Abstract
Objectives
This study aims to investigate the effect of modifying tricalcium silicate (TCS) cements on three key properties by adding ZrO2.
Materials and methods
TCS powders were prepared by adding ZrO2 at six different concentrations. The powders were mixed with 1 M CaCl2 solution at a 3:1 weight ratio. Biodentine (contains 5 wt.% ZrO2) served as control. To evaluate the potential effect on mechanical properties, the mini-fracture toughness (mini-FT) was measured. Regarding bioactivity, Ca release was assessed using ICP-AES. The component distribution within the cement matrix was evaluated by Feg-SEM/EPMA. Cytotoxicity was assessed using an XTT assay.
Results
Adding ZrO2 to TCS did not alter the mini-FT (p = 0.52), which remained in range of that of Biodentine (p = 0.31). Ca release from TSC cements was slightly lower than that from Biodentine at 1 day (p > 0.05). After 1 week, Ca release from TCS 30 and TCS 50 increased to a level that was significantly higher than that from Biodentine (p < 0.05). After 1 month, Ca release all decreased (p < 0.05), yet TCS 0 and TCS 50 released comparable amounts of Ca as at 1 day (p > 0.05). EPMA revealed a more even distribution of ZrO2 within the TCS cements. Particles with an un-reacted core were surrounded by a hydration zone. The 24-, 48-, and 72-h extracts of TCS 50 were the least cytotoxic.
Conclusions
ZrO2 can be added to TCS without affecting the mini-FT; Ca release was reduced initially, to reach a prolonged release thereafter; adding ZrO2 made TCS cements more biocompatible.
Clinical relevance
TCS 50 is a promising cement formulation to serve as a biocompatible hydraulic calcium silicate cement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydraulic calcium silicate cements have widely been used not only in various endodontic treatments, including pulp capping, pulpotomy, apexogenesis, repair of root perforation, treatment of root resorption, root-end filling, and apexification [1, 2], but also as material in replacement of dentin [3]. A great amount of studies reported the production of an apatite-rich surface layer on the cement upon contact with phosphate buffered saline (PBS) or simulated body fluid (SBF) [4–6]. The mechanism of action involves the production of calcium silicate hydrate upon hydrolysis of the cement, which in turn induces nucleation and crystallization of calcium-phosphate minerals [7, 8].
The deposition of such calcium-phosphate minerals was also observed in caries-like demineralized dentin [3, 9, 10]. In our previous study, calcium-phosphate minerals were formed within caries-like demineralized dentin upon 1-week SBF storage however, the re-mineralization zone stopped to become thicker when a thickness of approximately 40 μm was reached. Even upon 6-month SBF storage, dentin remained demineralized at larger depths. A very plausible explanation is that this phenomenon is caused by a fast and densely formed re-mineralization zone that hinders further infiltration of calcium deep into the remaining demineralized dentin; hence, re-mineralization remains incomplete. In an attempt to overcome this re-mineralization block and so to improve the re-mineralization potential of hydraulic calcium silicate cements, the release of calcium from the hydrated cement should be controlled better, i.e., slowed down and prolonged.
Hydraulic calcium silicate cements are mainly composed of Portland cement, of which the main component is tricalcium silicate (TCS) [11]. The percentage of TCS in Portland cement varies due to the use of different raw materials and differences in the manufacturing process. This variation is likely to induce different cement reactivity and related properties. In addition, the release of heavy metal elements from Portland cement, like arsenic and lead, remains a concern [12, 13]. To maintain stable performance and reliable quality, Portland cement in hydraulic calcium silicate cements can better be replaced with pure TCS since TCS can be manufactured from pure raw materials using a sol-gel process under controlled conditions [14–16]. In vitro bioactivity testing of TCS revealed generation of hydroxyapatite (HAp) upon contact with SBF [17]; dentinal tubules were occluded by HAp crystals [16]. The commercial TCS-based dentin-replacement cement Biodentine (Septodont, Saint-Maur-des-fosses, France) was shown to possess improved handling characteristics, reduced discoloration potential, as well as comparable or even superior physical and biological properties than those of the Portland cement-containing mineral trioxide aggregate (ProRoot MTA; Dentsply, Tulsa, OK, USA) [18–20]. As such, TCS has great potential as biomaterial to induce dentin re-mineralization and pulpal repair.
Bismuth oxide, as radiopacifier, is added to hydraulic calcium silicate cements, such as ProRoot MTA (Dentsply) and MTA Angelus (Angelus, Londrina, PR, Brazil) [21]. Several studies have, however, shown that bismuth oxide decreased compressive strength [22, 23], increased the relative porosity [24], and negatively affected the biocompatibility of ProRoot MTA cement (Dentsply) [25, 26]. In addition, bismuth oxide adversely affected the setting time of Portland cement; with 20 wt.% bismuth oxide, the setting time increased by 20 % [23]. Bismuth oxide was also documented to interact with collagen within dentin, thereby causing tooth discoloration, as was shown for MTA Angelus (Angelus) [27].
To avoid the abovementioned shortcomings, zirconium oxide (ZrO2) [23, 28–32], niobium oxide [33], barium sulfate [30], and zinc oxide [30] have been recommended as alternative radiopacifiers. Portland cement containing 30 wt.% ZrO2, added either at micro- or nano-particle size, revealed a radiopacity of above 3-mm thickness of aluminum, which corresponds to the recommended ISO standard 6876 for an endodontic material [23, 29]; 30–50 wt.% ZrO2 resulted in a radiopacity comparable to that of ProRoot MTA (Dentsply) [31]. ZrO2 is a bio-inert filler that does not affect the hydration reaction of Portland cement [28, 32]. On the contrary, ZrO2 added to Portland cement improved physical properties, like compressive strength, and triggered a better biological response than hydraulic calcium silicate cement to which bismuth oxide was added [32, 34]. In comparison with pure Portland cement, the Ca release from Portland cement with added ZrO2 was reduced [24]. This addition of ZrO2 to TCS may be a promising alternative to control the Ca release. Furthermore, the addition of ZrO2 reduces the relative concentration of TCS in hydraulic calcium silicate cements, thereby diminishing the high production cost.
The objective of this laboratory research was to investigate the effect of replacing TCS with varying percentages of ZrO2 on three properties of high relevance for hydraulic calcium silicate cements, which are strength, bioactivity, and biocompatibility. The null hypothesis tested was that adding ZrO2 does not affect the three key properties of TCS cements.
Materials and methods
Cement preparation
The materials used in present study included TCS powder (diameter of ±10 μm; Mineral Research Processing, Meyzieu, France) and ZrO2 (diameter of ±200 nm; Tosoh, Tokyo, Japan). Six different experimental cement powders were prepared by replacing TCS in part by ZrO2 at various concentrations: 0, 5, 10, 20, 30, and 50 wt.% (Table 1). Since calcium chloride (CaCl2) accelerates the setting time of TCS cement, 1 M CaCl2 aqueous solution was employed as the experimental cement liquid. The same powder to solution weight ratio of 3:1 was selected to achieve cements with a similar cement consistency, and thus similar handling and also similar surface-wetting characteristics. The different powders were mixed with 1 M CaCl2 aqueous solution for 30 s using a capsule mixer (RotoMix Capsule Mixer; 3M ESPE, Seefeld, Germany). Biodentine (Septodont), which contains 5 wt.% ZrO2, served as control. The powder and liquid of Biodentine were mixed according to the manufacturer’s instruction.
Mini-fracture toughness (mini-FT)
Ten rectangular bars (16 × 2 × 1.5 mm) were prepared per experimental material and Biodentine by filling the cement into custom-made silicon molds. Proper adaptation was achieved ultra-sonically using a dental ultra-sonic scaler equipped with a flat tip (MiniMaster Ultrasonic Scaler; Electro Medical Systems, Chemin de la Vuarpilliere, Switzerland). Specimens were stored at 37 °C and 100 % humidity for 1 week. A single mini-FT notch tip was positioned at the middle of each specimen under a stereo-microscope (Leica M715, Wetzlar, Germany); the notch was prepared under water-cooling using a 150-μm ultra-thin diamond blade (M1DO8; Struers, Ballerup, Denmark) at a feed speed of 0.015 mm/s and a wheel speed of 1000 rpm (Fig. 1a). The specimen was then transferred to the universal testing machine (Instron 5848 Micro Tester; Instron, Norwood, MA, USA) facing the specimen tip down in the test fixture. The so-called mini-FT (K Ic) was determined using a four-point bending test setup with a crosshead speed of 0.05 mm/min (Fig. 1b).
Schematic diagram explaining the mini-FT setup. a The single notch was prepared at the middle of each rectangular bar (1.5 × 2.0 mm). The notch width was smaller than 0.3 mm and the tip angle was 45°. The tip of the notch was located on the long surface at 0.24–0.48 mm (l 0) from the left bottom corner. The opposite part of the notch cut (l 1) did not touch the top part, but ended less than 0.2 mm from the top side. W specimen width, B specimen thickness. b The mini-FT of the specimen was measured using a four-point bending test. The inner and outer spans were 5 and 10 mm, respectively
After testing, all fractured specimens were air dried and gold-sputter coated, and the exact dimensions of the mini-FT notch were measured using a measuring microscope at a magnification of ×250, after which K Ic was calculated (MPa m1/2), according to the following equation from the ISO 24370:2005 standard:
with the minimum stress intensity factor coefficient Y*min being calculated from the following function:
where K Ic is the fracture toughness value in (MPa m1/2), F is the total force (N) (maximum force, F max, plus tare force, F Tare), S o is the outer span (mm) or 10.0, S i is the inner span (mm) or 5.0, B is the specimen thickness (mm) or 1.50 ± 0.1, W is the specimen width (mm) or 2.00 ± 0.1, Y*min is the stress intensity factor coefficient, l 0 is the position of the tip (mm) with 0.12 < l 0/W < 0.24, and l 1 is the position of the bottom part (mm) with 0.90 < l 1/W < 1.00 (Fig. 1a). The effect of adding ZrO2 on the K Ic value of the experimental cements was evaluated by calculating the Pearson’s correlation coefficient. As no correlation was found, the data from the experimental cements and Biodentine (Septodont) were compared by a t test. All statistical analyses were performed at a significance level of 0.05 using a software package (R3.01; R Foundation for Statistical Computing, Vienna, Austria).
Ca release
The experimental cements TCS 0, TCS 30, and TCS 50 were chosen to investigate the effect of adding ZrO2 on the Ca release from TCS cements. Biodentine (Septodont) and Dycal (Dentsply, Konstanz, Germany) served as controls. For each cement, five cylindrical cement blocks of 5-mm diameter and 1-mm thickness were prepared. Each specimen was stored at 37 °C for 24 h under 100 % humidity. A NaCl solution (133 mmol/L) was prepared and buffered to pH 7 using 50 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES). Each cylindrical cement block was immersed in 5-mL solution; the solution was collected and refreshed after 1 day, 1 week, and 1 month. Ca release was analyzed using Inductively Coupled Plasma Mass Spectrometry (ICP-AES; VISTA Pro, SII, Chiba, Japan). Prior to analysis, the solution was filtered and acidified using 5 mL of 10 % HNO3 solution. The data were statistically analyzed using a linear mixed effects model taking into account the time intervals and cements tested as fixed effects and the specimen origin as a random effect. The values were assessed by a Tukey multiple comparisons test.
Electron probe micro-analysis (EPMA)
The fractured mini-FT cement bars were air dried for 1 day and subsequently polished using an argon-ion-beam polisher (IB-09010CP Cross Section Polisher; JEOL, Tokyo, Japan) at 5.0 kV for 7 h to achieve an ion-beam polished cement surface of approximately 1 mm2. A 2-nm-thick Pt-Pd coating was applied to the cement surface by means of a turbomolecular-pumped coater (Q150T S; Quorum, East Sussex, UK). The hydration characteristics and the distribution of Ca, Si, and Zr along the cross-sections were investigated using Feg-SEM/EPMA (JXA-8530F; JEOL, Tokyo, Japan) at a high spatial resolution (±0.05 μm). X-ray profiles and quantification were performed at 15 kV and 15 μA (probe current) under high vacuum.
Cement cytotoxicity
Cell culture
Healthy human third molars (from patients at 15–25 years of age), extracted for orthodontic reasons, were gathered as approved by the Commission for Medical Ethics of KU Leuven under the file number S57622. The teeth were rinsed in PBS (Gibco, Carlsbad, CA, USA) with 200 U/mL penicillin and 200 μg/mL streptomycin (Invitrogen, Carlsbad, CA, USA); the periodontal ligament was removed with a blade, and the teeth were next mechanically split. From each tooth, pulpal tissue was gently separated using a forceps; the isolated pulpal tissue was cut with a scissor into approximately 1-mm3 fragments. The pulp-tissue fragments were seeded in six-well plates (Costar, Cambridge, MA, USA) filled with culture medium consisting of Dulbecco modified Eagle medium (Gibco) supplemented with 10 % fetal bovine serum (FBS, Gibco). When human pulp fibroblast cells (HPFs) reached 70–80 % confluence, the cells were harvested using 0.25 % Trypsin/EDTA (Sigma-Aldrich, St. Louis, MO, USA) and observed as passage 0. The HPFs (passage 4–10) were cultured in 175 cm2 cell culture flasks at 37 °C, 5 % CO2, and 100 % humidity. Experiments were performed with confluent cell monolayers at a density of 80–90 %.
Cement extracts
The experimental cements TCS 0 and TCS 50 were chosen to test the effect of adding ZrO2 on the cytotoxicity of TCS cements. To correctly estimate the level of biocompatibility of the experimental cement formulations, commercial products Biodentine (Septodont), TheraCal LC (Bisco, Schaumburg, IL, USA), Dycal (Dentsply), and Alganol (Kemdent, Swindon, UK) were selected as controls (Table 2). The experimental cements were prepared by mixing the powder with 1 M CaCl2 at a weight ratio of 3:1; the other cements were mixed according to the respective manufacturer’s instructions. The cements were applied to the bottom of each well in a 24-well plate; the thickness of the cylindrical cement disks was 1 mm. The resin-based light-curing calcium silicate cement TheraCal LC (Bisco) was light-cured for 20 s (Bluephase 20i, Ivoclar Vivadent, Schaan, Liechtenstein). A 300-μL culture medium was immediately added to the cement disk in each well. The 24-, 48-, and 72-h extracts were collected; each extract concentrate was diluted with fresh culture medium to 1:1, 1:3, 1:9, 1:27, and 1:81 of the original concentration.
XTT assay
A Cell Proliferation Kit II (Roche Diagnostic, Penzberg, Germany) was used to assess the metabolic activity of cells on the basis of cleavage of yellow tetrazolium salt 2, 3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) by mitochondrial enzymes in metabolically active cells to form a soluble orange formazan product. The production of formazan is directly related to the number of vital cells. HPFs were seeded at a concentration of 20,000 cells/well in 100-μL culture medium in 96-well plates. The cells were incubated at 37 °C and 5 % CO2 for 24 h. The culture medium was next removed and replaced by 50 μL of undiluted or diluted extracts. After 20 h of incubation and 4 h before photospectrometric analysis, 50 μL XTT labeling agent (in RPMI [Roswell Park Memorial Institute] without phenol red) and electron-coupling reagent (PMS [N-methyldibenzopyrazine methyl sulfate] in PBS) was added to each well. Quantification of the formazan production was performed photospectrometrically at a wavelength of 450 nm using a microplate reader (Multiskan Ascent 96/384; Thermo Scientific, Waltham, MA, USA). The metabolic activities of cells, which were exposed to fresh culture medium or 1 % Triton X-100, were used as positive and negative controls, respectively. Each extract was tested in triplicate per test and the XTT test was repeated four times. Absorbance values of the positive and negative controls were adjusted to 100 and 0 % and the relative formazan production was calculated. Subsequently, concentration/dose-effect models were fitted for each cement at each extraction time separately; the estimated median effective dose (ED50) was calculated. This data processing was conducted using a software package (R3.01, and drc, package version 2.3-96).
RESULTS
Mini-FT (Fig. 2)
Adding ZrO2 to TCS did not affect the mini-FT (K Ic) of the experimental cements (estimated effect of 0.0005/wt.%; p = 0.52). Overall, the experimental TCS cements exhibited a slightly but not significantly higher mean K Ic than Biodentine (0.45 ± 0.11 vs. 0.36 ± 0.06, respectively, p = 0.31).
Mini-FT (K Ic) of the experimental TSC cements (open circles) and Biodentine (Septodont; closed circles). The mini-FT did not significantly increase (p = 0.52) with a higher concentration of ZrO2 (estimated effect of 0.0005/wt.%). Overall, the experimental TCSs revealed a slightly higher but not significantly different (p = 0.31) mean K Ic of 0.45 ± 0.11 MPa m1/2 vs. 0.36 ± 0.06 MPa m1/2 recorded for Biodentine (Septodont)
Ca release (Table 3)
The concentrations of Ca ions released from each cement at 1 day, 1 week, and 1 month in distilled water are summarized in Table 3. At 1 day, Ca release among all experimental TCS cements did not differ statistically (p > 0.05). The initial Ca release at 1 day was slightly higher for Biodentine, while at 1 week Ca release from TCS 30 and TCS 50 was increased to a level that was significantly higher than that from Biodentine (p < 0.05). Ca release from all TCS cements and Biodentine significantly decreased after a 1-month storage period (p < 0.05), yet TCS 0 and TCS 50 remained to release comparable amounts of Ca as at 1 day (p > 0.05). The Ca release from Biodentine at 1 month was, however, significantly lower than the release at 1 day (p < 0.05). At all time intervals, Dycal revealed the lowest Ca release, and its Ca release did not statistically significantly differ for the different time intervals (p > 0.05).
Component distribution and hydration characteristics (Fig. 3)
ZrO2 particles with a small particle size (diameter of ±200 nm) were rather evenly distributed within the matrix of the experimental cements TCS 30 and TCS 50 (Fig. 3e, i, h, l). In contrast, ZrO2 particles were less homogenously dispersed in the Biodentine matrix, in which clearly some highly concentrated Zr islands could be detected (Fig. 3m, p, marked with #). Back-scattered electron (BSE) image of Biodentine showed particles with a thin rim, representing a light-gray hydration zone around the dark-gray un-reacted core (Fig. 3m, marked with ☆). Compared with the un-reacted core, the hydration zone was less rich in Ca (Fig. 3n) and Si (Fig. 3o), while the core was free of Zr (Fig. 3p). Similarly reacted hydration zones were observed in all experimental TCS-cement matrices (Fig. 3a–l, marked with *).
Back-scattered electron (BSE) SEM photomicrographs of the argon-ion-beam polished cement cross-sections along with EPMA chemical elemental mappings of calcium (Ca), silicon (Si), and zirconium (Zr), this for the experimental TCS cements “TCS 0” (a–d), “TCS 30” (e–h), and “TCS 50” (i–l), as well as for Biodentine (Septodont; m–p). The shallow dark-gray rim (marked with *) at the particle boundary represents a zone of hydration surrounding the particle core. The hydration zones have lower concentrations of Ca and Si than the un-reacted particle cores. No Zr was found within the particle core. Similarly reacted hydration zones were observed in Biodentine (marked with ☆). ZrO2 particles with a smaller particle size were more evenly distributed within the matrix of the experimental cements TCS 30 (h) and TCS 50 (l) compared to those ZrO2 particles (marked with #) in Biodentine (Septodont) matrix
Cytotoxicity
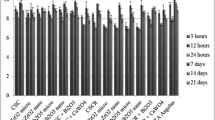
The metabolic activities of HPFs after exposure to the 24-, 48-, and 72-h cement extracts are summarized in Fig. 4. The 24-h undiluted extracts of all tested cements were cytotoxic, while TCS 50 exhibited the least cytotoxicity (Fig. 4a). ED50 of the 24-h undiluted extracts could be ranked in decreasing order as follows: TCS 50 > TCS 0 > Dycal > TheraCal LC > Biodentine > Alganol (Table. 4). Accordingly, the level of cytotoxicity of the 24 h undiluted cement extracts followed a reverse order. The 48- and 72-h undiluted experimental cements TCS 0 and TCS 50 appeared non-cytotoxic (Fig. 4b, c), and consequently the related ED50 could not be generated (Table 4). The cytotoxicity of 48- and 72-h undiluted Biodentine extract was lower in comparison to that of the 24-h undiluted cement extract; however, it was still more cytotoxic than the experimental TCS cements (Fig. 4). The 48- and 72-h undiluted extracts of the light-curing resin-based cement TheraCal LC were found to be cytotoxic, although the cytotoxicity decreased for the 72-h undiluted extract compared with the 48-h undiluted extract (Table 4). The undiluted Alganol extract, employed as negative control, demonstrated the highest cytotoxicity among all cements tested (Table 4).
Evaluation of relative formazan formation of human pulp fibroblasts (HPFs) after exposure to the 24 h (a), 48 h (b), and 72 h (c) undiluted (1:1) and diluted (1:3, 1:9, 1:27, 1:81) cement extracts using an XTT assay. a The 24-h undiluted (1:1) extracts of all cements tested appeared cytotoxic. The ranking of the 24-h cement extracts in order of highest to lowest cytotoxicity is Alganol (Kemdent) > Biodentine (Septodont) > Dycal (Dentsply) > TheraCal LC (Bisco) > TCS 0 > TCS 50. b The 48-h undiluted (1:1) TCS 50 and TCS 0 cement extracts were relatively non-cytotoxic. The ranking of the 48-h cement extracts in order of highest to lowest cytotoxicity changed slightly: Alganol (Kemdent) > Dycal (Dentsply) > TheraCal LC (Bisco) > Biodentine (Septodont) > TCS 0 > TCS 50. c The 72-h undiluted (1:1) TCS 50, TCS 0, and Biodentine (Septodont) cement extracts were relatively non-cytotoxic. The ranking of the 72-h cement extracts for cytotoxicity was the same as for the 48-h cement extracts in b
Discussion
The hypothesis that the addition of ZrO2 does not affect strength, bioactivity, and biocompatibility of TCS cements was partially rejected. The mini-FT was not statistically altered by adding ZrO2. Regarding Ca release, all experimental TCS cements released less Ca at 1 day as compared to Biodentine, and in particular the Ca release from TCS 50 was prolonged at a higher rate than that of Biodentine at 1 week and 1 month. Adding ZrO2 appeared an effective means to control Ca release with a slower early and longer higher rate. No effect of adding ZrO2 on the chemical hydration characteristics of TCS cements could be documented, this basically because ZrO2 was not involved in the hydration reaction of TCS. Finally, adding ZrO2 reduced the cytotoxicity of TCS cements, this as compared to four control Ca-releasing materials and with TSC 50 being least cytotoxic.
To assess mechanical strength of hydraulic calcium silicate cements, most commonly compressive strength is measured [19, 23, 24]. However, (ultimate) compressive strength is the capacity of a material to withstand compressive loads until complete failure; it thus also depends on the number and size of intrinsic flaws, e.g., pores, which hydraulic calcium silicate cements as powder-liquid mixtures certainly incorporate [35]. We therefore opted to measure fracture toughness, as it describes an intrinsic material property to resist crack propagation and eventually fracture. We employed a single-gradient notch beam (SGNB) FT method [36, 37]; compared with other FT methods, specimen preparation of SGNB FT is simpler and easier to miniaturize [37]. Special care was taken to prepare defect-poor specimen bars by ultra-sonically filling the silicon molds with cement. Upon complete setting for 1 week, a single notch was prepared as standardized defect according to an innovative method introduced by Pongprueksa et al. [37]; the notch area was carefully checked for absence of voids and other defects, upon which the notched specimen bars were loaded until failure in a four-point bending test to determine the mini-FT, thereby representing a reliable test to assess the cements’ mechanical performance [35]. Despite the addition of ZrO2 and irrespective of its concentration, the mechanical properties in terms of FT were not affected. The relative small size of the ZrO2 particles (diameter of ±200 nm) may have resulted in a superior filler packing with the ZrO2 particles filling up the spaces in between the TCS particles, thereby having resulted a refined microstructure. The fact that adding ZrO2 did not affect cement strength correlated well with previous studies in which the addition of ZrO2 to Portland cement did not harm its compressive strength [23, 31].
In terms of bioactivity, adding ZrO2 to TCS enabled to control Ca release with a lower early but a prolonged higher release, this as compared to the Ca release of the commercial cement Biodentine. This controlled Ca release may speculatively be ascribed to the refined microstructure of the experimental TCS cements, potentially preventing Ca to be initially resealed massively; the actual working mechanism behind this controlled Ca release, however, remains to be determined. Overall, the experimental TCS 50 cement demonstrated a continuous and constant release of Ca during a period of 1 month. Our Ca release assessment appeared valid, as the Ca release data for Dycal were in agreement with a previous study that showed a stable and low Ca release at neutral pH [38].
The distribution of the cement ingredients and the hydration characteristics of the different experimental TCS cements were evaluated by high-resolution Feg-SEM/EPMA chemical-element mapping and corresponding back-scattered electron (BSE) imaging of the ion-beam polished cement cross-sections. The ZrO2 particles were smaller and more evenly distributed within the experimental cements, as compared to the structure of Biodentine. All the experimental and commercial cements investigated exhibited similar hydration features, basically containing particles with an un-reacted core surrounded by a hydration zone, in which no precipitation of zirconium could be detected; these structural hydration effects were also identified in previous research [28]. Hence, besides not affecting mechanical properties, adding ZrO2 to TCS appeared also not to participate in/interfere with the hydration reaction of TCS.
Finally, the effect of adding ZrO2 to TCS was evaluated with respect to biocompatibility by subjecting the cements to a cytotoxicity test that involved the assessment of cell viability of HPFs after being exposed to extracts of cement blocks. The results from the mini-FT and Ca release tests revealed that TCS 50 is the most suitable cement formulation. Therefore, only TCS 0 and TCS 50 were selected for the cytotoxicity test; TCS 50 contains the largest amount of ZrO2, and TCS 0 contains pure TCS. It is therefore assumed that the other experimental cements would behave regarding biocompatibility somewhere in between that of TCS 50 and TCS 0. A series of diluted extracts were prepared to observe a possible dose-response relationship. HPFs were selected as test cells based on the clinical indication of using TCS cements for pulp capping. XTT assay showed that the viability of HPFs exposed to the cement extracts was highly dependent on the dilution rate. All the undiluted cement extracts exhibited initial (24 h) cytotoxicity, which decreased with a longer extraction time. Among all cements, TCS 50 appeared least cytotoxic regardless of the extraction time. Our results confirmed previous findings that showed that ZrO2 improved the in vitro biocompatibility of TCS cements [32, 34]. The difference in cytotoxicity found among the cements investigated in this study may be due to the specific chemical composition as well as solubility of each individual cement. TCS 0, which consists of pure TCS, was more biocompatible than Biodentine, indicating that other components contained in Biodentine, like calcium carbonate or iron oxide, may affect cell vitality of HPFs. A previous study using ICP-MS revealed a high level of iron release (711.7 ± 267.9 g/L) from Biodentine upon 7 days of storage in distilled water [19]. Moreover, traces of arsenic, lead, and chromium were detected in elutes from Biodentine [39]. The biocompatibility of the light-curing resin-based calcium silicate cement TheraCal LC was sparsely reported on in literature. Only one study reported that TheraCal LC presented with a low cytocompatibility in direct contact with rat odontoblast-like cells (MDPC-23), this as compared to Biodentine [40]. In that study, the 24-h undiluted TheraCal LC extracts were slightly less cytotoxic than the 24-h undiluted Biodentine. This difference may be due to the different evaluation method used; we investigated cement extracts versus cement blocks in the former study [40]. The cytotoxicity of resin-based materials is commonly attributed to leaching out of unpolymerized monomers due to incomplete polymerization [41, 42]. Polyethylene glycol dimethacrylate (PEG-DMA) is the organic component present in TheraCal LC. Calcium hydroxide has traditionally been the standard pulp-capping material [43, 44]. Its initial cytotoxic effect has been shown previously [40, 45–47] and should be ascribed to its high alkalinity (pH ≈ 12). Along with calcium hydroxide’s disinfection potential, the simultaneous induction of necrosis at the superficial pulp is thought to initiate an inflammatory response that, in the absence of bacteria, will heal by forming a “dentin bridge.”
Conclusion
In view of the above, ZrO2 can be added in relatively large quantity to TCS without affecting the mechanical properties in terms of mini-FT; Ca release was reduced initially, to reach a higher and longer release rate thereafter; adding ZrO2 made TCS cements more biocompatible. It is concluded that adding ZrO2 to TCS improved some key properties in function of its indication as dentin-replacement and/or pulp-repair material.
References
Torabinejad M, Chivian N (1999) Clinical applications of mineral trioxide aggregate. J Endod 25:197–205
Parirokh M, Torabinejad M (2010) Mineral trioxide aggregate: a comprehensive literature review—part III: clinical applications, drawbacks, and mechanism of action. J Endod 36:400–403. doi:10.1016/j.joen.2009.09.009
Qi YP, Li N, Niu LN, Primus CM, Ling JQ, Pashley DH, Tay FR (2012) Remineralization of artificial dentinal caries lesions by biomimetically modified mineral trioxide aggregate. Acta Biomater 8:836–842. doi:10.1016/j.actbio.2011.10.033
Bozeman TB, Lemon RR, Eleazer PD (2006) Elemental analysis of crystal precipitate from gray and white MTA. J Endod 32:425–428
Gandolfi MG, Taddei P, Siboni F, Modena E, Ciapetti G, Prati C (2011) Development of the foremost light-curable calcium-silicate MTA cement as root-end in oral surgery. Chemical-physical properties, bioactivity and biological behavior. Dent Mater 27:e134–e157. doi:10.1016/j.dental.2011.03.011
Camilleri J, Sorrentino F, Damidot D (2013) Investigation of the hydration and bioactivity of radiopacified tricalcium silicate cement, Biodentine and MTA Angelus. Dent Mater 29:580–593. doi:10.1016/j.dental.2013.03.007
Niu LN, Jiao K, Wang TD, Zhang W, Camilleri J, Bergeron BE, Feng HL, Mao J, Chen JH, Pashley DH, Tay FR (2014) A review of the bioactivity of hydraulic calcium silicate cements. J Dent 42:517–533. doi:10.1016/j.jdent.2013.12.015
Prati C, Gandolfi MG (2015) Calcium silicate bioactive cements: biological perspectives and clinical applications. Dent Mater 31:351–370. doi:10.1016/j.dental.2015.01.004
Chen Z, Cao S, Wang H, Li Y, Kishen A, Deng X, Yang X, Wang Y, Cong C, Wang H, Zhang X (2015) Biomimetic remineralization of demineralized dentine using scaffold of CMC/ACP nanocomplexes in an in vitro tooth model of deep caries. PLoS One 14:e0116553. doi:10.1371/journal.pone.0116553
Gandolfi MG, Taddei P, Siboni F, Modena E, De Stefano ED, Prati C (2011) Biomimetic remineralization of human dentin using promising innovative calcium-silicate hybrid “smart” materials. Dent Mater 27:1055–1069. doi:10.1016/j.dental.2011.07.007
Camilleri J, Montesin FE, Brady K, Sweeney R, Curtis RV, Ford TR (2005) The constitution of mineral trioxide aggregate. Dent Mater 21:297–303
Schembri M, Peplow G, Camilleri J (2010) Analyses of heavy metals in mineral trioxide aggregate and Portland cement. J Endod 36:1210–1215. doi:10.1016/j.joen.2010.02.011
Monteiro Bramante C, Demarchi AC, de Moraes IG, Bernadineli N, Garcia RB, Spangberg LS, Duarte MA (2008) Presence of arsenic in different types of MTA and white and gray Portland cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 106:909–913. doi:10.1016/j.tripleo.2008.07.018
Camilleri J (2011) Characterization and hydration kinetics of tricalcium silicate cement for use as a dental biomaterial. Dent Mater 27:836–844. doi:10.1016/j.dental.2011.04.010
Chen CC, Ho CC, David Chen CH, Ding SJ (2009) Physicochemical properties of calcium silicate cements for endodontic treatment. J Endod 35:1288–1291. doi:10.1016/j.joen.2009.05.036
Dong Z, Chang J, Deng Y, Joiner A (2011) Tricalcium silicate induced mineralization for occlusion of dentinal tubules. Aust Dent J 56:175–180. doi:10.1111/j.1834-7819.2011.01321.x
Zhao W, Wang J, Zhai W, Wang Z, Chang J (2005) The self-setting properties and in vitro bioactivity of tricalcium silicate. Biomaterials 26:6113–6121
Corral Nunez CM, Bosomworth HJ, Field C, Whitworth JM, Valentine RA (2014) Biodentine and mineral trioxide aggregate induce similar cellular responses in a fibroblast cell line. J Endod 40:406–411. doi:10.1016/j.joen.2013.11.006
Jang YE, Lee BN, Koh JT, Park YJ, Joo NE, Chang HS, Hwang IN, Oh WM, Hwang YC (2014) Cytotoxicity and physical properties of tricalcium silicate-based endodontic materials. Restor Dent Endod 39:89–94. doi:10.5395/rde.2014.39.2.89
Tran XV, Gorin C, Willig C, Baroukh B, Pellat B, Decup F, Opsahl Vital S, Chaussain C, Boukpessi T (2012) Effect of a calcium-silicate-based restorative cement on pulp repair. J Dent Res 91:1166–1171. doi:10.1177/0022034512460833
Song JS, Mante FK, Romanow WJ, Kim S (2006) Chemical analysis of powder and set forms of Portland cement, gray ProRoot MTA, white ProRoot MTA, and gray MTA-Angelus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 102:809–815
Coomaraswamy KS, Lumley PJ, Hofmann MP (2007) Effect of bismuth oxide radioopacifier content on the material properties of an endodontic Portland cement-based (MTA-like) system. J Endod 33:295–298
Antonijevic D, Medigovic I, Zrilic M, Jokic B, Vukovic Z, Todorovic L (2014) The influence of different radiopacifying agents on the radiopacity, compressive strength, setting time, and porosity of Portland cement. Clin Oral Investig 18:1597–1604
Hungaro Duarte MA, Minotti PG, Rodrigues CT, Zapata RO, Bramante CM, Tanomaru Filho M, Vivan RR, Gomes de Moraes I, Bombarda de Andrade F (2012) Effect of different radiopacifying agents on the physicochemical properties of white Portland cement and white mineral trioxide aggregate. J Endod 38:394–397. doi:10.1016/j.joen.2011.11.005
Camilleri J, Montesin FE, Papaioannou S, McDonald F, Pitt Ford TR (2004) Biocompatibility of two commercial forms of mineral trioxide aggregate. Int Endod J 37:699–704
Gomes Cornelio AL, Salles LP, Campos da Paz M, Cirelli JA, Guerreiro-Tanomaru JM, Tanomaru Filho M (2011) Cytotoxicity of Portland cement with different radiopacifying agents: a cell death study. J Endod 37:203–210. doi:10.1016/j.joen.2010.11.017
Marciano MA, Costa RM, Camilleri J, Mondelli RF, Guimaraes BM, Duarte MA (2014) Assessment of color stability of white mineral trioxide aggregate angelus and bismuth oxide in contact with tooth structure. J Endod 40:1235–1240. doi:10.1016/j.joen.2014.01.044
Camilleri J, Cutajar A, Mallia B (2011) Hydration characteristics of zirconium oxide replaced Portland cement for use as a root-end filling material. Dent Mater 27:845–854. doi:10.1016/j.dental.2011.04.011
Guerreiro Tanomaru JM, Storto I, Da Silva GF, Bosso R, Costa BC, Bernardi MI, Tanomaru-Filho M (2014) Radiopacity, pH and antimicrobial activity of Portland cement associated with micro- and nanoparticles of zirconium oxide and niobium oxide. Dent Mater J 33:466–470
Hungaro Duarte MA, de Oliveira El Kadre GD, Vivan RR, Guerreiro Tanomaru JM, Tanomaru Filho M, de Moraes IG (2009) Radiopacity of Portland cement associated with different radiopacifying agents. J Endod 35:737–740. doi:10.1016/j.joen.2009.02.006
Cutajar A, Mallia B, Abela S, Camilleri J (2011) Replacement of radiopacifier in mineral trioxide aggregate; characterization and determination of physical properties. Dent Mater 27:879–891. doi:10.1016/j.dental.2011.04.012
Li Q, Deacon AD, Coleman NJ (2013) The impact of zirconium oxide nanoparticles on the hydration chemistry and biocompatibility of white Portland cement. Dent Mater J 32:808–815
Silva GF, Tanomaru-Filho M, Bernardi MI, Guerreiro-Tanomaru JM, Cerri PS (2015) Niobium pentoxide as radiopacifying agent of calcium silicate-based material: evaluation of physicochemical and biological properties. Clin Oral Investig 19:2015–2025. doi:10.1007/s00784-015-1412-9
Silva GF, Bosso R, Ferino RV, Tanomaru-Filho M, Bernardi MI, Guerreiro-Tanomaru JM, Cerri PS (2014) Microparticulated and nanoparticulated zirconium oxide added to calcium silicate cement: evaluation of physicochemical and biological properties. J Biomed Mater Res A 102:4336–4345. doi:10.1002/jbm.a.35099
Zhang J, Liu W, Schnitzler V, Tancret F, Bouler JM (2014) Calcium phosphate cements for bone substitution: chemistry, handling and mechanical properties. Acta Biomater 10:1035–1049. doi:10.1016/j.actbio.2013.11.001
Wan D, Bao Y, Peng J, Zhou Y (2009) Fracture toughness determination of Ti3Si(Al)C2 and Al2O3 using a single gradient notched beam (SGNB) method. J Eur Cera Soc 29:763–771. doi:10.1016/j.jeurceramsoc.2008.06.031
Pongprueksa P, De Munck J, Karunratanakul K, Barreto BC, Van Ende A, Senawongse P, Van Meerbeek B (2016) Dentin bonding testing using a mini-interfacial fracture toughness approach. J Dent Res 95:327–333
Gandolfi MG, Siboni F, Prati C (2012) Chemical-physical properties of TheraCal, a novel light-curable MTA-like material for pulp capping. Int Endod J 45:571–579. doi:10.1111/j.1365-2591.2012.02013.x
Camilleri J, Kralj P, Veber M, Sinagra E (2012) Characterization and analyses of acid-extractable and leached trace elements in dental cements. Int Endod J 45:737–743. doi:10.1111/j.1365-2591.2012.02027.x
Poggio C, Arciola CR, Beltrami R, Monaco A, Dagna A, Lombardini M, Visai L (2014) Cytocompatibility and antibacterial properties of capping materials. Scientific World J 2014:181945. doi:10.1155/2014/181945
Caughman WF, Caughman GB, Shiflett RA, Rueggeberg F, Schuster GS (1991) Correlation of cytotoxicity, filler loading and curing time of dental composites. Biomaterials 12:737–740
Stanislawski L, Daniau X, Lauti A, Goldberg M (1999) Factors responsible for pulp cell cytotoxicity induced by resin-modified glass ionomer cements. J Biomed Mater Res 48:277–288
Schröder U (1985) Effects of calcium hydroxide-containing pulp-capping agents on pulp cell migration, proliferation, and differentiation. J Dent Res 64 Spec No:541–8
Cvek M (1978) A clinical report on partial pulpotomy and capping with calcium hydroxide in permanent incisors with complicated crown fracture. J Endod 4:232–237
Min KS, Kim HI, Park HJ, Pi SH, Hong CU, Kim EC (2007) Human pulp cells response to Portland cement in vitro. J Endod 33:163–166
Furey A, Hjelmhaug J, Lobner D (2010) Toxicity of flow line, Durafill VS, and Dycal to dental pulp cells: effects of growth factors. J Endod 36:1149–1153. doi:10.1016/j.joen.2010.03.013
Hirschman WR, Wheater MA, Bringas JS, Hoen MM (2012) Cytotoxicity comparison of three current direct pulp-capping agents with a new bioceramic root repair putty. J Endod 38:385–388. doi:10.1016/j.joen.2011.11.012
Acknowledgments
Xin Li’s study at KU Leuven is supported by the China Scholarship Council (File No. 201206270126). The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author Xin Li declares that she has no conflict of interest. Author Kumiko Yoshihara declares that she has no conflict of interest. Author Jan De Munck declares that he has no conflict of interest. Author Stevan Cokic declares that he has no conflict of interest. Author Pong Pongprueksa declares that he has no conflict of interest. Author Eveline Putzeys declares that she has no conflict of interest. Author Mariano Pedano declares that he has no conflict of interest. Author Zhi Chen declares that he has no conflict of interest. Author Kirsten Van Landuyt declares that she has no conflict of interest. Author Bart Van Meerbeek declares that he has no conflict of interest.
Funding
Drs. Xin Li’s study at KU Leuven is supported by the China Scholarship Council (File No. 201206270126).
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Li, X., Yoshihara, K., De Munck, J. et al. Modified tricalcium silicate cement formulations with added zirconium oxide. Clin Oral Invest 21, 895–905 (2017). https://doi.org/10.1007/s00784-016-1843-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-016-1843-y