Abstract
Objectives
The aim of this study is to investigate the performance and fracture resistance of different CAD/CAM ceramic and composite materials as implant- or tooth-supported single crowns with respect to the clinical procedure (screwed/bonded restoration).
Materials and methods
One hundred twenty crowns were fabricated on implants or human molar teeth simulating (a) chairside procedure ([CHAIR] implant crown bonded to abutment), (b) labside procedure ([LAB] abutment and implant crown bonded in laboratory, screwed chairside), and (c) reference ([TOOTH] crowns luted on human teeth). Four materials were investigated: ZLS (zirconia-reinforced lithium silicate ceramic; Celtra Duo, Degudent: polished (P)/crystallized (C)), RB (resin-based composite; Cerasmart, GC), and RIC (resin-infiltrated ceramic; Enamic, Vita-Zahnfabrik). LiS (lithiumdisilicate; Emax CAD, Ivoclar-Vivadent) served as reference. Combined thermal cycling and mechanical loading (TCML) was performed simulating a 5-year clinical situation. Fracture force was determined. Data were statistically analyzed (Kolmogorov-Smirnov test, one-way ANOVA; post hoc Bonferroni, α = 0.05).
Results
One crown of ZLS_C[LAB] (1,200,000 cycles) and RB[CHAIR] (890 cycles) failed during TCML. Fracture values varied between 977.7 N(RB) and 3070.4 N(LiS)[CHAIR], 1130.6 N(RB) and 2998.1 N(LiS)[LAB], and 1802.4 N(ZLS) and 2664.3 N(LiS)[TOOTH]. Significantly (p < 0.003) different forces were found between the materials in all three groups. ZLS_C, RIC, and RB showed significantly (p < 0.014) different values for the individual groups.
Conclusions
Partly ceramic and resin-based materials performed differently on implant or tooth abutments. The insertion of a screw channel reduced the stability for individual crown materials. Insertion of the screw channel should be performed carefully.
Clinical relevance
All restorations were in a range where clinical application seems not restricted, but insertion of a screw channel might reduce stability of individual materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The combination of intra-oral scanning with CAD/CAM enables an innovative work flow comprising the individual properties of a variety of CAD/CAM materials. CAM machinable materials are available as composite blocks, resin-infiltrated ceramic blocks, or different ceramic blocks (feldspar, lithiumdisilicate, zirconia-reinforced lithium silicate (ZLS), zirconia), in most cases for the use as single tooth or implant restorations. While the loading on tooth-supported crowns is buffered by the periodontal ligament, loading on implant-supported crowns is directly transferred to the bone without resilience. Therefore, the application of brittle materials on human teeth is considered unproblematic, whereas the use of these materials on implants may cause a number of mechanical in vivo complications like fracture or chipping [1]. For this reason, materials with different moduli of elasticity may be alternatively preferred for implant restorations due to a high stability (zirconia) or damping effects (resin-based materials) [2, 3].
Implants with preformed or custom abutments are state of the art for replacing missing teeth. But the success of cemented or bonded abutments may be limited by inflammatory effects caused by residual cement remaining especially in inaccessible areas. With the use of screwed bonding abutments, the situation is resolved, because bonding areas become distant from the sulcus. Alternatives may be bonding abutments with crowns that are laboratory-bonded in advance. These crowns enable a reversible and easy access to the screw for retightening or replacement. Titanium base and implant platform are synchronized, guaranteeing good fit and force-fit connections, excluding fitting inaccuracies, like reported for patient-specific CAD/CAM fabricated ceramic abutments or crowns [4–6]. With this design, bonding can be ideally performed under laboratory conditions (dry conditions, surface activation, and optimized polymerization) and bonding areas are located in iso- or supra-gingival regions: this might improve bonding durability and reduce allergy or inflammation effects. Certainly, the screw channel might reduce the stability of the crowns [7–10].
Although already clinically used, there is only little scientific information and even less clinical data that show the long-term applicability of implant-supported restorations [11]. Here, laboratory tests such as thermal cycling and mechanical loading may allow a first prediction of long-term mechanical performance and resistance against hydrolytic effects. These test methods might stimulate fatigue failures and provide detailed information of possibly appearing deficiencies. During oral application, aging and deterioration effects might occur, thus reducing strength and fracture resistance. In these cases, a subsequent static fracture test may help locate initiated weak points of the restorations. The hypothesis of this investigation was that posterior crowns show different in vitro performance and fracture resistance when (a) bonded to abutment chairside (group CHAIR), (b) bonded to abutments in the laboratory and screwed on implant chairside (group LAB), or (c) bonded to human teeth (group TOOTH).
Materials and methods
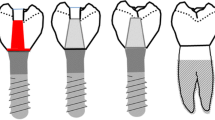
A total of 120 molar crowns were milled of five different CAD/CAM materials (n = 8 per material per group) (Table 1). Materials were resin-based composite, resin-infiltrated ceramic, lithiumdisilicate ceramic (reference), and ZLS. ZLS was used in two different variations: (P) polished after milling and (C) crystallized. The crystallized application is expected to show higher strength and stability. Three groups were designed to simulate the following clinical procedures (Fig. 1):
Group “CHAIR” chairside procedure: in a first step, implant and abutment were screwed together and then the implant crown was bonded to the abutment.
Group “LAB” labside procedure: a screw channel was milled into the crown (diamond red, 1.5 mm, water cooling), and the abutment and implant crown were bonded in the dental laboratory. Abutment and implant crown as one unit were screwed on the implant. The screw channel was restored with composite (Filtek Supreme; Elipar Trilight 40 s, 3 M Espe) after appropriate pre-treatment of the screw channel (Table 1).
Group “TOOTH” reference: crowns were luted on human teeth.
For simulating the posterior implant situation (groups CHAIR and LAB) replacing tooth, 46 10 × 8 implant-abutment dummies (Straumann, d = 4.1 mm, H = 12 mm, abutment length 6 mm, 6°) were vertically positioned in resin blocks (Palapress Vario, Heraeus-Kulzer, D).
For group “TOOTH,” extracted human molars (n = 8 per group) were prepared according to ceramic guidelines with a circular and occlusal anatomical reduction of about 1.5 mm and a preparation angle of ∼4°. The finishing line resulted in an approximately 1 mm deep circular shoulder with rounded inner angles at an isogingival height of the tooth cervix. Teeth were positioned in resin blocks (Palapress Vario, Heraeus-Kulzer, D) and resilience of the human periodontium was simulated by coating the roots of the teeth with a 1-mm polyether layer (Impregum, 3 M Espe, D). For achieving a constant layer, the roots were dipped in a wax bath, which was replaced by polyether in a second fabrication process, as described [12]. Loaded with 50 N, the layer allows a reproducible maximum mobility of the single tooth in axial and vertical direction of 0.1 mm [12, 13], which is within the physiological range of periodontal compression (0.03 to 0.15 mm) [14].
Implant situation and prepared teeth were digitalized (Cerec Omnicam, Sirona, D) and full-contour molar crowns (design youth) with identical outer dimensions were milled (Cerec, MCXL, Sirona, D, normal speed) at spacer settings of 100 μm. The occlusal and circular wall thickness of the crowns depended on the abutment, but in all cases was at least 1.5 mm (occlusal)/0.8 mm (circular). Abutments were pre-treated (110 μm Al2O3, 1.5 bar) and teeth were conditioned (ED Primer II; Panavia F 2.0, Kuraray, J; Elipar Trilight, 3 M Espe, USA; 3 × 60s). Inner sides of the crowns were treated as recommended by the individual manufacturers (Table 1). All bonding was done adhesively (Clearfil Ceramic Primer: 60s, Panavia F 2.0, Kuraray, J; Elipar Trilight, 3 M Espe, USA; 3 × 60s).
Thermal cycling and mechanical loading (TC 2 × 3000 cycles between 5 and 55 °C, distilled water, ML 50 N for 1.2 × 106 cycles; f = 1.6 Hz; mouth opening, 2 mm) with online failure-control were performed to stimulate and control fatigue failures. Twelve-millimeter steatite balls (CeramTec, Plochingen, D) served as standardized antagonists and were positioned in three point occlusal contact situation. Appearing failures were documented and failed specimens were excluded from the further simulation process. Parameters are based on literature data on zirconia and ceramic restorations, expressing that chewing simulations using these parameters might simulate 5 years of oral service [15, 16]. Restorations which failed during TCML were investigated in detail with scanning electron microscopy (SEM, Quanta FEG 400, FEI, USA). All restorations that survived were loaded to fracture (1446, Zwick, v = 1 mm/min). In analogy to chewing simulation, the load was occlusally applied with a steel sphere (d = 12 mm). A tin foil (0.25 mm, Dentaurum, D) between the crown and sphere prevented force peaks. All systems were optically examined after fracture testing, and the failure mode was documented. Calculations and statistical analysis were carried out using SPSS 22.0 for Windows (SPSS Inc., Chicago, IL, USA). Power calculation (G*Power 3.1.3, Kiel, D) provided an estimated power of >91 % using eight specimens per group. Distribution of the data was controlled with Kolmogorov-Smirnov test. Means and standard deviations were calculated and analyzed using one-way analysis of variance (ANOVA) and the Bonferroni test for post hoc analysis. The level of significance was set to α = 0.05.
Results
All crowns showed comparable wear traces on the occlusal contact areas after TCML. One crown of ZLS_C [LAB] (1,200,000 cycles) and RB [CHAIR] (890 cycles) failed during TCML. Failure pattern of the RB crown was due to a defect on the inner occlusal side of the crown (Fig. 2). SEM analysis indicated that the ZLS_C crown fractured from the occlusal side, due to defects which were initiated during the manual insertion of the screw channel (Fig. 3).
After TCML, fracture values in the three groups varied between 977.7 N (RB) and 3070.4 N (LiS) [CHAIR], 1130.6 N (RB) and 2998.1 N (LiS) [LAB], and 1802.4 N (ZLS) and 2664.3 N (LiS) [TOOTH]. Significantly (p < 0.003) different fracture forces were found between the materials in all three groups. In all groups, highest values were found for the reference LiS. The materials ZLS_C, RIC, and RB showed significantly (p < 0.014) different fracture values for the individual groups. Only ZLS_P and LiS crowns provided no differences between the individual groups. ZLS_P and LiS showed similar distribution of the fracture values comparing [CHAIR] and [LAB] groups (Table 2). Fracture of the crowns was characterized by a straight fracture, which followed the fissure line from mesial to distal (type A, Fig. 4), a curved fracture from mesial to oral (type B), or a chipping of an individual cusp (type C). All three fracture types were found for the tooth groups. Both implant groups provided only types A and B with minor different shares, indicating no differences between groups CHAIR or LAB (details see Table 3). For material RB, a different fracture performance could be found in the TOOTH group, and RB and LiS showed differences in the LAB group. In all fracture cases, main parts of the crown materials remained on the abutments.
Discussion
The hypothesis of this investigation that posterior crowns show different in vitro performance and fracture resistance when bonded to abutments chairside, screwed on implants chairside or bonded to human teeth was confirmed in parts. The results indicated a material-dependent in vitro performance and fracture force. Only two ceramic materials (ZLS_P, LiS) provided no differences in the in vitro performance and fracture resistance between the three different groups. These data are in agreement with investigations showing that screw-retained and cemented ceramic [7] or metal-ceramic crowns [17] provide no significant different fracture values, although some studies demonstrated higher fracture resistance for cemented metal-ceramic crowns [8, 10]. The influence of the type of ceramic on the fracture resistance of implant supported crowns was confirmed earlier [18]. Strong differences between ceramic and composite CAD/CAM crowns were shown [19], but failure patterns/analysis indicated contact-induced cracks in all materials. For composite crowns, a longer endurance limit for catastrophic failure was found in comparison to ceramic crowns [19].
Only one crown of the groups ZLS_C and RB on implants failed during TCML, but no crowns on human teeth. This was in contrast to the expectations that screwed implant-based systems might provide a higher number of chippings [9]. The failure was apparently not caused by weakening of the crown by the screw channel, as fractures were found in both groups (LAB and CHAIR). Failure analysis identified a fracture of the RB crown due to an occlusal defect in the crown, which also explained the early failure after <1.000 loading cycles. The defect seemed not to be material-dependent or caused by fatigue/aging but may be a result of the milling process or local overloading due to an ignored early contact. The ZLS_C crown showed a crack from the occlusal side, due to defects which might be initiated during the manual insertion of the screw channel. Every adaptation might cause sub-critical defects or cracks, which can cause failure of the restoration during loading. Preformed fittings like provided, for example, for bonding-bases might be preferred.
There was a trend to a correlation between in vitro performance and fracture results and the individual material properties: as expected materials with lower modulus of elasticity and flexural strength (RB, RIC) provided lower fracture resistance. Previous studies have shown that resin-based materials and composites have higher shock absorbing capacity than ceramics [20, 21]. However, a protective effect due to higher damping could not be established in the present study as most materials provided similar TCML performance. This was partly in agreement with findings by Kok et al. [3], but they found higher fracture values for materials with a lower modulus of elasticity. For resin-based materials with low modulus of elasticity a recent clinical study found high numbers of debonding between resin nano ceramic crowns and zirconia abutments, which were supposed to be a result of stress concentrations in the adhesive layer [22]. In the present study, LiS, the material with the highest flexural strength and modulus of elasticity, showed the highest fracture resistance, which can be confirmed by the results of previous studies [3, 23]. Higher flexural strength of crystallized ZLS (370 MPa) compared to polished ZLS (210 MPa) was reflected in higher fracture forces in two situations (CHAIR, TOOTH). Nevertheless, fracture resistance of ZLS_C was still inferior to LiS, which is in accordance to a study by Weyhrauch [24]. In contrast, another study found comparable resistance against TCML and comparable high fracture resistance for crystallized ZLS and LiS using human teeth in a similar experimental set-up [25]. Considering that the found fracture values exceeded maximum chewing forces in the posterior region, which are reported to reach up to 900 N, all groups have the potential to withstand physiological force peaks [26], but fracture stability of the crowns even may exceed the clinical stability of screwed connections.
Different fracture values for the implant restorations were found, showing lower values for four materials in the LAB situation. Only one material showed a much broader distribution of the values with inserted screw channel. These data might again indicate some pre-damaging due to the drilling of the screw channel. The drilling was done carefully with water cooling under identical conditions but regardless may influence the individual materials in a different manner. With a subsequent crystallization (LiS; ZLS_C), flexural strength increases and induced flaws are expected to be reduced, which may lead to a higher stability and resistance to fracture. Preformed screw-holes and fittings, as partly already available on the market, or standardized CAD/CAM drilling might be preferred in order to reduce possible failure initiation. In this context, it should be mentioned that none of the composite seals was damaged during TCML.
Based on the assumption of an altered loading situation with increased chewing forces by rigid implant bearing, a higher stability of the materials is generally required for implant restorations [1, 27]. Astonishingly and in contrast to these expectations [2, 3, 28], the modulus of elasticity of the abutment (comparison: groups CHAIR and TOOTH) showed an influence on the fracture results only for group RB: fracture values were about 50 % lower for the RB_CHAIR group. The rigid attaching of the abutments in contrast to the movable anchoring of the crowns with a simulated periodontium had no influence on the fracture resistance in the other groups. The results are partly in contrast to studies showing satisfying clinical results for all implant bonded restorations [1], but lower success rates for implant-supported restorations in comparison to tooth-supported FPDs [29, 30]. Differences between in vitro and in vivo data might be attributed to the lack of a tactile sensitivity [31–33] in the in vitro test. Clinical data of CAD/CAM-fabricated crowns after 3 years showed inferior wear resistance and decementation on cast posts for composite-based crowns [34].
Although crowns of the TOOTH group were individual in shape and geometry, similar fracture values were found between TOOTH and CHAIR groups. Results for ceramic materials were in the range of other investigations [25]. Higher standard variations of the fracture forces were expected in group TOOTH: fracture forces may have been influenced by the individual preparation and crown design with its occlusal variations and individual thickness. All teeth provided approximately similar occlusal design, but already slightly steeper cusps are expected to show higher wear and lower occlusal load ability. Influences of the different geometry, caused by a smaller occlusal support in case of the implanted supported crowns, or the thicker crowns in case of the implant group were not evident but may have influenced the results. Here, further tests are required for investigating these influences.
The study design has to be discussed critically: the influence of possibly clinically occurring screwing joints was completely eliminated by the one-piece implant dummy design, trying to focus on the investigation of the crown. Clinically, screwed connections often represent the weakest part of the implant-abutment-crown combination [35, 36], but any failures that may be influenced by screws were not considered in this study. Though, it was shown that defects induced by insertion of the screw channel have led to failure of the crown. A weakening effect of the screw channel may be observed for low-strength crown materials. However, the present results have shown that the mechanical stability of all crown materials investigated was high enough not to be influenced by the presence of a screw channel.
The artificial periodontal mobility simulates the clinical situation only in rudiments and of course the design allowed no feedback and control of the applied loading forces as under clinical conditions [31–33]. It should be kept in mind that the contact situation varies during TCML due to wear effects or, for the tooth group, due to the flexible bearing, which may cause a shift of the contacts under loading. However, no different failure patterns, wear traces, or fatigue effects were found between the groups with the elaborate failure analysis after TCML. A further limiting factor of the significance of this study may be that steatite antagonists and not human tooth antagonists were used for TCML. Although steatite spheres guaranteed a standardized antagonistic situation, they might have caused different wear and damage. It might be argued that the load distribution was changed with increasing destruction and flattening of the loading points during TCML. However, this is a phenomenon that naturally takes place in clinical service, too. The resistance between specimen and antagonist increases with increasing surface roughness by wear and a higher number of flaws further the development of cracks. In contrast, for fracture testing, it is important that the fracture results are not influenced by damage or deformation of the antagonist. Therefore, steel spheres with identical diameters were applied. Both the use of steatite and steel spheres as antagonists is well documented in literature [37, 38].
Besides mechanical performance, plaque accumulation and soft tissue response to the different CAD/CAM materials should be considered. In general, higher plaque accumulation and inflammation effects have been reported for resin-based materials and composites than for ceramics, further influenced by the surface roughness [39, 40]. Further investigations on the biological impact of new CAD/CAM materials are suggested.
Conclusion
Ceramic and resin-based materials partly performed differently on implant or tooth. For individual materials, the insertion of a screw channel reduced the stability of the crown, but all restorations were in a range where clinical application seems not restricted. Insertion of the screw channel should be performed carefully.
References
Pjetursson BE, Karoussis I, Bürgin W, Brägger U, Lang NP (2005) Patients’ satisfaction following implant therapy. A 10-year prospective cohort study. Clin Oral Implants Res 16:185–193
Magne P, Silva M, Oderich E, Boff LL, Enciso R (2013) Damping behavior of implant-supported restorations. Clin Oral Implants Res 24:143–148
de Kok P, Kleverlaan CJ, de Jager N, Kuijs R, Feilzer AJ (2015) Mechanical performance of implant-supported posterior crowns. J Prosthet Dent 114:59–66
Alikhasi M, Monzavi A, Bassir SH, Naini RB, Khosronedjad N, Keshavarz S (2013) A comparison of precision of fit, rotational freedom, and torque loss with copy-milled zirconia and prefabricated titanium abutments. Int J Oral Maxillofac Implants 28:996–1002
Gehrke P, Johannson D, Fischer C, Stawarczyk B, Beuer F (2015) In vitro fatigue and fracture resistance of one- and two-piece CAD/CAM zirconia implant abutments. Int J Oral Maxillofac Implants 30:546–554
Baldassarri M, Hjerppe J, Romeo D, Fickl S, Van Thompson P, Stappert CFJ (2012) Marginal accuracy of three implant-ceramic abutment configurations. Int J Oral Maxillofac Implants 27:537–543
Zarone F, Sorrentino R, Traini T, Di lorio D, Caputi S (2007) Fracture resistance of implant-supported screw- versus cement-retained porcelain fused to metal single crowns: SEM fractographic analysis. Dent Mater 23:296–301
Al-Omari WM, Shadid R, Abu-Naba’a L, El Masoud B (2010) Porcelain fracture resistance of screw-retained, cement-retained, and screw-cement-retained implant-supported metal ceramic posterior crowns. J Prosthodont 19:263–273
Karl M, Graef F, Taylor TD, Heckmann SM (2007) In vitro effect of load cycling on metal-ceramic cement- and screw-retained implant restorations. J Prosthet Dent 97:137–140
Torrado E, Ercoli C, Al Mardini M, Graser GN, Tallents RH, Cordaro L (2004) A comparison of the porcelain fracture resistance of screw-retained and cement-retained implant-supported metal-ceramic crowns. J Prosthet Dent 91:532–537
Patzelt SBM, Spies BC, Kohal RJ (2015) CAD/CAM-fabricated implant-supported restorations: a systematic review. Clin Oral Implants Res 26(Suppl 11):77–85
Rosentritt M, Behr M, Scharnagl P, Handel G, Kolbeck C (2011) Influence of resilient support of abutment teeth on fracture resistance of all-ceramic fixed partial dentures: an in vitro study. Int J Prosthodont 24:465–468
Rosentritt M, Kolbeck C, Handel G, Schneider-Feyrer S, Behr M (2011) Influence of the fabrication process on the in vitro performance of fixed dental prostheses with zirconia substructures. Clin Oral Investig 15:1007–1012
Parfitt GJ (1960) Measurement of the physiological mobility of individual teeth in an axial direction. J Dent Res 39:608–618
Rosentritt M, Behr M, Van der Zel JM, Feilzer AJ (2009) Approach for valuating the influence of laboratory simulation. Dent Mater 25:348–352
Rosentritt M, Siavikis G, Behr M, Kolbeck C, Handel G (2008) Approach for valuating the significance of laboratory simulation. J Dent 36:1048–1053
Derafshi R, Farzin M, Taghva M, Heidary H, Atashkar B (2015) The effects of new design of access hole on porcelain fracture resistance of implant-supported crowns. J Dent (Shīrāz) 16:61–67
Stona D, Burnett LH, Mota EG, Spohr AM (2015) Fracture resistance of computer-aided design and computer-aided manufacturing ceramic crowns cemented on solid abutments. J Am Dent Assoc 146:501–507
Shembish FA, Tong H, Kaizer M, Janal MN, Van Thompson P, Opdam NJ, Zhang Y (2016) Fatigue resistance of CAD/CAM resin composite molar crowns. Dent Mater. doi:10.1016/j.dental.2015.12.005
Menini M, Conserva E, Tealdo T, Bevilacqua M, Pera F, Signori A, Pera P (2013) Shock absorption capacity of restorative materials for dental implant prostheses: an in vitro study. Int J Prosthodont 26:549–556
Conserva E, Menini M, Tealdo T, Bevilacqua M, Ravera G, Pera F, Pera P (2009) The use of a masticatory robot to analyze the shock absorption capacity of different restorative materials for prosthetic implants: a preliminary report. Int J Prosthodont 22:53–55
Schepke U, Meijer HJ, Vermeulen KM, Raghoebar GM, Cune MS (2015) Clinical bonding of resin nano ceramic restorations to zirconia abutments: a case series within a randomized clinical trial. Clin Implant Dent Relat Res. doi:10.1111/cid.12382
Zesewitz TF, Knauber AW, Northdurft FP (2014) Fracture resistance of a selection of full-contour all-ceramic crowns: an in vitro study. Int J Prosthodont 27:264–266
Weyhrauch M, Igiel C, Scheller H, Weibrich G, Lehmann KM (2016) Fracture strength of monolithic all-ceramic crowns on titanium implant abutments. Int J Oral Maxillofac Implants 31:304–309
Preis V, Behr M, Hahnel S, Rosentritt M (2015) Influence of cementation on in vitro performance, marginal adaptation and fracture resistance of CAD/CAM-fabricated ZLS molar crowns. Dent Mater 31:1363–1369
Varga S, Spalj S, Lapter Varga M, Anic Milosevic S, Mestrovic S, Slaj M (2011) Maximum voluntary molar bite force in subjects with normal occlusion. Eur J Orthod 33:427–433
Romeo E, Lops D, Margutti E, Ghisolfi M, Chiapasco M, Vogel G (2004) Long-term survival and success of oral implants in the treatment of full and partial arches: a 7-year prospective study with the ITI dental implant system. Int J Oral Maxillofac Implants 19:247–259
Bijjargi S, Chowdhary R (2013) Stress dissipation in the bone through various crown materials of dental implant restoration: a 2-D finite element analysis. J Investig Clin Dent 4:172–177
Jung RE, Pjetursson BE, Glauser R, Zembic A, Zwahlen M, Lang NP (2008) A systematic review of the 5-year survival and complication rates of implant-supported single crowns. Clin Oral Implants Res 19:119–130
Baran G, Boberick K, McCool J (2001) Fatigue of restorative materials. Crit Rev Oral Biol Med 12:350–360
Kim Y, Oh T, Misch CE, Wang H (2005) Occlusal considerations in implant therapy: clinical guidelines with biomechanical rationale. Clin Oral Implants Res 16:26–35
Ferrario VF, Sforza C, Zanotti G, Tartaglia GM (2004) Maximal bite forces in healthy young adults as predicted by surface electromyography. J Dent 32:451–457
Earthman JC, Li Y, VanSchoiack LR, Sheets CG, Wu JC (2006) Reconstructive materials and bone tissue engineering in implant dentistry. Dent Clin North Am 50:229–44
Vanoorbeek S, Vandamme K, Lijnen I, Naert I (2010) Computer-aided designed/computer-assisted manufactured composite resin versus ceramic single-tooth restorations: a 3-year clinical study. Int J Prosthodont 23:223–230
Preis V, Kammermeier A, Handel G, Rosentritt M (2016) In vitro performance of two-piece zirconia implant systems for anterior application. Dent Mater 32:765–774
Rosentritt M, Hagemann A, Hahnel S, Behr M, Preis V (2014) In vitro performance of zirconia and titanium implant/abutment systems for anterior application. J Dent 42:1019–1026
Preis V, Behr M, Hahnel S, Handel G, Rosentritt M (2012) In vitro failure and fracture resistance of veneered and full-contour zirconia restorations. J Dent 40:921–928
Rosentritt M, Behr M, Gebhard R, Handel G (2006) Influence of stress simulation parameters on the fracture strength of all-ceramic fixed-partial dentures. Dent Mater 22:176–182
Vrochari AD, Petropoulou A, Chronopoulos V, Polydorou O, Massey W, Hellwig E (2015) Evaluation of surface roughness of ceramic and resin composite material used for conservative indirect restorations, after repolishing by intraoral means. J Prosthodont. doi:10.1111/jopr.12390
Teughels W, van Assche N, Sliepen I, Quirynen M (2006) Effect of material characteristics and/or surface topography on biofilm development. Clin Oral Implants Res 17(Suppl 2):68–81
Acknowledgments
We would like to thank the manufacturers for providing the materials.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
Prof. Dr. Martin Rosentritt declares that he has third-party research projects with all dental companies, including Degudent, D, GC, B, Ivoclar-Vivadent, FL., and Vita Zahnfabrik, D.
Prof. Dr. Sebastian Hahnel declares that he has no conflict of interest.
Dr. Frank Engelhardt declares that he has no conflict of interest.
Prof. Dr. Michael Behr declares that he has no conflict of interest.
Dr. Verena Preis declares that she has no conflict of interest.
Informed consent
For this type of study, formal consent is not required.
Funding
No.
Rights and permissions
About this article
Cite this article
Rosentritt, M., Hahnel, S., Engelhardt, F. et al. In vitro performance and fracture resistance of CAD/CAM-fabricated implant supported molar crowns. Clin Oral Invest 21, 1213–1219 (2017). https://doi.org/10.1007/s00784-016-1898-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-016-1898-9