Abstract
Objectives
The aim of the study was to compare the color change produced by tray-delivered carbamide peroxide [CP] versus hydrogen peroxide products [HP] for at-home bleaching through a systematic review and meta-analysis.
Materials and methods
MEDLINE via PubMeb, Scopus, Web of Science, Latin American and Caribbean Health Sciences Literature database (LILACS), Brazilian Library in Dentistry (BBO), and Cochrane Library and Grey literature were searched without restrictions. The abstracts of the International Association for Dental Research (IADR) and unpublished and ongoing trial registries were also searched. Dissertations and theses were explored using the ProQuest Dissertations and Periodicos Capes Theses databases. We included randomized clinical trials that compared tray-delivered CP versus HP for at-home dental bleaching. The color change in shade guide units (SGU) and ΔE were the primary outcomes, and tooth sensitivity and gingival irritation were the secondary outcomes. The risk of bias tool of the Cochrane Collaboration was used for quality assessment.
Data
After duplicate removal, 1379 articles were identified. However, only eight studies were considered to be at “low” risk of bias in the key domains of the risk bias tool and they were included in the analysis. For ΔE, the standardized mean difference was −0.45 (95 % CI −0.69 to −0.21), which favored tray-delivered CP products (p < 0.001). The color change in ΔSGU (p = 0.70), tooth sensitivity (p = 0.83), and gingival irritation (p = 0.62) were not significantly different between groups.
Conclusions
Tray-delivered CP gels showed a slightly better whitening efficacy than HP-based products in terms of ΔE, but they were similar in terms of ΔSGU. Both whitening systems demonstrated equal level of gingival irritation and tooth sensitivity.
Clinical significance
Tray-delivered CP gels have a slightly better whitening efficacy than HP-based products in terms of ΔE. This should be interpreted with caution as the data of ΔSGU did not show statistical difference between the products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, there is an increasing number of patients requesting treatment for dental discoloration due to recent health-related and esthetic demands [1, 2]. Among the therapies for dental discoloration, vital tooth bleaching is a safe and conservative approach. This procedure can be performed in the office by the dentist using high concentrations of hydrogen peroxide [HP] or can be applied by the patient at-home using low concentrations of carbamide [CP] and hydrogen peroxides [3].
Since the introduction of the at-home bleaching technique [3], successful results have been reported [4–6]. In a recent questionnaire-based survey conducted in the southern of Brazil, at-home bleaching was broadly preferred (78.1 %) for vital tooth bleaching compared with in-office therapy (21.9 %) [7]. This trend seems to occur worldwide. The advantages of the at-home technique include ease of application, reduced chair time and costs, high rates of success [8–11], and safety of materials used [10, 12]. Carbamide peroxide breaks down into HP and urea, with the HP concentration being approximately one third of the original CP percentage. There are many carbamide peroxide bleaching systems on the market with concentrations ranging from 10 to 22 % [13]. Irrespective of the concentration, these bleaching gels are recommended for periods ranging from 8 (overnight) to 2 h daily [6, 13–15].
Even though at-home bleaching is the most frequently recommended treatment, some patients do not adapt to the technique, because they need to wear the bleaching tray for longer periods of time to see effective results in 2 to 3 weeks. These patients might request a method with better comfort that reaches the same bleaching efficacy.
In view of this market need, some companies have introduced low-concentrated strips or tray-delivered HP products for at-home bleaching. They claim that these new systems are as effective as CP in equivalent concentrations with the advantage of requiring less time of use. However, the literature is still controversial as to which product is actually the most effective one [16–18]. Although an earlier systematic review of the literature has already evaluated the effectiveness of at-home bleaching agents [13], this review was published 8 years ago. Additionally, they did not aim to investigate a narrow research question as several comparisons were done, making the clinical decision harder.
Therefore, the aim of this systematic review of the literature was to answer the following focused question: Are there differences in color changes of tray-delivered CP versus HP for at-home dental bleaching in patients of any age group?
Material and methods
Protocol and registration
We registered the study protocol at the PROSPERO database under the number CRD42015008993, and we followed the recommendations of the PRISMA statement for the report of this systematic review [19].
Eligibility criteria
The controlled vocabulary (mesh terms) and free keywords in the search strategy (Table 1) were defined based on the following elements of the PICOS question:
-
1.
Population (P): patients with dental discoloration of any age group
-
2.
Intervention (I): at-home bleaching with HP-based products
-
3.
Comparison (C): at-home bleaching with the tray-delivered CP products
-
4.
Outcome (O): color change (primary outcome); Secondary outcomes (tooth sensitivity risk and gingival irritation) were also assessed
-
5.
Study design (S): randomized clinical trials (RCTs)
A validated filter from the Cochrane Handbook for Systematic reviews of Interventions 5.1.0 (http://handbook.cochrane.org) for RCTs was employed for the PubMed database. The strategy keys of intervention and comparison were combined. The O was not used in the search strategy to maximize the sensitivity over the specificity of the search strategy.
Only RCTs that compared the color change of tray-delivered CP with HP-based products in permanent dentition of patients of any age group were eligible. We included parallel or split-mouth design in clinical human trials (Table 1).
No minimum follow-up was required for inclusion as we were interested in the immediate color change reached by the products. The color change in ΔE or in shade guide units (ΔSGU) was the primary outcome of the study, and the risk of tooth sensitivity and gingival irritation were the secondary outcomes. No restrictions regarding settings (academic university department, dental hospital, primary care, private practice, etc.) were established.
Non-controlled clinical trials, editorial letters, pilot studies, historical reviews, in vitro studies, cohort, and observational and descriptive studies, such as case reports and case series, were excluded. Additionally, RCT studies were excluded if (1) the experimental group was not an at-home HP bleaching product, (2) a control tray-delivered CP was not used, (3) when HP products were compared to placebo gels, and (4) over-the-counter instead of tray-delivered CP was used as control.
Information sources and search
To identify trials to be included for this review, we searched on the electronic databases MEDLINE via PubMeb, Scopus, Web of Science, Latin American and Caribbean Health Sciences Literature database (LILACS), Brazilian Library in Dentistry (BBO), and Cochrane Library (Table 1). We hand-searched the reference lists of all eligible primary studies for additional relevant publications and the related article links of each eligible primary study in the PubMed database. No restrictions were placed on the publication date or language.
The abstracts of the annual conference of the International Association for Dental Research (IADR) and their regional divisions (1990–2014) were also searched, and authors of relevant abstracts were contacted for further information. The grey literature was explored using the database System for Information on Grey literature in Europe (SIGLE). Dissertations and theses were searched using the ProQuest Dissertations and Theses Full Text database as well as the Periódicos Capes Theses database.
To locate unpublished and ongoing trials related to the review question, the following trials registry were also searched: Current Controlled Trials (www.controlled-trials.com), International Clinical trials registry platform (http://apps.who.int/trialsearch/), the ClinicalTrials.gov (www.clinicaltrials.gov), Rebec (www.rebec.gov.br), and EU Clinical Trials Register (https://www.clinicaltrialsregister.eu).
The search strategies defined for the databases described above are listed in Table 1. The search strategy was appropriately modified for each database and performed by two reviewers (I.L.-M. and A.R.) to identify eligible studies. Full-text versions of the papers that appeared to meet the inclusion criteria were retrieved for further assessment and data extraction.
Study selection and data collection process
Initially, the articles were selected by title and abstracts according to the previously described search strategy (Table 1). Articles appearing in more than one database were considered only once. Full reports were also obtained when there was insufficient information in the title and abstract to make a clear decision. Subsequently, full-text articles were acquired and two reviewers (I.L.-M and A.R.) classified those who met the inclusion criteria. We gave a study identification number for each eligible study, combining first author and year of publication. The collection form was pilot tested using a sample of study reports to ensure that the criteria were consistent to the research question.
Data were extracted using customized extraction forms and the following data recorded for each included study:
-
Details of the study including year of publication, author(s), setting, and evaluation criteria for color change
-
Details of study method such as the study design and setting
-
Details of participants including age (mean and range), sex, and number of patients per group
-
Details of the bleaching products used, including concentration and bleaching protocol (bleaching daily time, time of bleaching treatment)
-
Details of the methods used for evaluation of adverse effects such as tooth sensitivity and gingival irritation
-
Details of the outcomes including tooth used for color evaluation, color change in ΔE and ΔSGU, and number of dropouts: When more than one bleaching gel of each group was investigated, their values were combined to make a single entry per outcome
-
If the study reported any conflict of interest
Risk of bias in individual studies
Quality assessments of the included trials were evaluated by two independent reviewers (I.L.-M and A.R.), using the Cochrane Collaboration’s tool for assessing risk of bias in randomized trials [20]. The assessment criteria contained six items: sequence generation, allocation concealment, blinding of the outcome assessors, incomplete outcome data, selective outcome reporting, and other possible sources of bias. All these domains were evaluated at the study level. During data extraction and risk of bias assessment, any disagreements between the reviewers were resolved through discussion, and if needed, by consulting a third reviewer (L.C.M.).
For each aspect of the quality assessment, the risk of bias of each domain was scored following the recommendations of the Cochrane Handbook for Systematic reviews of Interventions 5.1.0 (http://handbook.cochrane.org). The judgment for each entry involved recording “yes” indicating low risk of bias, “no” indicating high risk of bias, and “unclear” indicating either lack of information or uncertainty over the potential for bias.
For the ΔSGU, tooth sensitivity risk and gingival irritation, which are subjective measures, three (random sequence generation, allocation concealment, and blinding) out of the six domains from the Cochrane risk of bias tool were considered the key domains for the assessment of the risk of bias. For the ΔE, which is an objective measure that cannot be influenced by the examiner’s awareness of the group assignment, we only considered adequate sequence generation and allocation concealment as the key domains.
Studies were considered to be at “low” risk of bias if there was adequate sequence generation, allocation concealment, and evaluator blindness, being the latter only considered for the ΔSGU outcome. When the study was judged as “unclear” in their key domains, we tried to contact authors to obtain more information and allow a definitive judgment of “yes” or “no.”
Summary measures and synthesis of the results
Data from eligible studies were either continuous (color change) or dichotomous (tooth sensitivity and gingival irritation). We performed subgroup analysis according to the risk of bias of the included studies (“low” risk of bias and “unclear/high” risk of bias). Although subgroup analyses were not pre-specified in the research protocol registered at PROSPERO, it allowed us to evaluate the impact of the exclusion of “unclear/high risk” of bias studies on the overall results.
To summarize the color change for each study, we calculated the standardized mean difference with a 95 % confidence interval (CI). For the tooth sensitivity and gingival irritation, we calculated the risk ratio along with the 95 % CI. The random effects models were employed. Heterogeneity was assessed using the Cochran Q test and I 2 statistics. All analyses were conducted using RevMan (Review Manager, version 5.3 software, Cochrane Collaboration, Copenhagen, Denmark). We performed a sensitivity analysis whenever decisions about the data had to be done so that the impact of such decision on the overall conclusion could be evaluated.
Results
Study selection
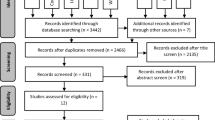
After the database screening and removal of duplicates, 1379 studies were identified (Fig. 1). After title screening, 195 studies remained and this number was reduced to 29 after careful examination of the abstracts. The full texts of these 29 studies were assessed to check if they were eligible. Among them, 16 were excluded due to the following reasons: (1) old systematic reviews [ [13, 21]], (2) case series studies [ [22]], (3) placebo-controlled studies [ [23–25]], (4) studies that did not compare PC and PH products [ [26–31]], and (5) studies that did not employ a control tray-delivered CP product [32–35].
Characteristics of included articles
The characteristics of the 13 selected studies are listed in Table 2. The parallel study design was predominantly used in these studies [9, 14, 15, 17, 18, 36–40]. Most of the studies whose setting was reported were conducted in universities [6, 9, 11, 14–18, 36, 40].
Eight out of the 13 studies employed a VITA shade guide for color evaluation [6, 9, 11, 14, 16–18, 36]. Four out of these eight studies added an objective instrument (spectrophotometer or colorimeter) for color assessment [11, 14, 16, 17]. Digital image analysis was employed in four studies [15, 36–38] and only one employed a fluorosis scale [40] for color change assessment.
The number of patients included in these studies ranged from 16 to 114 participants. The mean age of all participants included in the clinical trials was approximately 35.7 years [9, 14, 15, 17, 18, 36–40], except for three articles that did not report this information. In six [9, 15, 16, 18, 37, 40] out of the 13 studies, bleaching trays with reservoirs were fabricated (Table 2). Three studies [14, 17, 36] did not employ reservoirs, and four papers did not report this information [6, 11, 38, 39]. More than half of the studies were fully or partially supported by manufacturers, posing an important conflict of interest [11, 15, 16, 37–39] (Table 2).
Bleaching protocol
As shown in Table 2, nine of the selected studies used HP in strips [9, 15–17, 36–40] and four papers used HP delivered in a tray [6, 11, 14, 18] (Table 2). The concentration of the CP gels varied from 5 to 35 %, but most of the studies employed a 10 % CP gel [6, 9, 14, 15, 17, 37, 40]. For HP, the concentrations varied from 2.5 to 14 %, being 7.5 % the concentration most used [11, 14, 36, 40]. The studies selected showed a protocol of treatment from 2 to 28 days, whereas 14 days was the bleaching period most used [11, 14, 17, 18, 37, 38] (Table 2).
Assessment of the risk of bias
The assessment of the risk of bias of the selected studies is presented in Fig. 2. Few full-text studies reported the method of randomization employed and how the allocation concealment was performed in their full texts. As these two items were the key domains of the current systematic review, authors were contacted for further information. E-mails were sent to 11 corresponding authors of the 12 different studies [6, 9, 11, 14, 15, 17, 18, 37–40] to request further information, but only seven corresponding authors answered [6, 14, 15, 18, 37–39]. Eight [6, 11, 14–17, 36, 38] were considered to be at “low” risk of bias in these two domains and five [9, 18, 37, 39, 40] “unclear” for at least one of these two domains.
In relation to blinding of participants and evaluators, this domain was judged “unclear” in one full-text article [39] and at “high” risk of bias in another one [17]. Regarding selective reporting, all studies, except one [39] were considered at “low” risk of bias.
In summary, from the 13 studies, five [9, 18, 37, 39, 40] were considered to be at “high” risk of bias in the key domains of the Cochrane risk of bias tool, yielding 8 studies [6, 11, 14–17, 36, 38] which met the best requirement features (randomization, allocation concealment, and blinding) for meta-analysis of the color change in ΔE. From these eight studies classified as at low risk of bias, two [6, 15] did not report color change in terms of ΔE.
For ΔSGU, eight studies [6, 11, 14–17, 36, 38] met the best requirements (randomization and allocation concealment) for meta-analysis, but two [15, 38] did not measure the color change in shade guide units.
Meta-analyses
In all meta-analyses conducted, subgroup analyses with studies classified as at “low” risk of bias and those classified as at unclear/high risk of bias were performed. In none of the cases, the incorporation of the studies as at “unclear/high” risk of bias changed the conclusions reached with the studies classified as at “low” risk of bias. In light of that, the description of the results section will focus on the subgroup of studies as at “low” risk of bias.
Color change
For both meta-analyses (ΔE and ΔSGU), we included data for only those participants whose results were known (available case analysis). The impact of this decision was evaluated in a sensitivity analysis, where an intention-to-treat protocol was applied. No change in the overall significance was shown (data not shown).
The forest plot of the ΔE for the subgroup of studies at “low” risk of bias indicated that the data was not heterogenous (chi2 test p = 0.20; I 2 = 31 %; Fig. 3). For ΔSGU, the heterogeneity among the studies classified as at “low” risk of bias was significant (chi2 test; p < 0.001; I 2 = 69 %; Fig. 4). This heterogeneity was caused by inclusion of the study data of a single paper [17], as detected by means of a sensitivity analysis.
In three studies, two for the ΔE [37, 38] and another for ΔSGU [6], the standard deviations were not reported in the original articles and we imputed an arbitrary value based on the standard deviations of the other studies (Figs. 3 and 4). Through a sensitivity analysis, we observed that imputations of half, the same, or twice the mean did not produce significant changes in the model (data not shown).
For ΔE, the standardized mean difference for the subgroup of studies at “low” risk of bias was −0.45 (95 % CI −0.69 to −0.21) (Fig. 3), being statistically significant (p < 0.001). In other words, bleaching with tray-delivered CP provided more color change than HP gels. For ΔSGU (Fig. 4), the standardized mean difference for the subgroup at “low” risk of bias was 0.08 (−0.32 to 0.48) and it was not statistically significant (p = 0.70), meaning that both groups yielded similar color changes in terms of shade guide units.
Tooth sensitivity
Figure 5 shows the forest plot for the tooth sensitivity. Data was not heterogeneous for the subset of studies at “low” risk of bias (chi2 test, p = 0.90; I 2 = 0 %). The risk rate was 0.98 (95 % CI 0.78 to 1.23), and no significant difference was detected among the study groups (p = 0.83).
Gingival irritation
Figure 6 shows the forest plot for the gingival sensitivity. Data was not heterogeneous for the subset of studies at “low” risk of bias (chi2 test, p = 0.17; I 2 = 38 %). The risk rate was 1.18 (95 % CI 0.62 to 2.23), and no significant difference was detected among the study groups (p = 0.62).
Discussion
Meta-analysis takes the advantage of aggregating information with a higher statistical power for any measure of interest, as opposed to a less precise measure derived from a single study [20, 41]. However, this method presents some weaknesses. Meta-analysis cannot control for sources of bias of individual studies: a good meta-analysis of badly designed studies will still result in bad statistics. This means that only methodologically sound studies should be included in a meta-analysis in a practice called “best evidence synthesis” [20]. Although we have performed subgroup analyses with studies classified as at “low” risk of bias and “unclear/high” risk of bias, the main conclusions should be based only in the studies classified as at “low” risk of bias in regard to randomization and allocation concealment, opposed to earlier studies that based their conclusions on all included studies regardless of their risk of bias [13, 21]. Although the inclusion of studies at “high” risk of bias did not change the overall conclusions of the present investigation, this type of inclusion tends to overestimate the effect size [42].
Randomization, when correctly performed, guarantees that the chances of a patient being allocated in either test or control group are the same for all participants, which means that both known and unknown prognostic factors are balanced between groups [20]. As important as randomization, the allocation concealment is necessary to protect the randomization process, since the treatment to be allocated is not known before the patient is enrolled into the study [20]. The adequate management of these two domains (randomization and allocation concealment) minimizes selection bias, as clinical investigators in RCTs often find it difficult to maintain impartiality when knowing treatment allocation and this may produce non-comparable groups in baseline features.
Randomization and allocation concealment were poorly described in the included studies. Although some investigators described that the participants were randomly distributed, they rarely described the method of randomization (random number tables, computer random number generator, coin tossing, shuffling cards or envelopes, throwing a dice, etc.). This prevented the authors from performing a straightforward evaluation of the risk of the bias. Description of the allocation concealment was even more infrequent in the studies, which required contact with authors for further information.
Blinding the study participants and personnel may reduce the risk that knowledge, of which intervention was received, rather than the intervention itself, affects outcomes and outcome measurements. In other words, blinding avoids performance bias [20]. For the present systematic review, participants’ blinding was difficult to perform in some studies due to the differences in the at-home protocols (strips vs. trays, for instance) [9, 15–17, 36–38, 40, 43], which could be easily identified by the patients.
In light of that, and considering that the primary outcome of this study was not a patient-centered response (not susceptible to bias by the patient’s knowledge of the technique), we did not consider participants’ blinding a key domain for this systematic review. On the other hand, evaluator blinding was considered important when the color change was subjectively assessed in shade guide units. For the ΔE, which derive from objective numbers produced by a spectrophotometer, evaluator blinding was not considered a key domain.
An earlier systematic review [13] also compared the effectiveness of at-home bleaching products for dental bleaching, but the authors included many different comparisons, which made it difficult to reach an overall conclusion for clinical practice. The authors concluded that most of the available dentist-supervised or over-the-counter bleaching products available in the market for at-home bleaching are effective, but this conclusion was based on studies classified as at “moderate” and at “high” risk of bias. In the present systematic review, we opted to answer a narrower PICO question, comparing the widely used tray-delivered CP bleaching protocol [3] with the most recent strips or tray-delivered HP at-home bleaching products released in the market.
In terms of ΔE, this meta-analysis revealed that tray-delivered CP showed a higher whitening efficacy than HP-based products. Regardless of the bleaching product used, the active whitening component of both types of bleaching gels is the same HP (a 10 % solution of CP is roughly 3.5 % HP and 6.5 % urea). Thus, one could expect that a better whitening efficacy would be observed for groups when higher active HP was employed.
A closer evaluation of the bleaching products used in the studies included in the meta-analysis of ΔE (Fig. 3) revealed that, opposed to previous expectation, the concentration of the active HP was higher in the HP-based products rather than in the tray-delivered CP gels. Additionally, this reduced concentrated of active HP in tray-delivered CP was not compensated for extended application time in three out of the five studies [11, 14, 16].
This highlights that other factors apart from active HP concentration are responsible for the better whitening degree achieved with the tray-delivered CP products. While both CP and HP are used for whitening, their properties are quite different. HP-based products are very unstable and release all of its active hydrogen peroxide in 30 to 60 min [44, 45]. As oxidization of organic substance involve a series of consecutive steps and takes time to occur, there might be a limit to which the rapid release a chemical reagent (in this case, HP) leads to faster reaction rate. Thus, part of the fast HP released from the HP-based products may even not have time to contact the organic substance of the teeth, being partially lost at the dental surface. This hypothesis, however, requires in vitro and clinical evaluations.
On the other hand, the release of active HP in tray-delivered CP gels is slower than in HP-based products: about 50 % of its peroxide is released in the first 2 to 4 h, then the remainder over the next 2 to 6 h [44, 46]. In this way, there will be always available HP for oxidization due to the slow release, allowing better oxidization of the organic matrix of the dentin.
Additionally, when applied on the dental surfaces, CP breaks down into HP and urea. The HP further reduces to water and oxygen and the urea to ammonia and carbon dioxide. This has an additional advantage: the ammonia yield pH increase, which favors the dissociation of HP into free radicals. It is known that in an alkaline media, the dissociation of HP into free radicals is the highest as the dissociation constant (pKa) of the HP is around 11.5. In a pH of 9, HP dissociates 2.7 times more than in a pH of 4.4 [47]. Maximum effectiveness of bleaching was shown to occur under an alkaline pH of 9.
The pH of the media not only affects the decomposition kinetics but also the type of by-products produced. While in an acidic solution, free oxygen radicals and hydroxyl anions are produced; in an alkaline medium, there is a higher concentration of perhydroxyl ions [48]. Whether or not the different free radicals produced plays a role on the whitening outcome is yet to be investigated.
From a clinical perspective, these findings should be cautiously interpreted. The lack of statistical difference between at-home protocols in the studies included in the meta-analysis of ΔSGU may suggest that the differences observed in ΔE may not be clinically significant. A change in one shade guide unit can be clinically detectable, but only changes in ΔE equal to or higher than 3 are detectable by visual inspection [49]. Perhaps on the long run, this slight difference may result in less color rebound and more stable results. However, this requires further long-term clinical trials for investigation.
ΔE values are obtained from objective measures from spectrophotometer while ΔSGU values are obtained from visual matching with shade guide units. Randomized clinical trials usually employ these two instruments (spectrophotometer and shade guide units) for color evaluation in order to increase the confidence in the results of the study. However, although similar results are obtained when they are compared, some papers show significant differences when these two measurement instruments are compared [50–52]. Systematic reviews of the literature do not show new results but only express a summary of the results from primary RCTs, and this may be the reason of why the data of ΔE values reached significance and the ΔSGU did not. According to some studies, spectrophotometer readings provide more accurate results than visual shade matching with shade guides [53, 54], which is another reason for such difference between the two instruments in the present study.
Gingival irritation and tooth sensitivity, the most common side effects associated to bleaching therapy [13, 55] were not different among the at-home bleaching protocols. The gingival irritation associated with at-home bleaching is mainly related to two factors: trauma due to the tray/strip and the aggression of the hydrogen peroxide and derivates to gingiva tissue.
The use of a thick and rigid tray material, no scalloped (extended more than 1 mm onto the soft tissue), was responsible for gingival irritation at the past [31, 56]. Currently, many improvements in tray materials and designs have reduced gingival irritation, especially with the use of a soft, thin tray material [31, 56], which was used in the included clinical trials [6, 15–18, 36, 37, 39].
On the other hand, bleaching strips have a predefined form and the contact of the hydrogen peroxide occurs directly with the gingival tissue during treatment. Although there is a consensus that this might be responsible for some degree of gingival irritation, we have confirmed this in the present and earlier meta-analyses of the literature [13, 57].
Regarding the tooth sensitivity, the etiology of tooth sensitivity induced by bleaching is not fully understood. Since the hydrodynamic theory of dentin sensitivity has been widely accepted as the explanation of dentinal sensation, some authors have used this theory to explain tooth sensitivity due to bleaching [58]. However, pain during and following bleaching treatment can affect intact teeth lacking dentin exposure and this is in sharp contrast with the hydrodynamic theory [59].
Kielbassa et al. in a recent review of the literature suggested that the most probable cause of tooth sensitivity is a reversible pulpitis. There is some degree of pulpal inflammation due to some amount of hydrogen peroxide that reaches the pulp. It is widely known that hydrogen peroxide can pass easily through the enamel and dentin to the pulp [60] and can cause damage to the pulp cells [61]. The inflammatory reaction into the pulp after bleaching was demonstrated by the presence of inflammatory mediators such as the cell-derived factor adenosine triphosphate [62] and prostaglandins, which excite and sensitize pulpal nociceptors [63] causing the tooth sensitivity.
However, although the risk of bleaching-induced tooth sensitivity in the primary studies was quite variable (13.8 to 66 %), the overall risk of tooth sensitivity was 41 % (95 % CI 36.0 to 46.1), which is lower from that reported for in-office bleaching that uses a higher concentration of hydrogen peroxide (62.9 %; 95 % CI 56.9–67.3) [64]. In the study of Rezende et al. [64], the authors observed that at-home bleaching was associated with reduced risk and intensity of tooth sensitivity compared to in-office bleaching, which is in agreement with the results of the present study. This is in line with the results of an in vitro study that demonstrated that the damage to the pulp cells is directly correlated with the amount of HP that reaches the pulp chamber [65, 66].
Finally, this review highlights an important problem in the evidence base of bleaching products. More than half of the studies were fully or partially supported by manufacturers. Currently, manufacturers conduct their own evaluations of products or fund researchers to test their products. While this approach to product development is standard in all industries, it may pose a significant publication bias, as studies with results that do not favor the manufacturer would not be published. Therefore, there is an urgent need for independent studies using commercially available products that follow the current standards for design and reporting of randomized controlled trials [13].
Conclusions
Tray-delivered CP gels showed a slightly better whitening efficacy than HP-based products when the color change was evaluated with a spectrophotometer; such superiority, however, could not be detected with shade guide units. Both whitening systems demonstrated equal level of gingival irritation and tooth sensitivity.
References
Xiao J, Zhou XD, Zhu WC, Zhang B, Li JY, Xu X (2007) The prevalence of tooth discolouration and the self-satisfaction with tooth colour in a Chinese urban population. J Oral Rehabil 34:351–360. doi:10.1111/j.1365-2842.2007.01729.x
Alkhatib MN, Holt R, Bedi R (2004) Prevalence of self-assessed tooth discolouration in the United Kingdom. J Dent 32:561–566
Haywood VB, Heymann HO (1989) Nightguard vital bleaching. Quintessence Int 20:173–176
Haywood VB, Leonard RH, Nelson CF, Brunson WD (1994) Effectiveness, side effects and long-term status of nightguard vital bleaching. J Am Dent Assoc 125:1219–1226
Cibirka RM, Myers M, Downey MC, Nelson SK, Browning WD, Hawkins IK, Dickinson GL (1999) Clinical study of tooth shade lightening from dentist-supervised, patient-applied treatment with two 10% carbamide peroxide gels. J Esthet Dent 11:325–331
Alonso de la Pena V, Balboa Cabrita O (2006) Comparison of the clinical efficacy and safety of carbamide peroxide and hydrogen peroxide in at-home bleaching gels. Quintessence Int 37:551–556
Demarco FF, Conde MC, Ely C, Torre EN, Costa JR, Fernandez MR, Tarquinio SB (2013) Preferences on vital and nonvital tooth bleaching: a survey among dentists from a city of southern Brazil. Braz Dent J 24:527–531. doi:10.1590/0103-6440201302152
Meireles SS, Heckmann SS, Santos IS, Della Bona A, Demarco FF (2008) A double blind randomized clinical trial of at-home tooth bleaching using two carbamide peroxide concentrations: 6-month follow-up. J Dent 36:878–884. doi:10.1016/j.jdent.2008.07.002
Auschill TM, Hellwig E, Schmidale S, Sculean A, Arweiler NB (2005) Efficacy, side-effects and patients’ acceptance of different bleaching techniques (OTC, in-office, at-home). Oper Dent 30:156–163
Tay LY, Kose C, Herrera DR, Reis A, Loguercio AD (2012) Long-term efficacy of in-office and at-home bleaching: a 2-year double-blind randomized clinical trial. Am J Dent 25:199–204
Mokhlis GR, Matis BA, Cochran MA, Eckert GJ (2000) A clinical evaluation of carbamide peroxide and hydrogen peroxide whitening agents during daytime use. J Am Dent Assoc 131:1269–1277
Giachetti L, Bertini F, Bambi C, Nieri M, Scaminaci Russo D (2010) A randomized clinical trial comparing at-home and in-office tooth whitening techniques: a nine-month follow-up. J Am Dent Assoc 141:1357–1364
Hasson H, Ismail AI, Neiva G (2006) Home-based chemically-induced whitening of teeth in adults. Cochrane Database Syst Rev:CD006202. doi: 10.1002/14651858.CD006202
Alonso de la Pena V, Lopez Raton M (2014) Randomized clinical trial on the efficacy and safety of four professional at-home tooth whitening gels. Oper Dent 39:136–143. doi:10.2341/12-402-C
Ferrari M, Cagidiaco MC, Monticelli F, Kugel G, Barker ML, Gerlach RW (2007) Daytime use of a custom bleaching tray or whitening strips: initial and sustained color improvement. Am J Dent 20(Spec No A):19A–22A
da Costa JB, McPharlin R, Hilton T, Ferracane JI, Wang M (2012) Comparison of two at-home whitening products of similar peroxide concentration and different delivery methods. Oper Dent 37:333–339. doi:10.2341/11-053-C
Bizhang M, Chun YH, Damerau K, Singh P, Raab WH, Zimmer S (2009) Comparative clinical study of the effectiveness of three different bleaching methods. Oper Dent 34:635–641. doi:10.2341/08-069-C
Delgado E, Hernandez-Cott PL, Stewart B, Collins M, De Vizio W (2007) Tooth-whitening efficacy of custom tray-delivered 9% hydrogen peroxide and 20% carbamide peroxide during daytime use: a 14-day clinical trial. P R Health Sci J 26:367–372
Moher D, Altman DG, Liberati A, Tetzlaff J (2011) PRISMA statement. Epidemiology 22:128. doi:10.1097/EDE.0b013e3181fe7825, author reply 128
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods G, Cochrane Statistical Methods G (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928. doi:10.1136/bmj.d5928
Gerlach RW, Barker ML, Karpinia K, Magnusson I (2009) Single site meta-analysis of 6% hydrogen peroxide whitening strip effectiveness and safety over 2 weeks. J Dent 37:360–365. doi:10.1016/j.jdent.2009.01.005
Berga-Caballero A, Forner-Navarro L, Amengual-Lorenzo J (2006) At-home vital bleaching: a comparison of hydrogen peroxide and carbamide peroxide treatments. Med Oral Patol Oral Cir Bucal 11:E94–E99
Niederman R, Tantraphol MC, Slinin P, Hayes C, Conway S (2000) Effectiveness of dentist-prescribed, home-applied tooth whitening. A meta analysis. J Contemp Dent Pract 1:20–36
Swift EJ Jr, May KN Jr, Wilder AD Jr, Heymann HO, Wilder RS, Bayne SC (1997) Six-month clinical evaluation of a tooth whitening system using an innovative experimental design. J Esthet Dent 9:265–274
Xu X, Zhu L, Tang Y, Wang Y, Zhang K, Li S, Bohman LC, Gerlach RW (2007) Randomized clinical trial comparing whitening strips, paint-on gel and negative control. Am J Dent 20(Spec No A):28A–31A
Reid JS (1985) Patient assessment of the value of bleaching tetracycline-stained teeth. ASDC J Dent Child 52:353–355
Nathoo S, Stewart B, Petrone ME, Chaknis P, Zhang YP, DeVizio W, Volpe AR (2003) Comparative clinical investigation of the tooth whitening efficacy of two tooth whitening gels. J Clin Dent 14:64–69
Migliore S, Damiani MG, Faucher A (1991) Bleaching of vital teeth. Part one of a comparative study of two ambulatory methods. Inf Dent 73:1109–1114
Dawson PF, Sharif MO, Smith AB, Brunton PA (2011) A clinical study comparing the efficacy and sensitivity of home vs combined whitening. Oper Dent 36:460–466. doi:10.2341/10-159-C
Arzt AH (1981) Updating tetracycline-stained teeth bleaching technique. Quintessence Int Dent Dig 12:15–18
Haywood VB, Leonard RH, Dickinson GL (1997) Efficacy of six months of nightguard vital bleaching of tetracycline-stained teeth. J Esthet Dent 9:13–19
Wilson CF, Seale NS (1985) Color change following vital bleaching of tetracycline-stained teeth. Pediatr Dent 7:205–208
Karpinia K, Magnusson I, Barker ML, Gerlach RW (2003) Clinical comparison of two self-directed bleaching systems. J Prosthodont 12:242–248
Lo EC, Wong AH, McGrath C (2007) A randomized controlled trial of home tooth-whitening products. Am J Dent 20:315–318
Collins LZ, Maggio B, Gallagher A, York M, Schafer F (2004) Safety evaluation of a novel whitening gel, containing 6% hydrogen peroxide and a commercially available whitening gel containing 18% carbamide peroxide in an exaggerated use clinical study. J Dent 32(Suppl 1):47–50
Li Y, Lee SS, Cartwright SL, Wilson AC (2003) Comparison of clinical efficacy and safety of three professional at-home tooth whitening systems. Compend Contin Educ Dent 24:357–360, 362, 364 passim; quiz 378
Gerlach RW, Gibb RD, Sagel PA (2000) A randomized clinical trial comparing a novel 5.3% hydrogen peroxide whitening strip to 10%, 15%, and 20% carbamide peroxide tray-based bleaching systems. Compend Contin Educ Dent Suppl:S22–8; quiz S42–43
Donly KJ, Garcia-Godoy F, Segura A, Baharloo L, Rojas-Candelas E, X. Z (2002) Efficacy and safety of vital bleaching in teenagers using 6.5% hydrogen peroxide strips during the day or 10% carbamide tray system overnight. J Dent Res Sp Issue 1953
Gerlach RW, Shahidi H, Zhou X (2002) Clinical trial comparing whitening strips to a carbamide peroxide potassium nitrate tray system. J Dent Res Sp Issue 1952
Loyola-Rodriguez JP, Pozos-Guillen Ade J, Hernandez-Hernandez F, Berumen-Maldonado R, Patino-Marin N (2003) Effectiveness of treatment with carbamide peroxide and hydrogen peroxide in subjects affected by dental fluorosis: a clinical trial. J Clin Pediatr Dent 28:63–67
Schroeder M, Reis A, Luque-Martinez I, Loguercio AD, Masterson D, Maia LC (2015) Effect of enamel bevel on retention of cervical composite resin restorations: a systematic review and meta-analysis. J Dent 43:777–788. doi:10.1016/j.jdent.2015.02.017
Schulz KF, Chalmers I, Hayes RJ, Altman DG (1995) Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 273:408–412
Collins LZ, Maggio B, Liebman J, Blanck M, Lefort S, Waterfield P, Littlewood D, Naeeni M, Schafer F (2004) Clinical evaluation of a novel whitening gel, containing 6% hydrogen peroxide and a standard fluoride toothpaste. J Dent 32(Suppl 1):13–17
Matis BA (2000) Degradation of gel in tray whitening. Compend Contin Educ Dent Suppl:S28, S31-5; quiz S49
Al-Qunaian TA, Matis BA, Cochran MA (2003) In vivo kinetics of bleaching gel with three-percent hydrogen peroxide within the first hour. Oper Dent 28:236–241
Matis BA, Yousef M, Cochran MA, Eckert GJ (2002) Degradation of bleaching gels in vivo as a function of tray design and carbamide peroxide concentration. Oper Dent 27:12–18
Frysh H, Bowles WH, Baker F, Rivera-Hidalgo F, Guillen G (1995) Effect of pH on hydrogen peroxide bleaching agents. J Esthet Dent 7:130–133
Sun G (2000) The role of lasers in cosmetic dentistry. Dent Clin N Am 44:831–850
Chen H, Huang J, Dong X, Qian J, He J, Qu X, Lu E (2012) A systematic review of visual and instrumental measurements for tooth shade matching. Quintessence Int 43:649–659
Ontiveros JC, Paravina RD (2009) Color change of vital teeth exposed to bleaching performed with and without supplementary light. J Dent 37:840–847. doi:10.1016/j.jdent.2009.06.015
Gurgan S, Cakir FY, Yazici E (2010) Different light-activated in-office bleaching systems: a clinical evaluation. Lasers Med Sci 25:817–822. doi:10.1007/s10103-009-0688-x
Ontiveros JC, Eldiwany MS, Paravina R (2012) Clinical effectiveness and sensitivity with overnight use of 22% carbamide peroxide gel. J Dent 40(Suppl 2):e17–e24. doi:10.1016/j.jdent.2012.08.009
Alsaleh S, Labban M, AlHariri M, Tashkandi E (2012) Evaluation of self shade matching ability of dental students using visual and instrumental means. J Dent 40(Suppl 1):e82–e87. doi:10.1016/j.jdent.2012.01.009
Alshiddi IF, Richards LC (2015) A comparison of conventional visual and spectrophotometric shade taking by trained and untrained dental students. Aust Dent J 60:176–181. doi:10.1111/adj.12311
Kielbassa AM, Maier M, Gieren AK, Eliav E (2015) Tooth sensitivity during and after vital tooth bleaching: a systematic review on an unsolved problem. Quintessence Int 46:881–897. doi:10.3290/j.qi.a34700
Matis BA (2003) Tray whitening: what the evidence shows. Compend Contin Educ Dent 24:354–362
Gerlach RW, Zhou X (2001) Vital bleaching with whitening strips: summary of clinical research on effectiveness and tolerability. J Contemp Dent Pract 2:1–16
Swift EJ Jr (2005) Tooth sensitivity and whitening. Compend Contin Educ Dent 26:4–10, quiz 23
Markowitz K (2010) Pretty painful: why does tooth bleaching hurt? Med Hypotheses 74:835–840. doi:10.1016/j.mehy.2009.11.044
Cooper JS, Bokmeyer TJ, Bowles WH (1992) Penetration of the pulp chamber by carbamide peroxide bleaching agents. J Endod 18:315–317. doi:10.1016/S0099-2399(06)80479-6
Costa CA, Riehl H, Kina JF, Sacono NT, Hebling J (2010) Human pulp responses to in-office tooth bleaching. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontics 109:e59–e64. doi:10.1016/j.tripleo.2009.12.002
Cook SP, McCleskey EW (2002) Cell damage excites nociceptors through release of cytosolic ATP. Pain 95:41–47
Huynh MP, Yagiela JA (2003) Current concepts in acute pain management. J Calif Dent Assoc 31:419–427
Rezende M, Loguercio AD, Kossatz S, Reis A (2016) Predictive factors on the efficacy and risk/intensity of tooth sensitivity of dental bleaching: a multi regression and logistic analysis. J Dent 45:1–6. doi:10.1016/j.jdent.2015.11.003
Soares DG, Basso FG, Pontes EC, Garcia Lda F, Hebling J, de Souza Costa CA (2014) Effective tooth-bleaching protocols capable of reducing H(2)O(2) diffusion through enamel and dentine. J Dent 42:351–358. doi:10.1016/j.jdent.2013.09.001
Soares DG, Basso FG, Hebling J, de Souza Costa CA (2014) Concentrations of and application protocols for hydrogen peroxide bleaching gels: effects on pulp cell viability and whitening efficacy. J Dent 42:185–198. doi:10.1016/j.jdent.2013.10.021
Acknowledgments
This study was conducted during the doctoral stage of Issis Luque-Martinez under the supervision of the Prof. Lucianne Cople-Maia. The authors of this study would like to thank the following authors who kindly provided information not available in their full texts: Bruce Matis, Alonso de la Peña, Kevin James Donly, Mozhang Bizhang, and Marco Ferrari. This study was partially supported by National Council for Scientific and Technological Development from the Brazilian Government, under grants 304105/2013-9 and 305588/2014-1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Funding
This study was partially supported by CAPES and National Council for Scientific and Technological Development (CNPq) under grant nos. 304105/2013-9 and 305588/2014-1.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Luque-Martinez, I., Reis, A., Schroeder, M. et al. Comparison of efficacy of tray-delivered carbamide and hydrogen peroxide for at-home bleaching: a systematic review and meta-analysis. Clin Oral Invest 20, 1419–1433 (2016). https://doi.org/10.1007/s00784-016-1863-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-016-1863-7