.org/10.1007/s00784-015-1567-4
Eldeniz AU, Shehata M, Högg C, Reichl FX (2016) DNA double-strand breaks caused by new and contemporary endodontic sealers. Int Endod J 49:1141–1151. https://doi.org/10.1111/iej.12577
Martinho FC, Camargo SEA, Fernandes AMM, Campos MS, Prado RF, Camargo CHR, Valera MC (2018) Comparison of cytotoxicity, genotoxicity and immunological inflammatory biomarker activity of several endodontic sealers against immortalized human pulp cells. Int Endod J 51:41–57. https://doi.org/10.1111/iej.12785
Camargo CHR, Camargo SEA, Valera MC, Hiller KA, Schmalz G, Schweikl H (2009) The induction of cytotoxicity, oxidative stress, and genotoxicity by root canal sealers in mammalian cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 108:952–960. https://doi.org/10.1016/j.tripleo.2009.07.015
Baraba A, Zelježić D, Kopjar N et al (2011) Evaluation of cytotoxic and genotoxic effects of two resin-based root-canal sealers and their components on human leucocytes in vitro. Int Endod J 44:652–661. https://doi.org/10.1111/j.1365-2591.2011.01869.x
Darrag AM, Fayyad DM (2014) Genotoxicity of three endodontic sealers by single cell gel-electrophoresis/comet assay. Tanta Dent J 11:85–92. https://doi.org/10.1016/j.tdj.2014.06.001
Huang T, Yang J, Li H, Kao C (2002) The biocompatibility evaluation of epoxy resin-based root canal sealers in vitro. Biomaterials 23:77–83. https://doi.org/10.1016/S0142-9612(01)00081-3
Leyhausen G, Heil J, Reifferscheid G, Waldmann P, Geurtsen W (1999) Genotoxicity and cytotoxicity of the epoxy resin-based root canal sealer AH plus. J Endod 25:109–113. https://doi.org/10.1016/S0099-2399(99)80007-7
Schweikl H, Schmalz G, Federlin M (1998) Mutagenicity of the root canal sealer AHPlus in the Ames test. Clin Oral Investig 2:125–129. https://doi.org/10.1007/s007840050057
Jukić S, Miletić I, Anić I et al (2000) The mutagenic potential of AH+ and AH26 by Salmonella/microsome assay. J Endod 26:321–324. https://doi.org/10.1097/00004770-200006000-00003
Bin CV, Valera MC, Camargo SEA, Rabelo SB, Silva GO, Balducci I, Camargo CHR (2012) Cytotoxicity and genotoxicity of root canal sealers based on mineral trioxide aggregate. J Endod 38:495–500. https://doi.org/10.1016/j.joen.2011.11.003
Candeiro GTM, Moura-Netto C, D’Almeida-Couto RS et al (2016) Cytotoxicity, genotoxicity and antibacterial effectiveness of a bioceramic endodontic sealer. Int Endod J 49:858–864. https://doi.org/10.1111/iej.12523
Victoria-Escandell A, Ibañez-Cabellos JS, de Cutanda SB-S, Berenguer-Pascual E, Beltrán-García J, García-López E, Pallardó FV, García-Giménez JL, Pallarés-Sabater A, Zarzosa-López I, Monterde M (2017) Cellular responses in human dental pulp stem cells treated with three endodontic materials. Stem Cells Int 2017:1–14. https://doi.org/10.1155/2017/8920356
Marques EF, da Silva Benigno MB, Macedo CP, Bitencourt L (2020) Cytotoxicity and genotoxicity analysis of two endodontic cements in human fibroblast culture in vitro. Int J Adv Eng Res Sci 7:103–108. https://doi.org/10.22161/ijaers.71.13
Ribeiro DA, Duarte MAH, Matsumoto MA et al (2005) Biocompatibility in vitro tests of mineral trioxide aggregate and regular and white Portland cements. J Endod 31:605–607. https://doi.org/10.1097/01.don.0000153842.06657.e2
Ribeiro DA, Sugui MM, Matsumoto MA, Duarte MAH, Marques MEA, Salvadori DMF (2006) Genotoxicity and cytotoxicity of mineral trioxide aggregate and regular and white Portland cements on Chinese hamster ovary (CHO) cells in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101:258–261. https://doi.org/10.1016/j.tripleo.2005.02.080
Braz MG, Camargo EA, Salvadori DMF et al (2006) Evaluation of genetic damage in human peripheral lymphocytes exposed to mineral trioxide aggregate and Portland cements. J Oral Rehabil 33:234–239. https://doi.org/10.1111/j.1365-2842.2005.01559.x
Zeferino EG, Bueno CES, Oyama LM, Ribeiro DA (2010) Ex vivo assessment of genotoxicity and cytotoxicity in murine fibroblasts exposed to white MTA or white Portland cement with 15% bismuth oxide. Int Endod J 43:843–848. https://doi.org/10.1111/j.1365-2591.2010.01747.x
Ding SJ, Kao CT, Chen CL, Shie MY, Huang TH (2010) Evaluation of human osteosarcoma cell line genotoxicity effects of mineral trixoide aggregate and calcium silicate cements. J Endod 36:1158–1162. https://doi.org/10.1016/j.joen.2010.03.039
Naghavi N, Ghoddusi J, Sadeghnia HR et al (2014) Genotoxicity and cytotoxicity of mineral trioxide aggregate and calcium enriched mixture cements on L929 mouse fibroblast cells. Dent Mater 33:64–69. https://doi.org/10.4012/dmj.2013-123
Ko H, Jeong Y, Kim M (2017) Cytotoxicities and genotoxicities of cements based on calcium silicate and of dental formocresol. Mutat Res Toxicol Environ Mutagen 815:28–34. https://doi.org/10.1016/j.mrgentox.2017.01.001
Zakerzadeh A, Esnaashari E, Dadfar S (2017) In vitro comparison of cytotoxicity and genotoxicity of three vital pulp capping materials. Iran Endod J 12:419–425. https://doi.org/10.22037/iej.v12i4.15104
Ribeiro DA, Matsumoto MA, Duarte MAH, Marques MEA, Salvadori DMF (2005) In vitro biocompatibility tests of two commercial types of mineral trioxide aggregate. Braz Oral Res 19:183–187. https://doi.org/10.1590/S1806-83242005000300005
Ribeiro DA, Matsumoto MA, Duarte MAH, Marques MEA, Salvadori DMF (2006) Ex vivo biocompatibility tests of regular and white forms of mineral trioxide aggregate. Int Endod J 39:26–30. https://doi.org/10.1111/j.1365-2591.2005.01043.x
da Silva GN, Braz MG, de Camargo EA, Salvadori DMF, Ribeiro DA (2006) Genotoxicity in primary human peripheral lymphocytes after exposure to regular and white mineral trioxide aggregate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 102:50–54. https://doi.org/10.1016/j.tripleo.2006.02.032
Nair AV, Nayak M, Prasada LK et al (2018) Comparative evaluation of cytotoxicity and genotoxicity of two bioceramic sealers on fibroblast cell line: An in vitro study. J Contemp Dent Pract 19:656–661. https://doi.org/10.5005/jp-journals-10024-2315
Laurent P, Camps J, De Méo M et al (2008) Induction of specific cell responses to a Ca3SiO5-based posterior restorative material. Dent Mater 24:1486–1494. https://doi.org/10.1016/j.dental.2008.02.020
Nai GA, de Almeida Logar G, Mori GG et al (2016) Evaluation of the genotoxicity and mutagenicity of Ca3SiO5-based cement. Braz Oral Res 30:1–7. https://doi.org/10.1590/1807-3107BOR-2016.vol30.0097
Opačić-Galić V, Petrović V, Živković S, Jokanović V, Nikolić B, Knežević-Vukčević J, Mitić-Ćulafić D (2013) New nanostructural biomaterials based on active silicate systems and hydroxyapatite: Characterization and genotoxicity in human peripheral blood lymphocytes. Int Endod J 46:506–516. https://doi.org/10.1111/iej.12017
Koch MJ (1999) Formaldehyde release from root-canal sealers: nfluence of method. Int Endod J 32:10–16. https://doi.org/10.1046/j.1365-2591.1999.00173.x
Zhu C, Ju B, Ni R (2015) Clinical outcome of direct pulp capping with MTA or calcium hydroxide: a systematic review and meta-analysis. Int J Clin Exp Med 8:17055–17060
Arossi GA, Dihl RR, Lehmann M, Cunha KS, Reguly ML, de Andrade HHR (2008) In vivo genotoxicity of dental bonding agents. Mutagenesis 24:169–172. https://doi.org/10.1093/mutage/gen066
Blasiak J, Kasznicki J, Drzewoski J, Pawlowska E, Szczepanska J, Reiter RJ (2011) Perspectives on the use of melatonin to reduce cytotoxic and genotoxic effects of methacrylate-based dental materials. J Pineal Res 51:157–162. https://doi.org/10.1111/j.1600-079X.2011.00877.x
Lottner S, Shehata M, Hickel R, Reichl FX, Durner J (2013) Effects of antioxidants on DNA-double strand breaks in human gingival fibroblasts exposed to methacrylate based monomers. Dent Mater 29:991–998. https://doi.org/10.1016/j.dental.2013.07.005
Yang M-L (2014) The effects of cytotoxicity and genotoxicity induced by 2,2-bis{[}4-(acryloxypropoxy)phenyl]propane via caspases in human gingival fibroblasts. Toxicol Ind Health 30:755–764. https://doi.org/10.1177/0748233712462472
Almadi EM, Almohaimede AA (2018) Natural products in endodontics. Saudi Med J 39:124–130. https://doi.org/10.15537/smj.2018.2.21038
Modareszadeh MR, Chogle SA, Mickel AK, Jin G, Kowsar H, Salamat N, Shaikh S, Qutbudin S (2011) Cytotoxicity of set polymer nanocomposite resin root-end filling materials. Int Endod J 44:154–161. https://doi.org/10.1111/j.1365-2591.2010.01825.x
Ndong F, Sadhasivam S, Lin FH, Savitha S, Wen-Hsi W, Lin CP (2012) The development of iron-free partially stabilized cement for use as dental root-end filling material. Int Endod J 45:557–564. https://doi.org/10.1111/j.1365-2591.2012.02011.x
Yang W-K, Ko H-J, Kim M-R (2012) Evaluation of the rat tissue reaction to experimental new resin cement and mineral trioxide aggregate cement. Restor Dent Endod 37:194–200. https://doi.org/10.5395/rde.2012.37.4.194
Grossman L (1981) Endodontic practice, vol 10. Lea Febiger, Philadelphia, p 458
Ingle JI, Bakland LK, Baumgartner JC. (2008) Ingle's Endodontics 6th edition. Hamilton: BC Decker. p 1555
Orstavik DAG (2005) Materials used for root canal obturation: technical, biological and clinical testing. Endod Top 12:25–38. https://doi.org/10.1111/j.1601-1546.2005.00197.x
Grassi TF, Camargo EA, Salvadori DMF, Marques MEA, Ribeiro DA (2007) DNA damage in multiple organs after exposure to chlorhexidine in Wistar rats. Int J Hyg Environ Health 210:163–167. https://doi.org/10.1016/j.ijheh.2006.09.001
Saxena P, Pant A, Gupta S, Pant V (2012) Release and toxicity of dental resin composite. Toxicol Int 19:225–234. https://doi.org/10.4103/0971-6580.103652
Schweikl H, Spagnuolo G, Schmalz G (2006) Genetic and cellular toxicology of dental resin monomers. J Dent Res 85:870–877. https://doi.org/10.1177/154405910608501001
Trachootham D, Lu W, Ogasawara MA, Valle NRD, Huang P (2008) Redox regulation of cell survival. Antioxid Redox Signal 10:1343–1374. https://doi.org/10.1089/ars.2007.1957
Lodienė G, Kopperud HM, Ørstavik D, Bruzell EM (2013) Detection of leachables and cytotoxicity after exposure to methacrylate- and epoxy-based root canal sealers in vitro. Eur J Oral Sci 121:488–496. https://doi.org/10.1111/eos.12065
Marin-Bauza GA, Rached-Junior FJA, Souza-Gabriel AE, Sousa-Neto MD, Miranda CES, Silva-Sousa YTC (2010) Physicochemical properties of methacrylate resin-based root canal sealers. J Endod 36:1531–1536. https://doi.org/10.1016/j.joen.2010.05.002
Schweikl H, Hiller KA, Eckhardt A, Bolay C, Spagnuolo G, Stempfl T, Schmalz G (2008) Differential gene expression involved in oxidative stress response caused by triethylene glycol dimethacrylate. Biomaterials 29:1377–1387. https://doi.org/10.1016/j.biomaterials.2007.11.049
Urcan E, Scherthan H, Styllou M, Haertel U, Hickel R, Reichl FX (2010) Induction of DNA double-strand breaks in primary gingival fibroblasts by exposure to dental resin composites. Biomaterials 31:2010–2014. https://doi.org/10.1016/j.biomaterials.2009.11.065
Blasiak J, Synowiec E, Tarnawska J, Czarny P, Poplawski T, Reiter RJ (2012) Dental methacrylates may exert genotoxic effects via the oxidative induction of DNA double strand breaks and the inhibition of their repair. Mol Biol Rep 39:7487–7496. https://doi.org/10.1007/s11033-012-1582-3
Pawlowska E, Poplawski T, Ksiazek D, Szczepanska J, Blasiak J (2010) Genotoxicity and cytotoxicity of 2-hydroxyethyl methacrylate. Mutat Res Genet Toxicol Environ Mutagen 696:122–129. https://doi.org/10.1016/j.mrgentox.2009.12.019
Gallorini M, Cataldi A, di Giacomo V (2014) HEMA-induced cytotoxicity: Oxidative stress, genotoxicity and apoptosis. Int Endod J 47:813–818. https://doi.org/10.1111/iej.12232
Ghanaati S, Willershausen I, Barbeck M, Unger RE, Joergens M, Sader RA, Kirkpatrick CJ, Willershausen B (2010) Tissue reaction to sealing materials: different view at biocompatibility. Eur J Med Res 15:483–492. https://doi.org/10.1186/2047-783X-15-11-483
Torabinejad M, Parirokh M (2010) Mineral trioxide aggregate: a comprehensive literature review. Part II. Leakage and biocompatibility investigations. J Endod 36:190–202. https://doi.org/10.1016/j.joen.2009.09.010
Kaur M, Singh H, Dhillon JS et al (2017) MTA versus biodentine: review of literature with a comparative analysis. J Clin Diagn Res 11:ZG01–ZG05. https://doi.org/10.7860/JCDR/2017/25840.10374
Gomes-Cornélio AL, Rodrigues EM, Mestieri LB et al (2016) Cytotoxicity and genotoxicity of calcium silicate-based cements on an osteoblast lineage. Braz Oral Res 30:1–10. https://doi.org/10.1590/1807-3107BOR-2016.vol30.0048
Sidhu SK (2011) Glass-ionomer cement restorative materials: a sticky subject? Aust Dent J 56:23–30. https://doi.org/10.1111/j.1834-7819.2010.01293.x
Sidhu SK, Nicholson JW (2016) A review of glass-ionomer cements for clinical dentistry. https://doi.org/10.3390/jfb7030016
Dearfield KL, Gollapudi BB, Bemis JC, Benz RD, Douglas GR, Elespuru RK, Johnson GE, Kirkland DJ, LeBaron MJ, Li AP, Marchetti F, Pottenger LH, Rorije E, Tanir JY, Thybaud V, van Benthem J, Yauk CL, Zeiger E, Luijten M (2017) Next generation testing strategy for assessment of genomic damage: a conceptual framework and considerations. Environ Mol Mutagen 58:264–283. https://doi.org/10.1002/em.22045
Nesslany F (2017) The current limitations of in vitro genotoxicity testing and their relevance to the in vivo situation. Food Chem Toxicol 106:609–615. https://doi.org/10.1016/j.fct.2016.08.035
Acknowledgments
The authors would like to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq - MCT), process n. 304845/2015-9, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), process n. PVE1232013-UFRN-123/2013, for the financial support provided.
Funding
The work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq, proc.n.442329/2014-8, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) proc.n. PVE1232013, from Brazil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 25 kb).
Rights and permissions
About this article
Cite this article
dos Santos Costa, F., Fernandes, M. & Batistuzzo de Medeiros, S.R. Genotoxicity of root canal sealers: a literature review. Clin Oral Invest 24, 3347–3362 (2020). https://doi.org/10.1007/s00784-020-03478-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03478-z