Abstract
This study investigated the protective effect of long-term treatment with the bisphosphonate zoledronic acid on bone mass, structure, and strength in adult, estrogen-deficient rats. Rats were ovariectomized (OVX) at the age of 4 months and divided into four groups of 20 rats: one group of saline-treated OVX controls, and three groups of OVX rats treated with 0.3, 1.5, or 7.5 μg/kg/week s.c. zoledronic acid (ZOL). An additional group of sham-operated, saline-treated rats served as normal controls. Biochemical assays were performed after 16 and 51 weeks, respectively, and bone mineral density (BMD) determinations after 17 and 52 weeks, respectively. Before the end of the experiment animals were injected with tetracyclines for the determination of dynamic bone indexes. Finally, animals were sacrificed after 52 weeks, and vertebral bones (LV5) were subjected to mechanical compression testing. LV4 were used for histology and LV2 for microcomputed tomography. ZOL treatment abolished the rise of osteocalcin and reduced urinary deoxypyridinoline excretion. BMD was reduced in the OVX group in comparison to sham controls, and the decline was dose-dependently prevented by ZOL treatment. Tetracycline labeling showed a significant increase in bone formation rate (BFR) in OVX rats which was abolished by ZOL treatment. The same was observed for osteoid perimeter (Os.Pm) suggesting that ZOL diminished the high bone turnover associated with estrogen deficiency. Architectural parameters (BV/TV, Tb.Th*, Tb.N*, Tb.Sp*, SMI, CD) underwent the expected changes toward structural deterioration which was completely prevented by ZOL administration at doses of 1.5 and 7.5 μg/kg/week s.c. Similar results were obtained in compression testing: maximum stress fell significantly after OVX, and this effect was effectively prevented by ZOL treatment. Regression analysis suggests that in this rat model, SMI and Tb.Th* significantly contribute to compressive strength, albeit to a smaller degree than total cross-sectional area. The data further suggest that in the aged OVX rat, SMI and TB.Th* change in an interdependent way. ZOL prevents this process by inhibiting plate thinning and the transition into rod-shaped trabeculae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bisphosphonates are potent inhibitors of bone resorption and are therapeutically used in a variety of clinical conditions in which elevated osteoclastic activity is a major contributing factor. Among these conditions are hypercalcemia of malignancy, bone metastases, Paget’s disease of bone, and osteoporosis. Following the first-generation bisphosphonates etidronate and clodronate, more potent second-generation aminobisphosphonates such as pamidronate and alendronate were introduced into the clinic, and more recently, the third generation of highly potent nitrogen-containing heterocyclic bisphosphonates were developed [1]. ZOL is among these most potent heterocyclic bisphosphonates [2] and is already used to treat hypercalcemia of malignancy and bone metastases, regardless of tumour type [3]. Although ZOL has been widely investigated in a variety of animal models, no detailed study has yet been reported on the effects of chronic administration on vertebral bone in a rodent model of postmenopausal bone loss. The mature, ovariectomized (OVX) rat rapidly develops a high bone turnover state that leads to significant bone loss within weeks and continues for several months [4]. We used this model to investigate the effects of long-term administration of ZOL to OVX rats and report here on the structural changes and accompanying mechanical effects observed in their vertebrae. In essence, ZOL administration reduced the high bone turnover caused by estrogen deficiency as shown by a reduction in osteocalcin and deoxypyridinium levels in serum or urine, respectively, increased trabecular and cortical bone structural indexes in vertebrae, and significantly increased maximum stress in compression. Multiregression analysis revealed a significant correlation between mechanical strength and cross-sectional area, structure model index (SMI), and trabecular thickness. These results indicate that ZOL reduces the transition of trabecular plates to rods by inhibiting thinning and fenestrations as an end result of unabated high bone turnover.
Materials and methods
Animals
A total of 144 female Sprague Dawley rats, 3 months old, were housed under standard laboratory conditions (temperature 21±2ºC, humidity 55±10%, lighting cycle 12 h light/12 h dark). All animals had free access to tap water and were fed a diet containing 0.71% calcium, 0.5% phosphorus, and 600 IU/kg vitamin D3. Whole body BMD was determined by dual-energy X-ray absorptiometry (DXA), and those animals furthest from the mean were discarded to leave a pool of 100. These animals were then divided into five groups of 20 so that the group mean, whole body BMD values were equal. Ovariectomy on four groups (OVX), or a sham operation on the fifth group (SHAM), was performed under anesthesia (midazolam/fentanyl/fluanisone mixture i.p.) when the rats were 4 months old. Immediately after surgery, while the animals were still anesthetized, treatment of the four OVX groups was initiated with ZOL (dissolved in sterile saline) at doses of 0, 0.3, 1.5, and 7.5 µg/kg, injected s.c. (1 ml/kg) once a week for 52 weeks. The SHAM control group received saline injections at an identical frequency. Food consumption was recorded weekly for the SHAM group, and this amount of food was then fed to the OVX rats during the following week. Body weight was recorded at weekly intervals throughout the study, and the wet weight of the uterus was recorded at necropsy. Vertebrae were removed and freed of soft tissue, and the LV5 vertebrae were wrapped in gauze soaked in Ringer’s solution and stored frozen for later biomechanical testing. The LV4 and LV2 vertebrae, destined for histomorphometric or tomographic analysis, were stored in 70% ethanol.
DXA studies
Prior to surgery, the rats were subjected to a whole body as well as spine DXA scan using a Lunar DPX-L and associated small animal software. BMC and BMD were then calculated. Total body BMD was used for randomization and stratification so that initial group means were approximately equal. Rats received further total body and spine scans during week 17 and 52 after commencement of dosing. The interassay coefficient of variation (CV) was 2.5%. Accuracy was determined by correlation of spinal ash weight with spinal BMC yielding r 2=0.67, p<0.003.
Microcomputed tomography
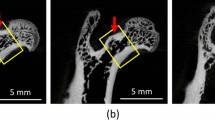
Lumbar vertebrae (LV2) of 10 rats were placed longitudinally into sample holders of a microcomputer tomograph (μCT-20, Scanco Medical, Bassersdorf, Switzerland) [5]. The vertebrae were scanned perpendicularly to their longitudinal axis by a fixed X-ray fan beam (10-μm spot-size tube, 0.1 mA, 50 kVp) while the holder rotated along its axis. For 3-D data accumulation, the sample was moved incrementally along its axis after each turn. A total of 500 slices (1,024×1,024 pixel matrix per slice) of 13-μm thickness were determined, yielding a voxel size of 13×13×13 μm3. The resulting scan length of 6.5 mm was sufficient to cover the secondary spongiosa between both growth plates of the vertebrae. The innermost growth plate areas at both ends of the vertebral bodies (“visible” as empty clefts) were used to position a core volume of interest for 3-D reconstruction. A carefully longitudinally centered cancellous core of 300 slices of 13-μm thickness (3.9-mm length) was finally evaluated with the μCT software of Scanco Medical to determine parameters of bone morphometry. By these means the primary spongiosa at both ends was excluded from measurement. The spatial resolution with the given settings of the instrument was 24 μm; precision of repeated measurements was below 1%. Threshold settings were optimized with histomorphometric methods [6]. Total vertebral bone cross-sectional area, and cortical and trabecular bone cross-sectional areas (CSA) were determined in slice 150 in the center of the vertebral body (Fig. 1) where its diameter and bone volume fraction are minimal [6]. In 3-D mode, TV, BV, BS, Tb.N*, Tb.Sp*, Tb.Th*, and structure model index (SMI) were measured in the volume of interest after removal of cortical bone with a semiautomatic morphing procedure (Fig. 1). Tb.N*, Tb.Sp*, and Tb.Th* denote model-independent 3-D calculations which have been developed by Hildebrand and Rüegsegger [7]. SMI is a measure for the prevalence of plate-like or rod-like trabecular structures, whereby 0 represents “plates and 3 rods,” respectively [8]. Connectivity density (CD) was derived from the trabecular 3-D structure according to Odgaard and Gundersen [9].
Microtomographic reconstruction of a lumbar vertebra. A The central, 3.9-mm long part of LV2 (corresponding to 300 sections of 13-μm thickness) is shown from the dorsocaudal side. B1 Trabecular bone core of a vertebral body of a sham-operated control animal showing dense, connected plates, whereas B2 shows a core of a OVX rat with looser and more rod-like structures. Corresponding midcore cross-sectional areas are shown in C. Trabeculae of OVX rat (C2) are thinner and more rod-shaped than trabeculae of a sham-operated control rat (C1)
Histology
For the measurement of longitudinal bone growth and bone formation rate, bone-seeking fluorescent markers were injected into all rats. One day before starting treatment, the animals received calcein (20 mg/kg i.p.) to label preexisting bone. Toward the end of the treatment period, another injection of calcein (20 mg/kg i.p.) was given 17 days before necropsy, followed by two injections of demeclocycline (20 mg/kg i.p.) at 14 and 4 days before necropsy. Histomorphometry was performed on lumbar vertebrae (LV4) from 10 animals of each group using the procedures described previously in a study on the effects of short-term ZOL treatment in young, intact rats [10]. In brief, serial sections of 8-μm thickness were cut on a Polycut S microtome (Reichert & Jung, Germany) from the methylmethacrylate-embedded vertebral material. Sections from undecalcified bone were stained by the method of Goldner and von Kossa, decalcified bone sections were stained with ponceau acid–fuchsin. Static (Os.Pm) and dynamic (sL.Pm, dL.Pm, BFR, MAR) histomorphometric parameters were determined for cancellous bone at the proximal and distal end of the fourth vertebrae at least 1 mm distal from the growth plate such that no primary spongiosa was included in the measurements. The evaluations were performed semiautomatically with a Leitz DMRBE microscope equipped with a TV camera and a Quantimet 500 image analysis system [10]. Fluorescence measurements were done with a Leica Microvid system connected to a Orthoplan fluorescence microscope of Leitz Microsystems (Switzerland). IrL.Wi was directly determined with the Microvid system. Osteoid and labeled perimeters were quantified by means of the intersection counting method using a Merz test grid (10 lines) as an eye piece reticule at ×250 magnification. Labeled perimeter [L.Pm=(sL.Pm/2)+sL.Pm] and BFR (μm3/μm2/day, surface referent) were calculated according to Frost et al. [11].
Biochemical measurements
For the determination of biochemical markers of bone metabolism, blood (serum and plasma) was collected prior to ovariectomy and during weeks 16 and 51 of ZOL treatment. Alkaline phosphatase (ALP) activity and creatinine were determined with a Technion RA-1000 using standard RA-1000 methodology. Osteocalcin was determined with a rat-specific RIA kit (Biogenesis, Poole, UK) according to the manufacturer’s instructions (inter- and intra-assay coefficient of variation 12% and 7%, respectively). Total deoxypyridinium crosslink determinations (interassay and intra-assay coefficient of variation 13% and 6%, respectively) were performed by the Health and Safety Executive, Occupational Medicine & Hygiene Laboratory, Sheffield, UK, by the method of Kollerup et al. [12] and a fully automated HPLC system based on Yoshimura et al. [13].
Mechanical testing
Prior to testing, the frozen bones (LV5) were equilibrated overnight in Ringer’s solution at room temperature. Spinous and transverse processes were removed from each vertebra using a diamond saw. In addition, the articular surfaces were removed in such a way as to provide two flat, parallel faces for compression testing [14]. The vertebrae were mounted between the faces of a compression jig and loaded to failure. All specimens were tested completely immersed in Ringer’s solution with a load applied at a constant rate of 2 mm/min by an Instron 1185 testing machine. The maximum stress σmax (Fmax/CSA) and stiffness were calculated from the stress-strain curve.
Compound
Zoledronic acid—(1-hydroxy-2-imidazol-1-yl-phosphonoethyl) phosphonic acid—was supplied by Novartis Pharma as the hydrated disodium salt and dissolved in sterile saline (dosing volume 1 ml/kg body weight) before subcutaneous (s.c.) administration.
Statistics
Data from the DXA scans, and biochemical and mechanical testing were analyzed using parametric methods. Normality of distribution was tested with the method of Shapiro-Wilks (values of W did not reach significance), followed by ANOVA to test for changes in the parameters of interest (SAS statistics software, SAS Institute, Cary, NC, USA). Variance stabilizing transformation was used as required. Histomorphometric data were analyzed by the method of Kruskal-Wallis followed by the Mann-Whitney test with the Bonferroni adjustment. Nonparametric tests, regressions, and correlations were calculated with the statistics software Prism 3 and Instat of GraphPad, San Diego, CA, USA.
Results
All rats were tested for completeness of ovariectomy by determination of plasma estradiol. In all groups, except the sham-operated animals, estradiol levels were significantly reduced or below the detection limit of the assay (data not shown). The body weights of OVX rats increased significantly within the first 5 weeks after surgery despite pair feeding (data not shown).
Biochemical markers
Sixteen weeks after ovariectomy, rats showed significantly increased levels of osteocalcin (Fig. 2A) and alkaline phosphatase activity (1.5-fold increase, data not shown) in their serum consistent with the increased bone turnover following estrogen deprivation. ZOL inhibited the elevation of osteocalcin levels to maintain approximately normal levels at all doses tested. At the end of the experiment at 51 weeks, no significant effects on the levels of osteocalcin (Fig. 2B) and alkaline phosphatase (data not shown) were seen under ZOL treatment. Bone resorption indexes (deoxypyridinium crosslinks / creatinine ratio, means ± SEM) were significantly elevated after 16 weeks from 0.32±0.05 in sham-operated rats to 0.87±0.08 in OVX rats. Treatment with increasing doses of ZOL dose-dependently and significantly (p<0.01) inhibited this increase to 0.42±0.03, 0.28±0.02, and 0.18±0.01, respectively. This pattern did not change throughout the experiment: ratios remained increased after 51 weeks from 0.34±0.03 in the sham-operated rats to 0.98±0.07 in OVX rats. ZOL reduced the levels significantly (p<0.01) with increasing doses to 0.34±0.02, 0.22±0.02, and 0.14±0.01, respectively. These data demonstrate a continuing antiresorptive effect of ZOL throughout the experiment.
Serum concentrations of osteocalcin 16 and 51 weeks after OVX. Weekly doses of zoledronic acid (Z) in μg/kg. Note the different scale of the y-axis in A and B. Statistics: means ± SEM (15–20 rats per group), ANOVA, and Dunnett post test; § p<0.05; §§ p<0.01 in comparison to sham-operated rats; *p<0.05; **p<0.01 in comparison to ovariectomized (OVX) rats
Dynamic bone histomorphometry
The enhanced bone turnover following ovariectomy was also reflected at the vertebral tissue level by fluorochrome labeling measurements (Table 1). Ovariectomy increased double-labeled perimeter, BFR, and osteoid perimeter (Fig. 3B), but MAR was not significantly changed (Fig. 3A). Weekly treatment for 52 weeks with ZOL dose-dependently diminished these parameters, but even at the highest dose of 7.5 μg/kg, no complete suppression of tetracycline labeling occurred. The data show a persistently increased organic matrix formation (Os.Pm) and mineral deposition (BFR) in 16-month-old ovariectomized rats which were dose-dependently inhibited by 52 weeks of ZOL administration.
Indexes of vertebral cancellous bone. A Mineral apposition rate (MAR), B osteoid perimeter (Os.Pm), C trabecular number (Tb.N*), D trabecular thickness (Tb.Th*), E trabecular separation (Tb.Sp*), F bone volume fraction (BV/TV), G structure model index (SMI), H connectivity density (Connectivity Dens.). Bars represent means ± SEM measured in LV2, 5–10 rats per group. Statistics: Kruskal-Wallis test. § p<0.05; §§ p<0.01; §§§ p<0.001 in comparison to sham-operated rats; *p<0.05; **p<0.01; ***p<0.001 in comparison to OVX rats
Vertebral bone mineral density
BMD increased during the initial 4 months of treatment and thereafter declined in sham-operated control rats as a consequence of aging (Fig. 4). OVX rats did not gain BMD for the first 17 weeks and lost BMD at later stages at a slightly higher rate than sham controls. ZOL treatment completely prevented the effects of OVX during the early phase irrespective of the dose given. After 17 weeks of treatment, however, a marked difference was noted in the lowest dose group of ZOL, which lost efficacy. The animals of this group lost vertebral BMD in parallel with the OVX group. The higher doses of ZOL completely prevented the loss of BMD at the later stage, and ZOL-treated rats retained the same BMD as sham-operated controls.
Time course of spinal bone mineral density. Baseline values were determined 1 week before zoledronic acid (ZOL) administration started. Sham-operated rats (circles); OVX rats (squares); ZOL-treated rats, 0.3 μg/kg (inverted triangles), 1.5 μg/kg (diamonds), 7.5 μg/kg (triangles). Means of 16–20 rats; error bars SEM. Statistics: Change in group mean results in comparison to predose scans; *p<0.05, **p<0.01, comparison to OVX rats; § p<0.05; §§ p<0.01; comparison to sham-operated rats
Vertebral architecture
Determination of growth plate thickness (means ± SEM: anterior 8.7 ± 0.2 μm; posterior 9.7 ± 0.4 μm in OVX rats) revealed no significant differences between OVX rats and sham-operated or ZOL-treated rats (data not shown). In the high dose ZOL-treated rats however, a portion of calcified primary spongiosa persisted to the end of the experiment indicating that the rats were still growing when treatment was started. These parts of the cancellous core were carefully excluded from our measurements. In the secondary spongiosa, evaluation of the 3-D trabecular structure revealed a 16% loss of trabecular thickness and a 40% loss of trabecular number in estrogen-deplete animals (Fig. 3C, D) which yielded an overall reduction in trabecular bone volume fraction of 54% and caused a correspondingly wider separation of trabeculae (Fig. 3E, F). In parallel to this, the structure model index (SMI) increased and connectivity density (CD) fell as a reflection of the transition from plate-shaped to rod-shaped trabecular structures with lower connectivity (Fig. 3G, H). The structural deterioration was significantly and dose-dependently counteracted by ZOL treatment: treated rats (1.5 and 7.5 μg/kg/week) maintained structural integrity at the same level as sham-operated control animals. The maximal effect was reached with 1.5 μg/kg/week, at the higher dosage of 7.5 μg/kg/week the effect reached a plateau and did not increase further. The lowest dosage of 0.3 μg/kg/week effected a differing pattern: Tb.Th* and SMI were not different from untreated OVX controls, whereas the other parameters were significantly improved (Fig. 3). The cross-sectional cortical area in the midportion of vertebrae increased significantly under ZOL treatment (Fig. 5A). This part of the vertebral body was not subjected to longitudinal growth during the experiment and is therefore clear evidence that the cortical geometry was positively affected by ZOL treatment in a nongrowing part of the vertebra.
Effect of zoledronic acid treatment (Z, μg/kg/week) on vertebral cross-sectional dimensions and mechanics. A cortical area, C trabecular area, B maximum stress in compression, D correlation of total area (Ct.Ar+Tb.Ar), and maximum stress of all samples. Data show means ± SEM, 5–10 rats per group. CSA was determined in LV2, maximum stress in LV5
Mechanical properties
Changes in maximum compressive stress (Fig. 5B), followed the same pattern as seen for the structural parameters BV/TV, Tb.Th*, and, inversely, SMI (Fig. 3). OVX diminished, and bisphosphonate treatment augmented, compressive strength but only at the intermediate and high doses, not at the low dose. Bone stiffness paralleled very closely the pattern seen with compressive maximum stress: stiffness (means ± SEM) fell from 4,817 ± 854 MPa in sham-operated rats to 2,954 ± 282 MPa in OVX rats. Treatment with 0.3, 1.5, or 7.5 μg/kg s.c. ZOL augmented stiffness to 3,249 ± 653, 5,769 ± 1,060, and 5,233 ± 919 MPa, respectively. The intermediate and high dose groups were statistically significantly different from OVX controls (p<0.05). The highest correlation of compressive stress with structural measures was found for total cross-sectional area (r=0.81) as determined in the center of the bone core where the rat vertebral body has its smallest diameter and lowest trabecular bone volume fraction [6]. This is obviously the mechanically weakest site within the vertebral body predisposed to compressive deformation and failure. The bone volume fraction of the fully reconstructed vertebral core showed a lower correlation (r=0.68) which is caused by the contribution of frontal and distal trabecular bone components which are wider and not located near the critical central position of section z150 (Fig. 1). Multiple regression analysis with maximum stress and the structural parameters revealed significant (p<0.0001) contributions of total CSA, SMI, and Tb.Th* with an overall fit r 2=71.6% (Table 2).
Our data show a positive effect of chronic ZOL administration on deteriorating architectural indexes in the estrogen-deprived rat. ZOL treatment dose-dependently maintained structural integrity and functional competence under compression in lumbar vertebrae.
Discussion
Ovariectomy-induced changes of bone metabolism were reflected by alterations in the serum levels of ALP and osteocalcin. Osteocalcin increased at 16 weeks and normalized at week 51. ZOL administration reduced the rise of osteocalcin in the early phase. In contrast to osteocalcin, levels of deoxypyridinoline were significantly suppressed at 16 and 51 weeks, showing that zoledronic acid exerted its antiresorptive effect throughout the entire time of the experiment.
A strong reduction of bone turnover and particularly of bone forming activity is often considered as potentially harmful because microfractures could accumulate leading to a mechanically inferior bone quality [15]. Our data show that in all cases a residual osteogenic activity was present in ZOL-treated animals (Table 1, Fig. 3), and the time course of spinal BMD (Fig. 4) demonstrated a continuing remodeling activity between 17 and 52 weeks as shown by a continuous and obviously physiological decline of BMD in ZOL-treated rats which paralleled the declining BMD of sham-operated and OVX controls, although at a significantly higher level.
Further evidence for a reduced bone turnover under ZOL treatment came from morphometric parameters obtained by histology and microtomography: all relevant measures indicating a high turnover after OVX, such as L.Pm, BFR, or Os.Pm, were significantly reduced by ZOL treatment, and the accompanying bone volume loss due to unabated osteoclastic activity was reverted (Table 1, Fig. 3B, F). This effect of ZOL was seen in both the cortical (Fig. 5) and cancellous bone fractions, thus increasing bone volume and mechanical strength. It is noteworthy that OVX induced only the loss of trabecular CSA but not cortical CSA. On the other hand, ZOL treatment augmented both measures significantly, therefore cortical thickness was also increased [6]. The lowest dose of ZOL however, exerted no significant effects on Tb.Th*, trabecular CSA, and SMI, and this went in parallel with a lack of effect on maximum stress (Figs. 3 and 5) pointing to a close relation between these parameters as demonstrated by multiple regression analysis (Table 2). CSA contributed most (r 2=65%) to compression strength, SMI and Tb.Th* added only 7% to the final r 2 of 71.6%. In this analysis, CD did not contribute significantly to maximum stress, although a low, but significant, correlation (r 2=0.112, p<0.04) was present between these parameters after 52 weeks. Kinney et al. [16] found a significant increase in rat vertebral trabecular connectivity 17 weeks after OVX which they explained as a consequence of plate perforation and fenestration. Thus connectivity density follows a biphasic pattern: after an initial increase, CD drops when parts of fenestrated plates dissociate into more isolated rods, as originally suggested by Parfitt et al. [17]. In consequence, CD appears to be less suited as a predictor of compressive strength, and a correspondingly more rapid deterioration of another structural element would have to be postulated which would compensate for the initial increase of CD. Maybe a similar situation has occurred in our low dose ZOL group: CD values were completely preserved and even slightly higher (+13%) than in sham-operated controls (Fig. 3H), however, loss of trabecular thickness and transition from plates to rods (SMI) were indistinguishable from OVX vertebrae (Fig. 3D, G), as was maximum stress (Fig. 5B). Therefore our data suggest a relevant role for SMI and Tb.Th* in compression strength, whereas CD appears to be less relevant. The finding is further corroborated by the fact that SMI and Tb.Th* correlate closely with trabecular bone volume, and Tb.Th* nearly linearly decreases with increasing SMI (Fig. 6). This shows a close relation between loss of Tb.Th* and shift toward rod-type structures. Nevertheless the data show a high prevalence of plates (SMI=0) in the aged rat vertebral body. Mitton et al. [18] found in vertebral cancellous bone of ewes a correlation (r=0.72) between maximum compressive strength and Tb.Th comparable to that reported here for rats, and, also in line with our findings, no correlation with Tb.N could be established.
A better correlation with maximum stress was found for the 2-D CSA (r 2=0.65, p<0.0001) derived from a single, 13-μm thin central slice than for the whole trabecular BV/TV (r 2=0.58, p<0.0001) derived from 300 slices (corresponding to a length of 3.9-mm vertebral bone; Fig. 1). The explanation is most probably that the total CSA includes the cortical component (which is the dominant part in compression testing), whereas BV/TV (Fig. 3F) comprises only trabecular bone which per se contributes only approximately 25% to the stiffness [16]. Furthermore, the data of this study were not derived from identical vertebrae: microtomography was done with LV2, and compression testing with LV5. This divergence adds some additional inaccuracy. Similar findings have been reported for other bisphosphonates: vertebrae of long-term alendronate-treated OVX rats demonstrated a significant correlation between ultimate load and bone area fraction [19, 20], and in lifelong ibandronate-treated rats, ultimate load, stiffness, and BMD increased dose-dependently [21].
We have used intact vertebrae instead of cancellous cores for mechanical testing. At an age of 1.5 years, OVX Sprague Dawley rats have very little cancellous bone in the central part of the vertebral body, particularly in the dorsal area adjacent to the neural canal, and it is therefore very difficult to obtain intact cores suitable for testing. Under compression, an intact vertebra restricts lateral movements of trabeculae and therefore affects strength in comparison to an isolate core. Further consideration should be given to the plano-parallel machining of both ends, a procedure which is frequently used for controlled force transmission in vertebrae [14]. High dose ZOL treatment resulted in a dense layer of remnant primary spongiosa which was very hard (as indicated by the difficulties during cutting of LV4 with the microtome). This part was mostly removed by the machining as it was of limited interest for our study. The cutting of trabeculae at the ends can lead to additional strain at the boundaries to the loading platens, potentially increasing the strain measured and finally reducing stiffness. Despite these methodological limitations, we consider our data as representative, as we were more interested in a comparison of identically treated specimens than in physical accuracy.
At the cancellous level, ZOL treatment effectively preserved structures by inhibiting plate fenestration and thinning, processes which also lead to mechanical weakening of human vertebrae. Thomsen et al. [22] investigated the relationship between maximum stress and static histomorphometric measurements in human vertebral bodies and found correlation values for BV/TV (r=0.86) higher than those that we found for CSA (r=0.81). In their data set, Tb.Th (r=0.2) was not significantly correlated with bone strength, but Tb.N (r=0.85) and CD (r=0.68) were. Our rat data showed a different relationship: maximum stress correlated equally well with Tb.Th* (r=0.67, p<0.0001) and with Tb.N* (r=0.66, p<0.0001), but CD (r=0.33, p<0.040) clearly had less functional relationship to compression strength. This points to a difference between human and rat trabecular structures: in human vertebrae, perforation and fenestration would lead to rod structures with a thickness similar to that of the original plates [23], whereas in the OVX rat, plate thinning and transition from plate to rod-shaped cancellous bone goes in parallel. These processes, leading to osteopenia and bone weakening, are effectively and dose-dependently suppressed by ZOL treatment, yielding vertebral structures more resistant to compression. Mechanistically, these effects are brought about by inhibition of osteoclastic bone resorption [1]. Nitrogen-containing bisphosphonates such as ZOL are powerful inhibitors of intra-cellular prenylation steps which are crucial for osteoclast function [1]. Appropriately dosed, bisphosphonates efficiently block the resorption process, and as a consequence, reduce accelerated bone turnover which follows estrogen deficiency. In recent years, several new bisphosphonates have been developed, such as alendronate, risedronate, and ibandronate. Comparable long-term studies in rats and mice show that these and other bisphosphonates improve mechanical properties in vertebrae [19, 20, 21] and long bones [24]. In our study, ZOL also showed positive effects on femoral strength in bending tests [25]. The latter effect is probably achieved by the inhibition of endosteal resorption while periosteal apposition is unaffected [24]. In summary, our data show that long-term zoledronic acid treatment effectively prevents the structural deterioration of vertebral bone in estrogen-depleted rats and significantly increases its compressive strength and stiffness.
References
Russell RGG, Rogers MJ (1999) Bisphosphonates: from the laboratory to the clinic and back again. Bone 25:97–106
Green JR, Müller K, Jaeggi KA (1994) Preclinical pharmacology of CGP42’446, a potent, heterocyclic bisphosphonate compound. J Bone Miner Res 9:745–751
Wellington K, Goa KL (2003) Zoledronic acid: a review of its use in the management of bone metastases and hypercalcaemia of malignancy. Drugs 63:417–437
Wronsky TJ, Dann LM, Horner (1989) Time course of vertebral osteopenia in ovariectomized rats. Bone 10:295–301
Rüegsegger P, Koller B, Müller R (1996) A microtomographic system for the nondestructive evaluation of bone architecture. Calcif Tissue Int 58:24–29
Glatt M. The bisphosphonate zoledronate prevents vertebral bone loss in mature estrogen-deficient rats as assessed by micro-computed tomography. Eur Cells Materials 2001;1:18–26. http://www.ecmjournal.org Cited 3 February 2004
Hildebrand T, Rüegsegger P (1997) A new method for the model independent assessment of thickness in three-dimensional images. J Microsc 185:67–75
Hildebrand T, Rüegsegger P (1997) Quantification of bone microarchitecture with the structure model index. Comp Meth Biomech Biomed Eng 1:15–23
Odgaard A, Gundersen HJG (1993) Quantification of connectivity in cancellous bone, with special emphasis on 3-D reconstructions. Bone 14:173–182
Pataki A, Müller K, Green JR, Ma YF, Li QN, Jee WSS (1997) Effects of short-term treatment with the bisphophonates zoledronate and pamidronate on rat bone: a comparative histomorphometric study on the cancellous bone formed before, during and after treatment. Anat Rec 249:458–468
Frost MA, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. J Bone Miner Res 6:595–610
Kollerup G, Thamsborg G, Bhatia H, Sorensen OH (1992) Quantitation of urinary hydroxypyridinium cross-links from collagen by high-performance liquid chromatography. Scand J Clin Lab Invest 52:657–662
Yoshimura Y, Ohnishi K, Hamamura M, Oda T, Sohda T (1993) Automated high-performance liquid chromatographic determination of hydroxylysyl-pyridinoline and lysylpyridinoline in urine using a column-switching method. J Chromatogr 613:43–49
Mosekilde L, Danielsen CC, Knudsen UB (1993) The effect of aging and ovariectomy on the vertebral bone mass and biomechanical properties of mature rats. Bone 14:1–6
15 Mashiba T, Hirano T, Turner CH et al (2000) Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res 15:621–625
16 Kinney JH, Haupt DL, Balooch M, Ladd AJC, Ryaby JT, Lane NE (2000) Three-dimensional morphometry of the L6 vertebra in the ovariectomized rat model of osteoporosis: biomechanical implications. J Bone Miner Res 15:1981–1991
Parfitt AM, Mathews CH, Villanueva AR, Kleerekoper M, Frame B, Rao DS (1983) Relationship between surface, volume, and thickness of iliac trabecular bone in aging and in osteoporosis: implications for the microanatomic and cellular mechanisms of bone loss. J Clin Invest 72:1396–1409
Mitton D, Cendre E, Roux J-P et al (1998) Mechanical properties of ewe vertebral cancellous bone compared with histomorphometry and high-resolution computed tomography parameters. Bone 22:651–658
Toolan BC, Shea M, Myers ER, Borchers ER, Seedor JG, Quartuccio H, Rodan G, Hayes WC (1992) Effects of 4-amino-1-hydroxybutylidene bisphosphonate on bone biomechanics in rats. J Bone Miner Res 7:1399–1406
Guy JA, Shea M, Peter CP, Morrissey R, Hayes WC (1993) Continuous alendronate treatment throughout growth, maturation, and aging in the rat results in increases in bone mass and mechanical properties. Clacif Tissue Int 53:283–288
Lalla S, Hothorn LA, Haag N, Bader R, Bauss F (1998) Lifelong administration of high doses of ibandronate increases bone mass and maintains bone quality of lumber vertebrae in rats. Osteoporos Int 8:97–103
Thomsen JS, Ebbesen EN, Mosekilde LI (2002) Predicting human vertebral bone strength by vertebral static histomorphometry. Bone 30:502–508
Hildebrand T, Laib A, Müller R et al (1999) Direct three-dimensional morphometric analysis of human cancellous bone: microstructural data from spine, femur, iliac crest, and calcaneus. J Bone Miner Res 14:1167–1174
Ferretti JL, Mondelo N, Capozza RF, Cointry GR, Zanchetta JR, Montuori E (1995) Effects of large doses of olpadronate (dimethyl-pamidronate) on mineral density, cross-sectional architecture, and mechanical properties of rat femurs. Bone 4S:285S–293S
Hornby SB, Evans GP, Hornby SL, Pataki A, Glatt M, Green JR (2003) Long-term zoledronic acid treatment increases bone structure and mechanical strength of long bones of ovariectomized adult rats. Calcif Tissue Int 72:519–527
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Glatt, M., Pataki, A., Evans, G.P. et al. Loss of vertebral bone and mechanical strength in estrogen-deficient rats is prevented by long-term administration of zoledronic acid. Osteoporos Int 15, 707–715 (2004). https://doi.org/10.1007/s00198-004-1588-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-004-1588-3