Abstract
We investigated the effects of biologics for rheumatoid arthritis (RA) patients on bone mineral density (BMD) and bone metabolic markers (BMM), retrospectively, and also clarified the effects of bisphosphonates (alendronate or risedronate 35 mg/week) and glucocorticoids. Participants in this study comprised 219 patients with RA, including 117 patients treated with biologics (infliximab, n = 90; etanercept, n = 27) and 102 patients with conventional disease-modifying anti-rheumatic drugs (DMARDs) for 1 year. Changes in BMD at the lumbar spine and total hip and BMMs [urinary type I collagen cross-linked N-telopeptide (NTX) and bone-specific alkaline phosphatase] were measured. BMD of the lumbar spine in both groups and total hip BMD in the biologics group were unchanged during treatment with biologics. However, BMD of the total hip was significantly decreased in the DMARDs group (from 0.731 ± 0.135 to 0.706 ± 0.135 g/cm2). Patients receiving glucocorticoids without bisphosphonates showed significant decrease in BMD of the total hip compared with patients not receiving glucocorticoids or receiving glucocorticoids with bisphosphonates in both biologics and DMARDs groups. Furthermore, BMD of the lumbar spine increased (p < 0.05) for patients in the biologics group who received bisphosphonates. NTX was significantly decreased only in the biologics group. Multiple regression analysis showed that BMD and bone metabolic marker levels correlated positively with bisphosphonate and biologics use and negatively with glucocorticoid use. BMD of the total hip was maintained in the patients using biologics without glucocorticoids or with bisphosphonates, but it was not maintained in the DMARDs patients, even without glucocorticoids or with bisphosphonates. Even if biologics have protective effect against bone loss of RA patients, we should consider reducing the dose of glucocorticoids and adding bisphosphonates for the treatment of osteoporosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease that is characterized by joint inflammation and joint destruction. RA is associated with subchondral bone erosion, cartilage degradation, and systemic bone loss [1, 2]. Osteoporosis is one of the complications in RA [3]. Previous studies have shown that the frequency of osteoporosis is 15–20 % at the hip and spine in patients with RA [4, 5]. Accelerated generalized bone loss often leads to an increased risk of hip [6–8] or vertebral fracture [8–10]. Oral glucocorticoids are known to have deleterious effects on bone [11–13] and are frequently used in the treatment of RA to suppress inflammation.
The mainstay of RA treatment is the application of disease-modifying anti-rheumatic drugs (DMARDs) [14–16]. DMARD treatment helps to suppress inflammatory activity, which is an important risk factor for osteoporosis in RA, and then might decrease susceptibility to osteoporosis. However, bone mineral density (BMD) has been shown to decrease among RA patients receiving treatment with DMARDs alone [17, 18]. In the past decade, there have been significant advances in treating rheumatoid arthritis, especially for patients whose arthritis does not respond to traditional DMARDs. The most important advance has been the development of a group of drugs called biologic response modifiers, or biologics. Therapy with biologics has been expected to prove efficacious in improving not only disease activity but also focal bone erosions in patients with RA [19]. Anti-tumor necrosis factor (TNF)-α antibodies are now commercially available and have been successfully used in halting both joint inflammation and joint destruction in RA patients [20, 21]. Some experimental investigation into the effects of TNF-α on RA-related osteoporosis has been undertaken. In fact, transgenic mice expressing soluble TNF-α receptor to neutralize TNF-α showed protection against estrogen deficiency-related bone loss [22]. Blockade of TNF-α might thus not only serve to block inflammation but also halt the erosive nature of RA and generalized and localized juxta-articular bone loss.
In this study, we compared changes in BMD between RA patients treated with biologics and RA patients treated using conventional DMARDs alone. BMDs of the lumbar spine and total hip and levels of bone metabolic markers [urinary type I collagen cross-linked N-telopeptide (NTX) and bone-specific alkaline phosphatase (BAP)] were measured at baseline and after about 1 year. The effects of bisphosphonates and glucocorticoids on changes in BMD and bone metabolic markers were analyzed and compared.
Materials and methods
Subjects
This study included a total of 219 patients (191 women, 28 men) with RA. All subjects fulfilled the American College of Rheumatology (ACR) criteria (1987) for adult RA [23]. Patients were enrolled from April 1, 2004, to December 31, 2010 at the Osaka City University Hospital and related satellite clinics. The DMARDs group included 102 patients (91 women, 11 men). The biologics group included 117 patients (100 women, 17 men) requiring biologics therapy for treatment of persistent active disease, despite DMARD treatment with methotrexate. Ninety patients in the biologics group received infliximab (3 mg/kg in weeks 0, 2, and 6, and every 8 weeks thereafter. If the effect of infliximab for RA was insufficient, the next dose up was allowed; 27 patients received etanercept (50 mg/week).
Fifty-six patients (26 %) were taking bisphosphonates (alendronate or risedronate, 35 mg/week) and 27 patients (12 %) were taking active vitamin D 1α(OH)D3. The proportion of patients on glucocorticoids and mean dose of glucocorticoids used were higher in the biologics group [DMARD group 2.1 ± 2.4 mg/day (n = 53), biologics group 3.7 ± 3.1 mg (n = 82)], but the proportions of patients using bisphosphonates and vitamin D did not differ between groups.
RA activity was measured using the disease activity score (DAS) composite index, applying a 28-joint score (DAS-28) [24]. This index included the number of swollen and tender joints, patient’s global assessment of disease activity, and erythrocyte sedimentation rate (ESR). Levels of C-reactive protein (CRP), matrix metalloproteinase (MMP)-3, and rheumatoid factor (RF) were also determined by standard methods. Functional disability was evaluated using the modified Health Assessment Questionnaire (mHAQ) [25].
BMD and markers of bone metabolism evaluation
At baseline and after about 1 year, BMD (g/cm2) was measured at the lumbar spine (second to fourth vertebrae, anteroposterior view) and left hip (total hip) by dual-energy X-ray absorptiometry using a QDR 4500 system (Hologic, Waltham, MA, USA). Quality control for the device was performed by daily assessment of a spine phantom. CV (coefficient of variation) with the lumbar spine was approximately 1 %.
At baseline and after 1 year, NTX and BAP were measured at the same time as BMD.
Statistical analysis
Changes were compared between values at entry and after 1 year for BMD, DAS28, BAP, urinary NTX, ESR, CRP, and MMP-3. In each group, data were compared using Student’s paired t test for continuous variables, between baseline and after 1 year. Data for both groups were compared using the unpaired Student’s t test for continuous variables. Steinblocker stage and class were compared using the Wilcoxon signed-rank test. Absolute changes were measured and presented as variation, defined as the final value minus the initial value. In multivariate analysis, multiple regression analysis was performed for all patients. Statistical tests were considered significant at the level of p < 0.05. All p values were two sided. All analyses were performed using SAS version 9.1 software (SAS Institute, Cary, NC, USA).
Role of the funding source
The sponsors of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Demographic and clinical characteristics
Demographic and clinical characteristics of the 219 patients included in the study are shown in Table 1. Mean (± standard deviation) age was 59.2 ± 10.2 years. Mean duration of disease was 11.3 ± 10.5 years. Mean DAS28 score was 4.74 ± 1.49. A total of 135 patients (62 %) were on glucocorticoids (mean dose, 3.0 ± 2.9 mg/day).
When the two groups were compared, Steinblocker stage, Steinblocker class, CRP, MMP-3, DAS28 ESR, and mHAQ were significantly higher in the biologics group than in the DMARDs group, but disease duration showed no significant difference between groups. This result shows that severity of RA activity was higher in the biologics group. The proportion of patients with osteoporosis (defined as BMD < 0.7 g/cm2 at the lumbar spine) was 21.6 % in the DMARDs group and 15.4 % in the biologics group.
Changes in BMD and markers of bone turnover
In the DMARDs group, lumbar spine BMD did not show any significant decrease after final follow-up. However, total hip BMD was decreased from 0.731 ± 0.135 to 0.706 ± 0.135 g/cm2 (p < 0.001) in the DMARDs group. Regarding markers of bone turnover, the DMARDs group showed a significant decrease in BAP but no significant change in NTX level between baseline and final follow-up (Fig. 1).
Changes in bone mineral density (BMD) and bone metabolic markers at baseline and final follow-up: lumbar spine BMD (a), total hip BMD (b), bone-specific alkaline phosphatase (BAP) (c); urinary type I collagen cross-linked N-telopeptide (NTX) (d). Values are expressed as mean ± standard deviation. For each parameter, data were compared using Student’s paired t test for continuous variables, between baseline and final follow-up, *p < 0.01. DMARDs disease-modifying anti-rheumatic drugs
In the biologics group, lumbar spine and total hip BMD exhibited no change between baseline and final follow-up. BAP was maintained, but NTX level was significantly decreased, from 61.1 ± 58.5 to 48.6 ± 31.0 nmol/mmol CRE (p < 0.01) (Fig. 1).
Effect of glucocorticoids and bisphosphonates on BMD
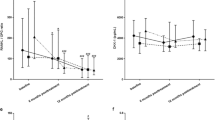
We examined the effects of glucocorticoids and bisphosphonates on BMD and markers of bone turnover. BMD of the total hip in patients receiving glucocorticoids without bisphosphonates showed a significant decrease (p < 0.01) compared with that in patients not receiving glucocorticoids or receiving glucocorticoids with bisphosphonates in both biologics and DMARDs groups (Fig. 2). Moreover, BMD of the lumbar spine was increased (p < 0.05) for patients in the biologics group who received bisphosphonates (Fig. 3).
Effect of glucocorticoids and bisphosphonates on bone mineral density of the lumbar spine. Changes in bone mineral density (BMD) of the lumbar spine at baseline and final follow-up: with or without glucocorticoids (G) (a), with or without bisphosphonates (B) (b), with or without glucocorticoids in patients without bisphosphonates (c), and with or without glucocorticoids in patients with bisphosphonates (d). In each group, data were compared between baseline and final follow-up using Student’s paired t test for continuous variables. NS not significant, *p < 0.05
Effect of glucocorticoids and bisphosphonates on bone mineral density of the total hip. Changes in bone mineral density (BMD) of the total hip at baseline and final follow-up: with or without glucocorticoids (G) (a), with or without bisphosphonates (B) (b), with or without glucocorticoids in patients without bisphosphonates (c), and with or without glucocorticoids in patients with bisphosphonates (d). In each group, data were compared between baseline and final follow-up using Student’s paired t test for continuous variables. NS not significant, *p < 0.05, † p < 0.01
In the biologics group, mean dose of glucocorticoid could be reduced in 32.4 % of patients, from 3.7 ± 3.1 to 2.5 ± 2.7 mg, and 12 patients (14.6 %) were able to cease glucocorticoid use by final follow-up. On the other hand, in the DMARDs group, mean dose could be reduced in 38.1 % of patients, from 2.1 ± 2.4 to 1.3 ± 2.0 mg, and 17 patients (32.0 %) were able to cease glucocorticoid use during the study period.
Multiple regression analysis
Multiple regression analysis was used to examine the specific effects of biologics, bisphosphonates, and glucocorticoid on bone metabolism. Explanatory variables included in the analysis were sex (categorical); age (continuous); body weight (continuous); duration of RA (continuous); baseline ESR (continuous); biologics use (categorical); bisphosphonate use (categorical); glucocorticoid use (categorical); and vitamin D use (categorical). Multiple regression analysis using lumbar BMD as a dependent variable identified bisphosphonate use as a positive independent variable (Table 2A). Biologic use and bisphosphonate use were associated with increased total hip BMD, whereas glucocorticoid use and vitamin D use were associated with decreases in total hip BMD (Table 2B). Bisphosphonate use was associated with decreases in both BAP (Table 2C) and NTX (Table 2D).
Relationship to biologics response
Sixty-six patients receiving biologics were classified as good responders, defined by an improvement ≥1.2 in DAS28 ESR score at final follow-up. The change in lumbar spine BMD was +0.2 % (0.870 ± 0.138 g/cm2 at baseline; 0.872 ± 0.132 g/cm2 at final follow-up) for non-responders and −0.2 % (0.883 ± 0.170 g/cm2 at baseline and 0.882 ± 0.164 g/cm2 at final follow-up) for responders. The change in BMD at the total hip was +0.6 % (0.746 ± 0.128 g/cm2 at baseline and 0.751 ± 0.119 g/cm2 at final follow-up) in non-responders and −2.1 % (0.715 ± 0.142 g/cm2 at baseline and 0.700 ± 0.142 g/cm2 at final follow-up) in responders. No significant difference in BMD change was seen between responders and non-responders. Similarly, no significant difference in bone metabolic markers BAP and NTX was apparent between responders and non-responders.
Discussion
Several biologics targeting cytokines, chemokines, and adhesion molecules have been developed for RA therapy. Biologics usually work quickly to relieve the symptoms and swelling associated with RA. Joint destruction is also prevented, and good influences on bone metabolism are expected. The causes of osteoporosis of RA patients are multifactorial and could be associated with the disease itself, or decreased physical activity [26], or treatment with glucocorticoids [27], or also common postmenopausal osteoporosis [26–28]. High BMD loss in RA patients is reportedly associated with joint damage progression, disease activity, functional disability, and immobility, even in early RA [2, 29–32]. However, several observational studies were performed without enrolled control groups. A 1-year case-control study was performed to compare changes in BMD between RA patients treated with infliximab and those not receiving this agent [33]. In that study, the control group (n = 99) showed a significant decrease in BMD (−3.4 % at total hip and −3.9 % at lumbar spine; p < 0.001), whereas no decrease was observed in the group treated using methotrexate and infliximab (n = 90). In our multiple regression analysis, use of biologics and bisphosphonates was positively correlated with BMD whereas glucocorticoid use was negatively correlated with BMD.
Bone remodeling is the result of two opposing activities: production of new bone matrix by osteoblasts and destruction of existing bone by osteoclasts. Biochemical markers of bone turnover are substances in the blood or urine that are produced or released during bone remodeling. Lange et al. [34] reported persistent increases in OC and decreases in CTX-I among 26 patients with RA treated using infliximab for 1 year. Another report noted that bone resorption was restrained and bone metabolism improved according to measured concentrations of CTX-I and ICTP during treatment with infliximab [35]. In our study, suppression of bone resorption was also confirmed in improvement of urinary NTX. However, in the bisphosphonates group, because bisphosphonates were used before the introduction of biologics, NTX already showed lower levels on the baseline. No change in NTX was therefore recognized.
These findings suggest that biologics can lower NTX levels and may exert positive influences on bone metabolism for the whole body.
Furthermore, the dose of glucocorticoid is a very important factor determining the intensity of influence in bone metabolism. In this study, mean doses of glucocorticoid at baseline were 2.1 ± 2.4 and 3.7 ± 3.1 mg/day in DMARDs and the biologics group, respectively. So, even at the low dose of glucocorticoid, less than 5 mg/day, glucocorticoid has harmful effects on BMD and bone metabolic markers.
Several limitations to the present study must be considered when interpreting the findings. In this study, we included patients with therapy that was not always constant, such as variations in methotrexate and glucocorticoid doses during the investigation period. Furthermore, mean dosages of methotrexate were substantially lower than those seen in Europe and America, because the upper limit for methotrexate in Japan was 8 mg/week until February 2011.
To the best of our knowledge, this represents the first study to examine the effects of biologics, DMARDs, glucocorticoids, and bisphosphonates on BMD in RA patients and to compare the findings with the results for a control group. In this study, BMD loss at the total hip was prevented by the use of biologics and bisphosphonates. However, biologics were unable to prevent BMD loss under glucocorticoid use. Reductions in the dosage of glucocorticoid or administration of bisphosphonates may be necessary to maintain or increase BMD during administration of biologics for RA.
References
Kroot EJ, Nieuwenhuizen MG, de Waal Malefijt MC, van Riel PL, Pasker-de Jong PC, Laan RF (2001) Change in bone mineral density in patients with rheumatoid arthritis during the first decade of the disease. Arthritis Rheum 44:1254–1260
Gough AK, Lilley J, Eyre S, Holder RL, Emery P (1994) Generalised bone loss in patients with early rheumatoid arthritis. Lancet 344:23–27
Deodhar AA, Woolf AD (1996) Bone mass measurement and bone metabolism in rheumatoid arthritis: a review. Br J Rheumatol 35:309–322
Lodder MC, Haugeberg G, Lems WF et al (2003) Radiographic damage associated with low bone mineral density and vertebral deformities in rheumatoid arthritis: the Oslo-Truro-Amsterdam (OSTRA) collaborative study. Arthrtis Rheum 49:209–215
Haugeberg G, Uhlig T, Falch JA, Halse JI, Kvien TK (2000) Bone mineral density and frequency of osteoporosis in female patients with rheumatoid arthritis: results from 394 patients in the Oslo County Rheumatoid Arthritis register. Arthritis Rheum 43:522–530
Cooper C, Coupland C, Mitchell M (1995) Rheumatoid arthritis, corticosteroid therapy and hip fracture. Ann Rheum Dis 54:49–52
Huusko TM, Korpela M, Karppi P, Avikainen V, Kautiainen H, Sulkava R (2001) Threefold increased risk of hip fractures with rheumatoid arthritis in Central Finland. Ann Rheum Dis 60:521–522
van Staa TP, Geusens P, Bijlsma JW, Leufkens HG, Cooper C (2006) Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum 54:3104–3112
Spector TD, Hall GM, McCloskey EV, Kanis JA (1993) Risk of vertebral fracture in women with rheumatoid arthritis. BMJ 306:558
Peel NF, Moore DJ, Barrington NA, Bax DE, Eastell R (1995) Risk of vertebral fracture and relationship to bone mineral density in steroid treated rheumatoid arthritis. Ann Rheum Dis 54:801–806
Van Staa TP, Abenhaim L, Cooper C, Zhang B, Leufkens HG (2000) The use of a large pharmacoepidemiological database to study exposure to oral corticosteroids and risk of fractures: validation of study population and results. Pharmacoepidemiol Drug Saf 9:359–366
Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C (2000) Use of oral corticosteroids and risk of fractures. J Bone Miner Res 15:993–1000
van Staa TP, Leufkens HG, Cooper C (2002) The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 13:777–787
Saag KG, Teng GG, Patkar NM et al (2008) American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum 59:762–784
Smolen JS, Landewé R, Breedveld FC et al (2010) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis 69:964–975
Smolen JS, Aletaha D, Bijlsma JW et al (2010) Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis 69:631–637
Lane NE, Pressman AR, Star VL, Cummings SR, Nevitt MC (1995) Rheumatoid arthritis and bone mineral density in elderly women. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res 10:257–263
Kröger H, Honkanen R, Saarikoski S, Alhava E (1994) Decreased axial bone mineral density in perimenopausal women with rheumatoid arthritis—a population-based study. Ann Rheum Dis 53:18–23
Døhn UM, Ejbjerg B, Boonen A et al (2011) No overall progression and occasional repair of erosions despite persistent inflammation in adalimumab-treated rheumatoid arthritis patients: results from a longitudinal comparative MRI, ultrasonography, CT and radiography study. Ann Rheum Dis 70:252–258
Keystone EC, Avanaugh AF, Sharp JT et al (2004) Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum 50:1400–1411
Smolen JS, Han C, Bala M et al (2005) Evidence of radiographic benefit of treatment with infliximab plus methotrexate in rheumatoid arthritis patients who had no clinical improvement: a detailed subanalysis of data from the anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study. Arthritis Rheum 52:1020–1030
Ammann P, Rizzoli R, Bonjour JP et al (1997) Transgenic mice expressing soluble tumor necrosis factor-receptor are protected against bone loss caused by estrogen deficiency. J Clin Invest 99:1699–1703
Arnett FC, Edworthy SM, Bloch DA et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Prevoo ML, van’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL (1995) Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 38:44–48
Fries JF, Spitz P, Kraines RG, Holman HR (1980) Measurement of patient outcome in arthritis. Arthritis Rheum 23:137–145
Laan RF, Buijs WC, Verbeek AL et al (1993) Bone mineral density in patients with recent onset rheumatoid arthritis: influence of disease activity and functional capacity. Ann Rheum Dis 52:21–26
Hall GM, Spector TD, Griffin AJ, Jawad AS, Hall ML, Doyle DV (1993) The effect of rheumatoid arthritis and steroid therapy on bone density in postmenopausal women. Arthritis Rheum 36:1510–1516
Haugeberg G, Ørstavik RE, Uhlig T, Falch JA, Halse JI, Kvien TK (2002) Bone loss in patients with rheumatoid arthritis: results from a population-based cohort of 366 patients followed up for two years. Arthritis Rheum 46:1720–1728
Shenstone BD, Mahmoud A, Woodward R et al (1994) Longitudinal bone mineral density changes in early rheumatoid arthritis. Br J Rheumatol 33:541–545
Cortet B, Guyot MH, Solau E et al (2000) Factors influencing bone loss in rheumatoid arthritis: a longitudinal study. Clin Exp Rheumatol 18:683–690
Forslind K, Keller C, Svensson B, Hafström I, Group BS (2003) Reduced bone mineral density in early rheumatoid arthritis is associated with radiological joint damage at baseline and after 2 years in women. J Rheumatol 30:2590–2596
Jensen T, Klarlund M, Hansen M et al (2004) Bone loss in unclassified polyarthritis and early rheumatoid arthritis is better detected by digital X ray radiogrammetry than dual X ray absorptiometry: relationship with disease activity and radiographic outcome. Ann Rheum Dis 63:15–22
Marotte H, Pallot-Prades B, Grange L, Gaudin P, Alexandre C, Miossec P (2007) A 1-year case-control study in patients with rheumatoid arthritis indicates prevention of loss of bone mineral density in both responders and nonresponders to infliximab. Arthritis Res Ther 9:R61
Lange U, Teichmann J, Müller-Ladner U, Strunk J (2005) Increase in bone mineral density of patients with rheumatoid arthritis treated with anti-TNF-alpha antibody: a prospective open-label pilot study. Rheumatology (Oxf) 44:1546–1548
Chopin F, Garnero P, le Henanff A et al (2008) Long-term effects of infliximab on bone and cartilage turnover markers in patients with rheumatoid arthritis. Ann Rheum Dis 67:353–357
Acknowledgments
We thank Atsuko Kamiyama for her special efforts as a research coordinator in recruiting subjects, collecting data, and managing the quality of data. We also thank Shinji Takahashi for his special efforts as a statistical adviser in analyzing this data.
Conflict of interest
Dr. Koike has received research grants and/or speaking fees from Takeda Pharmaceutical, Mitsubishi Tanabe Pharma Corporation, Chugai Pharmaceutical, Eisai, Abbott Japan, Teijin Pharma, Banyu Pharmaceutical, and Ono Pharmaceutical. The other authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Okano, T., Koike, T., Tada, M. et al. The limited effects of anti-tumor necrosis factor blockade on bone health in patients with rheumatoid arthritis under the use of glucocorticoid. J Bone Miner Metab 32, 593–600 (2014). https://doi.org/10.1007/s00774-013-0535-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-013-0535-9