Abstract
Although osteonecrosis of the femoral head is a known primary limitation of long-term or high-dose clinical administration of glucocorticoids, the mechanisms underlying this side effect remain unclear. Hypoxia is an important biological state under numerous pathological conditions. In this study, we investigated glucocorticoid-induced osteonecrosis under hypoxic conditions in the MC3T3-E1 osteoblast cell line using a cell cytotoxicity assay, flow cytometry, and western blotting. 6α-Methylprednisolone sodium succinate (MPSL) more effectively induced apoptosis and G0/G1 arrest of MC3T3-E1 osteoblasts under hypoxic conditions than under normoxic conditions. Correspondingly, MPSL more effectively upregulated cellular levels of cleaved caspase 3, p53, and its target p21, and downregulated cyclin D1 levels in hypoxia. Moreover, overexpression of Akt abrogated the MPSL activation of p53, p21, and cleaved caspase 3 and the attenuation of cyclin D1 expression and rescued osteoblasts from MPSL-induced cell cycle arrest and apoptosis, indicating that phosphatidylinositol 3-kinase (PI3K)/Akt signaling might play an essential role in MPSL-induced inhibition of osteoblasts. Furthermore, the suppression of PI3K/Akt signaling and upregualtion of cellular p85α monomer levels by MPSL were more pronounced under hypoxic conditions than under normoxic conditions. Finally, we found that the enhancement of the effects of MPSL under hypoxic conditions was attributed to hypoxia-upregulated glucocorticoid receptor activity. In conclusion, our results demonstrate that MPSL, a synthetic glucocorticoid receptor agonist, promotes the level of p85α and inhibits PI3K/Akt signaling to induce apoptosis and cell cycle arrest in osteoblasts, and that this effect is enhanced under hypoxic conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glucocorticoids (GCs) exert most of their biological functions, including their anti-inflammatory and immune-suppressive effects, by activating the GC receptor (GR) [1]. The use of GCs in the treatment of rheumatoid arthritis, inflammatory bowel disease, and severe acute respiratory syndrome is hampered by the occurrence of osteonecrosis [2, 3]. It has been reported that approximately 39 % of severe acute respiratory syndrome patients develop osteonecrosis within a few months of GC therapy [3]. A better understanding of osteonecrosis is required to develop strategies to prevent this unwanted side effect of GC therapy.
Most cases of nontraumatic osteonecrosis during the administration of GCs involve the femoral head, and in particular the upper third of the femur, where the blood supply is limited to a single branch of the obturator artery [4]. This light vascularity has been linked to the enhancement by lipopolysaccharide of the effect of GCs in a rabbit model of osteonecrosis [5], in which lipopolysaccharide-induced hypercoagulability is thought to aggravate ischemic hypoxia in bone tissue. These observations suggest that the detrimental effect of GCs on the skeleton is facilitated under hypoxic conditions, although the mechanism requires clarification.
The adult skeleton undergoes continuous bone remodeling, in which a homeostatic balance between osteoblast and osteoclast activity plays a pivotal role [6]. Recent studies have demonstrated that impairment of the osteoblast–osteoclast balance by inhibiting osteoblastogenesis, and promoting osteoblastic apoptosis, may underlie GC-induced osteonecrosis [7, 8]. Mechanisms invoked in this process include ischemia of bone marrow, altered transcription of proapoptotic and anti-apoptotic genes, and decreased expression of cyclin-dependent kinases [9–11]. The exact underlying cause of GC-induced apoptosis and cell cycle arrest in osteoblasts, however, remains unclear.
The phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway confers the antiapoptotic and prosurvival function of insulin-like growth factor 1 in a variety of cell types. For example, activation of PI3K/Akt protects intestinal stem cells from radiation-induced injury [12], whereas, in contrast, inhibition of PI3K/Akt has been shown to sensitize human sarcoma cells to doxorubicin in hypoxia [13]. However, the question of whether GCs stimulate apoptosis and inhibit proliferation of osteoblasts through suppression of PI3K/Akt signaling, specifically under hypoxic conditions, remains to be elucidated.
In this study, we examined the effects of GCs on osteoblasts under hypoxic conditions and, then investigated the molecular mechanisms for GC-induced osteoblastic apoptosis and cell cycle arrest under hypoxic conditions. We demonstrate that the inhibitory impact of GCs on osteoblasts is tightly associated with suppression of p85α–PI3K/Akt signaling. Interestingly, this effect is enhanced with potentiation of GR activity under hypoxic conditions.
Materials and methods
Cell culture and transfection
Mouse MC3T3-E1 osteoblasts were purchased from ATCC (Manassas, VA, USA; CRL-2593) and were cultured in minimum essential medium α supplemented with 10 % fetal bovine serum, 1 % 2 mM l-glutamine (Sigma), and 1 % penicillin/streptomycin (Gibco). Cells were grown either under normoxic (95 % air and 5 % CO2) or hypoxic (1 % O2, 94 % N2, and 5 % CO2) conditions at 37 °C. The confluent MC3T3-E1 cells used in this study were identified to be differentiated osteoblasts [14]. All experiments were performed using cells of passages 5 to 15 and were repeated at least three times.
Cells were cultured in the corresponding medium with 0.5 % fetal bovine serum for 24 h prior to treatment with 6α-methylprednisolone 21-hemisuccinate sodium salt (MPSL; Sigma M3781) under normoxic or hypoxic conditions. When required, 5 μM lactacystin was added 2 h prior to treatment with MPSL. Plasmids for hemagglutinin-tagged wild-type Akt and constitutively active Akt were a kind gift from Jin Wei-lin (Institute of Neurosciences, School of Life Sciences and Biotechnology, Shanghai Jiao Tong University, China). Cells were transfected with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions.
Cytotoxicity assay and living cell count
Cytotoxicity was measured using the quantitative 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay in 96-well plates. Briefly, MTT solution (5 mg/mL) was added to each well and the plates were incubated for 4 h at 37 °C. The absorbance was read at 570 nm using a microplate spectrophotometer (Multiscan FC, Thermo Scientific, USA). Cytotoxicity (%) was calculated as [(absorbance of normal cells − absorbance of MPSL and/or hypoxia-treated cells)/absorbance of normal cells] × 100 [15].
A trypan blue exclusion assay was performed to estimate the number of living cells using a cell counter. Harvested cells (100 μL) were mixed with trypan blue (100 μL), and the number of living cells was calculated as (total cell number − dead cell number) × 104/4 × dilution.
4′,6-Diamidino-2-phenylindole staining
Cells were washed twice with phosphate-buffered saline and fixed with 4 % paraformaldehyde for 20 min at room temperature. After they had been washed twice, the cells were mounted using 4′,6-diamidino-2-phenylindole and were visualized under a fluorescence microscope (Leica, Germarny). Nuclear condensation is characteristic of apoptotic cells.
Apoptosis and cell cycle analysis
Apoptosis was measured using an annexin V–fluorescein isothiocyanate apoptosis detection kit (Invitrogen). Briefly, cells were resuspended in annexin binding buffer, after which annexin V and propidium iodide (100 μg/mL) were added. After incubation for 15 min at 25 °C, the cells were analyzed using a flow cytometer (Becton, Dickinson, USA).
For cell cycle analysis, the cells were fixed with 70 % ethanol for 2 h at 25 °C. After centrifugation, the cells were incubated with 0.5 mL of phosphate-buffered saline containing RNase (100 g/μL) and propidium iodide (5 μg/mL) for 30 min at 37 °C (Sigma). Cell cycle kinetics were analyzed by measuring the DNA content using a flow cytometer (Beckman Coulter, USA).
Western blotting
The harvested cells were suspended in radioimmunoprecipitation assay buffer containing 1 % NP-40, 0.5 % sodium deoxycholate, 0.1 % sodium dodecyl sulfate, and protease inhibitor cocktails for 1 h (Sigma), and supernatants were collected. The protein concentration was determined using a bicinchoninic acid protein assay kit (Applygen, Beijing, China). After separation on 8–12 % polyacrylamide gels, the proteins were transferred onto a poly(vinylidene difluoride) membrane and blocked with 5 % bovine serum albumin in tris(hydroxymethyl)aminomethane-buffered saline containing 0.1 % Tween 20 for 1 h at 25 °C. Membranes were incubated with the corresponding primary antibody (Table S1) at 4 °C overnight. After they had been washed, the membranes were incubated with horseradish peroxidase conjugated secondary antibody (Table S1). β-Actin was used as an internal control. Proteins were detected using enhanced chemiluminescence reagent (Applygen, Beijing, China).
Quantitative real-time PCR analysis
Total RNA was extracted from cells using an RNA prep kit (TIGNGEN, Beijing, China), and first-strand complementary DNA was synthesized using a Fast Quant RT kit (TIGNGEN). The complementary DNA was diluted and used to perform real-time quantitative PCR using an Applied Biosystems model 7500 real-time PCR system with TaqMan PCR master mix (Applied Biosystems, Foster City, CA, USA). Messenger RNA (mRNA) levels were normalized using the comparative C T method. GAPDH expression was used for internal normalization. Primer sequences are listed in Table S2.
Statistical analysis
The results are presented as the mean ± standard error of the mean and were analyzed using Student’s t test between two groups or one-way ANOVA among groups with SPSS 15.0 (SPSS, Chicago, IL USA). P ≤ 0.05 was considered statistically significant.
Results
Hypoxia enhances inhibition of cell proliferation and induction of cell death in MC3T3-E1 osteoblasts
MC3T3-E1 cells were exposed to a range of MPSL concentrations for 24 h or to 2 μM MPSL for different durations, under either hypoxia or normoxia. Whereas culturing cells under hypoxic conditions alone for 24 h had no significant effect on cytotoxicity as measured by the MTT assay, the cytotoxic effect of MPSL was significantly increased under hypoxic conditions in a time- and dose-dependent manner (Fig. 1a). Consistent with this, the trypan blue exclusion assay showed that, compared with MPSL treatment alone, the living MC3T3-E1 population was significantly smaller after MPSL treatment under hypoxic conditions in a time- and dose-dependent manner (Fig. 1b). These observations suggest that enhancement of MPSL inhibition of osteoblasts under short-term hypoxic conditions likely results from a potentiation of MPSL effects, rather than from additional hypoxia-induced injury.
6α-Methylprednisolone 21-hemisuccinate sodium salt (MPSL) more significantly exerts an inhibitory effect on MC3T3-E1 cells under hypoxic conditions. MC3T3-E1 cells were incubated with different MPSL concentrations for 24 h (left panel) or with 2 μM MPSL for various treatment durations (right panel) under normoxic or hypoxic conditions. a The cytotoxic effect of MPSL was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. b Living cells were measured with a cell counter using the trypan blue exclusion assay. The data are expressed as the mean ± standard error of the mean for at least three determinations compared with MPSL treatment in normoxia. * p < 0.05, ** p < 0.01
MPSL more significantly induces apoptosis of osteoblasts and cell cycle arrest under hypoxic conditions than under normoxic conditions
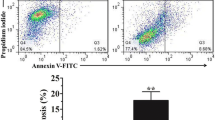
To elucidate the effect of MPSL on death in MC3T3-E1 osteoblasts, we next conducted an apoptosis assay. Treatment with 2 μM MPSL for 24 h under hypoxic conditions resulted in more apparent nuclear condensation in MC3T3-E1 cells than under normoxic conditions, indicating MPSL plus hypoxia induced more apoptotic cells (Fig. 2a). Moreover, the percentage of MPSL-induced apoptotic cells was higher under hypoxic conditions, assessed by cytometry (Fig. 2b, c). Correspondingly, the levels of apoptosis-related p53 and cleaved caspase 3 were significantly increased after treatment with MPSL plus hypoxia (Fig. 2d).
MPSL significantly induces apoptosis in MC3T3-E1 cells under hypoxic conditions. MC3T3-E1 cells were incubated with 2 μM MPSL for 24 h under hypoxic or normoxic conditions. a The arrows signify apoptotic cells assessed by condensed chromatin after 4′,6-diamidino-2-phenylindole staining (bar 20 μm). b Apoptosis was determined by flow cytometry followed by annexin V–propidium iodide (PI) double staining. Annexin V positive cells were considered as apoptotic cells. c The percentage of apoptotic cells measurement by cytometry was higher for cells treated with MPSL plus hypoxia than for cells treated with MPSL only (* p < 0.05, ** p < 0.01). d The levels of cleaved caspase 3 and p53 were significantly increased after treatment with MPSL plus hypoxia than after treatment with MPSL alone. The total cell extracts were collected and prepared for western blotting
We further analyzed the effect of MPSL on cellular proliferation and the cell cycle. The percentage of G0/G1 phase MC3T3-E1 cells was significantly increased in cells treated with MPSL plus hypoxia for 16 h, compared with MPSL-treated cells (Fig. 3a, b). Moreover, both downregulation of cyclin D1 and upregulation of p21 in MC3T3-E1 cells were more evident after treatment with MPSL plus hypoxia than after treatment with MPSL only (Fig. 3c). We found that pretreatment of MPSL-treated MC3T3-E1 cells with lactacystin, an inhibitor of the ubiquitin–proteasome system, abrogated MPSL-induced degradation of cyclin D1 in both hypoxia and normoxia (Fig. S1). Collectively, these results suggest that although hypoxia alone had little effect on osteoblast survival, the apoptotic and cell cycle arrest effect of MPSL might be exaggerated under hypoxic conditions.
MPSL significantly induces cell cycle arrest in MC3T3-E1 cells under hypoxic conditions. MC3T3-E1 cells were incubated with 2 μM MPSL for 16 h under hypoxic or normoxic conditions. a Cell cycle arrest was determined by flow cytometry with PI staining. b The percentage of the G0/G1 phase cells obtained using cytometry, which were induced by MPSL treatment, was significantly higher under hypoxic conditions than under normoxic conditions (** p < 0.01). c Downregulation of cyclin D1 levels and upregulation of p21 levels were more evident after treatment with MPSL plus hypoxia than after treatment with MPSL alone
Hypoxia enhances the inhibitory effects of MPSL on osteoblasts via the PI3K/Akt signaling pathway
The PI3K/Akt signaling pathway plays a pivotal role in cellular events, including cell cycle progression, differentiation, survival, and angiogenesis. It has been reported that p85α monomer, a target of GR in C2C12 myoblasts, inhibits Akt activity by competing for the receptor tyrosine kinase binding site with p110/p85 heterodimers [16]. We found that accompanied by upregulation of p85α monomer levels, the levels of phosphorylated Akt (p-Akt), the activated form of Akt, were significantly decreased after 24 h of treatment with MPSL, although overall Akt levels were unchanged (Fig. 4a). Hypothesizing that PI3K/Akt signaling might play a role in MPSL-induced p53 activation and cyclin D1 degradation, we next probed this pathway in MPSL-treated MC3T3-E1 cells (Fig. 4b–d). Moreover, transfection of wild-type or constitutively active forms of Akt abrogated the MPSL-induced increases in p53, p21, and cleaved caspase 3 levels, and restored cellular levels of cyclin D1 in the presence of MPSL (Fig. 4b). Furthermore, MPSL-induced apoptosis and cell cycle arrest were significantly blocked by constitutively active Akt in osteoblasts (Fig. 4c, d). Collectively, these results indicate that reduced PI3K/Akt signaling contributes to MPSL-induced apoptosis and cell cycle arrest.
MPSL induced apoptosis and cell cycle arrest through the phosphatidylinositol 3-kinase (PI3K)/Akt pathway in MC3T3-E1 cells. MC3T3-E1 osteoblasts were incubated with 2 μM MPSL for 24 h. a Upregulation of p85α by MPSL inhibited the phosphorylated Akt (p-Akt) activity. b Activated Akt suppressed MPSL induction of p53, p21, and cleaved-caspase 3 and MPSL repression of cyclin D1 levels. The cells were collected for western blotting analysis with the treatments indicated following transfection with hemagglutinin (HA)-tagged wild-type Akt (WT Akt) or constitutively active Akt (Con. Akt) plasmids for 24 h. c Active Akt rescued osteoblasts from MPSL-induced apoptosis. d Active Akt restored osteoblastic cell cycle progression
To investigate the mechanism underlying the exacerbation of GC-induced apoptosis and cell cycle arrest under hypoxic conditions, we next evaluated the status of PI3K/Akt signaling in MC3T3-E1 cells under hypoxic and normoxic conditions. No differences in the levels of p85α, p-Akt, and the downstream PI3K/Akt signaling were observed between hypoxic and normoxic cells until after 8 h of treatment with MPSL. A difference in induction of p85α between the MPSL plus hypoxia and MPSL-alone groups was evident at 8 h, at which time inactivation of p-Akt was also observed. Activation of p53 and p21 and degradation of cyclin D1 were significantly enhanced in hypoxic cells compared with normoxic cells after 16 h of MPSL treatment; and these differences were further enhanced between the two cell groups after 24 h of treatment with MPSL (Fig. 5).
MPSL significantly inhibits PI3K/Akt signaling under hypoxic conditions. The levels of p85α, p-Akt, p53, p21, cleaved caspase 3, and cyclin D1 in MC3T3-E1 cells were measured under normoxic or hypoxic conditions in the presence of 2 μM MPSL for 2, 8, 16, or 24 h. MPSL plus hypoxia more effectively promoted p85α levels and attenuated p-Akt activity than MPSL alone under normoxic conditions after 8 h. Induction of p53, p21, and cleaved caspase 3 and degradation of cyclin D1 were more noticeable after 16 h of MPSL treatment under hypoxic conditions than under normoxic conditions, and the synergistic effect of MPSL under hypoxic conditions was more significant after 24 h
Finally, we found that levels of GR phosphorylated at Ser21, biomarkers for activated GR in vivo [17, 18], and nuclear GR were higher in hypoxic cells than in normoxic cells after 4 h of MPSL treatment (Fig. 6). To further confirm the enhancement of GC-dependent GR transcriptional activity by hypoxia, we investigated the mRNA level of GR target genes, including interleukin-10 and osteopontin [11]. We found that MPSL-induced mRNA levels of interleukin-10 and osteopontin were significantly enhanced under hypoxic conditions. Collectively, these data support the conclusion that hypoxia enhances MPSL-induced osteoblast apoptosis and G0/G1 arrest via the p85α–PI3K/Akt signaling pathway.
Increased glucocorticoid receptor (GR) activity under hypoxic conditions. a The levels of GR (phosphorylated at Ser211) and nuclear GR were compared after 4 h of MPSL treatment under normoxic and hypoxic conditions. b The messenger RNA (mRNA) levels of interleukin-10 (IL-10), osteopontin (OPN), and glyceraldehyde 3-phosphate dehydrogenase were determined by real-time PCR. MPSL-induced expression levels of IL-10 and OPN were significantly enhanced under hypoxic conditions (* p < 0.05, ** p < 0.01)
Discussion
Although treatment with GCs is known to be associated with nontraumatic osteonecrosis, the underlying molecular mechanisms of this side effect are unclear. Although earlier studies invoked changes in the bone microenvironment, including intramedullary hemorrhage, vascular thrombosis, and fat embolism [19, 20], more recently attention has been focused on the direct actions of GCs on osteoblasts. Our current study demonstrates that hypoxia enhances the effects of MPSL, a synthetic GR agonist, on apoptosis and G0/G1 arrest in osteoblasts. Moreover, MPSL inhibition of osteoblasts was potentiated under hypoxic conditions, which further increased levels of p85α and inhibited PI3K/Akt signaling activity.
The protein p53, which is referred to as the “guardian of the genome,” activates a broad range of genes that mediate diverse cellular responses to cellular stress, including DNA repair, apoptosis, and senescence [21]. Our results showed that MPSL-induced apoptosis in osteoblasts was enhanced under hypoxic conditions, which was related to a more efficient induction of cleaved caspase 3 and p53 (Fig. 2). Moreover, the patterns of p53 overexpression occurred in parallel with increased levels of its transcriptional target p21, which has been shown to impede the transition from G1 phase to S phase in the cell cycle [22]. The increased percentage of G1 phase cells following MPSL treatment was accompanied by the downregulation of cyclin D1 (Fig. 3), a regulator of cyclin-dependent kinases that is required for G1/S transition [23]. In addition, the proteasome inhibitor lactacystin abrogated MPSL-induced degradation of cyclin D1 (Fig S1), suggesting that p53 was not directly involved in the transcriptional regulation of cyclin D1 expression in response to treatment with MPSL.
To ascertain whether a common mechanism exists for regulation of osteoblast proliferation and apoptosis via modulation of the levels of p53 and cyclin D1 by MPSL, we analyzed the PI3K/Akt signaling pathway. Previous studies have reported that Akt, a serine/threonine kinase, directly blocks glycogen synthase kinase 3β activity to protect cyclin D1 from degradation by the proteasome [24], and promotes the interaction between MDM2 and p53 to limit intracellular p53 levels [25]. Activation of Akt in T-cell acute lymphoblastic leukemia has been shown to effectively block GC-induced apoptosis [26] and, conversely, inhibition of PI3K or Akt has been shown to enhance dexamethasone induction of apoptosis in human follicular lymphoma cells [27]. PI3K, a kinase upstream of Akt, is composed of a regulatory p85 subunit and a catalytic p110 subunit. An interaction between p85α and receptor tyrosine kinase causes a conformational change that results in activation of p110, which in turn initiates conversion of phosphoinositide to activate a variety of cellular signaling networks [28]. Elevated levels of the p85α monomer have been postulated to compete with p110/p85 heterodimers for the binding site of the tyrosine receptor, resulting in inactivation of Akt signaling [29]. On the basis of this finding, Wei et al. [12] achieved suppression of Akt activity by transfection of PI3K (p85) expression plasmid in colorectal cancer cells. We observed induction of cellular p85α levels and inhibition of cellular p-Akt levels in response to treatment with MPSL (Fig. 4a). Moreover, overexpression of a constitutively active form of Akt resulted in the stabilization of cyclin D1 and abrogated MPSL induction of p53, p21, and cleaved caspase 3 (Fig. 4b). In addition, activated Akt abrogated MPSL-induced osteoblastic apoptosis and cell cycle arrest (Fig. 3c, d). Furthermore, MPSL induction of p85α and inactivation of p-Akt were more evident in response to treatment with MPSL under hypoxic conditions than under normoxic conditions, with increased p53, p21, and cleaved caspase 3 levels, and decreased cyclin D1 levels (Fig. 5). In concert, these data indicate that the acceleration of PI3K/Akt signaling suppression might have a more pronounced impact on aggravation of osteoblastic apoptosis and cell cycle arrest under hypoxic conditions.
A final important finding in this study is that the GR activity was potentiated in hypoxia (Fig. 6). It is well characterized that activation of GR in response to binding of GC ligand results in dissociation of the GR–heat shock protein complex in the cytosol, followed by translocation to the nucleus, where activated GR interacts with GC response elements in the promoters of its target genes [30]. The GR target p85α has been shown to inhibit the antiapoptotic effects of insulin-like growth factors by impairing PI3K/Akt signaling in myotubes [16, 27], and our own study confirmed that MPSL induced p85α levels in a time-dependent manner in osteoblasts (Fig. 5). The enhanced effect of MPSL during hypoxia was mediated by potentiation of GR activity, indicating that increased GR–p85α activity had a more pronounced impact on inhibition of PI3K/Akt signaling under hypoxic conditions. Indeed, similar mechanisms may contribute to enhancement of GC-dependent GC response element transcriptional activity in hypoxic human renal proximal tubular epithelial cells [31]. The immunophilin FKBP52-mediated dissociation and trafficking of the GR–heat shock protein 90 complex might be involved in this enhanced GR sensitivity under hypoxic conditions [32], although further investigation is needed.
Hypoxia, which occurs when the oxygen requirements for normal cellular ATP synthesis exceed the vascular supply, is characteristic of a variety of pathological conditions, including severe infection and toxic shock [33]. In this context, it is tempting to speculate that hypoxia resulting from limited blood supply makes the femoral head more sensitive to GC-induced osteonecrosis. It has been reported that osteocytes exposed to high-dose dexamethasone appear to be more sensitive to apoptosis when cultured in a hypoxic environment [34]. GCs can impair hypoxia-mediated neutrophil survival [35]. Similarly, our data show that osteoblasts survived for 24 h under hypoxic conditions and, moreover, the levels of all factors we assayed were unchanged under hypoxic conditions compared with normoxic conditions. Many processes during mammalian embryogenesis take place at an O2 concentration of 1–5 % [36], and the resistance of tumor cells to anticancer drugs under hypoxic conditions has been extensively documented [37, 38].
In summary, we identified suppression of PI3K/Akt signaling as a common mechanism underlying GC-induced osteoblastic apoptosis and cell cycle arrest. Moreover, we found that this effect of GCs was enhanced with promotion of GR activity and p85α expression under hypoxic conditions. We presumed that targeting PI3K/Akt signaling and improving local blood supply to ameliorate ischemia and anoxia could reduce the risk of adverse skeleton events induced by GCs, especially in the femoral head.
References
Manolides AS, Cullen DM, Akhter MP (2008) Effects of glucocorticoid treatment on bone strength. J Bone Miner Metab 28:532–539
Weinstein RS (2012) Glucocorticoid-induced osteoporosis and osteonecrosis. Endocrinol Metab Clin North Am 41:595–611
Lv H, de Vlas SJ, Liu W, Wang TB, Cao ZY, Li CP, Cao WC, Richardus JH (2009) Avascular osteonecrosis after treatment of SARS: a 3-year longitudinal study. Trop Med Int Health 14:79–84
Lafforgue P (2006) Osteonecrosis of the femoral head. Rev Prat 56:817–825
Yamamoto T, Hirano K, Tsutsui H, Sugioka Y, Sueishi K (1995) Corticosteroid enhances the experimental induction of osteonecrosis in rabbits with Shwartzman reaction. Clin Orthop Relat Res 316:235–243
Olney RC (2009) Mechanisms of impaired growth: effect of steroids on bone and cartilage. Horm Res 72:30–35
Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC (1998) Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. J Clin Invest 102:274–282
Kim HJ (2010) New understanding of glucocorticoid action in bone cells. BMB Rep 43:524–529
Wang TY, Avlonitis EG, Relkin R (1988) Systemic necrotizing vasculitis causing bone necrosis. Am J Med 84:1085–1086
Asada T, Kushida T, Umeda M, Oe K, Matsuya H, Wada T, Sasai K, Ikehara S, Iida H (2008) Prevention of corticosteroid-induced osteonecrosis in rabbits by intra-bone marrow injection of autologous bone marrow cells. Rheumatology 47:591–596
Moutsatsou P, Kassi E, Papavassiliou AG (2012) Glucocorticoid receptor signaling in bone cells. Trends Mol Med 18:348–359
Qiu W, Leibowitz B, Zhang L, Yu J (2010) Growth factors protect intestinal stem cells from radiation-induced apoptosis by suppressing PUMA through the PI3K/AKT/p53 axis. Oncogene 29:1622–1632
Kilic-Eren M, Boylu T, Tabor V (2013) Targeting PI3K/Akt represses Hypoxia inducible factor-1α activation and sensitizes rhabdomyosarcoma and Ewing’s sarcoma cells for apoptosis. Cancer Cell Int 13:36
Kósa JP, Kis A, Bácsi K, Balla B, Nagy Z, Takács I, Speer G, Lakatos P (2011) The protective role of bone morphogenetic protein-8 in the glucocorticoid-induced apoptosis on bone cells. Bone 48:1052–1057
Elia U, Flescher E (2008) PI3K/Akt pathway activation attenuates the cytotoxic effect of methyl jasmonate toward sarcoma cells. Neoplasia 10:1303–1313
Kuo T, Lew MJ, Mayba O, Harris CA, Speed TP, Wang JC (2012) Genome-wide analysis of glucocorticoid receptor-binding sites in myotubes identifies gene networks modulating insulin signaling. Proc Natl Acad Sci U S A 109:11160–11165
Wang Z, Frederick J, Garabedian MJ (2002) Deciphering the phosphorylation code of the glucocorticoid receptor in vivo. J Biol Chem 277:26573–26580
Blind RD, Garabedian MJ (2008) Differential recruitment of glucocorticoid receptor phospho-isoforms to glucocorticoid-induce genes. J Steroid Biochem Mol Biol 109:150–157
Jones JJ (1993) Fat embolism, intravascular coagulation, and osteonecrosis. Clin Orthop Relat Res 292:294–308
Wang TY, Avlonitis EG, Relkin R (1988) Systemic necrotizing vasculitis causing bone necrosis. Am J Med 84:1085–1086
Purvis JE, Karhohs KW, Mock C, Batchelor E, Loewer A, Lahav G (2012) p53 dynamics control cell fate. Science 336:1440–1444
Heber-Katz E, Zhang Y, Bedelbaeva K, Song F, Chen X, Stocum DL (2013) Cell cycle regulation and regeneration. Curr Top Microbiol Immunol 367:253–276
Lim S, Kaldis P (2013) Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development 140:3079–3093
Diehl JA, Cheng M, Roussel MF, Sherr CJ (1998) Glycogen synthase kinase-3β regulates cyclin D1 proteolysis and subcellular localization. Genes Dev 12:3499–3511
Levine AJ, Feng Z, Mak TW, You H, Jin S (2006) Coordination and communication between the p53 and IGF-1-AKT-TOR signal transduction pathways. Genes Dev 20:267–275
Piovan E, Yu J, Tosello V, Herranz D, Ambesi-Impiombato A et al (2013) Direct reversal of glucocorticoid resistance by AKT inhibition in acute lymphoblastic leukemia. Cancer Cell 24:766–776
Nuutinen U, Postila V, Mättö M, Eeva J, Ropponen A, Eray M, Riikonen P, Pelkonen J (2006) Inhibition of PI3-kinase-Akt pathway enhances dexamethasone-induced apoptosis in a human follicular lymphoma cell line. Exp Cell Res 312:322–330
Leis H, Page A, Ramírez A, Bravo A, Segrelles C, Paramio J, Barettino D, Jorcano JL, Pérez P (2004) Glucocorticoid receptor counteracts tumorigenic activity of Akt in skin through interference with the phosphatidylinositol 3-kinase signaling pathway. Mol Endocrinol 18:303–311
Backer JM (2010) The regulation of class IA PI 3-kinases by inter-subunit interactions. Curr Top Microbiol Immunol 346:87–114
Alangari AA (2010) Genomic and non-genomic actions of glucocorticoids in asthma. Ann Thorac Med 5:133–139
Leonard MO, Godson C, Brady HR, Taylor CT (2005) Potentiation of glucocorticoid activity in hypoxia through induction of the glucocorticoid receptor. J Immunol 174:2250–2257
Grad I, Picard D (2007) The glucocorticoid responses are shaped by molecular chaperones. Mol Cell Endocrinol 275:2–12
Huang LE, Bunn HF (2003) Hypoxia-inducible factor and its biomedical relevance. J Biol Chem 278:19575–19578
Tsuji M, Ikeda H, Ishizu A, Miyatake Y, Hayase H, Yoshiki T (2006) Altered expression of apoptosis-related genes in osteocytes exposed to high-dose steroid hormones and hypoxic stress. Pathobiology 73:304–309
Marwick JA, Dorward DA, Lucas CD, Jones KO, Sheldrake TA, Fox S, Ward C, Murray J, Brittan M, Hirani N, Duffin R, Dransfield I, Haslett C, Rossi AG (2014) Oxygen levels determine the ability of glucocorticoids to influence neutrophil survival in inflammatory environments. J Leukoc Biol 94:1285–1292
Gregg L (2012) Hypoxia-inducible factors in physiololgy and medicine. Cell 148:399–408
Kim HS, Wannatung T, Lee S, Yang WK, Chung SH, Lim JS, Choe W, Kang I, Kim SS, Ha J (2012) Quercetin enhances hypoxia-mediated apoptosis via direct inhibition of AMPK activity in HCT116 colon cancer. Apoptosis 17:938–949
Kilic M, Kasperczyk H, Fulda S, Debatin KM (2007) Role of hypoxia inducible factor-1 alpha in modulation of apoptosis resistance. Oncogene 26:2027–2038
Kodama T, Shimizu N, Yoshikawa N, Makino Y, Ouchida R, Okamoto K, Hisada T, Nakamura H, Morimoto C, Tanaka H (2003) Role of the glucocorticoid receptor for regulation of hypoxia-dependent gene expression. J Biol Chem 278:33384–33391
Acknowledgments
This study was supported by the National Science Foundation of China (81300285, 31371220, 31271290, 81101769), the Specialized Research Fund for the Doctoral Program of Higher Education of China (20111107110012, 20121107120020), the Scientific Research Key Program of Beijing Municipal Commission of Education (KZ201310025020), and the Funding Project for Academic Human Resources Development in Institutions of Higher Learning under the Jurisdiction of Beijing Municipality (PHR201007113).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
774_2014_627_MOESM1_ESM.tif
Supplementary material 1 Ubiquitination-mediated cyclin D1 degradation in osteoblasts participates in MPSL regulation of the cell cycle. After pretreatment with or without 5 μM lactacystin proteasome inhibitor for 2 h, MC3T3-E1 cells were cultured with 2 μM MPSL under hypoxic or normoxic conditions for 16 h. Degradation of cyclin D1 by the ubiquitin–proteasome system was more evident after MPSL plus hypoxia treatment than after treatment with MPSL alone for 16 h.(TIFF 1106 kb)
About this article
Cite this article
Zou, W., Yang, S., Zhang, T. et al. Hypoxia enhances glucocorticoid-induced apoptosis and cell cycle arrest via the PI3K/Akt signaling pathway in osteoblastic cells. J Bone Miner Metab 33, 615–624 (2015). https://doi.org/10.1007/s00774-014-0627-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-014-0627-1