Abstract
Objectives
The objective of this prospective clinical study was to evaluate the clinical performance of chair-side generated monolithic lithium disilicate crowns after 72 months.
Materials and methods
Forty-one posterior full contour crowns made of lithium disilicate ceramic were inserted in 34 patients with a chair-side CAD/CAM technique. One crown per patient was randomly selected for evaluation at baseline, 6, 12, 24, 36, 48, 60 and 72 months according to the modified US Public Health Service criteria.
Results
After a mean examination time of 73.2 months (SD ± 1.7 months), 25 crowns were available for re-examination. Within the observation period, three failures occurred due to one crown fracture after 2.9 years, an abutment fracture after 6.0 years, and one severe endodontic problem after 6.1 years. One lithium disilicate crown showed a loss of retention after 2 years but could be reinserted. There were two events of caries below the crown margin, one after 24 and another one after 48 months. Both teeth received cervical adhesive composite fillings. Two abutment teeth changed their sensibility perception from positive to negative within the first 13 months. The failure-free rate was 87.6%, and the complication-free rate was 70.1% after 6 years according to the Kaplan-Meier analysis.
Conclusions
Due to the fact that there was only one severe technical complication and the severe biological complications were in a normal range, the clinical performance of monolithic lithium disilicate crowns in the posterior region was completely satisfying.
Clinical relevance
The chair-side application of monolithic lithium disilicate crowns can be recommended.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
All-ceramic restorative materials are used routinely in patients’ treatment for crowns and fixed dental prostheses (FDP) nowadays. In daily dental practice, the patients’ desire for natural tooth-coloured teeth in a short period of time is attainable, since the introduction of chair-side computer-aided design/computer-aided manufacturing (CAD/CAM). This technology offers the opportunity to produce restorations within one appointment and covers the requirements for natural coloured teeth by milling glass ceramic blocks, such as lithium disilicate. This material combines a high mechanical strength up to 360 ± 60 MPa with a fracture toughness between 2.0 and 2.5 MPa × m0.5 [1] and translucent characteristics appropriate for tooth-coloured restorations [2]. Besides, the chair-side CAD/CAM technology is time-saving since no temporary restorations are required [3]; additionally, the fresh ground dentin provides the best adhesive bond [4]. Furthermore, an immediate evaluation of the preparation and the margin is possible based on the digital impression procedure [5].
In addition to lithium disilicate blocks, other materials like feldspathic ceramics, leucite-reinforced glass ceramics, zirconia-reinforced lithium silicate ceramics, and hybrid ceramics are available for chair-side generated CAD/CAM crowns. For the majority of these materials, no clinical efficacy has been proven [6]. The estimated 12-year survival rate of chair-side CAD/CAM feldspathic crowns depending on the location of the abutment tooth and the preparation design extends from 75 to 95% [7].
While there are some published data for the short-time performance of chair-side fabricated monolithic lithium disilicate restorations [8, 9], long-term results are limited to one study about laboratory-fabricated pressable, veneered lithium disilicate FDPs [10]. The veneering technique seems to be sensible in the posterior area because of its proneness to chipping. To the authors’ knowledge, no medium- or long-term studies are available for the clinical performance of chair-side generated monolithic lithium disilicate crowns.
Therefore, the purpose of this prospective study was to evaluate the 6-year clinical performance of monolithic lithium disilicate crowns in posterior teeth. The working hypothesis was that this kind of chair-side treatment is equal to other methods for the fabrication of single crowns in the posterior area regarding failure and complication rate.
Material and methods
Between June 2006 and February 2007 in 34 patients (mean age 46.5 years ±13.1 years; min. 26.2 years; max. 73.8 years; 62% female), optical impressions were taken and they were provided with 41 chair-side milled and sintered lithium disilicate crowns (IPS e.max CAD LT, Ivoclar Vivadent, Schaan, Liechtenstein). Within the study group, seven patients received two crowns but only one crown per patient was selected randomly for further clinical observation [11]. Twenty-seven crowns were inserted on molars and seven crowns on premolars, whereas 17 teeth had been successfully endodontically treated. Thereby, 11 teeth received fibre posts and five were restored with an adhesive core built-up before crown preparation. One tooth kept its metal post and core.
According to the study protocol, the following criteria had to be fulfilled: healthy patient, vital abutment tooth or a successful root canal treatment at least 6 months before definitive restoration, healthy periodontal conditions of the tooth to be treated and the adjacent teeth (pocket depths ≤3.5 mm, no signs of inflammation). Patients with xerostomia, patients with temporomandibular disorders, and pregnant women were excluded from the study. The requirements of Helsinki Declaration were observed, and the patients gave their signed informed consent. The study was approved by the ethics committee of the University of Leipzig (no. 103–2006).
Fourteen crowns were chair-side manufactured in a private dental practice, and 27 crowns were done in the Department of Prosthodontics and Materials Science of the University of Leipzig. The crown preparation according to the study protocol required the following: a shoulder preparation or a distinct chamfer of 1.0 mm width as finish line and a minimum ceramic thickness in the cusp area and at the fissure line of at least 2.0 and 1.5 mm, respectively. Optical impressions were done with Cerec 3 units (Sirona, Salzburg, Austria), and the crowns were fabricated in the Articulation mode (Software version 2.9). The restorations were milled (Cerec 3 milling unit, Sirona) from a lithium disilicate block (e.max CAD LT, Ivoclar Vivadent). Within the metasilicate status, all crowns were evaluated clinically and corrected in proximal, internal, and occlusal fit, if necessary. Afterwards, staining and glazing (IPS e.max CAD Crystall./Glaze Paste, Ivoclar Vivadent) on the blue surface were followed by crystallization and stain/glaze firing in one step (Programat CS, Ivoclar Vivadent). This was associated with a shrinkage of 0.2% and a transition from a bluish to a tooth-coloured restoration. Before adhesive cementation, the intaglio surface of the crowns was etched with hydrofluoric acid (IPS Empress etch, Ivoclar Vivadent) for 20 s, and a silane coupling agent was applied for 60 s (Monobond S, Ivoclar Vivadent). The tooth surface was cleaned mechanically with pumice and hand instruments. The restorations were inserted adhesively with the dual cure self-adhesive resin cement (Multilink Sprint, Ivoclar Vivadent). More detailed information are available in the 4-year publication [12].
Within the prospective clinical trial, the crowns were examined at a baseline-, 6, 12, 24, 36, 48, 60 and 72-month recall according to the modified US Public Health Service (USPHS) criteria by two independent examiners. In case of discrepancies of the ratings, the differences were subsequently discussed and an agreement was found. The examiners were not involved in the treatment procedures.
The primary outcome variables were the failure-free rate and the complication-free rate. All events were subdivided into biological and technical complications. The biological ones were caries below the crown margin, abutment fracture and endodontic interventions. The technical complications comprised crown fracture, loss of retention and chipping of the ceramic. Failures were defined as events that led to the refabrication of the crown or the extraction of the abutment tooth. Statistics including Kaplan-Meier analysis were done (IBM SPSS Statistics 22, IBM, Ehningen, Germany), and the level of significance was set to p < 0.05.
Results
At the 6-year re-examination (mean observation time 73.2 months; SD ± 1.7 months; min. 70.1 months; max. 78.3 months), 25 out of 34 crowns were available. The mean patients’ age was 52.2 years (SD ± 12.3 years; min. 32.9 years; max. 79.9 years; 68% female). Six patients were lost during the 6-year recall program: two patients had died and three patients had moved. One patient refused to take part in the examination again. This patient had a loss of retention of the lithium disilicate crown after 2.9 years, which could be recemented. It was rated as a complication and included in the study group again. Three crowns could not be examined during the 72-month recall, since they had shown severe failure within the observation period. The following parameters were evaluated according to the modified USPHS criteria: surface, colour, adhesive gap, tooth and crown integrity, proximal contact, endodontic complication, occlusion, complaints and compliance. All results from baseline up to 6 years are shown explicitly in Tables 1 and 2. Except of two crowns with endodontic complications, all crowns scored Alfa or Bravo after 72 months. For each USPHS criterion, the change of scores between baseline and 6-year recall were statistically analysed. Significant differences were found for the criteria surface (p = 0.007, Wilcoxon signed-rank test) as well as for the complaints (p = 0.008, Wilcoxon signed-rank test).
Biological complications
One abutment tooth received endodontic treatment after 1.1 years, whereby the crown could be removed without any damage and reinserted after a root canal treatment. Another tooth did not react on a sensitivity test at baseline. The patient was observed until the 72-month recall without any need for endodontic treatment, but the sensitivity was still uncertain. A dental x-ray after 72 months showed no apical radiolucency. The tooth was included as endodontic complication in the survival statistics. There were two severe biological complications after 6 years: one tooth needed to be extracted because of an apical infection and another tooth had an abutment fracture caused by caries. Thus, the crown could not be reinserted again. Both events were regarded as a failure.
One patient showed different events of caries near the crown margin for the vestibular side (24 months) as well as for the oral side (36, 48, 60 months). After 48 months, another patient had caries below the crown margin. Each caries was removed and an adhesive composite filling was applied.
Technical complications
One molar crown showed loss of retention after 2 years. The abutment was caries free and the restoration could be recemented. After the removal of residual cement, the crown was etched for 20 s with hydrofluoric acid, and after silane application, it was inserted with a self-adhesive resin cement (RelyX Unicem, 3M Espe, Seefeld, Germany). This was regarded as a complication. After 2.8 years, a fracture of a lithium disilicate crown occurred to another molar. This was regarded as a failure.
Fifty percent of all complications appeared in endodontically treated teeth. Within all complications, a percentage of 25% occurred in crowns inserted in a private practice. For more detail, see Table 3.
Kaplan-Meier analysis
The failure-free rate after 6 years was 87.6%. The complication-free rate after 6 years was 70.1% considering all biological and technical events. The Kaplan-Meier analysis for the failure-free and the complication-free rate are represented in Fig. 1.
The cumulative survival rates for crowns with positive sensitivity at insertion were 69.2% and 70.0% for endodontically treated abutment teeth. The relative complication rate for loss of abutment tooth vitality (n = 2) was 8.2%.
Within the setting group, the estimated survival was 83.3% for private practice and 57.1% for university. No significant differences have been detected (p = 0.214, log rank test) (Table 3).
Discussion
The failure-free rate for monolithic lithium disilicate crowns in this prospective clinical study was 87.6% and the complication-free rate was 70.1% after 6 years. During the last 24 months, two new events occurred. Both of them were due to severe biological complications.
According to the authors’ knowledge, only two prospective studies are available that refer to the clinical performance of chair-side fabricated lithium disilicate crowns [8, 9]. Both describe the results of a 2-year observation period. Because of the lack of publications with resembling procedures and a similar monitoring time, we compared our data with studies of other designs, fabrication techniques or materials.
A retrospective study monitoring the survival of monolithic lithium disilicate crowns reported a failure-free rate of 99.1% in up to 4 years [13]. The data of a laboratory database system tracked the number of returned restorations in need for a remake of the crowns. Therefore, it is likely that some biological or technical events were missed.
A prospective study which monitored 121 veneered lithium disilicate crowns placed predominantly on anterior teeth reported a failure-free rate of 87.1% in up to 9 years [14]. Another prospective study on 104 laboratory-fabricated layered lithium disilicate crowns placed in anterior and posterior teeth indicated a survival rate of 94.8% after 8 years. The most reported complication was chipping by 3.3% [15]. Therefore, chair-side manufactured monolithic crowns milled from lithium disilicate are similarly successful in the medium run.
In our study, the relative complication rate for loss of abutment tooth vitality (8.2%) was comparatively high when compared to the 5-year results for other materials [16]. This might be due to the fact that one crown, which did not react on the sensitivity test at recall, was regarded as a complication. If endodontic complications were counted as crowns in need of endodontic treatment, only one crown would have been taken into account. Therefore, the relative complication rate decreases to 4.5%, which is competitive to the 5-year results of feldspathic/silica-based ceramic crowns. On the other hand, the occlusal tooth substance removal according to the preparation design of our study was relatively high when applying 2.0-mm ceramic thickness in the cusp area. This might have led to a higher risk of endodontic problems. Recent in vitro studies indicate that a minimum wall thickness of 1.0–1.5 mm for lithium disilicate crowns is conceivable [17, 18] and might reduce the risk of endodontic complications.
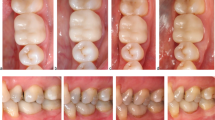
The ranking of the USPHS criteria in our study compared to our previous study showed an increase of the rating Bravo for compliance and complaints. One patient commented that the observation period was too long, and another patient complained about problems with food retention. In this case, the proximal contact was found to be a little too weak (Alpha 2, distance 50 μm). The patient refused any treatment. In the 4-year observation period, two restorations were ranked Delta for the adhesive gap due to caries below the crown margin. After the removal of the caries, an adhesive composite filling was done. At the 6-year recall, the marginal integrity was sufficient. The surface and the colour of all evaluated posterior crowns received only Alpha scores, which is competitive to the medium-term results of veneered lithium disilicate crowns [14, 19]. Exemplarily, Fig. 2 shows the clinical pictures of one lithium disilicate crown (FDI 36) at baseline and 72-month recall.
Regarding the changes of the USPHS scores from baseline to 6-year examination, statistically significant differences for the criterion surface (Alpha 1 to Alpha 2) might indicate wear of the lithium disilicate crowns. Recently, in vitro studies confirmed the wear values for lithium disilicate close to human enamel [20–22].
The different settings of manufacturing facilitate more general clinical results. The patients were chosen consecutively. A higher complication rate was found for crowns inserted at a university rather than at a private practice. This effect might be due to chance in the subgroups and is not statistically significant.
All in all, these results confirm that chair-side manufactured monolithic lithium disilicate crowns can be used with no aesthetic shortcomings in the posterior region. This fabrication technique provides stable crowns with no risk of chipping or fracture of the veneering material in a simple and fast way.
Strengths of this study are the prospective character, the assessment by independent examiners, and the inclusion of patients in a general dental practice setting.
However, there are some limitations, too. All crowns in this study were placed adhesively. Thus, the results cannot be generalized to a conventional cementation method. Nonetheless, studies using adhesive as well as conventional cementation for lithium disilicate crowns could not find any difference regarding the survival [15]. Furthermore, the minimum ceramic thickness suggested by the manufacturer may not be met in all cases as shown by the examination of the fractured crown [12]. The real occlusal thickness after the adjustment procedure has not been recorded.
Conclusion
Within the limitations of this prospective clinical study, monolithic lithium disilicate crowns (e.max CAD LT, Ivoclar Vivadent) had a 6-year survival rate of 87.6%. This is similar to those of other all-ceramic restorations. The use of monolithic restorations in the posterior area provides no esthetical shortcomings. In contrast to veneered crowns, no chipping occurs. The chair-side CAD/CAM fabrication of posterior single crowns with monolithic lithium disilicate can be recommended for medium-term use.
References
Wiedhahn K (2007) From blue to white: new high-strength material for Cerec—IPS e.max CAD LT. Int J Comput Dent 10(1):79–91
Beuer F, Schweiger J, Edelhoff D (2008) Digital dentistry: an overview of recent developments for CAD/CAM generated restorations. Br Dent J 204(9):505–511. doi:10.1038/sj.bdj.2008.350
Baroudi K, Ibraheem SN (2015) Assessment of chair-side computer-aided design and computer-aided manufacturing restorations: a review of the literature. J Int Oral Health 7(4):96–104
Sailer I, Oendra, Hernandez AE, Stawarczyk B et al (2012) The effects of desensitizing resin, resin sealing, and provisional cement on the bond strength of dentin luted with self-adhesive and conventional resincements. J Prosthet Dent 107(4):252–260. doi:10.1016/S0022-3913(12)60070-5
Poticny DJ, Klim J (2010) CAD/CAM in-office technology: innovations after 25 years for predictable, esthetic outcomes. J Am Dent Assoc 141(Suppl 2):5S–59
Reich S (2015) Tooth-colored CAD/CAM monolithic restorations. Int J Comput Dent 18(2):131–146
Otto T, Mormann WH (2015) Clinical performance of chairside CAD/CAM feldspathic ceramic posterior shoulder crowns and endocrowns up to 12 years. Int J Comput Dent 18(2):147–161
Seydler B, Schmitter M (2015) Clinical performance of two different CAD/CAM-fabricated ceramic crowns: 2-year results. J Prosthet Dent 114(2):212–216. doi:10.1016/j.prosdent.2015.02.016
Fasbinder DJ, Dennison JB, Heys D et al (2010) A clinical evaluation of chairside lithium disilicate CAD/CAM crowns: a two-year report. J Am Dent Assoc 141(Suppl 2):10S–114
Kern M, Sasse M, Wolfart S (2012) Ten-year outcome of three-unit fixed dental prostheses made from monolithic lithium disilicate ceramic. J Am Dent Assoc 143(3):234–240
Hickel R, Roulet J, Bayne S et al (2007) Recommendations for conducting controlled clinical studies of dental restorative materials. Clin Oral Invest 11(1):5–33. doi:10.1007/s00784-006-0095-7
Reich S, Schierz O (2013) Chair-side generated posterior lithium disilicate crowns after 4 years. Clin Oral Investig 17(7):1765–1772. doi:10.1007/s00784-012-0868-0
Sulaiman TA, Delgado AJ, Donovan TE (2015) Survival rate of lithium disilicate restorations at 4 years: a retrospective study. J Prosthet Dent. doi:10.1016/j.prosdent.2015.04.011
Toman M, Toksavul S (2015) Clinical evaluation of 121 lithium disilicate all-ceramic crowns up to 9 years. Quintessence Int 46(3):189–197. doi:10.3290/j.qi.a33267
Gehrt M, Wolfart S, Rafai N et al (2013) Clinical results of lithium-disilicate crowns after up to 9 years of service. Clin Oral Investig 17(1):275–284. doi:10.1007/s00784-012-0700-x
Sailer I, Makarov NA, Thoma DS et al (2015) All-ceramic or metal-ceramic tooth-supported fixed dental prostheses (FDPs)? A systematic review of the survival and complication rates. Part I: single crowns (SCs). Dent Mater 31(6):603–623. doi:10.1016/j.dental.2015.02.011
Seydler B, Rues S, Muller D et al (2014) In vitro fracture load of monolithic lithium disilicate ceramic molar crowns with different wall thicknesses. Clin Oral Investig 18(4):1165–1171. doi:10.1007/s00784-013-1062-8
Bindl A, Luthy H, Mormann WH (2006) Strength and fracture pattern of monolithic CAD/CAM-generated posterior crowns. Dent Mater 22(1):29–36. doi:10.1016/j.dental.2005.02.007
Valenti M, Valenti A (2009) Retrospective survival analysis of 261 lithium disilicate crowns in a private general practice. Quintessence Int 40(7):573–579
Nakashima J, Taira Y, Sawase T (2016) In vitro wear of four ceramic materials and human enamel on enamel antagonist. Eur J Oral Sci 124(3):295–300. doi:10.1111/eos.12272
Stawarczyk B, Liebermann A, Eichberger M et al (2015) Evaluation of mechanical and optical behavior of current esthetic dental restorative CAD/CAM composites. J Mech Behav Biomed Mater 55:1–11. doi:10.1016/j.jmbbm.2015.10.004
Mormann WH, Stawarczyk B, Ender A et al (2013) Wear characteristics of current aesthetic dental restorative CAD/CAM materials: two-body wear, gloss retention, roughness and Martens hardness. J Mech Behav Biomed Mater 20:113–125. doi:10.1016/j.jmbbm.2013.01.003
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author AR declares no conflict of interest. The author SR has held oral presentations receiving a separate and appropriate honorarium. SR and OS held courses that were supported with material by Ivoclar Vivadent.
Funding
This study was supported by Ivoclar Vivadent, Schaan, Principality of Liechtenstein.
Ethical approval
The study was approved by the ethics committee of the University of Leipzig (no. 103-2006).
Informed consent
The requirements of the Helsinki Declaration were observed, and the patients gave their signed informed consent.
Rights and permissions
About this article
Cite this article
Rauch, A., Reich, S. & Schierz, O. Chair-side generated posterior monolithic lithium disilicate crowns: clinical survival after 6 years. Clin Oral Invest 21, 2083–2089 (2017). https://doi.org/10.1007/s00784-016-1998-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-016-1998-6