Abstract
Dexamethasone (Dex), a glucocorticoid with strong anti-inflammatory and immunosuppressive activities, has been shown to exhibit marked cytotoxicity and apoptosis in osteoblasts, but the underlying mechanisms have not yet been comprehensively investigated. P21Waf1/Cip1 (p21) plays a critical role in the regulation of cell cycle progression and apoptosis. The present study aims to investigate the role of p21 in Dex-induced apoptosis in osteoblastic MC3T3-E1 cells, and to explore its mechanisms. Results demonstrated that Dex-induced apoptosis decreased the phosphorylation of Akt in a concentration-dependent manner. Moreover, LY294002, an inhibitor of the PI3K/Akt pathway enhanced the Dex-induced apoptosis of osteoblasts. On the contrary, insulin-like growth factor-1 (IGF-1), an activator of PI3K/Akt, attenuated the apoptosis of Dex in MC3T3-E1 cells. The protein level of p21 was downregulated by shortening its half-life, which was associated with inhibition of the PI3K/Akt pathway by Dex. Furthermore, depletion of p21 by siRNA enhanced Dex-induced caspase-3 activation and ROS generation, and promoted apoptosis of MC3T3-E1 cells. In addition, suppression of p21 led to a reduction of Dex-induced upregulation of nuclear Nrf2 and heme oxygenase-1 (HO-1) protein levels. These findings demonstrate that p21 depletion promotes Dex-induced apoptosis of MC3T3-E1 cells by inhibiting the antioxidant Nrf2/HO-1 pathway, which highlights the anti-apoptotic effect of p21 in MC3T3-E1 cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dexamethasone (Dex) represents a widely used glucocorticoid for the treatment of inflammatory diseases, cancers, pulmonary, gastrointestinal and autoimmune disorders (Schäcke et al. 2002; Spoorenberg et al. 2014). However, long term and/or excessive dexamethasone application has substantial adverse effects and may lead to different degrees of bone damage, including secondary osteoporosis and osteonecrosis (den Uyl et al. 2011; Kerachian et al. 2009; Weinstein 2011). It has been documented that about one-third of patients with long term intake of Dex suffered from bone fractures. Apoptosis of osteoblasts and osteocytes have been discussed as it is a major mechanism contributing to Dex-induced osteoporosis (den Uyl et al. 2011). Evidence suggests that Dex exerts direct cytotoxic effects to cultivated osteoblasts, triggers G1-phase cell cycle arrest, and induces apoptosis in osteoblasts via generation of reactive oxygen species (ROS) and induction of oxidative DNA damage (Li et al. 2012; Sato et al. 2015). Moreover, several studies demonstrated that Dex causes apoptosis of MC3T3-E1 cells, which is associated with an imbalance in the anti- and pro-apoptotic proteins, i.e., Bcl-2 and Bax, respectively (Li et al. 2012; Zhu et al. 2011). Although apoptosis of osteoblasts caused by Dex has been identified as an important contributor to the development of osteoporosis, the responsible molecular mechanisms still remain elusive. Therefore, further studies focusing on the underlying molecular mechanisms of Dex-induced osteoblasts apoptosis are required.
P21 (also known as p21Waf1/Cip1), a prototypical member of the cyclin-dependent kinase (CDK) inhibitor family, has been shown to negatively modulate cell cycle progression and induce cell cycle arrest in response to DNA damage by inhibiting the activity of cyclin/Cdk2 complexes (Bertoli et al. 2013; Wang et al. 2011). In addition, it also blocks DNA replication by binding to proliferating cell nuclear antigen (PCNA) (Prives and Gottifredi 2008). It also plays a central role in the regulation of apoptosis (Karimian et al. 2016). Accumulating data demonstrate that the anti-apoptotic function of p21 is associated with multiple pro-apoptotic molecules or the apoptosis signal-regulating kinase 1 (Ask1), which blocks activation of caspase-3 and inhibits the pro-apoptotic protein Ask1 (Zhan et al. 2007; Zhang et al. 1999). P21 null osteoblasts differentiate faster than wild-type cells and undergo increased differentiation-related apoptosis, suggesting that p21 acts a pro-apoptotic factor in osteoblastic cells (Bellosta et al. 2003).
Nuclear factor-erythroid 2-related factor 2 (Nrf2) plays an essential role in the cellular defense against osteoporosis. It represents a transcription factor that binds to antioxidant response elements and activates the expression of antioxidant-responsive genes and induces phase II detoxifying enzymes, such as heme oxygenase-1 (HO-1) (Ibáñez et al. 2014; Loboda et al. 2016). In response to oxidative stress, induction of Nrf2 and p21 supports cell survival and protects cells from oxidative damage (Chen et al. 2009). However, the underlying mechanisms remain unclear.
Although p21 has been shown to play an important role in regulating cell proliferation and apoptosis, the mechanisms how p21 modulates osteoblast responses to Dex has not been fully clarified. Therefore, the aim of present study was to investigate the role of p21 in Dex-induced apoptosis in MC3T3-E1 cells, and to explore the mechanisms underlying p21-mediated protection against Dex with special focus on the antioxidant Nrf2/HO-1 pathway.
Materials and methods
Reagents and antibodies
Minimum essential medium (α-MEM), Opti-MEM and fetal bovine serum (FBS) were purchased from Gibco (Grand island, NY, USA). Dexamethasone (Dex), 3- (4, 5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), cycloheximide (CHX) and insulin-like growth factor-1 (IGF-1) were purchased from Sigma-Aldrich Chemical (Sigma, USA). Oltipraz was purchased from Selleck (Shanghai, China). Primary antibodies to HO-1 (cat #ab13243) and Nrf2 (cat #ab62352) were obtained from Abcam (Abcam, USA). Antibody for β-actin (cat #AC004) was purchased from ABclonal Technology (Wuhan, China). LY294002 (cat #9901) and antibodies against p21 (cat #2947), p-Akt (#4060), cleaved caspase-3 (cat #9664) and histone H3 (cat #4499) were obtained from Cell Signaling Technology (Danvers, MA, USA).
Cell culture
The mouse osteoblastic cell line MC3T3-E1 were purchased from American Type Culture Collection (ATCC, Rockville, MD, USA), and maintained in α-MEM medium in the presence of 10% (v/v) FBS and 1% antibiotics (100 U/mL penicillin and 100 µg/mL streptomycin) at 37 °C in a humidified atmosphere of 5% CO2.
RNA interference and transfection of cells
Specific small interfering RNA (siRNA) of p21 was purchased from Ribobio, Co., Ltd (Guangdong, China). MC3T3-E1 cells grown on 6-well plates were transfected with 50 nM p21 siRNA and negative control (control siRNA) using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) in Opti-MEM without serum according to the manufacturer’s instructions. The cells were harvested 48 h after transection.
MTT assay
MC3T3-E1 cells were cultured with α-MEM containing 10% FBS in 96-well plates at a density of 2 × 104 cells per well. After reaching 90% confluency, the cells were treated according to the experimental design given in the results section. Subsequently, the medium was removed, and cells were cultured in 100 μL fresh medium containing 10% MTT for 4 h at 37 °C. The supernatant was removed, and the formazan crystals were dissolved in 150 μL of DMSO. Absorbance was recorded at 570 nm using a microplate reader (Bio-Rad, California, USA).
Flow cytometric analysis for apoptosis
Apoptotic cell counting was detected using the Annexin V-FITC apoptosis detection kit (KeyGEN, Nanjing, China). After various treatments, cells were harvested with 0.25% trypsin without EDTA and washed twice with ice-cold PBS. Then, cells were re-suspended in 500 μL of 1 × binding buffer and transferred to a sterile flow cytometry glass tube. Five μL of Annexin V-FITC and 5 μL of propidium iodide (PI) were added and incubated at room temperature in dark conditions for 15 min. Finally, apoptosis was detected using flow cytometric analysis according to the manufacturer’s instructions (Beckman, Fullerton, California, USA).
Reactive oxygen species (ROS) measurement
The ROS detection kit (Beyotime, Haimen, China) was used to measure intracellular ROS formation. MC3T3-E1 cells were washed twice with PBS and then incubated with 5 μM 2′, 7′-dichlorofluorescein diacetate (DCFH-DA) for 30 min at 37 °C. Fluorescence assays were measured using a flow cytometer (Beckman, Fullerton, California, USA) at excitation/emission of 488/525 nm.
MitoSOX™ Red mitochondrial superoxide indicator (MitoSOX™ Red) assay
MitoSOX™ Red mitochondrial superoxide indicator (Invitrogen Detection Technologies, USA) is a novel fluorogenic probe for highly selective detection of superoxide in the mitochondria of live cells. In brief, MC3T3-E1 cells were plated on coverslips in 12-well plates. After incubation and treatment, cells were washed twice with warm HBSS/Ca/Mg buffer and then stained with 1 mL of 5 μM MitoSOX™ reagent working solution for 10 min at 37 °C in the dark. After washed gently three times with warm HBSS/Ca/Mg buffer, MC3T3-E1 cells were counterstained and mounted for imaging with a fluorescence microscope at excitation/emission of 510/580 nm.
Confocal laser scanning microscopy (confocal LSM)
MC3T3-E1 cells were grown on coverslips. After treatments, cells were washed with ice-cold PBS, fixed with 4% paraformaldehyde for 30 min at room temperature, dried at room temperature for 10 min, permeabilized with 1% Triton X-100 in PBS for 10 min, and then blocked with 3% bovine serum albumin (BSA) in PBS with Tween 20 (PBST) for 1 h. After washing, the cells were incubated with anti-p21 antibody overnight at 4 °C, followed by mouse anti-rabbit IgG Cy3 (Beyotime, Haimen, China) in the dark at room temperature for 2 h. The nuclei were stained with diamidino-2-phenylindole (DAPI) dye (Beyotime, Haimen, China) for 5 min in the dark. Finally, the coverslips were washed and mounted on slides with anti-fade mounting medium (Beyotime, Haimen, China). The images were captured using a laser scanning confocal microscope (Olympus, Tokyo, Japan).
Western blot analysis
MC3T3-E1 cells were incubated with the indicated agents according to the experimental design. Total cytoplasmic and nuclear protein of MC3T3-E1 cells were prepared using the total protein extraction kit (KeyGEN, Nanjing, China), and cytoplasmic and nuclear extraction kit (KeyGEN, Nanjing, China), respectively. The protein contents were quantified using a bicinchoninic acid (BCA) protein assay kit (Beyotime, Haimen, China) according to the manufacturer’s instructions. Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a poly-vinylidene difluoride membrane (PVDF, Millipore, MA, USA). After being blocked, the membranes were incubated over night at 4 °C with specific primary antibodies. Then membranes were probed with HRP-conjugated secondary antibody for 1 h at room temperature and detection was performed by an enhanced chemiluminescence system (ECL, Beyotime, Haimen, China). The results were normalized to β-actin and histone H3 analyzed using Image J (National Institutes of Mental Health, Bethesda, MD, USA).
Protein stability assay
Stability of p21 was determined by treating MC3T3-E1 cells with Dex followed by 40 μg/mL of cycloheximide (CHX). Cells were harvested at the indicated time points (0, 0.5, 1, 3, 6 h), and protein lysates were analyzed by western blotting.
Statistical analysis
All results were obtained from at least three independent experiments performed with technical triplicates. Data were expressed as mean ± standard deviation (SD), and assessed by two-tailed Student’s t test for two groups and one-way analysis of variance (ANOVA) followed by post hoc Tukey’s test for more than two groups using SPSS 19.0 (SPSS Inc., Chicago, IL, USA). A p value less than 0.05 was considered statistically significant.
Results
Dex induces apoptosis in MC3T3-E1 cells
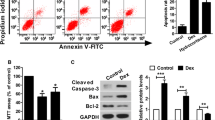
The apoptosis rate was tested by Annexin V-FITC/PI double staining following treatment with 1 µM Dex for 24 h. Flow cytometric analysis demonstrated that the apoptotic osteoblastic cells to the total cells ratio was significantly increased in the presence of Dex (17.83 ± 2.78%) as compared to 3.89 ± 1.13% in the control group (Fig. 1).
Effects of dexamethasone on apoptosis in MC3T3-E1 cells. MC3T3-E1 cells were incubated with 1 μM dexamethasone for 24 h, and apoptosis detected by flow cytometry with Annexin-FITC/PI staining. Data represent mean ± SD of three independent experiments. **p < 0.01 indicates an extremely significant difference compared to the control group
Dex decreases Akt phosphorylation in MC3T3-E1 cells
Akt is a critical kinase that regulates a variety of biological processed, such as cell survival, proliferation and apoptosis (Cunha et al. 2016). To investigate the role of PI3K/Akt pathway in Dex-induced apoptosis, MC3T3-E1 cells were incubated with Dex for 24 h in a concentration range between 0.25 and 1 µM and the phosphorylated level of Akt was measured. Western blot analysis showed a concentration-dependent decrease of phosphorylated Akt (Fig. 2). Already 0.25 µM Dex caused a small but statistically significant decrease. The highest tested concentration of 1 µM amounted to an approximately 50% decrease of p-Akt compared to controls.
Effects of dexamethasone on the phosphorylation of Akt in MC3T3-E1 cells. MC3T3-E1 cells were incubated with 0, 0.25, 0.5, and 1 μM of dexamethasone for 24 h, and cell lysates were subjected to western blot analysis using an anti-p-Akt antibody. Beta-actin was used as loading control. Data represent mean ± SD of three independent experiments. *p < 0.05 indicates a significant difference compared to the control group and **p < 0.01 indicates an extremely significant difference compared to the control group
PI3K/Akt pathway is involved in Dex-induced apoptosis
To further determine if Dex-induced apoptosis in MC3T3-E1 cells was mediated through suppressing PI3K/Akt signaling pathway, MC3T3-E1 cells were incubated with Dex in combination with LY294002 or IGF-1, respectively. The MTT assay and flow cytometry analyses showed that co-incubation of MC3T3-E1 cells with LY294002 slightly but significantly enhanced Dex-induced cytotoxicity (Fig. 3a) and apoptosis (Fig. 3b). Moreover, LY294002 slightly but significantly enhanced the inhibitory effect of Dex on p-Akt (Fig. 3c). In contrast, co-treatment with IGF-1 attenuated Dex-induced reduction of cell viability (Fig. 4a) and apoptosis (Fig. 4b), and antagonized suppressive effect of Dex on p-Akt levels (Fig. 4c).
LY294002 increases dexamethasone-induced apoptosis in MC3T3-E1 cells through inhibiting the PI3K/Akt signaling pathway. Cells were pre-incubated with LY294002 (20 μM) for 1 h, and then co-incubated with 1 μM dexamethasone for 24 h. a Cell viability was determined by the MTT assay. b Apoptosis was measured by flow cytometry, followed by Annexin V-FITC (FL 1 channels) and PI (FL 2 channels) double staining. c Expression of p-Akt was detected by western blot analysis. Data represent mean ± SD of three separate experiments. **p < 0.01 indicates an extremely significant difference compared to controls; # p < 0.05 indicates a significant difference compared to the dexamethasone-incubated samples and ## p < 0.01 indicates an extremely significant difference compared to the dexamethasone-incubated samples
IGF-1 attenuates dexamethasone-induced apoptosis in MC3T3-E1 cells through activating the PI3K/Akt signaling pathway. Cells were pre-incubated with IGF-1 (50 ng/mL) for 1 h, and then co-incubated with 1 μM dexamethasone for 24 h. a Cell viability was determined by the MTT assay. b Apoptosis was measured by flow cytometry, followed by Annexin V-FITC (FL 1 channels) and PI (FL 2 channels) double staining. c The expression of p-Akt was detected by western blot analysis. Data represent mean ± SD of three separate experiments. **p < 0.01 indicates an extremely significant difference compared to controls; # p < 0.05 indicates a significant difference compared to the dexamethasone-incubated samples and ## p < 0.01 indicates an extremely significant difference compared to dexamethasone-exposed samples
Dex downregulates p21 protein expression in MC3T3-E1 cells
P21 plays multiple roles not only as a cell cycle regulator, but also as a regulator of apoptosis (Li et al. 2012). In a next step, we studied the influence of Dex on p21 expression and its subcellular localization. Western blot analysis demonstrated that incubation with 1 μM Dex for 24 h significantly decreased the protein level of p21 in MC3T3-E1 cells by approximately 50% compared to controls (Fig. 5a). Confocal laser scanning fluorescence microscopy revealed that p21 visualized by red fluorescence decreased after Dex incubation compared to controls, which is consistent with the western blot analysis. In addition, incubation with Dex altered the subcellular localization of p21 in MC3T3-E1 cells, whereby the cytoplasmic fluorescence of p21 was reduced, but the nuclear p21 was significantly increased (Fig. 5b). After incubation with 40 μg/mL CHX and block protein synthesis, the half-life of p21 was analyzed at the indicated time points by western blotting. The results showed that the protein level of p21 was downregulated in Dex-exposed cells as compared to the control group (Fig. 5c), which suggests that Dex decreased the p21 protein level by shortening its half-life.
Effects of dexamethasone on protein level of p21 in MC3T3-E1 cells. a MC3T3-E1 cells were exposed to 1 μM dexamethasone for 24 h, and then proteins were extracted for western blot analysis using anti-p21 antibody. b The effect of dexamethasone on the subcellular distribution of p21 was analyzed by immunofluorescent staining. c After dexamethasone incubation for 24 h, MC3T3-E1 cells were exposed to 40 μg/mL CHX for indicated time periods (0, 30, 60, 120 min) and p21 protein level was examined by western blotting. Data represent mean ± SD of three separate experiments. *p < 0.05 indicates a significant difference compared to the controls and **p < 0.01 indicates an extremely significant difference compared to controls; ## p < 0.01 indicates an extremely significant difference compared to dexamethasone-exposed samples
PI3K/Akt is involved in Dex-induced downregulation of p21
Previous studies demonstrated that p21 could be phosphorylated by Akt to modulate its stability and subcellular location (Li et al. 2002). To further investigate whether PI3K/Akt pathway is involved in Dex-induced downregulation of p21 in MC3T3-E1 cells, LY294002 and IGF-1 were used to inhibit or promote Akt activation, respectively. Western blot analysis demonstrated that combined incubation of either LY294002 or IGF-1 with Dex enhanced or suppressed the Dex-induced downregulation of p21, respectively (Fig. 6a, b). LY294002 or IGF-1 incubation alone did not affect the levels of p21 in MC3T3-E1 cells (Fig. 6a, b). Moreover, the protein expression of nuclear p21 was decreased and cytoplasmic p21 was increased after incubation with Dex and IGF-1 compared to Dex-treated group. In the contrary, incubation of Dex and LY294002 could effectively increase the nuclear content of p21 and decrease the cytoplasmic content of p21 (Fig. 6c). Incubation of MC3T3-E1 cells with Dex led to a significantly decreased half-life of p21, IGF-1 antagonized this decrease, while LY294002 further shortened the half-life of p21 (Fig. 6d). This demonstrates that Dex decreases p21 protein level in MC3T3-E1 cells by destabilizing the p21 protein through inhibition of the PI3K/Akt pathway.
Dexamethasone-induced downregulation of p21 protein levels is mediated by the PI3K/Akt pathway. MC3T3-E1 cells were pre-incubated with IGF-1 (50 ng/mL) or LY294002 (20 μM) for 1 h, and then co-incubated with 1 μM dexamethasone for 24 h. a–c Expression of p21 was detected by western blot analysis. d Cells were incubated with dexamethasone alone or in combination with IGF-1 or LY294002, respectively, for 24 h, 40 μg/mL CHX was added to culture medium and p21 protein levels were detected at the indicated times (0, 30, 60, 120 min) by western blotting. Data represent mean ± SD of three separate experiments. **p < 0.01 indicates an extremely significant difference compared to controls; # p < 0.05 indicates a significant difference compared to the dexamethasone-incubated samples and ## p < 0.01 indicates an extremely significant difference compared to dexamethasone-exposed samples
P21 depletion increases Dex-induced cell death and ROS generation
Previous reports found that Dex caused cell growth inhibition and apoptosis in MC3T3-E1 cells (Fan et al. 2017; Li et al. 2012). To study the influence of p21 on Dex-induced cell death, knockdown experiments were performed by siRNA. Western blot analysis showed that protein expression of p21 was decreased to less than 30% in p21 siRNA-transfected cells as compared to control siRNA-transfected cells (Fig. 7). Next, viability and apoptosis of transfected cells exposed to Dex were determined by MTT and flow cytometry, respectively. The results demonstrated that p21 knockdown by siRNA in Dex-treated cells significantly reduced cell viability, although the difference was small (Fig. 8a). In addition, apoptosis (Fig. 8b) and activation of caspase-3 (Fig. 8c) were enhanced by p21 knockdown compared to that of control siRNA-transfected cells incubated with Dex. Furthermore, levels of intracellular ROS (Fig. 8d) and superoxide in the mitochondria of live cells (Fig. 8e) were increased by p21 knockdown after incubation with Dex for 3 h.
Protein levels of p21 in siRNA-transfected MC3T3-E1 cells. MC3T3-E1 cells were transfected with control siRNA or p21 siRNA for the indicated time; western blotting was used to analyze the protein levels of p21. Data represent mean ± SD of three separate experiments. **p < 0.01 indicates an extremely significant difference compared to control siRNA-transfected cells
Knockdown of p21 affects dexamethasone-induced cytotoxicity, apoptosis and ROS generation. MC3T3-E1 cells were transfected with control siRNA or p21 siRNA prior to dexamethasone exposure. a Effect of p21 depletion on cytotoxicity induced by dexamethasone analyzed by the MTT assays. b, c Effect of p21 knockdown on dexamethasone-induced apoptosis and activation of caspase-3 analyzed by flow cytometry with Annexin V-FITC/PI double staining and western blot, respectively. d Effect of p21 depletion on dexamethasone-induced intracellular ROS generation detected by flow cytometry with DCFH-DA probe. e Effect of p21 depletion on dexamethasone-induced superoxide in the mitochondria of live cells detected using MitoSOX™ Red assay and imaging with a fluorescence microscope. Data represent mean ± SD of three separate experiments. *p < 0.05 indicates a significant difference compared to control siRNA-transfected cells and **p < 0.01 indicates an extremely significant difference compared to control siRNA-transfected cells; # p < 0.05 indicates a significant difference compared to the dexamethasone-incubated control siRNA-transfected cells (color figure online)
Dex increases nuclear Nrf2 and HO-1
Nrf2 is well known to play a critical role in resisting oxidative stress by transcriptional activation of anti-oxidative enzymes such as HO-1, which could decrease ROS, and alleviate cell damage (Huang et al. 2013). To study the influence of Dex on nuclear protein levels of Nrf2 and HO-1, MC3T3-E1 cells were time-dependently incubated with 1 µM Dex and nuclear as well as total protein were analyzed by western blot. Dex significantly increased nuclear protein levels of Nrf2 (Fig. 9a) and HO-1 (Fig. 9b) after 1 h of incubation of MC3T3-E1 cells and later, indicating that Nrf2/HO-1 pathway was activated which protected cells from oxidative damage by Dex. In contrast, no significant changes were obtained for total Nrf2 levels (Fig. 9a).
Effects of dexamethasone on the protein levels of Nrf2 and HO-1 in MC3T3-E1 cells. a, b MC3T3-E1 cells were incubated with 1 μM dexamethasone for 0, 0.5, 1, 3, 6 h, and cell lysates were subjected to western blot analysis with anti-Nrf2 and anti-HO-1 antibodies. Histone H3 and β-actin were used as loading controls. Data represent mean ± SD of three independent experiments. *p < 0.05 indicates a significant difference compared to controls and **p < 0.01 indicates an extremely significant difference compared to controls
P21 depletion inhibits Dex-induced activation of the Nrf2/HO-1 pathway
To study if the increase in nuclear Nrf2 and HO-1 depends on p21, a siRNA knockdown technique was applied as shown in Fig. 7. Western blot analysis results demonstrated that knockdown of p21 efficiently suppresses Dex-induced upregulation of nuclear Nrf2 (Fig. 10a) and HO-1 (Fig. 10b). This indicates that p21 is involved in Dex-mediated upregulation of the nuclear protein levels of Nrf2 and its downstream gene HO-1 to activate an antioxidant response to Dex.
Knockdown of p21 affects dexamethasone-induced Nrf2/HO-1 activation in MC3T3-E1 cells. Cells were transfected with control siRNA or p21 siRNA. Subsequently, transfected cells were incubated with 1 μM dexamethasone for 3 h. a, b Expression of Nrf2 and HO-1 was detected by western blotting. Histone H3 and β-actin were used as loading controls. Data represent mean ± SD of three separate experiments. *p < 0.05 indicates a significant difference compared to control siRNA-transfected cells and **p < 0.01 indicates an extremely significant difference compared to control siRNA-transfected cells; # p < 0.05 indicates a significant difference compared to the dexamethasone-incubated control siRNA-transfected cells and ## p < 0.01 indicates an extremely significant difference compared to dexamethasone-exposed control siRNA-transfected cells
Activation of Nrf2 and depletion of p21 cause no obvious change on Dex-induced apoptosis in MC3T3-E1 cells
To further investigate whether depletion of p21 downregulates the protein levels of Nrf2 and HO-1 to promote Dex-induced apoptosis, MC3T3-E1 cells were first transfected with p21 depletion, and then treated with oltipraz (an Nrf2 activator) in combination with Dex. Flow cytometric analysis showed that knockdown of p21 significantly increased the apoptosis induced by Dex. Furthermore, depletion of p21 in both oltipraz and Dex-treated MC3T3-E1 cells obviously decreased apoptotic rate compared to that of control siRNA-transfected cells induced by Dex. However, when the cells were transfected with p21 and co-incubated with oltipraz and Dex, the apoptotic rate did not evidently change relative to that of control siRNA-transfected cells incubated with Dex (Fig. 11). These findings indicated that Nrf2/HO-1 pathway indeed plays a significant role in the p21 mediation of Dex-induced apoptosis in MC3T3-E1 cells.
Effects of p21 depletion and Nrf2 activation on dexamethasone-induced apoptosis in MC3T3-E1 cells. Cells were transfected with control siRNA or p21 siRNA. Subsequently, transfected cells were pre-incubated with oltipraz (20 μM) for 1 h, and then co-incubated with 1 μM dexamethasone for 24 h. Apoptosis was measured by flow cytometry, followed by Annexin V-FITC/PI double staining. Data represent mean ± SD of three separate experiments. **p < 0.01 indicates an extremely significant difference compared to control siRNA-transfected cells; ## p < 0.01 indicates an extremely significant difference compared to dexamethasone-exposed control siRNA-transfected cells; && p < 0.01 indicates an extremely significant difference compared to dexamethasone-exposed p21 siRNA-transfected cells
Discussion
Phosphatidylinositol 3-kinase (PI3K)/Akt is a key pro-survival signaling pathway in governing the cellular defense system against oxidative stress, and regulating cell proliferation, survival and apoptosis (Cunha et al. 2016; Fresno Vara et al. 2004). As the crucial kinase of the PI3K/Akt pathway, activation of Akt may effectively protect osteoblasts from oxidative stress and apoptosis caused by Dex (Li et al. 2016). In our study, Dex decreased the phosphorylation of Akt of MC3T3-E1 cells in a concentration-dependent manner. It should be considered that concentrations of Dex were used that range in the order of magnitude of human blood concentrations typically reached in clinical routine (Spoorenberg et al. 2014). Moreover, co-incubation with LY294002 significantly enhanced cytotoxicity and apoptosis caused by Dex through further suppressing the phosphorylation of Akt. Conversely, activation of Akt by IGF-1 alleviated the toxicity and apoptotic effects of Dex, which suggests that PI3K/Akt is involved in Dex-induced apoptosis in MC3T3-E1 cells.
It has been reported that Dex can upregulate p53 in the MC3T3-E1 osteoblast cell line, thereby resulting in p21 upregulation, which was paralleled by MC3T3-E1 cell apoptosis and cell cycle arrest (Li et al. 2012). Gene expression profiling displayed that Dex caused a significant upregulation of p21 mRNA in MC3T3-E1 cells (Leclerc et al. 2004). In the present study, Dex reduced protein levels of p21 by shortening its protein half-life. This suggests that a post-translational mechanism is involved in Dex-mediated downregulation of p21 in MC3T3-E1 cells.
The cytoprotective function of Akt has been attributed to its ability to involve in the phosphorylation of different cell proliferation factors such as p21, p27 and glycogen synthase kinase-3 (GSK3) (Dai et al. 2017; Jain et al. 2015; Zhang et al. 2015). It is well established that phosphorylation of p21 by Akt affects cellular distribution of p21, leads to its shift from the nucleus to the cytoplasm; thus promoting the transition from G0 to S phase (Pérez-Tenorio et al. 2006; Zhou et al. 2001). In contrast, Akt inhibition decreases cytoplasmic p21 expression (Li et al. 2002; Zhou et al. 2001). Moreover, Akt-mediated phosphorylation of p21 increases p21 protein stability (Li et al. 2002). In the present study, LY294002 enhanced the Dex-induced decrease of p21 protein levels and reduced its stability in MC3T3-E1 cells. In contrast, the PI3K/Akt activator IGF-1 further increased protein levels of p21 and prolonged its protein half-life.
Inhibition of the PI3K/Akt signaling may reduce phosphorylation of both Akt and p21, and induce nuclear translocation of p21 (Chen et al. 2017; Zhou et al. 2001). Recent studies have confirmed that Dex can induce apoptosis and inhibit proliferation of MC3T3-E3 cells through suppression of PI3K/Akt signaling (Li et al. 2015, 2016; Yao et al. 2016). Therefore, modulation of p21 by the PI3K/Akt pathway may play a role in Dex-induced apoptosis in MC3T3-E1 cells. P21 is a potent inhibitor of proliferation and apoptosis that is regulated through p53-dependent and p53-independent mechanisms (Abbas and Dutta 2009; Bellosta et al. 2003; Soria and Gottifredi 2010). The function of p21 in apoptosis is still under debate (Cmielová and Rezáčová 2011; Karimian et al. 2016; Nakamura et al. 2004). Knockdown of p21 by siRNA increased Dex-induced reduction of cell viability and apoptosis in MC3T3-E1 cells, which indicates that p21 may act as an anti-apoptotic factor in osteoblastic MC3T3-E1 cells in the presence of Dex. Furthermore, activation of caspase-3 by Dex could be promoted by p21 suppression, which demonstrated that the anti-apoptotic function of p21 is associated with inhibition of caspase-3. Our findings suggest that p21 shows an anti-apoptotic effect, and knockdown of p21 enhances the induction of caspase 3-mediated apoptosis in MC3T3-E1 cells by Dex.
Dex induced significant ROS production and oxidative stress in osteoblastic cells, which may contribute to subsequent cell death (Fan et al. 2017; Li et al. 2016; Lin et al. 2015; Suwanjang et al. 2016). Previous reports revealed that p21 effectively inhibits the generation of ROS in HepG2 cells (Deng et al. 2016). Suppression of p21 enhanced Dex-induced ROS generation compared to controls. Scavenging of ROS has been shown to block apoptosis and to efficiently rescue osteoblasts from Dex (Li et al. 2016; Lin et al. 2015). This shows that generation of ROS by p21 depletion may also contribute to the activation of caspase-3 and apoptosis to some extent. Upon generation of intracellular ROS, the antioxidant response pathway is activated by nuclear translocation of Nrf2 (Loboda et al. 2016; Min et al. 2011). It has been demonstrated that the antioxidant function of p21 is mediated through activation of Nrf2 by stabilizing the Nrf2 protein (Toledano 2009). Stabilization of Nrf2 is a consequence of direct interaction between p21 and Nrf2 (Chen et al. 2009; Toledano 2009). Nrf2 has emerged as a master regulator of an intracellular antioxidant response through transcriptional activation of antioxidants and phase II detoxifying enzymes, e.g., HO-1 (Loboda et al. 2016; Min et al. 2011). Upregulation of HO-1 may lead to reduced ROS and may contribute to the cellular defense in response to oxidative insults both in vitro and in vivo (Sun et al. 2017; Yu et al. 2016). The present study showed that Dex treatment significantly increased protein levels of Nrf2 and HO-1, suggesting that the Nrf2/HO-1 pathway was activated to protect MC3T3-E1 cells from Dex-induced oxidative stress. Moreover, knockdown of p21 partially reversed the increase in Nrf2 and HO-1 in the presence of Dex. These data clearly demonstrate that knockdown of p21 enhances ROS generation and reduces antioxidant capability of MC3T3-E1 cells through inhibiting the Nrf2/HO-1 pathway.
The current findings suggest a scenario where Dex induces the generation of ROS and inhibits the PI3K/Akt pathway (Fig. 12). Suppression of Akt phosphorylation by Dex led to decreased protein level of p21 by reducing its stability. However, further investigations are needed to study, whether the same mechanisms are responsible in vivo.
Conclusion
The present study concluded that Dex dramatically decreased the half-life of p21 by reducing the phosphorylation of Akt. Furthermore, depletion of p21 increased Dex-induced cell death and ROS generation through inhibition of the Nrf2/HO-1 signaling pathway.
References
Abbas T, Dutta A (2009) P21 in cancer: intricate networks and multiple activities. Nat Rev Cancer 9(6):400–414
Bellosta P, Masramon L, Mansukhani A, Basilico C (2003) P21WAF1/CIP1 acts as a brake in osteoblast differentiation. J Bone Miner Res 18(5):818–826
Bertoli C, Skotheim JM, de Bruin RA (2013) Control of cell cycle transcription during G1 and S phases. Nat Rev Mol Cell Biol 14(8):518–528
Chen W, Sun Z, Wang XJ, Jiang T, Huang Z, Fang D et al (2009) Direct interaction between Nrf2 and p21Cip/WAF1 upregulates the Nrf2-mediated antioxidant response. Mol Cell 34(6):663–673
Chen YJ, Liu WH, Chang LS (2017) Hydroquinone-induced FOXp3-ADAM17-Lyn-Akt-p21 signaling axis promotes malignant progression of human leukemia U937 cells. Arch Toxicol 91(2):983–997
Cmielová J, Rezáčová M (2011) P21Cip1/Waf1 protein and its function based on a subcellular localization. J Cell Biochem 112(12):3502–3506
Cunha MP, Budni J, Ludka FK, Pazini FL, Rosa JM, Oliveira A et al (2016) Involvement of PI3K/Akt signaling pathway and its downstream intracellular targets in the antidepressant-like effect of creatine. Mol Neurobiol 53(5):2954–2968
Dai P, Mao Y, Sun X, Li X, Muhammad I, Gu W et al (2017) Attenuation of oxidative stress-induced osteoblast apoptosis by curcumin is associated with preservation of mitochondrial functions and increased Akt-GSK3β signaling. Cell Physiol Biochem 41(2):661–677
den Uyl D, Bultink IE, Lems WF (2011) Advances in glucocorticoid-induced osteoporosis. Curr Rheumatol Rep 13(3):233–240
Deng S, Tang S, Dai C, Zhou Y, Yang X, Li D et al (2016) P21Waf1/Cip1 plays a critical role in furazolidone-induced apoptosis in HepG2 cells through influencing the caspase-3 activation and ROS generation. Food Chem Toxicol 88:1–12
Fan JB, Liu W, Zhu XH, Yi H, Cui SY, Zhan JN et al (2017) microRNA-25 targets PKCζ and protects osteoblastic cells from dexamethasone via activating AMPK signaling. Oncotarget 8(2):3226–3236
Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M (2004) PI3K/Akt signaling pathway and cancer. Cancer Treat Rev 30(2):193–204
Huang CS, Lii CK, Lin AH, Yeh YW, Yao HT, Li CC et al (2013) Protection by chrysin, apigenin, and luteolin against oxidative stress is mediated by the Nrf2-dependent up-regulation of heme oxygenase 1 and glutamate cysteine ligase in rat primary hepatocytes. Arch Toxicol 87(1):167–178
Ibáñez I, Ferrándiz ML, Brines R, Guede D, Cuadrado A (2014) Alcaraz MJ (2014) Effects of Nrf2 deficiency on bone microarchitecture in an experimental model of osteoporosis. Oxid Med Cell Longev 5:390–392
Jain MV, Jangamreddy JR, Grabarek J, Schweizer F, Klonisch T, Cieślar-Pobuda A et al (2015) Nuclear localized Akt enhances breast cancer stem-like cells through counter-regulation of p21Waf1/Cip1 and p27kip1. Cell Cycle 14(13):2109–2120
Karimian A, Ahmadi Y, Yousefi B (2016) Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair 42:63–71
Kerachian MA, Seguin C, Harvey EJ (2009) Glucocorticoids in osteonecrosis of the femoral head: a new understanding of the mechanisms of action. J Steroid Biochem Mol Biol 114(3–5):121–128
Leclerc N, Luppen CA, Ho VV, Nagpal S, Hacia JG, Smith E et al (2004) Gene expression profiling of glucocorticoid-inhibited osteoblasts. J Mol Endocrinol 33(1):175–193
Li Y, Dowbenko D, Lasky LA (2002) AKT/PKB phosphorylation of p21Cip/WAF1 enhances protein stability of p21Cip/WAF1 and promotes cell survival. J Biol Chem 277(13):11352–11361
Li H, Qian W, Weng X, Wu Z, Li H, Zhuang Q et al (2012) Glucocorticoid receptor and sequential P53 activation by dexamethasone mediates apoptosis and cell cycle arrest of osteoblastic MC3T3-E1 cells. PLoS One 7(6):e37030
Li H, Li T, Fan J, Li T, Fan L, Wang S et al (2015) miR-216a rescues dexamethasone suppression of osteogenesis, promotes osteoblast differentiation and enhances bone formation, by regulating c-Cbl-mediated PI3K/AKT pathway. Cell Death Differ 22(12):1935–1945
Li ST, Chen NN, Qiao YB, Zhu WL, Ruan JW, Zhou XZ (2016) SC79 rescues osteoblasts from dexamethasone though activating Akt-Nrf2 signaling. Biochem Biophys Res Commun 479(1):54–60
Lin H, Gao X, Chen G, Sun J, Chu J, Jing K et al (2015) Indole-3-carbinol as inhibitors of glucocorticoid-induced apoptosis in osteoblastic cells through blocking ROS-mediated Nrf2 pathway. Biochem Biophys Res Commun 460(2):422–427
Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J (2016) Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci 73(17):3221–3247
Min KJ, Lee JT, Joe EH, Kwon TK (2011) An IκBα phosphorylation inhibitor induces heme oxygenase-1(HO-1) expression through the activation of reactive oxygen species (ROS)-Nrf2-ARE signaling and ROS-PI3K/Akt signaling in an NF-κB-independent mechanism. Cell Signal 23(9):1505–1513
Nakamura K, Arai D, Fukuchi K (2004) Identification of the region required for the antiapoptotic function of the cyclin kinase inhibitor, p21. Arch Biochem Biophys 431(1):47–54
Pérez-Tenorio G, Berglund F, Esguerra Merca A, Nordenskjöld B, Rutqvist LE, Skoog L et al (2006) Cytoplasmic p21WAF1/CIP1 correlates with Akt activation and poor response to tamoxifen in breast cancer. Int J Oncol 28(5):1031–1042
Prives C, Gottifredi V (2008) The p21 and PCNA partnership: a new twist for an old plot. Cell Cycle 7(24):3840–3846
Sato AY, Tu X, McAndrews KA, Plotkin LI, Bellido T (2015) Prevention of glucocorticoid induced-apoptosis of osteoblasts and osteocytes by protecting against endoplasmic reticulum (ER) stress in vitro and in vivo in female mice. Bone 73(6):60–68
Schäcke H, Döcke WD, Asadullah K (2002) Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther 96(1):23–43
Soria G, Gottifredi V (2010) PCNA-coupled p21 degradation after DNA damage: the exception that confirms the rule? DNA Repair 9(4):358–364
Spoorenberg SM, Deneer VH, Grutters JC, Pulles AE, Voorn GP, Rijkers GT et al (2014) Pharmacokinetics of oral vs. intravenous dexamethasone in patients hospitalized with community-acquired pneumonia. Br J Clin Pharmacol 78(1):78–83
Sun Z, Hu W, Yin S, Lu X, Zuo W, Ge S et al (2017) NGF protects against oxygen and glucose deprivation-induced oxidative stress and apoptosis by up-regulation of HO-1 through MEK/ERK pathway. Neurosci Lett 641:8–14
Suwanjang W, Abramov AY, Charngkaew K, Govitrapong P, Chetsawang B (2016) Melatonin prevents cytosolic calcium overload, mitochondrial damage and cell death due to toxically high doses of dexamethasone-induced oxidative stress in human neuroblastoma SH-SY5Y cells. Neurochem Int 97:34–41
Toledano MB (2009) The guardian recruits cops: the p53-p21 axis delegates prosurvival duties to the Keap1-Nrf2 stress pathway. Mol Cell 34(6):637–639
Wang Y, Fisher JC, Mathew R, Ou L, Otieno S, Sublet J et al (2011) Intrinsic disorder mediates the diverse regulatory functions of the Cdk inhibitor p21. Nat Chen Biol 7(4):214–221
Weinstein RS (2011) Glucocorticoid-induced bone disease. N Engl J Med 365(1):62–70
Yao S, Zhang Y, Wang X, Zhao F, Sun M, Zheng X et al (2016) Pigment epithelium-derived factor (PEDF) protects osteoblastic cell line from glucocorticoid-induced apoptosis via PEDE-R. Int J Mol Sci 17(5):730
Yu J, Wang Y, Li Z, Dong S, Wang D, Gong L et al (2016) Effect of heme oxygenase-1 on mitofusin-1 protein in LPS-induced ALI/ARDS in rats. Sci Rep 6:36530
Zhan J, Easton JB, Huang S, Mishra A, Xiao L, Lacy ER et al (2007) Negative regulation of ASK1 by p21Cip1 involves a small domain that includes serine 98 that is phosphorylated by ASK1 in vivo. Mol Cell Biol 27(9):3530–3541
Zhang Y, Fujita N, Tsuruo T (1999) Caspase-mediated cleavage of p21Waf1/Cip1 converts cancer cells from growth arrest to undergoing apoptosis. Oncogene 18(5):1131–1138
Zhang S, Lu Z, Mao W, Ahmed AA, Yang H, Zhou J et al (2015) CDK5 regulates paclitaxel sensitivity in ovarian cancer cells by modulating AKT activation, p21Cip1- and p27Kip1-mediated G1 cell cycle arrest and apoptosis. PLoS One 10(7):e0131833
Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC (2001) Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol 3(3):245–252
Zhu ZH, Gao YS, Zeng BF, Zhang CQ (2011) The effect of dexamethasone and hypoxic stress on MC3T3-E1 cells. Front Biosci 16(3):2747–2755
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (nos. 31572587, 31272623 and 3151101034).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there is no conflict of interest regarding the publication of this paper.
Rights and permissions
About this article
Cite this article
Han, D., Gao, J., Gu, X. et al. P21Waf1/Cip1 depletion promotes dexamethasone-induced apoptosis in osteoblastic MC3T3-E1 cells by inhibiting the Nrf2/HO-1 pathway. Arch Toxicol 92, 679–692 (2018). https://doi.org/10.1007/s00204-017-2070-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-017-2070-2