Abstract
The objective of this study was to analyse fluoride uptake and microhardness alteration of carious-like demineralised enamel after application of differently concentrated acidulated sodium fluoride gels and to determine the effect of fluoridation on enamel resistance against subsequent demineralisation. Artificial caries-like lesions of bovine enamel specimens were treated with sodium fluoride gels of different concentration (group A: 1.25%, group B: 0.62%, group C: 0.31%, group D: 0.15%; n=20 each group) for 5 min and stored in artificial saliva for 24 h. This cycle was carried out three times. Subsequently, KOH-soluble and structurally bound fluoride (determined at depths of 30, 60, and 90 μm) were analysed. In the second part of the study, for each 12 enamel specimens surface microhardness was determined before and after demineralisation, after fluoridation with the differently concentrated gels A–D, and after a second demineralisation. With all groups uptake of KOH-soluble and structurally bound fluoride resulted in higher levels than baseline content. Statistical analysis revealed significant differences between fluoride uptake among the groups, with highest uptake for the 1.25% gel and lowest for the 0.15% gel. Moreover, with all gels highest uptake was observed in the outermost enamel layer (P<0.05). Microhardness values after second demineralisation increased with increased concentration of the applied sodium fluoride gel. Increasing concentration of the applied gel implies better protection of the enamel specimens against subsequent demineralisation (P<0.05). It is concluded that differently concentrated acidulated sodium fluoride gels resulted in concentration-related significant uptake of fluoride in carious-like demineralised enamel, leading to a better demineralisation protection with increasing fluoride concentration in the gel.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The caries-inhibiting effect of fluoride was initially thought to be primarily due to incorporation of fluoride into the crystal lattice during development of the teeth. Today, it is suggested that post-eruptive interaction of fluoride with enamel is more important for caries protection. The caries-preventive effects of fluoride are attributed to fluoride-induced enhancement of remineralisation, inhibition of demineralisation, and reduction of dissolution after fluoride incorporation into the enamel crystallites [35]. For caries prevention it seems to be essential to achieve high amounts of KOH-soluble fluoride on the enamel surface [28]. However, the formation of fluoridated apatite also has a limited, but measurable cariostatic effect [25].
Topical fluoridation can be achieved with dentifrices, varnishes, or gels. Acidulated phosphate fluoride (APF) gels containing amine fluoride or sodium fluoride reduced caries prevalence successfully in both adults and children [22, 36]. Delbem and Cury [10] showed that administration of acidulated fluoride gel led to greater fluoride deposition in enamel than did neutral gel. They also demonstrated that acidulated fluoride gel is also more effective in reducing the demineralisation of enamel submitted to a cariogenic challenge than is neutral fluoride gel.
However, most clinical studies evaluated the effects of 1.1–1.25% APF gels on caries inhibition [22, 36]. Because of the toxicological risks, application of 1.25% fluoride gel is not recommended for children up to 6 years [33]. Children tend to swallow substantial amounts of topically applied fluoride [12, 32]. Thereby, even latent systemic uptake of fluoride beyond a level of 0.05–0.1 mg kg−1 body weight may result in enamel fluorosis [16, 19]. The acute toxic dose of fluoride is probably 5 mg kg−1 body weight [37]. That means that ingestion of 5 mL of 1.25% fluoride gel containing 62.5 mg fluoride may cause life-threatening side-effects in a child of 10 kg body weight.
Therefore, it seems reasonable to scrutinize the efficacy of fluoride gels with lower fluoride concentrations which may possibly be applied in children also. Topical fluoridation with highly concentrated fluoride regimes results in an increase of both KOH-soluble fluoride and structurally bound fluoride in enamel [3, 5, 27]. The application of APF gels with different fluoride concentrations (0.11–1.23% fluoride) on sound enamel revealed no differences in structurally bound fluoride. Only the 1.23% APF gel led to a significant increase of KOH-soluble fluoride [11]. However, currently there is no information about the effects of APF gels of different concentration on carious enamel and resistance to further enamel demineralisation.
Consequently, the aim of this study was, first, to determine fluoride uptake in the form of KOH-soluble and structurally bound fluoride in carious-like demineralised enamel after application of sodium fluoride gels of different concentration. The second objective was to test whether application of these fluoride gels increases the resistance of dental enamel against subsequent demineralisation. For this purpose surface microhardness of enamel lesions treated with the different APF gels was assessed before and after a carious-like challenge.
Materials and methods
Preparation of specimens for fluoride analysis
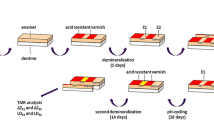
Five enamel cylinders (3 mm in diameter) were prepared from the buccal surface of each of 20 freshly extracted bovine incisors. The top surface of each cylinder was ground flat and polished with water-cooled carborundum discs (1,200 grit waterproof silicon carbide paper; Struers, Erkrath, Germany), thereby removing about 200 μm enamel. The thickness of the removed enamel layer was monitored with a micrometer (Digimatic, Micrometer, Mitutoyo, Tokyo, Japan). The specimens were covered with fluoride-free wax (Rosa Modellierwachs, Orbis Dental, Offenbach, Germany) except for the polished top surfaces. To produce demineralised lesions the samples were stored in acidic hydroxyethylcellulose (HEC, pH 4.8) for three days, in accordance with the method of Amaechi et al. [1].
One specimen from each tooth was assigned to each of the four experimental groups A–D. The fifth specimen was used for determination of the baseline fluoride content of the respective tooth. This specimen was not treated with a fluoride gel. The enamel surfaces of the remaining samples were covered with 1 mL fluoride gels of different concentration (group A: 1.25% sodium fluoride, group B: 0.62% sodium fluoride, group C: 0.31% sodium fluoride, group D: 0.15% sodium fluoride). The pH of these gels was 5.0. After 5 min the gel was carefully removed with a soft toothbrush and the samples were stored in 50 mL artificial saliva for 24 h. The saliva was mixed according to the formulation of Klimek at al. [21]. For each specimen fluoridation and storage in artificial saliva was carried out three times.
The fluoride gels were mixed according to the following formulation (as an example the composition (wt.%) of the 1.25% gel is given): 1.9% hydroxyethylcellulose, 2.72% sodium fluoride, 1.15% phosphoric acid (85%), 94.23% distilled water. To achieve a constant pH of 5.0 in the different concentrated fluoride gels the 1.25% fluoride gel was diluted with fluoride-free gel of the same pH in ratios of 1:2, 1:4 and 1:8.
Fluoride analysis
The respective cylinders were analysed for KOH-soluble and structurally bound fluoride. The amount of KOH-soluble fluoride was determined by the method of Caslavska et al. [7]. For this purpose the samples were stored in 1 mL 1 mol L−1 KOH. After 24 h the solution was neutralized with 1 mL 1 mol L−1 HNO3 and buffered with 1 mL TISAB II (Orion Research, Cambridge, USA) resulting in a pH of 5.8.
Afterwards the wax was removed from the specimens and structurally bound fluoride was determined in three successive layers of 30 μm each. The layers were ground off by a special technique using silicone carbide strips (3M, Neuss, Germany) which had previously been washed for 8 h in 3% HClO4 to remove traces of fluoride. After drying of the strips the enamel layers were removed by grinding the specimens with a special device which enabled the thickness of the enamel specimens to be controlled [18]. A fresh strip was used for each layer. The enamel particles worn off and the strip were immersed in 0.5 mL 0.5 mol L−1 HClO4 for 1 h. Subsequently the solution was buffered with 2.5 mL TISAB II resulting in a pH of 5.2. Fluoride in the solutions (KOH and structurally bound fluoride) was assayed by use of a specific fluoride electrode (Orion Research, Cambridge, USA). This electrode is designed for fluoride measurements in solutions of pH 5.0–6.0. Fluoride uptake of the respective sample was calculated according to the equation: Fluoride uptake=(measured fluoride value)−(baseline content of the tooth).
Preparation of specimens for microhardness testing
Forty-eight freshly extracted bovine incisors were stored in 10% thymol solution until required. The teeth were embedded in acrylic resin (Palavit G; Kulzer Wehrheim, Germany). The labial surfaces were ground flat and polished with water-cooled carborundum discs (1,200 grit waterproof silicon carbide paper; Struers) and polished with diamond paste (15 and 5 μm, Diamond paste; Struers) thereby removing approximately 200 μm of the outermost enamel layer. The thickness of the removed enamel layer was monitored with a micrometer (Digimatic, Micrometer, Mitutoyo, Tokyo, Japan).
The final area of the specimen used for microhardness measurement amounted to about 4 mm2. For all samples the surface was demineralised by immersion of the samples in HEC for 72 h (=first demineralisation). The specimens were then evenly distributed among four experimental groups according to the treatments with the differently concentrated gels as described above. The samples were treated with the respective gel for 5 min and subsequently stored in artificial saliva for 24 h. Finally all specimens were stored again in HEC for 72 h (=second demineralisation).
Microhardness analysis
Baseline microhardness of the enamel surface was determined for each specimen initially before artificial demineralisation. After the first carious-like attack, after fluoridation, and finally after the second demineralisation the microhardness of the samples were analysed. A digital microhardness tester (Leitz Miniload 2, Wild Leitz, Wetzlar, Germany) fitted with a Vickers diamond and a 1,961 N load was used to make indentations in the enamel surface. The loaded diamond was allowed to rest on the surface for 30 s. Five indentations were performed on each specimen for every measurement and results were averaged.
Statistical analysis
The t-test for dependent samples (software package Statistica 6.0/statsoft, Tulsa, USA) was used to analyse differences in mean fluoride concentration and hardness among the experimental groups. Bonferroni corrections for multiple comparisons were applied to the data. Level of significance was set at P<0.05.
Results
KOH-soluble fluoride
Data from KOH-soluble fluoride analysis are presented in Table 1. Statistical analysis revealed a significant difference between the four experimental groups. For all groups uptake of KOH-soluble fluoride resulted in higher levels than baseline content. Uptake of KOH-soluble fluoride correlated with the fluoride concentration of the gel applied, i.e. the higher the concentration of the fluoride gel, the higher was the retention of KOH-soluble fluoride (P<0.05).
Structurally bound fluoride
Data showing uptake of the structurally bound fluoride fraction are listed in Table 2. Statistical analysis revealed a significant difference between all experimental groups (P<0.05). In all groups a significant difference was observed between the fluoride uptake of the different enamel layers (P<0.05). In groups A–C the uptake of structurally bound fluoride was distinctly higher than for both group D and resulted in levels higher than the baseline value. Especially in groups A (1.25%) and B (0.62%) fluoridation resulted in a distinct fluoride uptake in the outermost layer.
Microhardness
Table 3 presents data from microhardness determination in the respective groups. The data show that after the first demineralisation the decline in microhardness in the different experimental groups was nearly equal. However, after fluoridation and second demineralisation microhardness of the specimens were highest for those treated with the 1.25% gel. Nevertheless, the specimens treated with either 0.62 or 0.31% fluoride also had increased resistance (P<0.05) against the second demineralisation compared with the specimens treated with 0.15% gel. The microhardness values imply increased protection of the enamel specimens with increasing concentration of applied fluoride gel (P<0.05).
Discussion
In this study bovine enamel specimens were used to determine the effect of a fluoridation agent on enamel fluoride uptake and microhardness. Several authors have used bovine enamel to evaluate mineral changes and fluoride uptake of enamel and demineralisation behaviour [3, 6], because its chemical composition is similar to that of human enamel [9, 30]. Although in-vitro caries experiments revealed a threefold faster rate of lesion progression in bovine compared with human enamel [14], bovine enamel is a suitable substitute for assessing mineral changes and fluoridation behaviour in dental enamel [23]. Demineralisation of the samples was performed according to the method of Ruben et al. [29] and Amaechi et al. [1], who showed that demineralisation with acidified hydroxyethylcellulose gel leads to superficial mineral loss with formation of a lesion body beneath, thereby closely resembling an initial carious lesion. However, exposure to acidified hydroxyethylcellulose for only three days might produce caries-like lesions but also only a softened or demineralised enamel surface without the formation of a subsurface lesion and a surface layer [1]. However, microhardness alterations are directly associated with mineral changes in superficial layers [15]. Kielbassa et al. [20] found a clear relationship between microhardness and the mineral content of in-situ-induced enamel lesions.
Consequently, in the present study microhardness determination was performed to evaluate the capacity of fluoride regimes to reharden demineralised enamel and to prevent further demineralisation, as has also been performed in other recent studies [10, 26].
For caries prevention it seems important to obtain sufficient amounts of KOH-soluble fluoride which appear as a calcium fluoride-like layer with fluoride adsorbed on to apatite crystallites [2, 8, 24]. Increased time of exposure, increased concentration, low pH, pre-treatment with calcium or the presence of free calcium will also effectively contribute to increasing calcium fluoride deposition [28]. The application of 0.11–1.23% APF gels on sound enamel revealed a significant increase of KOH-soluble fluoride for the 1.23% fluoride gel only [11]. However, the results of the present study showed that even the application of the low fluoridated gel (0.15% APF gel) resulted in an increase of KOH-soluble fluoride in demineralised enamel. Moreover, the amount of KOH-soluble fluoride in demineralised enamel increased with increasing fluoride concentration in the experimental gels.
Differences between the formation of calcium fluoride-like material on sound and demineralised enamel is related to their different microstructure. Bruun and Givskov [4] measured the formation of a calcium fluoride-like layer on sound and artificial caries lesions after 1 min exposure to 2% sodium fluoride solution. Calcium fluoride precipitation on caries-like lesions amounted to 30 μg F cm−2 whereas KOH-soluble fluoride on sound enamel amounted to 1 μg F cm−2 only. The enhanced formation of calcium fluoride-like material in early caries lesions is believed to be because of the increased surface area available for reaction with fluoride within the micropores of the lesion [4]. Compared with the study of Bruun and Givskov [4] fluoride treatment of bovine samples in the present study resulted in larger amounts of KOH-soluble fluoride. This might be because of the higher concentration and the longer duration of fluoridation in the present study. Furthermore, bovine enamel is known to be slightly more porous than human enamel [13]; this may affect fluoride accumulation.
Fluoridation with the experimental gels also resulted in an increase of structurally bound fluoride. These observations are in agreement with data from a previous study in which uptake of structurally bound fluoride in artificial initial enamel lesions was observed after application of 1% F− gel or 2.3% F− varnish [17]. The results of the present study show that this is also true for application of APF gels of lower concentration. In demineralised enamel, the amount of structurally bound fluoride increases with increasing concentration of fluoride in the gel. It has been suggested that structurally bound fluoride reduces the acid susceptibility of enamel and increases enamel resistance to lesion formation. It is, therefore, assumed that fluoridated apatite also has a limited cariostatic effect [34].
Application of experimental gels also led to concentration-related resistance of demineralised enamel against a subsequent demineralisation. Fluoride application in all experimental groups was performed for 5 min each, thereby simulating intraoral conditions during application of a fluoride gel with a tray. Whereas Seppä [31] suggested that the efficacy of fluoride varnish was not proportional to the fluoride concentration but rather to the number of applications on presoftened enamel, our findings showed that protection from enamel microhardness loss due to a demineralisation depends on fluoride concentration of the gel applied. Although the experimental gels differed in their ability to prevent further demineralisation during the second carious-like attack, microhardness determination revealed no difference in their potential to reharden the softened enamel surface after the first demineralisation. However, the gels were neither able to reharden the demineralised enamel surface completely nor to prevent lesion progression.
To minimize the toxicological risk of ingestion of fluoride gel fractions during application, 0.62 and 0.32% APF gels may be utilized for caries prevention in children, even though caries-preventive effects are not as high as after treatment with 1.25% APF gel. Further investigations are necessary to analyse the caries-preventive effects of APF gels of different concentration on deciduous enamel.
However, clinical studies are recommended to evaluate whether the results of this in-vitro study are also confirmed in vivo.
Conclusion
This study shows that application of differently concentrated acidulated sodium fluoride gels resulted in concentration-dependent fluoride uptake by demineralised enamel which was highest for the 1.25% gel and lowest for the 0.15% gel. Also, the ability to protect demineralised enamel against subsequent demineralisation increased with increasing concentration of the applied gels.
References
Amaechi BT, Higham SM, Edgar WM (1998) Factors affecting the development of carious lesions in bovine teeth in vitro. Arch Oral Biol 43:619–628
Arends J, Christoffersen J (1990) Nature and role of loosely bound fluoride in dental caries. J Dent Res 69:601–605
Attin T, Hartmann O, Hilgers RD, Hellwig E (1995) Fluoride retention of incipient enamel lesions after treatment with a calcium fluoride varnish in vivo. Arch Oral Biol 40:169–174
Bruun C, Givskov H (1993) Calcium fluoride formation in enamel from semi- or low-concentrated F agents in vitro. Caries Res 27:96–99
Bruun C, Thylstrup A, Uribe E (1983) Loosely bound fluoride extracted from natural carious lesions after topical application of APF in vitro. Caries Res 17:458–460
Buchalla W, Attin T, Schulte-Mönting J, Hellwig E (2002) Fluoride uptake, retention, and remineralization efficacy of a highly concentrated fluoride solution on enamel lesions in situ. J Dent Res 81:329–333
Caslavska V, Moreno EC, Brudevold F (1975) Determination of the calcium fluoride formed from in vitro exposure of human enamel to fluoride solutions. Arch Oral Biol 20:333–339
Christoffersen J, Christoffersen MR, Kibalczyc W, Perdok WG (1988) Kinetics of dissolution and growth of calcium fluoride and effects of phosphate. Acta Odontol Scand 46:325–336
Davidson CL, Boom G, Arends J (1973) Calcium distribution in human and bovine surface enamel. Caries Res 7:349–359
Delbem AC, Cury JA (2002) Effect of application time of APF and NaF gels on microhardness and fluoride uptake of in vitro enamel caries. Am J Dent 15:169–172
Dijkman AG, Tak J, Arends J (1982) Comparison of fluoride uptake by human enamel from acidulated phosphate fluoride gels with different fluoride concentrations. Caries Res 16:197–200
Ekstrand J, Koch G, Lindgren LE, Petersson LG (1981) Pharmacokinetics of fluoride gels in children and adults. Caries Res 15:213–220
Esser M, Tinschert J, Marx R (1998) Material characteristics of the hard tissue of bovine versus human teeth. Dtsch Zahnärztl Z 53:713–717
Featherstone JD, Mellberg JR (1981) Relative rates of progress of artificial carious lesions in bovine, ovine and human enamel. Caries Res 15:109–114
Featherstone JD, ten Cate JM, Shariati M, Arends J (1983) Comparison of artificial caries-like lesions by quantitative microradiography and microhardness profiles. Caries Res 17:385–391
Forsman B (1977) Early supply of fluoride and enamel fluorosis. Scand J Dent Res 85:22–30
Hellwig E, Attin T, Metke W (1993) Does the application of a fluoride lacquer modify the de- and remineralization of initial carious lesions in vitro? Schweiz Monatsschr Zahnmed 103:150–155
Hellwig E, Klimek J (1984) Fluoride loss from demineralized enamel after the application of different concentrations of NaF and Na-MFP solutions in an artificial mouth. Dtsch Zahnärztl Z 39:892–898
Hetzer G, Haftenberger M, Viergutz G, Neumeister V (2003) Fluoride intake in pre-school children. Oralprophylaxe 25:27–30
Kielbassa AM, Wrbas KT, Schulte-Mönting J, Hellwig E (1999) Correlation of transversal microradiography and microhardness on in situ-induced demineralization in irradiated and nonirradiated human dental enamel. Arch Oral Biol 44:243–251
Klimek J, Hellwig E, Ahrens G (1982) Fluoride taken up by plaque, by the underlying enamel and by clean enamel from three fluoride compounds in vitro. Caries Res 16:156–161
Marinho VC, Higgins JP, Logan S, Sheiham A (2003) Systematic review of controlled trials on the effectiveness of fluoride gels for the prevention of dental caries in children. J Dent Educ 67:448–458
Mellberg JR (1992) Hard-tissue substrates for evaluation of cariogenic and anti-cariogenic activity in situ. J Dent Res 71:913–919
Øgaard B, Rølla G, Ruben J, Arends J (1990) Relative cariostatic effects of KOH-soluble and KOH-insoluble fluoride in situ. J Dent Res 69:1505–1507
Øgaard B, Rølla G, Ruben J, Dijkman AG, Arends J (1988) Microradiographic study of demineralization of shark enamel in a human caries model. Scand J Dent Res 96:209-211
Paes Leme AF, Tabchoury CP, Zero DT, Cury JA (2003) Effect of fluoridated dentifrice and acidulated phosphate fluoride application on early artificial carious lesions. Am J Dent 16:91–95
Retief DH, Bradley EL, Holbrook M, Switzer P (1983) Enamel fluoride uptake, distribution and retention from topical fluoride agents. Caries Res 17:44–51
Rølla G, Saxegaard E (1990) Critical evaluation of the composition and use of topical fluorides, with emphasis on the role of calcium fluoride in caries inhibition. J Dent Res 69:780–785
Ruben J, Arends J, Christoffersen J (1999) The effect of window width on the demineralization of human dentine and enamel. Caries Res 33:214–219
Ruse ND, Smith DC, Torneck CD, Titley KC (1990) Preliminary surface analysis of etched, bleached, and normal bovine enamel. J Dent Res 69:1610–1613
Seppä L (1988) Effects of a sodium fluoride solution and a varnish with different fluoride concentrations on enamel remineralization in vitro. Scand J Dent Res 96:304–309
Shulman JD, Wells LM (1997) Acute fluoride toxicity from ingesting home-use dental products in children, birth to 6 years of age. J Public Health Dent 57:150–158
Stößer L, Hellwig E, Bößmann K, Schoeniger-Peters O (2002) Fluorides for community prevention programs or for individuals. Oralprophylaxe 24:125–128
Takagi S, Liao H, Chow LC (2000) Effect of tooth-bound fluoride on enamel demineralization/remineralization in vitro. Caries Res 34:281–288
ten Cate JM, Featherstone JD (1991) Mechanistic aspects of the interactions between fluoride and dental enamel. Crit Rev Oral Biol Med 2:283–296
van Rijkom HM, Truin GJ, ‘t Hof MA (1998) A meta-analysis of clinical studies on the caries-inhibiting effect of fluoride gel treatment. Caries Res 32:83–92
Whitford GM (1990) The physiological and toxicological characteristics of fluoride. J Dent Res 69:539–549
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wiegand, A., Krieger, C., Attin, R. et al. Fluoride uptake and resistance to further demineralisation of demineralised enamel after application of differently concentrated acidulated sodium fluoride gels. Clin Oral Invest 9, 52–57 (2005). https://doi.org/10.1007/s00784-005-0306-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-005-0306-7