Abstract
Autosomal dominant osteopetrosis type II (ADO-II) is a heritable bone disorder characterized by osteosclerosis, predominantly involving the spine (vertebral end-plate thickening, or rugger-jersey spine), the pelvis (“bone-within-bone” structures) and the skull base. Chloride channel 7 (CLCN7) has been reported to be the causative gene. In this study, we aimed to identify the pathogenic mutation in four Chinese families with ADO-II. All 25 exons of the CLCN7 gene, including the exon–intron boundaries, were amplified and sequenced directly in four probands from the Chinese families with ADO-II. The mutation site was then identified in other family members and 250 healthy controls. In family 1, a known missense mutation c.296A>G in exon 4 of CLCN7 was identified in the proband, resulting in a tyrosine (UAU) to cysteine (UGU) substitution at p.99 (Y99C); the mutation was also identified in his affected father. In family 2, a novel missense mutation c.865G>C in exon 10 was identified in the proband, resulting in a valine (GUC) to leucine (CUC) substitution at p.289 (V289L); the mutation was also identified in her healthy mother and sister. In family 3, a novel missense mutation c.1625C>T in exon 17 of CLCN7 was identified in the proband, resulting in an alanine (GCG) to valine (GUG) substitution at p.542 (A542V); the mutation was also identified in her father. In family 4, a hot spot, R767W (c.2299C>T, CGG>TGG), in exon 24 was found in the proband which once again proved the susceptibility of the site or the similar genetic background in different races. Moreover, two novel mutations, V289L and A542V, occurred at a highly conserved position, found by a comparison of the protein sequences from eight vertebrates, and were predicted to have a pathogenic effect by PolyPhen-2 software, which showed “probably damaging” with a score of approximately 1. These mutation sites were not identified in 250 healthy controls. Our present findings suggest that the novel missense mutations V289L and A542V in the CLCN7 gene were responsible for ADO-II in the two Chinese families.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autosomal dominant osteopetrosis type II (ADO-II) (OMIM 166600), also known as “Albers–Schönberg disease”, is a rare disease characterized by osteosclerosis, predominantly involving the spine (vertebral end-plate thickening, or rugger-jersey spine), the pelvis (“bone-within-bone” structures) and the skull base [1]. The gene encoding the phenotype was identified as chloride channel 7 (CLCN7) in 2001 [2]. The CLCN7 gene, which encodes the 803-amino-acid chloride channel protein 7 (ClC-7), is highly expressed in the osteoclast ruffled membrane and provides the chloride conductance necessary for the osteoclast-mediated degradation of bone tissue [3]. Homozygosity for CLCN7 mutations also accounts for autosomal recessive osteopetrosis (ARO) and intermediate autosomal recessive osteopetrosis (IAO) [3–5]. To date, more than 20 mutations have been identified in the CLCN7 gene in families with ADO-II [2, 4, 6–11]. Among them, nine mutations of CLCN7, reported in our previous studies [8–10], have been identified in Chinese patients. In the current study, we identified another two novel CLCN7 gene mutations in four Chinese families with ADO-II.
Materials and methods
Patients
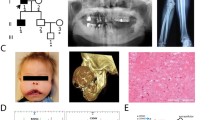
The study was approved by the Ethics Committee of the Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. All the subjects involved in the study were recruited by the Department of Osteoporosis and Bone Diseases and signed informed consent documents before joining the study. Altogether, four families were included (Fig. 1); they were all of Han ethnicity.
In family 1, a 5-year-old male (proband, III1) was the only son of a non-consanguineous and healthy mother and affected father. He was born of a full-term pregnancy with normal delivery and a normal birth weight and length. His current weight and height were also in the normal range. He had fallen from his bike while playing 1 year ago and was diagnosed with a fracture of the right clavicle. X-ray also revealed a diffuse increase in bone density. X-rays of spine and pelvis revealed a generalized increase in bone density with evidence of typical “sandwich” appearance in the vertebrae and “bone-in-bone” appearance in the iliac wings (Fig. 2a). The bone mineral density (BMD) of the proband and his parents was shown to be increased using dual-energy X-ray absorptiometry (DXA, Lunar Corp., Madison, WI, USA). Laboratory data showed normal blood routine, serum calcium, phosphorus and alkaline phosphate (ALP). Creatine kinase (CK) was increased at 546 U/L (reference range 21–190), its MB isoenzyme (CK-MB) was 757 U/L (reference range 0.0–25.0), and lactate dehydrogenase (LDH) was 510 U/L (reference range 114–240). ADO-II was suspected, in view of the mode of inheritance. The BMD of his mother was normal, but that of his father was increased (Table 1). The subsequent skeletal radiograph of spine and pelvis of his father also revealed typical “sandwich” appearance in the vertebrae and “bone-in-bone” appearance in the iliac wings (Fig. 2b), which verified our suspicion.
In family 2, a 35-year-old female (proband, II4) was the second daughter of non-consanguineous and healthy parents. X-ray revealed increased bone density on one incidental physical examination. There was no other discomfort. Further X-rays revealed similar typical “sandwich” appearance in the vertebrae and “bone-in-bone” appearance in the iliac wings (Fig. 2c). The BMD of the proband was found to be increased on DXA measurement. Laboratory data showed normal blood routine, serum calcium, phosphorus and ALP. CK was normal at 116 U/L, CK-MB was increased at 65 U/L, and LDH was normal at 147 U/L. Her older sister had a history of anemia. ADO-II was suspected, so we measured the BMD of her family members (Table 1).
In family 3, an 8-month-old female (proband, III1) was the only daughter of non-consanguineous and healthy parents. She was born of a full-term pregnancy with normal delivery, birth weight and length but with varus deformity of the left hip. Physical examination revealed that flexion and abduction of the left hip was restricted, the lower extremities were unequal and the left was 1 cm shorter than the right. X-ray examination of the hip unexpectedly found the bone density increased (Fig. 2d), but the patient was too young to be measured using DXA. In addition, we failed to obtain the CK, CK-MB and LDH data due to the limitation of blood volume. Considering the normal blood routine and liver function of the proband, ADO-II was suspected and the BMD of her parents was measured by DXA.
In family 4, a 27-year-old female (proband, II1) was the daughter of non-consanguineous and healthy parents. The patient saw a doctor because of neck discomfort. Cervical X-ray revealed increased bone density. The subsequent X-rays of vertebrae, hip and head revealed typical “sandwich” appearance and “bone-in-bone” appearance in the iliac wings (Fig. 2e). The BMD measurement also showed high bone density. Besides bone phenotype, the proband also stated a history of anemia since the age of 16. Laboratory data showed a low level of hemoglobin (78 g/L, reference range 113–172 g/L), normal serum calcium, phosphorus and ALP. Except that her mother had a history of anemia, the other family members had no other symptoms. Because the patient’s family members did not live in the locality and refused to provide blood samples for the research, we could not analyze the blood and perform mutation analysis.
Mutation analysis
Informed consent was obtained before blood sampling and DNA analyses. The proband (III1) and his parents (II2, II3) in family 1, the proband (II4), her parents (I1, I2), sister (II1) and daughter (III4) in family 2, the proband (III1), her parents (II1, II2) in family 3, and the proband (II1) in family 4 were available for DNA sequencing. The 250 healthy donors were also included in the study. Altogether, 262 DNA samples were obtained. Genomic DNA was extracted from peripheral white blood cells using conventional methods. All 25 exons of the CLCN7 gene, including the exon–intron boundaries, were sequenced in the probands from the four families with ADO-II, as previously described [8]; the mutation sites were then identified in the other family members and 250 healthy controls. We used BLAST (Basic Local Alignment Search Tool) to perform a homology analysis of Y99C, V289L and A542 V sites in eight vertebrates (http://www.ncbi.nlm.nih.gov/tools/cobalt/cobalt.cgi?link_loc=BlastHomeAd) using the Universal Protein Resource (http://www.uniprot.org/). The potential causal effects of the novel p.Trp319Arg and p.Ala518Val missense mutations were predicted by using PolyPhen-2 software (http://genetics.bwh.harvard.edu/pph2/) [12]. PolyPhen-2 can use structural and comparative evolutionary considerations to predict the possible impact of amino acid substitutions on the stability and function of human proteins. It estimates the probability of the missense mutation being damaging based on performing functional annotation of single nucleotide polymorphisms (SNPs), mapping coding SNPs to gene transcripts, extracting protein sequence annotations and structural attributes, and building conservation profiles. The output of the PolyPhen-2 prediction pipeline is a prediction of “probably damaging”, “possibly damaging” or “benign” along with a numerical score ranging from 0.0 (benign) to 1.0 (damaging). A prediction of “probably damaging” means that the mutation is predicted to be damaging with high confidence [13].
Genetic analysis of CLCN7 gene in three families with ADO-II. (a) Direct DNA sequencing of the proband in family 1; arrow indicates a heterozygous A-to-G transition at c.296 in exon 4 (Y99C). (b) Direct DNA sequencing of the proband in family 2; arrow indicates a heterozygous G-to-C transition at c.865 in exon 10 (V289L). (c) Direct DNA sequencing of the proband in family 3; arrow indicates a heterozygous C-to-T transition at c.1625 in exon 17 (A542V)
Results
In family 1, a known missense mutation c.296A>G in exon 4 of the CLCN7 gene (Fig. 3a) which had already been reported in Chinese and European people was identified in the proband and his affected father, resulting in a tyrosine (UAU) to cysteine (UGU) substitution at p.99 (Y99C) [14, 15]. In family 2, a novel missense mutation c.865G>C in exon 10 of the CLCN7 gene was identified in the proband, resulting in a valine (GUC) to leucine (CUC) substitution at p.289 (V289L) (Fig. 3b); the mutation was also identified in her healthy mother and older sister. In family 3, a novel missense mutation c.1625C>T in exon 17 of CLCN7 was identified in the proband, resulting in an alanine (GCG) to valine (GUG) substitution at p.542 (A542V) (Fig. 3c); the mutation was also identified in his healthy father. In family 4, a hot spot mutation R767W (c.2299C>T, CGG>TGG) in exon 24 of CLCN7 was found in the proband, which was consistent with previous studies [16–18]. The four mutation sites were not found in the 250 healthy controls.
Moreover, the known mutation Y99C and two novel mutations V289L and A542V occurred at a highly conserved position, according to a comparison of the protein sequences from eight vertebrates (Fig. 4). The missense mutations Y99C, V289L and A542V in CLCN7 were predicted to have a pathogenic effect by PolyPhen-2, which showed “probably damaging” with a score of 1 (sensitivity: 0.00; specificity: 1.00), “probably damaging” with a score of 1 (sensitivity: 0.00; specificity: 1.00) and “probably damaging” with a score of 0.999 (sensitivity: 0.14; specificity: 0.99), respectively.
Discussion
In this study, we reported four families with ADO-II; the probands all had generalized increase in bone density, typical “sandwich-like” sclerosis of the vertebral endplates and “bone-within-bone” appearance (mainly in iliac wings), but this is not typical for infants’ bones because they are not fully mature. The onset of the disease is not only in adulthood, but also in childhood or infancy (as in family 1 and 3), most often diagnosed on the basis of fractures (as seen in patient 1) [19]. A great variety of phenotypes is present, even within families in which the same mutation is carried by several individuals. Moreover, it is likely that asymptomatic members were also present. For a better and clearer understanding of the relationship between phenotypes and genotypes, we list the laboratory data of the probands and several family members in Table 2.
In this study, direct sequencing of the CLCN7 gene in the four families with ADO-II revealed two novel mutations, V289L and A542V, and two known mutations Y99C and R767W. They all occurred at a highly conserved position among different species and were predicted to have a pathogenic effect by PolyPhen-2, which showed “probably damaging”. Although the functional analysis of the newly-identified mutations was not done because of the limitations of this study, the prediction of PolyPhen-2 was acceptability with high accuracy.
The penetrance of ADO-II is 56–90 % in different studies [20], with a highly variable phenotype. It is very difficult to establish a correlation between genotype and phenotype. Chu et al. [21] found that the polymorphisms rs12926089 (V418M) of exon 15 and rs960476 of promoter region may affect the disease status and severity of ADO-II. Campos-Xavier et al. [22] also suggests that V418M may act as an allelic modifier of the R674Q mutation. Osteoclasts from the unaffected gene carriers functioned normally in cell culture, in contrast to those from the clinically affected subjects; this supports the hypothesis that intrinsic osteoclast factors determine disease expression in ADO-II in unaffected gene carriers [23]. DNA methylation plays an important role in regulation of gene expression and silencing of gene transcription [24], so we presumed that patients with ADO-II and asymptomatic mutational carriers presented different DNA methylation in CLCN7; however, we failed to find semimethylation in a CpG island of CLCN7 in two probands and three asymptomatic carriers in a previous study [9].
In conclusion, novel V289L and A542V mutations in the CLCN7 gene were responsible for the two Chinese families with ADO-II in our study, and the known Y99C and R767W mutations which were reported in Chinese and European populations revealed the homology of the genetic background. This may provide some clue to the correlation between the genotype and phenotype of this disease.
References
Johnston CC Jr., Lavy N, Lord T, Vellios F, Merritt AD, Deiss WP Jr (1968) Osteopetrosis. A clinical, genetic, metabolic, and morphologic study of the dominantly inherited, benign form. Medicine 47:149–167
Cleiren E, Benichou O, Van Hul E, Gram J, Bollerslev J, Singer FR, Beaverson K, Aledo A, Whyte MP, Yoneyama T, deVernejoul MC, Van Hul W (2001) Albers-Schonberg disease (autosomal dominant osteopetrosis, type II) results from mutations in the ClCN7 chloride channel gene. Hum Mol Genet 10:2861–2867
Kornak U, Kasper D, Bosl MR, Kaiser E, Schweizer M, Schulz A, Friedrich W, Delling G, Jentsch TJ (2001) Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell 104:205–215
Frattini A, Pangrazio A, Susani L, Sobacchi C, Mirolo M et al (2003) Chloride channel ClCN7 mutations are responsible for severe recessive, dominant, and intermediate osteopetrosis. J Bone Miner Res 18:1740–1747
Campos-Xavier AB, Saraiva JM, Ribeiro LM, Munnich A, Cormier-Daire V (2003) Chloride channel 7 (CLCN7) gene mutations in intermediate autosomal recessive osteopetrosis. Hum Genet 112:186–189
Waguespack SG, Koller DL, White KE, Fishburn T, Carn G, Buckwalter KA, Johnson M, Kocisko M, Evans WE, Foroud T, Econs MJ (2003) Chloride channel 7 (ClCN7) gene mutations and autosomal dominant osteopetrosis, type II. J Bone Miner Res 18:1513–1518
Letizia C, Taranta A, Migliaccio S, Caliumi C, Diacinti D, Delfini E, D’Erasmo E, Iacobini M, Roggini M, Albagha OM, Ralston SH, Teti A (2004) Type II benign osteopetrosis (Albers-Schonberg disease) caused by a novel mutation in CLCN7 presenting with unusual clinical manifestations. Calcif Tissue Int 74:42–46
Zhang ZL, He JW, Zhang H, Hu WW, Fu WZ, Gu JM, Yu JB, Gao G, Hu YQ, Li M, Liu YJ (2009) Identification of the CLCN7 gene mutations in two Chinese families with autosomal dominant osteopetrosis (type II). J Bone Miner Metab 27:444–451
Wang C, Zhang H, He JW, Gu JM, Hu WW, Hu YQ, Li M, Liu YJ, Fu WZ, Yue H, Ke YH, Zhang ZL (2012) The virulence gene and clinical phenotypes of osteopetrosis in the Chinese population: six novel mutations of the CLCN7 gene in twelve osteopetrosis families. J Bone Miner Metab 30:338–348
Zheng H, Zhang Z, He JW, Fu WZ, Wang C, Zhang ZL (2013) Identification of two novel CLCN7 gene mutations in three Chinese families with autosomal dominant osteopetrosis type II. Joint Bone Spine 81:188–189
Rashid BM, Rashid NG, Schulz A, Lahr G, Nore BF (2013) A novel missense mutation in the CLCN7 gene linked to benign autosomal dominant osteopetrosis: a case series. J Med Case Rep 7:7
Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR (2010) A method and server for predicting damaging missense mutations. Nat Methods 7:248–249
Adzhubei I, Jordan DM, Sunyaev SR (2013) Predicting Functional Effect of Human Missense Mutations Using PolyPhen-2. Curr Protoc Hum Genet 7:2
Sui W, Ou M, Liang J, Ding M, Chen J, Liu W, Xiao R, Meng X, Wang L, Pan X (2013) Rapid gene identification in a Chinese osteopetrosis family by whole exome sequencing. Gene 516:311–315
Del Fattore A, Peruzzi B, Rucci N, Recchia I, Cappariello A et al (2006) Clinical, genetic, and cellular analysis of 49 osteopetrotic patients: implications for diagnosis and treatment. J Med Genet 43:315–325
Wang C, Zhang H, He JW, Gu JM, Hu WW, Hu YQ, Li M, Liu YJ, Fu WZ, Yue H, Ke YH, Zhang ZL (2012) The virulence gene and clinical phenotypes of osteopetrosis in the Chinese population: six novel mutations of the CLCN7 gene in twelve osteopetrosis families. J Bone Miner Metab 30:338–348
Zhang ZL, He JW, Zhang H, Hu WW, Fu WZ, Gu JM, Yu JB, Gao G, Hu YQ, Li M, Liu YJ (2009) Identification of the CLCN7 gene mutations in two Chinese families with autosomal dominant osteopetrosis (type II). J Bone Miner Metab 27:444–451
Waguespack SG, Koller DL, White KE, Fishburn T, Carn G, Buckwalter KA, Johnson M, Kocisko M, Evans WE, Foroud T, Econs MJ (2003) Chloride channel 7 (ClCN7) gene mutations and autosomal dominant osteopetrosis, type II. J Bone Miner Res 18:1513–1518
Pangrazio A, Pusch M, Caldana E, Frattini A, Lanino E et al (2010) Molecular and clinical heterogeneity in CLCN7-dependent osteopetrosis: report of 20 novel mutations. Hum Mutat 31:E1071–E1080
Benichou O, Laredo J, De Vernejoul M (2000) Type II autosomal dominant osteopetrosis (Albers-Schönberg disease): clinical and radiological manifestations in 42 patients. Bone 26:87–93
Chu K, Koller DL, Snyder R, Fishburn T, Lai D, Waguespack SG, Foroud T, Econs MJ (2005) Analysis of variation in expression of autosomal dominant osteopetrosis type 2: searching for modifier genes. Bone 37:655–661
Campos-Xavier AB, Casanova JL, Doumaz Y, Feingold J, Munnich A, Cormier-Daire V (2005) Intrafamilial phenotypic variability of osteopetrosis due to chloride channel 7 (CLCN7) mutations. Am J Med Genet A 133A:216–218
Chu K, Snyder R, Econs MJ (2006) Disease status in autosomal dominant osteopetrosis type 2 is determined by osteoclastic properties. J Bone Miner Res 21:1089–1097
Sulewska A, Niklinska W, Kozlowski M, Minarowski L, Naumnik W, Niklinski J, Dabrowska K, Chyczewski L (2007) DNA methylation in states of cell physiology and pathology. Folia Histochem Cytobiol 45:149–158
Acknowledgments
The study was supported by the National Natural Science Foundation of China (81370978, 81270964), National Basic Research Program of China (973 Program) (2014CB942903), the Science and Technology Commission of Shanghai municipality (14JC1405000), Shanghai Municipal Commission of Health and Family Planning (2014ZYJB0009), and Shanghai Leading Talent Plan (051).
Conflict of interest
All authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
H. Zheng, C. Shao and Y. Zheng contributed equally to this work.
About this article
Cite this article
Zheng, H., Shao, C., Zheng, Y. et al. Two novel mutations of CLCN7 gene in Chinese families with autosomal dominant osteopetrosis (type II). J Bone Miner Metab 34, 440–446 (2016). https://doi.org/10.1007/s00774-015-0682-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-015-0682-2