Abstract
Osteopetrosis is a heritable bone disorder resulting from a deficiency of or a functional defect in osteoclasts. We aimed to characterize the molecular defects and clinical manifestations in Chinese patients with osteopetrosis by studying 12 unrelated osteopetrosis families. The entire coding region and adjacent splice sites of the CLCN7, TCIRG1, LRP5 and SOST genes were amplified and directly sequenced. X-rays of hip and lumbar spine, bone mineral density and bone turnover markers were examined simultaneously. Family history and fracture history were collected using a questionnaire. Among 12 unrelated families, 10 families were diagnosed with autosomal dominant osteopetrosis type II (ADOII) with 10 probands and 3 affected subjects. Two individuals in the other two families were diagnosed with uncategorized osteopetrosis because no mutations were detected in any of the four studied genes. Eight mutations, including two reported mutations (R767W and E798FS) and six novel mutations (E313K, A316G, R743W, G741R, W127G and S290F), were detected in the CLCN7 gene from 12 living ADOII patients. Among them, R767W and R743W mutations were two common mutations that were each found in 20% of 10 ADOII probands. In CLCN7-related ADOII patients, long bone fractures and elevated serum CK level were two major clinical phenotypes, especially in patients younger than 18 years. Further functional studies of the above eight mutations in the CLCN7 gene are needed in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteopetrosis is a heritable disease characterized by bone resorption disorder and high bone mineral density resulting from a deficiency of or functional defect in osteoclasts. Autosomal recessive osteopetrosis (ARO), intermediate autosomal osteopetrosis (IAO) and autosomal dominant osteopetrosis (ADO) are three major modes of this disease. ADOI and ADOII are two subtypes of ADO. ADOII, also termed Albers–Schönberg disease, occurs in 1 in 20,000 births. ADOII is characterized by late onset and highly variable symptoms including fractures, hip osteoarthritis and radiographic signs of “sandwich vertebrae” and “bone-within-bone” [1]. The Chloride channel 7 gene (CLCN7), the major disease-causing gene for ADOII [2], encodes an 803-amino-acid protein that functions to provide the chloride conductance required for efficient proton pumping in the osteoclast-ruffled membrane. Mutations in the CLCN7 gene may disrupt acidification of the osteoclast resorption lacunae, resulting in impaired bone degradation [3]. On the other hand, ADOI is associated with mutations in the low-density lipoprotein receptor-related protein 5 gene (LRP5), which regulates osteoblast function [4, 5]. ARO occurs in 1 in 250,000 births and causes severe disease including life-threatening bone marrow failure, hepatosplenomegaly, growth retardation and neural defects (blindness and deafness, among others). The most important molecular defects of ARO are mutations in the T-cell, immune regulator 1 gene (TCIRG1), which encodes the osteoclast-specific a3 subunit of the vacuolar proton pump [1]. Furthermore, the pleckstrin homology domain-containing family M member 1 gene (PLEKHM1) and the carbonic anhydrase II gene (CA2) have also been reported to be causally linked to IAO [6]. In addition, cases of grey lethal mutation (OSTM1 gene)-induced ARO have been reported by Chalhoub et al. [7] and Pangrazio et al. [8]. However, all previously known genes associated with the different types of osteopetrosis only account for approximately 70% of cases [1]. The variable disease severity and incomplete penetrance are two common phenomena described by studies of ADOII which focused on subjects from different ethnic backgrounds.
In recent decades, scientists from Japan, Korea and Hong Kong have reported sporadic cases of CLCN7-related infantile malignant osteopetrosis [9], osteopetrosis due to carbonic anhydrase II deficiency [10] and one ARO patient with a homozygous mutation (p.I261F) in the CLCN7 gene [11]. In our previous study [12], we observed two heterozygous mutations in the CLCN7 gene and incomplete penetrance in Chinese ADOII families. A large family study is needed to discover the molecular genetic mechanism, the typical clinical markers and the correlation between genotype and phenotype of osteopetrosis in East Asia.
In the current study, we aimed to characterize the clinical manifestations and molecular defects in Chinese patients associated with osteopetrosis and to investigate the phenotype–genotype correlations to improve our understanding of this heritable sclerosing bone disorder by studying 12 unrelated osteopetrosis families.
Materials and methods
Patients
Twelve unrelated Chinese osteopetrosis families comprising 78 individuals were recruited. Each family included at least one patient. All patients had non-consanguineous parents. We diagnosed 13 subjects with ADOII (families 1–10) and 2 subjects with uncategorized osteopetrosis (families 11 and 12) based on radiographic and biochemical findings as described previously [13, 14]. All subjects involved in the study were recruited by the Department of Osteoporosis and Bone Diseases outpatient clinic. This study was approved by the Ethics Committee of the Sixth People’s Hospital affiliated with Shanghai Jiao Tong University. Written consent for publication was obtained from the patients and their relatives. Clinical phenotypes, including clinical symptoms, auditory acuity, medical history including fracture history, family history; developmental history and childbearing history, were collected by questionnaire and medical examination at the first visit.
One hundred age- and sex-matched healthy donors were recruited as controls for mutation analysis [12].
Biochemical and radiographic examination
The full blood count, the levels of serum calcium (Ca), phosphonium (P), alkaline phosphatase (ALP), blood urea nitrogen (BUN), serum creatinine (Scr), creatine kinase (CK) and its MB isoenzyme (CK-MB), lactate dehydrogenase (LDH) and parathyroid hormone (PTH) were measured in patients and their family members. X-ray radiography of thoracic and lumbar vertebrae, limbs, hips and skulls were taken individually.
The biochemical parameters of two healthy carriers, FM8-1 and FM8-2, are unavailable due to the patient’s refusal of the test.
BMD measurements
The BMDs of the anteroposterior lumbar spine 1–4 (L1–4) and the left proximal femur, including the femoral neck and total hip, were measured using a Lunar Prodigy dual energy X-ray absorptiometry (DXA) densitometer (Lunar Corp., Madison, WI, USA). The data were analyzed using Prodigy enCORE software (ver. 6.70, standard-array mode; GE Healthcare, Madison, WI, USA). The machine was calibrated daily, and the coefficient of variability (CV) values of the DXA measurements obtained from triplicate measurements of the same 15 individuals at L1–4, total hip, femoral neck and trochanter were 1.39, 0.70, 2.22 and 1.41%, respectively [15]. The long-term reproducibility of our DXA data during the trial based on weekly repeated phantom measurements was 99.55% [16]. Height and body weight were measured using standardized equipment.
Two pediatric patients (FM3-2 and FM9-2) were too young to finish DXA examination.
Mutation analysis
Genomic DNA was isolated from peripheral blood leukocytes using the conventional phenol–chloroform extraction method. The entire coding region and adjacent splice sites of the CLCN7 (GenBank Accession Number RefSeq NG_007567.1), TCIRG1 (GenBank Accession Number RefSeq NG_007878.1), LRP5 (GenBank Accession Number RefSeq NG_015835.1) and Sclerostin (SOST) (GenBank Accession Number RefSeq NG_008078.2) genes were amplified and sequenced directly from all 14 living patients, 64 recruited family members and 100 healthy donors. We classified affected ADO patients and unaffected mutation carriers based on radiographic and/or biochemical findings and clinical manifestations of the disease.
Results
Characteristics of patients with ADOII
In the current study, 13 of 15 patients with osteopetrosis (86.7%) were diagnosed with ADOII. The age at diagnosis had an average of 18 years and a range of 2–34 years. Six of 13 patients (46%) were female, aged 13 to 34 years. The youngest ADOII patient was diagnosed at 2 years old on the basis of an abnormal chest radiographic examination when he suffered from bronchitis. The oldest patient was 34 years of age and had experienced several years of lumbodorsal pain.
Mutations in CLCN7-related ADOII patients
A total of eight heterozygous missense mutations in the CLCN7 gene were detected in 12 living ADOII patients from 10 families (families 1–10) and are summarized in Table 1. Six novel mutations (75%) are marked with asterisks. The affected ADO patients were distinguished from unaffected mutation carriers by radiographic and/or biochemical findings and clinical manifestations. The previously reported R767W and the novel R743W mutation were each identified in two ADOII probands (20%, 2 out of 10 probands). The E798 frameshift (FS) [12], E313K, A316G, G741R, W127G and S290F mutations were each only detected in one proband. Moreover, the S290F mutation in family 10 was a de-novo mutation because the parents of this patient had normal wild-type genotype.
Clinical and radiographic manifestations of CLCN7-related ADOII
The detailed clinical manifestations of all 13 ADOII patients are summarized in Table 2. Almost half of the patients (6 of 13, 46%), most of them female (5 of 6, 83.33%), had lumbodorsal pain. Mild to severe anemia was detected in three patients (23%). One patient from family 1 (FM1-4) with severe anemia, thrombocytopenia, splenomegaly and typical X-ray findings died at the age of 20. The female adult patient FM6-1 had mild anemia accompanied by nerve deafness, and another female adult patient FM4-2 had both mild anemia and mandibular osteomyelitis. The radiographic signatures of “sandwich vertebrae” and “iliac wing” were detected in each patient by X-ray examination (Fig. 1).
Fractures are the most common clinical complication in patients with ADOII. As shown in Table 2, nine patients (69%) had at least one limb fracture. Of these patients, five persons ranging from 8 to 21 years old had broken bones between two and nine times. The common fracture sites were femur, humerus, ulna, radius, ankle, metatarsal bone, digitus minimus and digitus medius. No fractures of vertebra were found in any of the ADOII patients.
Biochemical parameters of CLCN7-related ADOII
The levels of bone turnover markers, renal function markers, serum Ca and P of 12 CLCN7-related patients are summarized in Table 3. In five adult patients, the serum CK level was only elevated in patient FM1-2 (267 U/L), while CK-MB exceeded the reference range in patients FM1-2, FM4-2 and FM7-1 (60%). In seven young patients aged from 2 to 17 years, serum CK and CK-MB levels were simultaneously increased in patients FM3-2, FM4-3, FM5-1, FM8-3 and FM10-1 (71%). The 17-year old patient (FM2-2) had normal serum CK level (171 U/L) but higher CK-MB level (116 U/L). The excessive serum LDH levels in the adult patient (FM1-2) and two pediatric patients (FM5-1 and FM10-1) were 432, 608 and 491 U/L, respectively. The mildly elevated serum PTH levels in the adult patient (FM6-1) and pediatric patient (FM4-3) were 65.75 and 88.10 ng/L, respectively, while both of them had normal serum Ca levels. Only one adult patient (FM1-2) had an elevated Ca value (2.81 mmol/L) but with normal serum PTH (60 ng/L). The serum creatinine level was normal in all patients.
BMD of CLCN7-related ADOII
The BMD values of 10 CLCN7-related patients at L1–4 and hip sites are shown in Table 3 too. The BMD at each site of the five adult patients was significantly higher compared with the standard age- and sex-matched adult mean reference value. The Z scores of the L1–4, femoral neck, trochanter and total hip were 11.44 ± 3.39 SD, 11.58 ± 5.28 SD, 14.88 ± 7.13 SD and 12.82 ± 4.53 SD, respectively. The Z scores of five patients aged 8–17 years were calculated by comparison with BMD measurements from age-matched Chinese children and adolescents [17, 18]. The Z scores of the L1–4, femoral neck and total hip were 9.00 ± 3.51 SD, 7.54 ± 1.12 SD and 8.65 ± 0.70 SD, respectively.
Characteristics of unaffected CLCN7 mutation carriers
In the current study, seven subjects were defined as being unaffected carriers. Of the mutations present, R767W and G741R were each detected in two individuals (28.6%, 2 of 7) from families 1 and 8, while E789FS, E313K and W127G were each found only in one subject (Table 1). The incomplete penetrance rates of R767W, G741R, E789FS, E313K and W127G were 40, 66.7, 50, 33.3 and 50%, respectively.
The biochemical parameters and BMD values are summarized in Table 4. Compared with CLCN7-related ADOII patients, only one person (FM1-1) had elevated serum CK level (208 U/L) but normal CK-MB level (23 U/L). Although FM1-1 had higher BMD at total hip (Z score = 2.4 SD) and FM4-1 had higher BMD at L1–4 (Z score = 2.6 SD) and femoral neck (Z score = 2.1 SD), both of them had no radiographic signatures such as “sandwich vertebrae” and “bone-within-bone”.
Clinical manifestations and gene mutation of uncategorized osteopetrosis patients
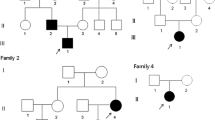
In families 11 and 12, the two probands were diagnosed with osteopetrosis due to osteodynia, high BMD and generalized sclerosing skeleton (Figs. 2, 3) without family history. The biochemical parameters and BMD in two uncategorized osteopetrosis patients are present in Table 5. The parents, three siblings and son of proband 11 had normal BMD and radiological signatures, and this was also true for the parents and son of proband 12. The serum ALP levels of proband 11 and 12 were 3 times the upper limit of normal (347 U/L) and 7 times the upper limit of normal (819 U/L), respectively. No evidence of anemia, hepatosplenomegaly or typical radiographic signs of “sandwich vertebrae” and “iliac wings” were found in either of these two subjects, although proband 12 had experienced multiple fractures. We failed to detect any mutation of the CLCN7, TCIRG1, LRP5 and SOST genes in these two patients and their family members. We classified these patients into the uncategorized osteopetrosis group owing to lack of evidence of a genetic basis for the disease.
Healthy donors
The CLCN7 gene mutations were analyzed in 100 age–sex-matched healthy donors. None of the above-mentioned mutations were detected in this cohort.
Phenotype–genotype correlation
In the current study, the three most common mutations—R767W, R743W and E313K—were present in 25, 16.7 and 16.7% of the 12 ADOII patients (Table 1), respectively. Nonetheless, the same CLCN7 mutations presented with divergent disease severity. Symptoms included fracture, mild to severe anemia, lumbodorsal pain, splenomegaly and nerve deafness. The single patient with the E798FS (8.3%), G741R (8.3%) or S290F (8.3%) mutation appeared to have more than three multiple limb fractures. Although each CLCN7-related ADOII patient had the typical radiographic “sandwich vertebrae” and “bone-within-bone” markers and although the BMD, biochemical markers and X-rays were also assessed for the unaffected carriers, it is still difficult to statistically address the existence of robust phenotype–genotype correlations.
Discussion
ADOII is the major subtype of ADO caused by mutations in the CLCN7 gene. In this study, eight mutations, including six novel mutations, in the CLCN7 gene were detected in 12 living patients with ADOII. R767W and R743W were two common mutations in the CLCN7 gene, although R767W has been already reported by other studies in Caucasian populations [14, 19, 20]. Therefore, we confirmed that mutations of the CLCN7 gene were disease-causing mutations in the Chinese patients with ADOII.
In the present study, seven subjects from families 1 through 10 harboring mutations in the CLCN7 gene without any clinical, biochemical or radiographic manifestations of osteopetrosis were defined as being unaffected carriers. Although ADO is an autosomal dominant disease, the penetrance ranges from 60 to 90% in families with ADOII [14]. Incomplete penetrance has been reported in many studies from 1968 [21] to the present. Waguespack and colleagues [20] revealed that only two-thirds of the individuals who had inherited mutations in the CLCN7 gene had typical ADOII phenotypes. Other studies [22, 23] have suggested that modifier genes play an important role in regulating disease progression in addition to the CLCN7 gene itself. Chu and colleagues [22] reported the presence of a modifier gene located in chromosome 9q21-22 that is able to affect disease status and severity. Furthermore, they suggested that osteoclast-specific properties determine the different statuses of the carriers. However, the mechanism remains elusive. In our previous study [12], we failed to explain the incomplete penetrance by analyzing semi-methylation of a CpG island of CLCN7. In agreement with Waguespack’s study [20], our unaffected mutation carriers had higher BMD values in comparison with age- and sex-matched mean reference values. Because of the late onset of ADOII, a follow-up study should determine whether the unaffected mutation carriers, especially the young carriers, become ADOII patients in the future.
To date, the difference in CLCN7 gene expression in patients with osteopetrosis and unaffected mutation carriers is still indistinct. In vitro study observed an increased acid-activated Cl− currents in HEK293 cells with wild-type human CLCN7, while these Cl− currents disappeared in HEK293 cells with the G215R mutation associated with ADOII [24]. The difference in osteoclast function in CLCN7-related ADOII patients, unaffected mutation carriers and normal controls has been investigated by Chu and colleagues [23]. By using osteoclasts derived from human peripheral blood mononuclear cells (PBMCs), they concluded that osteoclasts from the unaffected mutation carriers functioned normally in vitro, while defective osteoclast function was observed in osteoclasts from CLCN7-related ADOII patients. Because obtaining either PBMCs or bone biopsy samples from our patients is very difficult, a subsequent functional study of these six novel mutations by using cell lines may be a possible solution.
The clinical manifestations of ADOII comprise non-traumatic fractures, scoliosis, hip osteoarthritis and osteomyelitis of the mandible and other bones. The diagnosis of ADOII usually depends on the changes detected in radiographs that demonstrate “sandwich vertebrae” and “bone-within-bone” (iliac wings) [2]. In the current study, approximately 50% of patients suffered from lumbodorsal pain, while dental myelitis and hip osteoarthritis were rare in the ADOII cohort.
Fracture, especially of long bones, is the most likely complication of ADOII independent of gender differences [20]. In the ADOII cohort, we observed a rate of fracture of 69%, which is lower than the rate of 84% reported by Waguespack et al. [20]. They suggested that fractures occur more frequently in adults than in children. However, in our study, five patients with multiple fractures were between 8 and 21 years old. This finding may suggest that the earlier onset manifested in a higher fracture risk in the Chinese patients with ADOII. The mechanism responsible for the high prevalence of fractures in ADO patients has been previously studied. The main hypotheses include impaired bone remodeling due to defective osteoclast function, unsuccessful repair of microdamage and abnormal biomechanics of the bones of ADO patients [25].
Elevated CK and CK-MB levels were observed in more than 60% of CLCN7-related ADOII patients, including three who had simultaneously increased serum LDH levels. Although CK-BB is a sensitive biochemical marker for ADOII which has been previously validated by several studies [26–28], we had to measure CK-MB levels instead because of laboratory limitations. The serum CK-MB levels were measured using Roche/Hitachi CK-MB reagent. According to the manufacturer’s instructions, the cross-reactivity between CK-BB and CK-MB is only 0.1%. Therefore, our findings suggest that elevated levels of CK-MB are also a valuable biological marker in the diagnosis of ADOII if the measurement of CK-BB levels is unavailable. In addition to increased levels of CK and its isozyme, increased serum levels of LDH, BALP and osteocalcin and an increased N-terminal type I collagen telopeptide/creatinine ratio were reported by Letizia and colleagues [19] in an ADOII patient harboring a novel mutation in exon 25 of the CLCN7 gene. In addition, CLCN7-related osteopetrosis might be distinguished from other sclerosing bone disorders by elevated serum LDH and AST levels [29].
Two adult patients from families 11 and 12 had elevated serum ALP values and generalized sclerosing skeletons even though they did not exhibit “sandwich vertebrae” sign and “iliac wings”. No mutations of the LRP5, CLCN7, SOST or TCIRG1 genes were detected. We had to diagnose them with uncategorized osteopetrosis owing to the lack of evidence of a genetic basis for the disease. Further studies should analyze more candidate genes to verify the diagnosis and to identify novel causative genes.
To date, it has been very difficult for researchers to draw a clear correlation between genotype and phenotype for osteopetrosis. In the present study, we failed to establish a correlation between genotype and phenotype in Chinese patients with ADOII due to the variable clinical, biochemical and genetic findings.
In summary, R767W and R743W were two common mutations that cause CLCN7-related ADOII in China. Long bone fractures and elevated serum CK level were the two major manifestations. CLCN7-related ADOII patients are distinguished from unaffected mutation carriers by the radiographic “sandwich vertebrae” and “bone-within-bone” signatures and/or biochemical and clinical phenotypes, including non-traumatic fractures, elevated serum CK and CK-MB levels and a history of anemia. Unfortunately, no phenotype–genotype correlation was identified. Genetic analysis is necessary to diagnose osteopetrosis.
Further studies should focus on the following topics: (1) the functional study of mutations in the CLCN7 gene; (2) the causative mutation(s) in the two uncategorized osteopetrosis patients; (3) a follow-up study on unaffected carriers to observe whether they eventually show disease; (4) a follow-up study of ADOII patients to determine the natural disease progression; and (5) a possible explanation for the incomplete penetrance of CLCN7-related ADOII.
References
Stark Z, Savarirayan R (2009) Osteopetrosis. Orphanet J Rare Dis 4:5
Cleiren E, Benichou O, Van Hul E, Gram J, Bollerslev J, Singer FR, Beaverson K, Aledo A, Whyte MP, Yoneyama T, deVernejoul MC, Van Hul W (2001) Albers-Schonberg disease (autosomal dominant osteopetrosis, type II) results from mutations in the ClCN7 chloride channel gene. Hum Mol Genet 10:2861–2867
Tolar J, Teitelbaum SL, Orchard PJ (2004) Osteopetrosis. N Engl J Med 351:2839–2849
Henriksen K, Gram J, Hoegh-Andersen P, Jemtland R, Ueland T, Dziegiel MH, Schaller S, Bollerslev J, Karsdal MA (2005) Osteoclasts from patients with autosomal dominant osteopetrosis type I caused by a T253I mutation in low-density lipoprotein receptor-related protein 5 are normal in vitro, but have decreased resorption capacity in vivo. Am J Pathol 167:1341–1348
Van Wesenbeeck L, Cleiren E, Gram J, Beals RK, Benichou O, Scopelliti D, Key L, Renton T, Bartels C, Gong Y, Warman ML, De Vernejoul MC, Bollerslev J, Van Hul W (2003) Six novel missense mutations in the LDL receptor-related protein 5 (LRP5) gene in different conditions with an increased bone density. Am J Hum Genet 72:763–771
Del Fattore A, Cappariello A, Teti A (2008) Genetics, pathogenesis and complications of osteopetrosis. Bone 42:19–29
Chalhoub N, Benachenhou N, Rajapurohitam V, Pata M, Ferron M, Frattini A, Villa A, Vacher J (2003) Grey-lethal mutation induces severe malignant autosomal recessive osteopetrosis in mouse and human. Nat Med 9:399–406
Pangrazio A, Poliani PL, Megarbane A, Lefranc G, Lanino E, Di Rocco M, Rucci F, Lucchini F, Ravanini M, Facchetti F, Abinun M, Vezzoni P, Villa A, Frattini A (2006) Mutations in OSTM1 (grey lethal) define a particularly severe form of autosomal recessive osteopetrosis with neural involvement. J Bone Miner Res 21:1098–1105
Shin YJ (2004) Chloride channel CICN7 mutations in a Korean patient with infantile malignant osteopetrosis initially presenting with neonatal thrombocytopenia. J Perinatol 24:312–314
Aramaki S, Yoshida I, Yoshino M, Kondo M, Sato Y, Noda K, Jo R, Okue A, Sai N, Yamashita F (1993) Carbonic anhydrase II deficiency in three unrelated Japanese patients. J Inherit Metab Dis 16:982–990
Lam CW, Tong SF, Wong K, Luo YF, Tang HY, Ha SY, Chan MH (2007) DNA-based diagnosis of malignant osteopetrosis by whole-genome scan using a single-nucleotide polymorphism microarray: standardization of molecular investigations of genetic diseases due to consanguinity. J Hum Genet 52:98–101
Zhang ZL, He JW, Zhang H, Hu WW, Fu WZ, Gu JM, Yu JB, Gao G, Hu YQ, Li M, Liu YJ (2009) Identification of the CLCN7 gene mutations in two Chinese families with autosomal dominant osteopetrosis (type II). J Bone Miner Metab 27:444–451
Taranta A, Migliaccio S, Recchia I, Caniglia M, Luciani M, De Rossi G, Dionisi-Vici C, Pinto RM, Francalanci P, Boldrini R, Lanino E, Dini G, Morreale G, Ralston SH, Villa A, Vezzoni P, Del Principe D, Cassiani F, Palumbo G, Teti A (2003) Genotype-phenotype relationship in human ATP6i-dependent autosomal recessive osteopetrosis. Am J Pathol 162:57–68
Waguespack SG, Koller DL, White KE, Fishburn T, Carn G, Buckwalter KA, Johnson M, Kocisko M, Evans WE, Foroud T, Econs MJ (2003) Chloride channel 7 (ClCN7) gene mutations and autosomal dominant osteopetrosis, type II. J Bone Miner Res 18:1513–1518
Gao G, Zhang ZL, Zhang H, Hu WW, Huang QR, Lu JH, Hu YQ, Li M, Liu YJ, He JW, Gu JM, Yu JB (2008) Hip axis length changes in 10,554 males and females and the association with femoral neck fracture. J Clin Densitom 11:360–366
Zhang ZL, He JW, Qin YJ, Hu YQ, Li M, Zhang H, Hu WW, Liu YJ, Gu JM (2008) Association between myostatin gene polymorphisms and peak BMD variation in Chinese nuclear families. Osteoporos Int 19:39–47
Wu XP, Liao EY, Zhang H, Dai RC, Shan PF, Cao XZ, Liu SP, Jiang Y (2004) Determination of age-specific bone mineral density and comparison of diagnosis and prevalence of primary osteoporosis in Chinese women based on both Chinese and World Health Organization criteria. J Bone Miner Metab 22:382–391
Wu XP, Yang YH, Zhang H, Yuan LQ, Luo XH, Cao XZ, Liao EY (2005) Gender differences in bone density at different skeletal sites of acquisition with age in Chinese children and adolescents. J Bone Miner Metab 23:253–260
Letizia C, Taranta A, Migliaccio S, Caliumi C, Diacinti D, Delfini E, D’Erasmo E, Iacobini M, Roggini M, Albagha OM, Ralston SH, Teti A (2004) Type II benign osteopetrosis (Albers-Schonberg disease) caused by a novel mutation in CLCN7 presenting with unusual clinical manifestations. Calcif Tissue Int 74:42–46
Waguespack SG, Hui SL, Dimeglio LA, Econs MJ (2007) Autosomal dominant osteopetrosis: clinical severity and natural history of 94 subjects with a chloride channel 7 gene mutation. J Clin Endocrinol Metab 92:771–778
Johnston CC Jr, Lavy N, Lord T, Vellios F, Merritt AD, Deiss WP Jr (1968) Osteopetrosis. A clinical, genetic, metabolic, and morphologic study of the dominantly inherited, benign form. Medicine (Baltimore) 47:149–167
Chu K, Koller DL, Snyder R, Fishburn T, Lai D, Waguespack SG, Foroud T, Econs MJ (2005) Analysis of variation in expression of autosomal dominant osteopetrosis type 2: searching for modifier genes. Bone 37:655–661
Chu K, Snyder R, Econs MJ (2006) Disease status in autosomal dominant osteopetrosis type 2 is determined by osteoclastic properties. J Bone Miner Res 21:1089–1097
Kajiya H, Okamoto F, Ohgi K, Nakao A, Fukushima H, Okabe K (2009) Characteristics of ClC7 Cl− channels and their inhibition in mutant (G215R) associated with autosomal dominant osteopetrosis type II in native osteoclasts and hClcn7 gene-expressing cells. Pflugers Arch 458:1049–1059
Mashiba T, Hirano T, Turner CH, Forwood MR, Johnston CC, Burr DB (2000) Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res 15:613–620
Whyte MP, Chines A, Silva DP Jr, Landt Y, Ladenson JH (1996) Creatine kinase brain isoenzyme (BB-CK) presence in serum distinguishes osteopetroses among the sclerosing bone disorders. J Bone Miner Res 11:1438–1443
Yoneyama T, Fowler HL, Pendleton JW, Sforza PP, Gerard RD, Lui CY, Eldridge TH, Iranmanesh A (1992) Elevated serum levels of creatine kinase BB in autosomal dominant osteopetrosis type II—a family study. Clin Genet 42:39–42
Yoneyama T, Fowler HL, Pendleton JW, Sforza PP, Lui CY, Iranmanesh A, Gerard RD (1989) Elevated levels of creatine kinase BB isoenzyme in three patients with adult osteopetrosis. N Engl J Med 320:1284–1285
Whyte MP, Kempa LG, McAlister WH, Zhang F, Mumm S, Wenkert D (2010) Elevated serum lactate dehydrogenase isoenzymes and aspartate transaminase distinguish Albers-Schonberg disease (chloride channel 7 deficiency osteopetrosis) among the sclerosing bone disorders. J Bone Miner Res 25:2515–2526
Acknowledgments
We thank the patients and their family members for their invaluable cooperation. The study was supported by the National Natural Science Foundation of China (NSFC) (No. 30771019, 30800387, 81070692); Program of Shanghai Chief Scientist (Project No. 08XD1403000) and STCSM10DZ1950100; Shanghai Science and Technology Development Fund (Project No. 08411963100 and 11ZR1427300); Shanghai Rising-Star program (11QA1404900).
Conflict of interest
The authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Wang, C., Zhang, H., He, JW. et al. The virulence gene and clinical phenotypes of osteopetrosis in the Chinese population: six novel mutations of the CLCN7 gene in twelve osteopetrosis families. J Bone Miner Metab 30, 338–348 (2012). https://doi.org/10.1007/s00774-011-0319-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-011-0319-z