Abstract
Background
Considering the increasing number of elderly people, dementia has gained an important role in today’s society. Although the contributing factors for dementia have not been fully understood, chronic periodontitis (CP) seems to have a possible link to dementia.
Aim
To conduct a systematic review including meta-analysis in order to assess potential differences in clinical periodontal variables between patients with dementia and non-demented individuals.
Methods
The following focused question was evaluated: is periodontitis associated with dementia? Electronic searches in two databases, MEDLINE and EMBASE, were conducted. Meta-analysis was performed with the collected data in order to find a statistically significant difference in clinical periodontal variables between the group of dementia and the cognitive normal controls.
Results
Forty-two articles remained for full text reading. Finally, seven articles met the inclusion criteria and only five studies provided data suitable for meta-analysis. Periodontal probing depth (PPD), bleeding on probing (BOP), gingival bleeding index (GBI), clinical attachment level (CAL), and plaque index (PI) were included as periodontal variables in the meta-analysis. Each variable revealed a statistically significant difference between the groups. In an attempt to reveal an overall difference between the periodontal variables in dementia patients and non-demented individuals, the chosen variables were transformed into units that resulted in a statistically significant overall difference (p < 0.00001).
Conclusion
The current findings indicate that compared to systemically healthy individuals, demented patients show significantly worse clinical periodontal variables. However, further epidemiological studies including a high numbers of participants, the use of exact definitions both for dementia and chronic periodontitis and adjusted for cofounders is warranted.
Clinical relevance
These findings appear to support the putative link between CP and dementia. Consequently, the need for periodontal screening and treatment of elderly demented people should be emphasized.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodontitis is defined as a destruction of the supporting tissues of the teeth as a result of an inflammatory process induced by a microbial biofilm [1]. A measurable contribution of periodontal bacteria and inflammatory mediators to the systemic inflammation is underlined by the fact that those can enter the bloodstream and disseminate systemically [1]. According to recently published data, 743 million people or 10.8% of the population were affected by severe chronic periodontitis (CP) worldwide. The age-standardized prevalence and incidence of severe chronic periodontitis in 2010 was 11.2% (95% CI 10.5–12.0) [2].
Dementia was defined by the American Psychiatric Association in 2013 as follows: a neurodegenerative process leading to a significant decline of memory which interferes with daily activities, such as recognizing and identifying objects and persons, thinking abstractly or performing complex tasks [3]. Regarding to the meta-analysis of Prince et al. in 2013, the estimated global prevalence of dementia in a population > 60 years of age was between 5 and 7%. The estimation of demented people in 2010 was 35.6 millions worldwide and according to further calculations, the number will be doubled every 20 years which means an approximated total of 65.7 million affected people in 2030 and of 115.4 million in 2050 [4].
With 60–80% cases of the dementia patients, Alzheimer’s disease (AD) is nowadays the most common type of dementia and has gained importance during the past 30 years [3]. Histopathologically, two main hypotheses explain the neurodegenerative process in AD: the amyloid-beta peptide hypothesis and the tau hypothesis [5]. The amyloid-beta hypothesis is based on an abnormal process of cutting of the extracellular domains from the transmembrane amyloid precursor protein by the β-secretase and the γ-secretase into insoluble peptides called AbpE2-42. These insoluble peptides form the senile plaque with its potential of self-aggregation into fibrils [6]. According to the tau hypothesis, the microtubule-associated tau protein is abnormally hyperphosphorylated and is able to form neurofibrillary tangles leading to destruction of the neurons [6]. The in vivo study with mice by Bloom et al. [5] in April 2014 showed a possible interaction between tau protein and amyloid-beta and thus a connection between both hypotheses. But as in many chronic diseases there are multiple factors rather than a single cause of AD. Another possible mechanism suggested by Jawhar et al. in 2011 [7] might be a crucial role of the glutaminyl cyclase. The glutaminyl cyclase catalyzes the formation of AbpE2-42 and following an overexpression of these peptides, it induces a severe decline of neurons [7]. Three genes (APP, PSEN1, and PSEN2) and one genetic risk factor APOEε4 allele have been associated with autosomal dominant familial AD so far [8].
On the other hand, theories assuming that microorganisms from the human mouth are able to spread over the whole body are discussed [1]. Nowadays, considering the focal infection theory experts have made the suggestion of periodontal disease as a possible cause of systematic disease, such as AD [9].
There have been recent reviews reporting a putative association between CP and dementia. Most of the studies reported the number of teeth, tooth loss or the decayed, and missing and filled teeth (DMFT) [10,11,12]. In 2015, Foley et al. [13] published a systematic review comparing individuals with dementia and without analyzing the number of teeth, the DMFT record and the number of carious teeth. Individuals with dementia had significantly fewer teeth (mean difference − 1.25, 95% CI − 0.832; − 5.89, p < 0.0001; n = 8 studies), a significantly higher number of decayed, missing, and filled teeth, and a higher number of carious teeth [13]. In the systematic review published in 2016, the association between oral health (assessed mainly by the number of teeth) and cognitive status was reported [14]. Also in the most recent review by Tonsekar et al. [15] in 2017, tooth loss and the possible association between periodontal disease and dementia was discussed.
Until now, to the best of our knowledge, no systematic review is available including meta-analysis comparing the clinical periodontal variables of patients with dementia and without as assessed in cross-sectional studies. Therefore, the aim of this systematic review including meta-analysis was to assess potential differences in clinical periodontal variables between patients with dementia and non-demented individuals.
Material and methods
Sources
An electronic search was conducted by two reviewers (A.M. and S.E.). All studies were included in this review published until the 12th of September 2016:
National Library of Medicine (MEDLINE by PubMed) and EMBASE using the following MeSH terms: (Alzheimer’s disease OR Alzheimer OR cognitive decline OR dementia) AND (periodontal disease OR periodontitis).
No restrictions were applied in any search.
Search Strategy
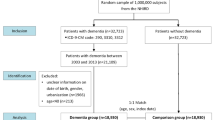
Electronic database search revealed 476 articles (Fig. 1); 290 titles were found in EMBASE and 186 in MEDLINE. After excluding 138 duplicates, 338 titles remained for the focused screening; 231 titles and 65 abstracts dropped out after the first screening meeting the following exclusion criteria:
-
Titles and abstracts not focusing on the association between dementia and periodontitis
-
Titles and abstracts only investigating the relationship between tooth loss or number of teeth in demented patients
-
Reviews, commentaries, replies, and posters
-
No abstract and/or text available
-
Animal studies.
If only one searcher has excluded a title or an abstract, the full article was retrieved and analyzed. The reviewers reached a kappa value of 0.96.
Data analysis for the systematic review
Forty-two abstracts remained for full text reading and full data recording. Only seven studies [16,17,18,19,20,21,22] complied with the following inclusion criteria and were considered for the systematic review:
-
Studies in English
-
Human studies
-
Studies reporting periodontal indices or measurements
-
All indices or measurements assessing the mean value and its standard deviation (SD) and/or standard error (SE) and/or range
-
Studies comparing a case to a control group
-
At least a total of ten participants in each group
-
Age of the participants ≥ 50 years.
Data analysis for meta-analysis
The seven articles included in this systematic review are listed in Table 1. The collected data of the two papers of Zenthöfer et al. [18, 21] were assessed as one study in the further meta-analyses. The 35 excluded articles after full text screening were neither a case-control schema nor reported a mean value with SD, SE, or range were reported or could be extracted accurately from the data. The excluded papers are listed in Tables 2 and 3.
After data extraction, it became obvious that various periodontal variables were used to show an association to dementia. It was decided to focus on the most common five clinical variables: plaque index (PI); bleeding on probing (BOP); gingival bleeding index (GBI); periodontal probing depth in millimeter (PPD); and the clinical attachment loss in millimeter (CAL). Five studies reported the mean and the standard deviations (SD) of the clinical variables. One study [22] only compared the mean number of teeth with periodontal pocket depth ≥ 4 mm between AD patients and non-demented individuals. Although the study fulfilled the inclusion criteria, the way of presenting a difference between AD patients and control group was not comparable to the other five studies and it was excluded from meta-analysis. The community index of periodontal treatment needs (CIPTN) was recorded only in one study [18, 21] and following not considered in the meta-analysis. Finally, five studies were included to the meta-analysis. In Table 4, the five variables (BOP, PI, GBI, PPD, and CAL) with mean values and the standard deviations (SD) are listed.
Quality assessment of the studies
In order to assess the quality of the five chosen studies, a modified Newcastle-Ottawa Scale (NOS) for case-control and cross-sectional studies was used [54]. Qualitative points were given in terms of the selection and comparability of the study groups and the assessment of the outcome and the exposure (Table 4).
Statistical evaluation
The first step of statistical calculation consisted of screening and completing of all data for further analysis. The data (mean value and standard deviation) of each periodontal variable were pooled using a weighted average and weighted standard deviation. After that, the weighted mean difference from each periodontal variable was calculated and presented in forest plots (Fig. 2a–e).
Not every study included all five variables. Thus, the effect size of a single variable was weighted independently for the same study in a second step. An overall difference in the periodontal variables between the dementia patients and individuals was calculated. In order to fulfill a comparison, each variable was standardized, meaning a conversion to unit-less effect sizes. Standardization of the effect size is needed when the units are not the same [55] (Fig. 3). The data preparation was performed in EXCEL© and for the statistical calculation and illustration, REVIEW MANAGER 5.3© was chosen.
Results
Study characteristics
The five studies included for meta-analysis were three case-control studies [16, 17, 20], a cohort study [18, 21], and a cross-sectional study [19]. In all studies, the individuals were ≥ 50 years and included between 52 and 409 study participants. In four of five studies [16, 18,19,20,21], a definition for CP and for dementia were found. Three [16, 19, 20] of the five studies chose the diagnosis criteria of the Diagnostic and Statistical Manual of Mental Disorders-IV for dementia from the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association [56]. One study [18, 21] screened participants for dementia using the Mini Mental State Examination (MMSE) developed by Folstein et al. [57] and another one [19] used the practical guide of Mungas et al. [58]. All studies reported at least one or more clinical periodontal variables. All variables were documented in mean values and the standard deviation (SD).
Qualitative assessment results
Two of the case-control studies [16, 20] accomplished the full score. The third case-control study [17] achieved only 6 points as neither inclusion or exclusion criteria for the case group and for the control group nor the assessment tool in order to define dementia were reported. Even though the study of Zenthöfer et al. [18, 21] was described as a prospective cohort study, no follow-up was made and it was therefore assessed with the cross-sectional schema of NOS. Both studies [18, 21] obtained 6 out of 7 points. Martande et al. [19] could not accomplish a satisfactory number of participants (≥ 200 participants). And the study of Zenthöfer et al. [18, 21] was conducted in nursing homes in Germany, and therefore, it represented only a particular group and not the general populations. Only residents in nursing homes were considered. The results of the NOS Assessment are shown in Table 4.
Statistical analysis
Only articles where the mean values and the SD could be extracted accurately from the reported periodontal measurements proceeded to meta-analysis. As mentioned above, from the seven studies included to the systematic review, only five studies remained in the two-step statistical analysis (Table 4; Figs. 2 and 3). The comparison between dementia patients and controls revealed statistically significant mean differences in both steps. The first step of analysis showed a significant weighted mean difference (Fig. 2a–e) of 35.72% (95% CI 31.95–39.50, p < 0.001) in BOP; of 2.53 mm in CAL (95% CI 2.42–2.63, p < 0.001); of 6.98% in GBI (95% CI – 0.11–14.07, p = 0.05); of 15.95% in PI (95% CI 8.26–23.64, p < 0.001); and of 1.46 mm in PPD (95% CI 1.30–1.62, p < 0.001). All analyzed clinical periodontal variables were significantly higher in the dementia groups (Table 5; Fig. 2).
In the second step of analysis, the whole data with standardized mean differences was analyzed. Here, the overall mean difference between dementia group and non-dementia group showed a positive result of 0.53 (95% CI 0.44, 0.62). The Z-value of the test for overall effect reached 11.07 (p < 0.001) (Fig. 3).
Discussion
According to our present knowledge, a meta-analysis comparing the differences in clinical periodontal indices between patients with dementia and non-demented individuals has been published up to now. This review and meta-analysis focused on the possible differences in clinical periodontal variables between demented patients and non-dementia controls. Validated neuropsychological tests were used to distinguish both groups. After running an electronic research in the databases of MEDLINE and EMBASE, five studies were included for the meta-analysis.
The results showed that periodontal parameters were significantly higher in patients with dementia than in subjects without cognitive decline. First analysis resulted in statistically significant mean differences between the dementia group and the control group in BOP, CAL, PI, PPD, and GBI.
The second analysis provided the overall mean difference (p < 0.001) between the periodontal indices of the dementia group and non-demented group. In order to evaluate an overall mean difference, each periodontal variable was turned into a unit-less effect size and weighted independently for each study. This result is in line with that of an AD study showing that clinical periodontal variables in cognitively normal healthy patients are positively associated with the load of amyloid-beta protein in the brain [32].
However, when interpreting the results, there are a few limitations that have to be considered. A considerable heterogeneity exists among the studies regarding the definition of dementia and CP. The cognitive status of the patients was validated by using different neuropsychological tests. In most of the studies, the MMSE scores were crucial for distinguish the “demented” and “non-demented” groups. In the study cohort analyzed by Zenthöfer et al. [18, 21], subjects were considered suffering from dementia scoring equal or below 20 in the MMSE score. AD was diagnosed by a neurologist, according to the NINCDS-ADRDA criteria, in three different studies [16, 19, 20]. Two of them completed the AD diagnosis not only with the NINCDS-ADRDA but also with a structural neuroimaging [16, 19]. It has to be mentioned that three of the included studies only considered the Alzheimer type of dementia [16, 19, 20] and the other two do not differ between dementia types [17, 18, 21]. Moreover, the definition of chronic periodontitis varied among the studies. Gil-Montaya et al. [16] evaluated the degree of periodontitis by the percentage of sites with CAL > 3 mm, Rai et al. [17] defined periodontitis as a CAL of 6 mm and more at least one site, while Zenthöfer et al. [18, 21] used the scoring of the community periodontal index of treatment needs (CPITN) to diagnose periodontitis. It can be assumed that the authors were aware about the limitation of CPITN using only PPD and not CAL and differentiated between gingival overgrowth and periodontal destruction.
Another aspect interfering with a cause-related association between CP and dementia is the incomplete adjustment for confounders. Besides age, smoking is considered as a common risk factor for dementia [59] as well as for periodontitis [60]. Tobacco status was reported in two studies [16, 17] while no information was given in the other studies [18,19,20,21]. Furthermore, the implications of cognitive impairment on oral health must be considered as well. Previous studies reported that patients with dementia might be less capable to perform sufficient oral hygiene [50]. A recent study in residential aged care facilities showed that oral hygiene status in residents with dementia was worse although those received assistance in oral care [26]. That result was explained with the resistive behavior of demented patients towards oral hygiene care [26]. As a further limitation, the low number of studies included in the meta-analysis has to be considered. Only five studies were accurate for meta-analysis.
Possible pathomechanisms for periodontitis to contribute to dementia were postulated. First, bacteria being associated with periodontitis may spread from the periodontal region to the blood system and into other organs in the body. Second, microbial toxins and inflammatory mediators enter and damage the vascular system [61]. Few studies showed that TNF-alpha levels were significantly higher in dementia and periodontitis subjects than in controls [17, 38]. And recent studies support an invasive infection where Porphyromonas gingivalis passed the blood-brain-barrier and invaded the AD brain [62,63,64]. Increased antibody levels also against other oral bacteria were reported. For example, patients with increased Actinomyces naeslundii serum IgG had a higher risk of developing AD than the controls [46] and elevated antibody levels to Fusobacterium nucleatum and Prevotella intermedia were assessed in the AD subjects compared to the controls.
Only few longitudinal studies followed demented patients receiving oral care and reported the relation to the cognitive status [39, 59, 65]. One study showed a statistically significant improvement (p < 0.05) in the MMSE score after 24 months in the oral care group compared to the group not receiving oral care. Both groups started with a similar MMSE score at baseline [65]. A 4-year prospective cohort study of older Japanese people reported an increased risk for developing dementia when not visiting regularly a dentist and not taking care of dental health [59]. The hazard ratios revealed 1.44 (95% CI 1.04–2.01) for patients not visiting the dentist and 1.76 (95% CI 0.96–3.20) for patients not looking after oral health at all [59]. Another study revealed in a 32-year follow-up of 597 community-dwelling men that the risk for a low MMSE score increased by 2–5% for each tooth with progressed loss of alveolar bone or progressed probing pocket depth [39]. A loss in bone height was considered when loosing at least 40% from baseline and a progression in probing pocket depth was defined as an increase of at least 2-mm probing pocket depth [39]. In the view of these results, it may be anticipated that an adequately performed periodontal therapy and maintenance may be beneficial to reduce the risk for dementia.
However, the limited number of patients included in the studies does not provide representative epidemiological data, and therefore, more epidemiological studies including a high numbers of participants using exact definitions both for dementia and chronic periodontitis and adjusted for cofounders are warranted.
Conclusion
In summary, the present data indicate that demented patients show significantly worse clinical periodontal variables as compared to systematically healthy individuals and appear to support the putative link between CP and dementia. Consequently, the need for periodontal screening and treatment of elderly demented people should be emphasized.
References
Van Dyke TE, van Winkelhoff AJ (2013) Infection and inflammatory mechanisms. J Clin Periodontol 40(Suppl 14):S1–S7. https://doi.org/10.1111/jcpe.12088
Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, Marcenes W (2014) Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. J Dent Res 93:1045–1053. https://doi.org/10.1177/0022034514552491
Alzheime’s A (2014) 2014 Alzheimer’s disease facts and figures. Alzheimers Dement 10:e47–e92
Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP (2013) The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 9:63–75 e2. https://doi.org/10.1016/j.jalz.2012.11.007
Bloom GS (2014) Amyloid-beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol 71:505–508. https://doi.org/10.1001/jamaneurol.2013.5847
Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, Harvey D, Jack CR, Jagust W, Liu E, Morris JC, Petersen RC, Saykin AJ, Schmidt ME, Shaw L, Shen L, Siuciak JA, Soares H, Toga AW, Trojanowski JQ, Alzheimer’s Disease Neuroimaging I (2013) The Alzheimer’s Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement 9:e111–e194. https://doi.org/10.1016/j.jalz.2013.05.1769
Jawhar S, Wirths O, Schilling S, Graubner S, Demuth HU, Bayer TA (2011) Overexpression of glutaminyl cyclase, the enzyme responsible for pyroglutamate A{beta} formation, induces behavioral deficits, and glutaminyl cyclase knock-out rescues the behavioral phenotype in 5XFAD mice. J Biol Chem 286:4454–4460. https://doi.org/10.1074/jbc.M110.185819
Bekris LM, Yu CE, Bird TD, Tsuang DW (2010) Genetics of Alzheimer disease. J Geriatr Psychiatry Neurol 23:213–227. https://doi.org/10.1177/0891988710383571
Pizzo G, Guiglia R, Lo Russo L, Campisi G (2010) Dentistry and internal medicine: from the focal infection theory to the periodontal medicine concept. Eur J Intern Med 21:496–502. https://doi.org/10.1016/j.ejim.2010.07.011
Gatz M, Mortimer JA, Fratiglioni L, Johansson B, Berg S, Reynolds CA, Pedersen NL (2006) Potentially modifiable risk factors for dementia in identical twins. Alzheimers Dement 2:110–117. https://doi.org/10.1016/j.jalz.2006.01.002
Chen JH, Lin KP, Chen YC (2009) Risk factors for dementia. J Formos Med Assoc 108:754–764. https://doi.org/10.1016/S0929-6646(09)60402-2
Hopcraft MS, Morgan MV, Satur JG, Wright FA, Darby IB (2012) Oral hygiene and periodontal disease in Victorian nursing homes. Gerodontology 29:e220–e228. https://doi.org/10.1111/j.1741-2358.2010.00448.x
Foley NC, Affoo RH, Martin RE (2015) A systematic review and meta-analysis examining pneumonia-associated mortality in dementia. Dement Geriatr Cogn Disord 39:52–67. https://doi.org/10.1159/000367783
Wu B, Fillenbaum GG, Plassman BL, Guo L (2016) Association between oral health and cognitive status: a systematic review. J Am Geriatr Soc 64:1752. https://doi.org/10.1111/jgs.14572
Tonsekar PP, Jiang SS, Yue G (2017) Periodontal disease, tooth loss and dementia: is there a link? A systematic review. Gerodontology 34:151–163. https://doi.org/10.1111/ger.12261
Gil-Montoya JA, Sanchez-Lara I, Carnero-Pardo C, Fornieles F, Montes J, Vilchez R, Burgos JS, Gonzalez-Moles MA, Barrios R, Bravo M (2015) Is periodontitis a risk factor for cognitive impairment and dementia? A case-control study. J Periodontol 86:244–253. https://doi.org/10.1902/jop.2014.140340
Rai B, Kaur J, Anand SC (2012) Possible relationship between periodontitis and dementia in a North Indian old age population: a pilot study. Gerodontology 29:e200–e205. https://doi.org/10.1111/j.1741-2358.2010.00441.x
Zenthofer A, Baumgart D, Cabrera T, Rammelsberg P, Schroder J, Corcodel N, Hassel AJ (2017) Poor dental hygiene and periodontal health in nursing home residents with dementia: an observational study. Odontology 105:208–213. https://doi.org/10.1007/s10266-016-0246-5
Martande SS, Pradeep AR, Singh SP, Kumari M, Suke DK, Raju AP, Naik SB, Singh P, Guruprasad CN, Chatterji A (2014) Periodontal health condition in patients with Alzheimer’s disease. Am J Alzheimers Dis Other Demen 29:498–502. https://doi.org/10.1177/1533317514549650
Cestari JA, Fabri GM, Kalil J, Nitrini R, Jacob-Filho W, Tesseroli de Siqueira JT, Siqueira SR (2016) Oral infections and cytokine levels in patients with Alzheimer’s disease and mild cognitive impairment compared with controls. J Alzheimers Dis 54:845. https://doi.org/10.3233/JAD-169006
Zenthofer A, Schroder J, Cabrera T, Rammelsberg P, Hassel AJ (2014) Comparison of oral health among older people with and without dementia. Community Dent Health 31:27–31
Syrjala AM, Ylostalo P, Ruoppi P, Komulainen K, Hartikainen S, Sulkava R, Knuuttila M (2012) Dementia and oral health among subjects aged 75 years or older. Gerodontology 29:36–42. https://doi.org/10.1111/j.1741-2358.2010.00396.x
Lewis S, Jagger RG, Treasure E (2001) The oral health of psychiatric in-patients in South Wales. Spec Care Dentist 21:182–186
Wu B, Plassman BL, Liang J, Wei L (2007) Cognitive function and dental care utilization among community-dwelling older adults. Am J Public Health 97:2216–2221. https://doi.org/10.2105/AJPH.2007.109934
Noble JM, Borrell LN, Papapanou PN, Elkind MS, Scarmeas N, Wright CB (2009) Periodontitis is associated with cognitive impairment among older adults: analysis of NHANES-III. J Neurol Neurosurg Psychiatry 80:1206–1211. https://doi.org/10.1136/jnnp.2009.174029
Philip P, Rogers C, Kruger E, Tennant M (2012) Oral hygiene care status of elderly with dementia and in residential aged care facilities. Gerodontology 29:e306–e311. https://doi.org/10.1111/j.1741-2358.2011.00472.x
Kamer AR, Morse DE, Holm-Pedersen P, Mortensen EL, Avlund K (2012) Periodontal inflammation in relation to cognitive function in an older adult Danish population. J Alzheimers Dis 28:613–624. https://doi.org/10.3233/JAD-2011-102004
Naorungroj S, Slade GD, Beck JD, Mosley TH, Gottesman RF, Alonso A, Heiss G (2013) Cognitive decline and oral health in middle-aged adults in the ARIC study. J Dent Res 92:795–801. https://doi.org/10.1177/0022034513497960
Cicciu M, Matacena G, Signorino F, Brugaletta A, Cicciu A, Bramanti E (2013) Relationship between oral health and its impact on the quality life of Alzheimer’s disease patients: a supportive care trial. Int J Clin Exp Med 6:766–772
Farhad SZ, Amini S, Khalilian A, Barekatain M, Mafi M, Barekatain M, Rafei E (2014) The effect of chronic periodontitis on serum levels of tumor necrosis factor-alpha in Alzheimer disease. Dent Res J (Isfahan) 11:549–552
Naorungroj S, Schoenbach VJ, Wruck L, Mosley TH, Gottesman RF, Alonso A, Heiss G, Beck J, Slade GD (2015) Tooth loss, periodontal disease, and cognitive decline in the Atherosclerosis Risk in Communities (ARIC) study. Community Dent Oral Epidemiol 43:47–57. https://doi.org/10.1111/cdoe.12128
Kamer AR, Pirraglia E, Tsui W, Rusinek H, Vallabhajosula S, Mosconi L, Yi L, McHugh P, Craig RG, Svetcov S, Linker R, Shi C, Glodzik L, Williams S, Corby P, Saxena D, de Leon MJ (2015) Periodontal disease associates with higher brain amyloid load in normal elderly. Neurobiol Aging 36:627–633. https://doi.org/10.1016/j.neurobiolaging.2014.10.038
Ide M, Harris M, Stevens A, Sussams R, Hopkins V, Culliford D, Fuller J, Ibbett P, Raybould R, Thomas R, Puenter U, Teeling J, Perry VH, Holmes C (2016) Periodontitis and cognitive decline in Alzheimer’s disease. PLoS One 11:e0151081. https://doi.org/10.1371/journal.pone.0151081
Iwasaki M, Yoshihara A, Kimura Y, Sato M, Wada T, Sakamoto R, Ishimoto Y, Fukutomi E, Chen W, Imai H, Fujisawa M, Okumiya K, Taylor GW, Ansai T, Miyazaki H, Matsubayashi K (2016) Longitudinal relationship of severe periodontitis with cognitive decline in older Japanese. J Periodontal Res 51:681–688. https://doi.org/10.1111/jre.12348
Ship JA, Puckett SA (1994) Longitudinal study on oral health in subjects with Alzheimer’s disease. J Am Geriatr Soc 42:57–63
Chalmers JM, Carter KD, Spencer AJ (2003) Oral diseases and conditions in community-living older adults with and without dementia. Spec Care Dentist 23:7–17
Kim JM, Stewart R, Prince M, Kim SW, Yang SJ, Shin IS, Yoon JS (2007) Dental health, nutritional status and recent-onset dementia in a Korean community population. Int J Geriatr Psychiatry 22:850–855. https://doi.org/10.1002/gps.1750
Kamer AR, Craig RG, Pirraglia E, Dasanayake AP, Norman RG, Boylan RJ, Nehorayoff A, Glodzik L, Brys M, de Leon MJ (2009) TNF-alpha and antibodies to periodontal bacteria discriminate between Alzheimer’s disease patients and normal subjects. J Neuroimmunol 216:92–97. https://doi.org/10.1016/j.jneuroim.2009.08.013
Kaye EK, Valencia A, Baba N, Spiro A 3rd, Dietrich T, Garcia RI (2010) Tooth loss and periodontal disease predict poor cognitive function in older men. J Am Geriatr Soc 58:713–718. https://doi.org/10.1111/j.1532-5415.2010.02788.x
Hatipoglu MG, Kabay SC, Guven G (2011) The clinical evaluation of the oral status in Alzheimer-type dementia patients. Gerodontology 28:302–306. https://doi.org/10.1111/j.1741-2358.2010.00401.x
Arrive E, Letenneur L, Matharan F, Laporte C, Helmer C, Barberger-Gateau P, Miquel JL, Dartigues JF (2012) Oral health condition of French elderly and risk of dementia: a longitudinal cohort study. Community Dent Oral Epidemiol 40:230–238. https://doi.org/10.1111/j.1600-0528.2011.00650.x
Miranda Lde P, Silveira MF, Oliveira TL, Alves SF, Junior HM, Batista AU, Bonan PR (2012) Cognitive impairment, the Mini-Mental State Examination and socio-demographic and dental variables in the elderly in Brazil. Gerodontology 29:e34–e40. https://doi.org/10.1111/j.1741-2358.2011.00541.x
Sparks Stein P, Steffen MJ, Smith C, Jicha G, Ebersole JL, Abner E and Dawson D, 3rd (2012) Serum antibodies to periodontal pathogens are a risk factor for Alzheimer’s disease. Alzheimers Dement 8:196–203. doi: https://doi.org/10.1016/j.jalz.2011.04.006
Chen X, Clark JJ, Naorungroj S (2013) Oral health in nursing home residents with different cognitive statuses. Gerodontology 30:49–60. https://doi.org/10.1111/j.1741-2358.2012.00644.x
Stewart R, Weyant RJ, Garcia ME, Harris T, Launer LJ, Satterfield S, Simonsick EM, Yaffe K, Newman AB (2013) Adverse oral health and cognitive decline: the health, aging and body composition study. J Am Geriatr Soc 61:177–184. https://doi.org/10.1111/jgs.12094
Noble JM, Scarmeas N, Celenti RS, Elkind MS, Wright CB, Schupf N, Papapanou PN (2014) Serum IgG antibody levels to periodontal microbiota are associated with incident Alzheimer disease. PLoS One 9:e114959. https://doi.org/10.1371/journal.pone.0114959
de Souza Rolim T, Fabri GM, Nitrini R, Anghinah R, Teixeira MJ, de Siqueira JT, Cestari JA, de Siqueira SR (2014) Oral infections and orofacial pain in Alzheimer’s disease: a case-control study. J Alzheimers Dis 38:823–829. https://doi.org/10.3233/JAD-131283
Bramanti E, Bramanti A, Matacena G, Bramanti P, Rizzi A, Cicciu M (2015) Clinical evaluation of the oral health status in vascular-type dementia patients. A case-control study. Minerva Stomatol 64:167–175
Stewart R, Stenman U, Hakeberg M, Hagglin C, Gustafson D, Skoog I (2015) Associations between oral health and risk of dementia in a 37-year follow-up study: the prospective population study of women in Gothenburg. J Am Geriatr Soc 63:100–105. https://doi.org/10.1111/jgs.13194
Chu CH, Ng A, Chau AM and Lo EC (2015) Oral health status of elderly Chinese with dementia in Hong Kong Oral health Prev Dent 13:51–57. doi: https://doi.org/10.3290/j.ohpd.a32343
Lee YT, Lee HC, Hu CJ, Huang LK, Chao SP, Lin CP, Su EC, Lee YC, Chen CC (2017) Periodontitis as a modifiable risk factor for dementia: a nationwide population-based cohort study. J Am Geriatr Soc 65:301–305. https://doi.org/10.1111/jgs.14449
Tzeng NS, Chung CH, Yeh CB, Huang RY, Yuh DY, Huang SY, Lu RB, Chang HA, Kao YC, Chiang WS, Chou YC, Chien WC (2016) Are chronic periodontitis and gingivitis associated with dementia? A nationwide, retrospective, matched-cohort study in Taiwan. Neuroepidemiology 47:82–93. https://doi.org/10.1159/000449166
Shin HS, Shin MS, Ahn YB, Choi BY, Nam JH, Kim HD (2016) Periodontitis is associated with cognitive impairment in elderly Koreans: results from the Yangpyeong cohort study. J Am Geriatr Soc 64:162–167. https://doi.org/10.1111/jgs.13781
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605. https://doi.org/10.1007/s10654-010-9491-z
Israel H, Richter RR (2011) A guide to understanding meta-analysis. J Orthop Sports Phys Ther 41:496–504. https://doi.org/10.2519/jospt.2011.3333
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34:939–944
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Mungas D (1991) In-office mental status testing: a practical guide. Geriatrics 46 63(66):54–58
Yamamoto T, Kondo K, Hirai H, Nakade M, Aida J, Hirata Y (2012) Association between self-reported dental health status and onset of dementia: a 4-year prospective cohort study of older Japanese adults from the Aichi Gerontological Evaluation Study (AGES) Project. Psychosom Med 74:241–248. https://doi.org/10.1097/PSY.0b013e318246dffb
Ebersole JL, Steffen MJ, Thomas MV, Al-Sabbagh M (2014) Smoking-related cotinine levels and host responses in chronic periodontitis. J Periodontal Res 49:642–651. https://doi.org/10.1111/jre.12146
Li X, Kolltveit KM, Tronstad L, Olsen I (2000) Systemic diseases caused by oral infection. Clin Microbiol Rev 13:547–558
Olsen I, Singhrao SK (2015) Can oral infection be a risk factor for Alzheimer’s disease? J Oral Microbiol 7:29143. https://doi.org/10.3402/jom.v7.29143
Singhrao SK, Harding A, Poole S, Kesavalu L, Crean S (2015) Porphyromonas gingivalis periodontal infection and its putative links with Alzheimer’s disease. Mediat Inflamm 2015:137357–137310. https://doi.org/10.1155/2015/137357
Poole S, Singhrao SK, Kesavalu L, Curtis MA, Crean S (2013) Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J Alzheimers Dis 36:665–677. https://doi.org/10.3233/JAD-121918
Yoneyama T, Yoshida M, Ohrui T, Mukaiyama H, Okamoto H, Hoshiba K, Ihara S, Yanagisawa S, Ariumi S, Morita T, Mizuno Y, Ohsawa T, Akagawa Y, Hashimoto K, Sasaki H, Oral Care Working G (2002) Oral care reduces pneumonia in older patients in nursing homes. J Am Geriatr Soc 50:430–433
Funding
This study was supported by the European Commission (FP7-Health 2012-306029 “TRIGGER”).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Maldonado, A., Laugisch, O., Bürgin, W. et al. Clinical periodontal variables in patients with and without dementia—a systematic review and meta-analysis. Clin Oral Invest 22, 2463–2474 (2018). https://doi.org/10.1007/s00784-018-2523-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2523-x