Abstract
This study aims to investigate the effect of iguratimod, a novel disease-modifying antirheumatic drug, alone or combined with methotrexate (MTX), on the serum levels of regulators of bone remodeling (receptor activator of nuclear factor-κB ligand (RANKL), osteoprotegerin (OPG), and Dickkopf-1 (DKK-1)) and bone remodeling markers (C-telopeptide of type I collagen (CTX-I) and procollagen type I N-terminal propeptide (PINP)) in patients with rheumatoid arthritis (RA). Patients with RA were treated with iguratimod, MTX, or their combination for 12 months. Serum samples were collected before treatment and 6 and 12 months afterwards. RANKL, OPG, DKK-1, CTX-I, and PINP levels were measured, and radiographic progression was assessed. The serum RANKL levels decreased after treatment for 6 and 12 months with iguratimod (median: baseline 565.00 pmol/L vs. 6 months 411.00 pmol/L vs. 12 months 212.00 pmol/L), MTX (median: baseline 562.50 pmol/L vs. 6 months 399.50 pmol/L vs. 12 months 163.50 pmol/L), and their combination (median: baseline 971.00 pmol/L vs. 6 months 272.50 pmol/L vs. 12 months 241.50 pmol/L). Combination therapy showed greater effects 6 months post-treatment compared to single-drug therapy. PINP levels increased significantly 12 months post-treatment with all therapies, but only the combination therapy led to decreased CTX-I levels. OPG and DKK-1 levels showed no significant changes. The three treatments showed no significant differences in radiographic progression. Iguratimod could stimulate bone formation and regulate the RANKL/RANK/OPG system rather than DKK-1levels. Its effects are comparable to those of MTX, and combination therapy showed stronger effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by synovitis and ultimately leads to the destruction of cartilage and bone in multiple joints [1]. Bone loss may appear in three different forms: bone erosion, periarticular bone loss, and systematic osteoporosis, and all three of these forms share similar mechanisms [2, 3]. Homeostasis of bone metabolism is regulated by the processes of resorption (mediated by osteoclasts) and formation (mediated by osteoblasts), which are regulated by many cytokines and signaling pathways [4, 5]. Receptor activator of nuclear factor-κB ligand (RANKL), osteoprotegerin (OPG), and Dickkopf-1 (DKK-1) are some of the major regulators of bone remodeling [6, 7]. C-telopeptide of type I collagen (CTX-I) and procollagen type I N-terminal propeptide (PINP) are two of the most widely used bone remodeling markers [2].
The RANKL/RANK/OPG system plays a major role in bone metabolism [7]. RANKL is a member of the tumor necrosis factor (TNF) superfamily. It can bind with its receptor, RANK, and promote the activation and development of osteoclasts. Its decoy receptor, OPG, protects against bone resorption by inhibiting the interaction between RANKL and RANK [2, 8]. Overexpression of RANKL or underexpression of OPG promotes RANKL-mediated bone loss. The serum ratio of RANKL/OPG is higher in patients with RA [9]. The Wnt signaling pathway is also a critical regulator of many aspects of bone physiology. Activation of the Wnt pathway increases bone mass through induction of osteoblastogenesis [10]. DKK-1 is an important soluble inhibitor of the Wnt pathway that can block osteoblast function by inhibiting the Wnt pathway [6, 11]. Serum DKK-1 levels are upregulated in patients with RA and are correlated with bone erosion and inflammation [12]. In addition, there might be a complex interaction between the Wnt pathway and RANKL/RANK/OPG system [6]. CTX-I is a very sensitive and specific marker of bone degradation [13]. On the contrary, PINP is a biomarker of bone formation. The levels of CTX-I and PINP reflect the activation of bone resorption and formation and are susceptible to regulation by the RANKL/RANK/OPG system and Wnt pathway [2].
Iguratimod, a novel disease-modifying antirheumatic drug (DMARD) that is an effective treatment for RA, has a positive effect on bone protection. Previous studies showed that iguratimod could promote osteoblast differentiation and inhibit osteoclast differentiation [14, 15]. It has been proven to suppress local RANKL expression in joint tissues [16, 17]. In addition, our previous research found that iguratimod could suppress the serum RANKL/OPG ratio after short-term treatment. However, its effects on DKK-1, bone remodeling markers, and the RANKL/RANK/OPG system after longer-term therapy have not been assessed. The purpose of the present study was to evaluate the effects of iguratimod and methotrexate (MTX), alone or in combination, on serum levels of regulators of bone remodeling and bone remodeling markers in patients with RA in order to discover the possible roles of these drugs on bone metabolism.
Materials and methods
Patients
The study population included 67 patients who were diagnosed with RA between 2013 and 2015 and treated for at least 1 year at the Department of Rheumatology and Immunology of China-Japan Union Hospital of Jilin University in China. All of the patients fulfilled the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria for RA. None of the patients had been treated with iguratimod, MTX, glucocorticoids (GCs), or other DMARDs including biological DMARDs (bDMARDs). Patients were divided into three groups according to their medication: iguratimod (n = 21, group A); MTX (n = 22, group B); and iguratimod + MTX (n = 24, group C). Iguratimod was administered at a dose of 25 mg, twice daily, and MTX was administered at a dose of 10 mg, once weekly. All treatments were administered based on the situations of the patients according to the Chinese Rheumatology Association (CRA) recommendations (2010) for the management of RA and our clinical experience. The exclusion criterion was the use of GCs and other DMARDs, including bDMARDs, during the 12-month treatment period. Clinical and laboratory data, serum samples, and radiographs of the hands of the patients were collected before treatment, 6 months after treatment, and 12 months after treatment. The study was conducted in accordance with the Helsinki Declaration and was approved by the Ethics Committee of China-Japan Union Hospital. Informed consent was given by all patients.
Clinical and laboratory assessments
Clinical characteristics included sex, age, RA duration, morning stiffness, health assessment questionnaire (HAQ), swollen joint count (SJC), tender joint count (TJC), 28-joint disease activity score (DAS28) based on erythrocyte sedimentation rate (ESR), and DAS28 based on C-reactive protein (CRP). Laboratory characteristics included ESR, CRP, rheumatoid factor (RF), and anti-cyclic citrullinated peptide (CCP) antibody.
Serum collection and assessments
All serum samples were centrifuged and stored at −80 °C without freeze/thaw cycles before they were analyzed. Serum CTX-I and PINP levels were measured with the β-CrossLaps/serum kit and total PINP kit (Roche Diagnostics, Mannheim, Germany), which employed an electrochemiluminescence immunoassay (ECLIA). Serum RANKL, OPG, and DKK-1 levels were measured using the enzyme-linked immunosorbent assay (ELISA) kit for RANKL and OPG (Biovendor, Brno, Czech Republic) and DKK-1 (R&D Systems, Minneapolis, MN, USA) according to the manufacturers’ instructions. Each serum sample was tested in triplicate wells.
Radiographic assessments
Radiographs of the hands of patients with RA were collected, and two specialists independently assessed the radiological damage using the van der Heijde modification of the Sharp score (SHS). The readers were blind to patient’s identity and treatment, and for each radiograph, the scores of the two readers were averaged as the final result. Total Sharp score (TSS; range, 0 to 280), erosion score (ES; range, 0 to 160), and joint space narrowing score (JSN; range, 0 to 120) were collected.
Statistical analysis
Values were presented as the mean ± standard deviation (SD) or median (interquartile range (IQR)) unless otherwise indicated. First, the Shapiro-Wilk test was performed to test whether the variable distribution could be described as normal or non-normal. Non-normal variables were log-transformed to be normally distributed. Fisher’s exact test was used to describe categorical variables. To compare the serum regulators and marker levels and DAS28 before and after treatment among the three groups, repeated-measures analysis of variance (ANOVA) was used for general analysis and for specific analysis, one-way ANOVA was used to compare values among groups, and the paired t test was used to compare values between times within each group. For the paired t test, the Bonferroni correction was applied and the significance level was adjusted to 0.05/2. To analyze the differences in baseline characteristics and radiological score changes among the three groups, ANOVA or the Kruskal-Wallis test was used for normally or non-normally distributed values, respectively. Pearson’s or Spearman’s correlation analysis was used to test for correlation between changes in DAS28 and changes in bone metabolism parameters for normally or non-normally distributed values. All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) version 21.0. GraphPad Prism 6 was used to create the graph. p values <0.025 for paired t test and p values <0.05 for other comparisons were considered significant.
Results
Clinical characteristics
A total of 67 patients with RA were enrolled in the study. Demographic, clinical, laboratory, and radiological characteristics at baseline are summarized in Table 1. Patients in group C had higher HAQ, CRP, and anti-CCP antibody levels compared to those in groups A and B. No significant differences were observed for other characteristics.
Disease activity measured by DAS28-ESR and DAS28-CRP decreased significantly in all three groups in general (both p < 0.001) but no significant difference was found among groups (both p > 0.05). There was an interaction between treatment time and treatment plan for both DAS28-ESR and DAS28-CRP (both p < 0.01). Specifically, DAS28-ESR decreased significantly 6 and 12 months after treatment (baseline vs. 6 months vs. 12 months: group A = 6.74 ± 1.03 vs. 4.93 ± 1.05 vs. 3.56 ± 1.02; group B = 6.25 ± 1.26 vs. 4.96 ± 1.55 vs. 3.41 ± 1.76; and group C = 6.85 ± 1.26 vs. 4.11 ± 1.42 vs. 2.94 ± 1.72; p < 0.001 at both points compared with baseline for all groups), as did DAS28-CRP (baseline vs. 6 months vs. 12 months: group A = 6.19 ± 0.94 vs. 4.62 ± 0.93 vs. 3.28 ± 0.87; group B = 5.97 ± 1.07 vs. 4.76 ± 1.47 vs.3.49 ± 1.39; and group C = 6.63 ± 1.05 vs. 4.02 ± 1.13 vs. 2.97 ± 1.44; p < 0.001 at both points compared with baseline for all groups). Analysis between groups showed significantly different DAS28-ESR and DAS28-CRP levels between groups A and C (p = 0.017 and p = 0.047, respectively) and between groups B and C (p = 0.025 and p = 0.036, respectively) 6 months after treatment. No difference was noticed 12 months after treatment.
Serum regulators of bone remodeling and bone remodeling marker levels
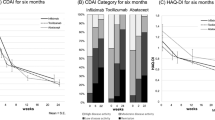
There was no significant difference in the levels of all the serum regulators of bone remodeling and bone remodeling markers at baseline among the three groups. Generally, all groups showed a significant decrease in RANKL after treatment (p < 0.001) but there was no significant difference among groups (p > 0.05). There was also an interaction between the two factors that affected the RANKL levels: treatment time and plan (p < 0.001). This indicated that the change in RANKL levels after treatment differed because of the different treatment plans. Specifically, RANKL levels were decreased in all three groups 6 months after treatment (baseline vs. 6 months: group A = 565.00 [229.00, 950.50] pmol/L vs. 411.00 [224.50, 654.00] pmol/L, p = 0.002; group B = 562.5 [229.25, 1527.25] pmol/L vs. 399.50 [224.00, 1111.75] pmol/L, p = 0.005; and group C = 971.00 [456.75, 1609.75] pmol/L vs. 272.50 [151.00, 535.00] pmol/L, p < 0.001) and 12 months after treatment (baseline vs. 12 months: group A = 565.00 [229.00, 950.50] pmol/L vs. 212.00 [62.00, 357.00] pmol/L; group B = 562.50 [229.25, 1527.25] pmol/L vs. 163.50 [26.00, 404.25] pmol/L; and group C = 971.00 [456.75, 1609.75] pmol/L vs. 241.50 [105.00, 390.25] pmol/L; all p < 0.001). Group C showed lower RANKL levels compared with the other two groups at 6 months after treatment (groups A vs. C, p = 0.042; groups B vs. C, p = 0.044), but this difference was not sustained at 12 months after treatment (Fig. 1a). OPG levels did not change significantly after treatment in all three groups (Fig. 1b), and this led to a change in the ratio of RANKL/OPG similar to that in RANKL. Generally, the ratio of RANKL/OPG decreased after treatment (p < 0.001), and there was an interaction between treatment time and plan (p < 0.01), but no significant difference was found among groups (p > 0.05). Specifically, the RANKL/OPG ratio decreased significantly only in group C at 6 months post-treatment (baseline vs. 6 months: 205.17 [100.10, 378.12] vs. 56.76 [29.23, 127.49], p < 0.001) whereas in the other two groups, the decrease was not significant. All groups showed a significant decrease 12 months post-treatment (baseline vs. 12 months: group A = 141.10 [56.95, 295.43] vs. 47.24 [8.63, 100.20]; group B = 108.23 [63.90, 341.75] vs. 47.34 [8.03, 114.67]; and group C = 205.17 [100.10, 378.12] vs. 44.56 [21.92, 104.62]; all p < 0.001). Six months after the treatment, the RANKL/OPG ratio of group C was significantly lower than that of the other two groups (groups A vs. group C, p = 0.029; groups B vs. C, p = 0.019), but then, it tended to stay stable and showed no significant difference at 12 months post-treatment (Fig. 1c). Serum levels of DKK-1 did not change significantly after treatment in any of the three treatment groups (Fig. 1d).
Serum levels of RANKL (a), OPG (b), the ratio of RANKL/OPG (c), DKK-1 (d), PINP (e), and CTX-I (f) were measured at baseline, 6 and 12 months after treatment with iguratimod (group A), MTX (group B) or their combination (group C) in patients with RA. Values are presented as median (IQR), # p < 0.05, ## p < 0.01, ### p < 0.001 compared with baseline, and *p < 0.05 compared with Group C
The serum PINP levels in general increased after treatment (p < 0.001), but there was no interaction between treatment time and plan (p > 0.05), and there was no significant difference among the three groups (p > 0.05). Specifically, PINP levels tended to increase 6 months post-treatment and showed significant differences at 12 months post-treatment in all three groups (baseline vs. 12 months: group A = 48.85 [34.67, 79.16] ng/mL vs. 61.64 [49.91, 80.05] ng/mL, p = 0.007; group B = 41.66 [34.03, 84.75] ng/mL vs. 52.69 [33.33, 141.33] ng/mL, p = 0.0249; and group C = 44.22 [28.75, 68.19] ng/mL vs. 67.98 [51.38, 87.19] ng/mL, p < 0.001). No significant post-treatment difference was found among the three groups (Fig. 1e). A general analysis showed that CTX-I changed after treatment (p < 0.05), and these changes were affected by the treatment plan (p < 0.05), but no significant difference was found among the three groups (p > 0.05). Specifically, only group C showed a significant decrease at 6 and 12 months after treatment (baseline vs. 6 months vs. 12 months: 0.47 [0.22, 0.60] ng/mL vs. 0.31 [0.21, 0.49] ng/mL vs. 0.27 [0.14, 0.39] ng/mL; p = 0.023 and p < 0.001, compared with baseline, respectively), whereas the other two groups did not experience a significant change. However, no significant post-treatment difference among the three groups was noticed (Fig. 1f).
Possible associations between changes in disease activity and changes in regulators of bone remodeling and bone remodeling markers were also examined. ΔDAS28-ESR correlated with ΔCTX-I positively and with ΔPINP negatively (r = 0.577, p = 0.006 and r = −0.443, p = 0.044, respectively) and ΔDAS28-CRP also correlated positively with ΔCTX-I (r = 0.625, p = 0.001) in group A after 6 months of treatment. In group C, ΔDAS28-ESR and ΔDAS28-CRP correlated negatively with ΔPINP (r = −0.433, p = 0.034 and r = −0.554, p = 0.005, respectively) at 12 months after treatment. No significant correlations between other changes in values were noticed.
Radiological assessment
Radiological score changes from baseline to 6 and 12 months after treatment were assessed to define the difference in radiological progression among different medications (Table 2). No significant differences among groups were noticed.
Discussion
We evaluated the effects of iguratimod and MTX, alone or in combination, on serum levels of regulators of bone remodeling and bone remodeling markers and on radiographic assessment of patients with RA to discover the possible roles of these drugs in bone protection. MTX, which is a classic DMARD, is used as the first-line drug for the treatment of patients with RA. Comparing the effects of iguratimod and combination therapy with MTX may further support the use of iguratimod in clinical practice.
Iguratimod is a small-molecule DMARD that is widely used in China and Japan. A previous study showed that its clinical efficacy was comparable to that of salazosulfapyridine [18]. Other studies showed that combined use of iguratimod and MTX resulted in better clinical outcomes than did the use of either drug alone [19,20,21]. In our study, patients were treated based on the disease condition and according to the CRA recommendations (2010) for the management of RA and our clinical experience. Patients in the combination treatment group had higher HAQ, CRP, and anti-CCP antibody levels, which corresponded to more severe disease activity. MTX was administered 10 mg/week because, in our clinical practice, this dose could efficiently suppress inflammation and decrease the disease activity without causing too many adverse effects. After treatment, DAS28, which is widely used to represent disease activity, decreased in all three groups. Moreover, in patients treated with combination therapy, DAS28 showed an even greater decrease 6 months post-treatment, suggesting quicker and stronger effects. In addition, iguratimod has shown effects on bone protection in mice with collagen-induced arthritis and on promoting osteoblast differentiation and inhibiting osteoclast differentiation in vitro [14,15,16].
The RANKL/RANK/OPG system and Wnt pathway are two major systems that regulate the homeostasis of bone metabolism. RANKL and macrophage colony-stimulating factor are essential in osteoclast differentiation. RANKL can be expressed as a membrane protein on osteoblasts, T cells and other cells, or as a soluble form that can be detected in serum and synovial fluid. RANKL interacts with RANK, its receptor located on osteoclast precursor cells and thereby promotes the differentiation of osteoclast precursors into mature osteoclasts, which are pivotal cells for bone resorption [7]. However, this interaction can be counterbalanced by OPG, an endogenous antagonist of RANKL [2]. The balance of RANKL and OPG is critical for osteoclastogenesis modulation and the homeostasis of bone metabolism [4]. Serum and synovial fluid RANKL levels are higher in patients with RA than in healthy people or those with osteoarthritis (OA) [22, 23]. The serum ratio of RANKL/OPG has been suggested as a predictor of bone damage progression and may contribute to the loss of bone mass [9, 22]. In our study, we found that serum RANKL levels decreased significantly after treatment with iguratimod, MTX, or their combination; OPG levels were stable and there was a corresponding similar significant reduction in the RANKL/OPG ratio. A previous study of ours and other studies on treatment with MTX or bDMARDs support our results [24,25,26]. Specific analysis suggested that only combination therapy showed a significant decrease in the RANKL/OPG ratio 6 months post-treatment while the ratio tended to decrease in the other two groups, but not significantly. Moreover, compared to therapy with a single agent, combination therapy had a stronger effect on RANKL and the RANKL/OPG ratio at 6 months after treatment, but this difference was not sustained at the 12-month follow-up. These all suggested that all three treatments may eventually achieve similar levels of RANKL and the RANKL/OPG ratio, but the combination of iguratimod and MTX was able to more quickly downregulate RANKL and the RANKL/OPG ratio in the serum of patients with RA compared to any single drug. There was, therefore, a synergistic effect between iguratimod and MTX.
The Wnt pathway promotes bone formation by stimulating osteoblast differentiation, and by inhibiting osteoblast apoptosis and osteoclastogenesis [10]. Among the regulators of the Wnt pathway, DKK-1, an endogenous inhibitory factor that binds to the Wnt co-receptor LRP5/6 to block Wnt signaling, plays a critical role [11]. Previous studies have shown that in patients with RA, serum DKK-1 levels at baseline may predict joint structural progression and might be a new structural biomarker [12, 27]. In addition, studies of animal models indicate that DKK-1 is a regulator of bone mass, and a therapy targeting DKK-1 showed that bone mineral density (BMD) in mice increased after treatment [28]. Serum DKK-1 levels are higher in patients with RA than in healthy controls and may decrease after treatment with bDMARDs such as infliximab, anakinra, and tocilizumab [12, 29]. However, in our study, we were the first to find that iguratimod had no significant effect on serum DKK-1 levels in patients with RA. All three groups showed no significant change in serum DKK-1 levels before and after treatment. In a study by Swierkot et al., patients who responded well to MTX treatment had significantly decreased serum DKK-1 levels 6 months after treatment [24]; however, our study showed no significant difference in the MTX treatment group. We suppose the difference may be due to differences in patient characteristics and different doses of MTX. Our patient cohort was composed of both males and females with shorter disease duration, and they were treated with a smaller dose of MTX. There was also an interaction between the RANKL/RANK/OPG system and Wnt pathway. The decrease of DKK-1 may lead to the increase of OPG [6]. This might help explain why neither OPG nor DKK-1 showed a significant change during our study.
CTX-I and PINP are two of the most widely used bone remodeling markers. They are recommended as markers of bone resorption and bone formation, respectively [30]. In our study, in all three groups, serum PINP levels showed a significant increase 12 months after treatment. For CTX-I, only combination therapy showed a significant decrease 6 and 12 months after treatment. These findings suggested that all three therapeutic strategies could stimulate bone formation, but only the combination of iguratimod and MTX could efficiently prevent bone resorption.
We also investigated the associations between changes in disease activity and changes in regulators of bone remodeling and bone remodeling markers. No correlation between DAS28 and RANKL, OPG, the RANKL/OPG ratio or DKK-1 was noticed. This indicated that iguratimod could directly affect the RANKL/RANK/OPG system and that the effects were independent of the control of inflammatory activity. However, changes in CTX-I and PINP significantly correlated with the change in DAS28 in the iguratimod and combination therapy group, suggesting that iguratimod could not only regulate the RANKL/RANK/OPG system, but also could participate in bone resorption and formation by suppressing inflammation.
The homeostasis of bone metabolism is based on the balance of bone resorption and bone formation. Bone loss, whether periarticular or systemic, appears in RA, and one of the targets of treatment is to regain the homeostasis of bone metabolism to prevent joint deformities and secondary osteoporosis. In our study, iguratimod as a novel DMARD that showed great effects on stimulating bone formation, but it had limited effects on preventing bone resorption. Its effects were most likely achieved through regulating the RANKL/RANK/OPG system rather than through regulating DKK-1 levels. The effects of iguratimod on regulators of bone remodeling and bone remodeling markers were comparable with those of MTX. Moreover, the combination therapy also had an effect not only on stimulating bone formation, but also on preventing bone resorption, and resulted in a faster decrease in serum RANKL levels and the RANKL/OPG ratio. This suggested that the combination of iguratimod and MTX had stronger effects than any single drug.
Bone erosion may be evaluated by radiographic assessment. In a study conducted by Hara et al., iguratimod showed no significant effects on the progression of articular destruction compared with salazosulfapyridine or placebo 6 months after treatment in a group of RA patients with a long disease duration [18]. In our study, iguratimod showed comparable effects to those of MTX on the progression of articular destruction. We were also the first to evaluate the effects of iguratimod on radiographic assessment 12 months after treatment. Stronger effects of the combination therapy group was expected, but there was no significant difference. We suppose this might be because the sample size in our study was not large and there was not a very high rate of progression because of low scores of damage at baseline and a good disease control after only 12-month treatment. In future studies, larger sample sizes with longer observation times could enable better assessment of the inhibitory effects of iguratimod on articular destruction.
Our study had limitations, which arise from the small study group, observation of only Chinese patients diagnosed and treated at only one hospital. In the future, studies involving larger groups of patients including patients with RA from different centers would do great help to confirm our results.
In conclusion, our study is the first to show the effects of iguratimod, used alone or in combination with MTX, on circulating regulators of bone remodeling, bone remodeling markers, and radiographic progression in patients with RA during a 1-year study. Iguratimod could stimulate bone formation and show effects on regulating the RANKL/RANK/OPG system but not DKK-1 levels. Its effects are comparable with those of MTX and the combination therapy shows even stronger effects. These results imply that iguratimod participates in bone metabolism and may protect against bone destruction and even potential secondary osteoporosis. They may further support the use of iguratimod in clinical practice.
References
Smolen JS, Aletaha D, McInnes IB (2016) Rheumatoid arthritis. Lancet. doi:10.1016/S0140-6736(16)30173-8
Fardellone P, Sejourne A, Paccou J, Goeb V (2014) Bone remodelling markers in rheumatoid arthritis. Mediat Inflamm 2014:484280. doi:10.1155/2014/484280
Roux C (2011) Osteoporosis in inflammatory joint diseases. Osteoporosis Int 22(2):421–433. doi:10.1007/s00198-010-1319-x
Amarasekara DS, Yu J, Rho J (2015) Bone loss triggered by the cytokine network in inflammatory autoimmune diseases. J Immunol Res 2015:832127. doi:10.1155/2015/832127
Schett G, Gravallese E (2012) Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol 8(11):656–664. doi:10.1038/nrrheum.2012.153
Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, Korb A, Smolen J, Hoffmann M, Scheinecker C, van der Heide D, Landewe R, Lacey D, Richards WG, Schett G (2007) Dickkopf-1 is a master regulator of joint remodeling. Nat Med 13(2):156–163. doi:10.1038/nm1538
Kearns AE, Khosla S, Kostenuik PJ (2008) Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev 29(2):155–192. doi:10.1210/er.2007-0014
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423(6937):337–342. doi:10.1038/nature01658
van Tuyl LH, Voskuyl AE, Boers M, Geusens P, Landewe RB, Dijkmans BA, Lems WF (2010) Baseline RANKL:OPG ratio and markers of bone and cartilage degradation predict annual radiological progression over 11 years in rheumatoid arthritis. Ann Rheum Dis 69(9):1623–1628. doi:10.1136/ard.2009.121764
Krishnan V, Bryant HU, Macdougald OA (2006) Regulation of bone mass by Wnt signaling. J Clin Invest 116(5):1202–1209. doi:10.1172/JCI28551
Daoussis D, Andonopoulos AP (2011) The emerging role of Dickkopf-1 in bone biology: is it the main switch controlling bone and joint remodeling? Semin Arthritis Rheu 41(2):170–177. doi:10.1016/j.semarthrit.2011.01.006
Wang SY, Liu YY, Ye H, Guo JP, Li R, Liu X, Li ZG (2011) Circulating Dickkopf-1 is correlated with bone erosion and inflammation in rheumatoid arthritis. J Rheumatol 38(5):821–827. doi:10.3899/jrheum.100089
Chopin F, Garnero P, le Henanff A, Debiais F, Daragon A, Roux C, Sany J, Wendling D, Zarnitsky C, Ravaud P, Thomas T (2008) Long-term effects of infliximab on bone and cartilage turnover markers in patients with rheumatoid arthritis. Ann Rheum Dis 67(3):353–357. doi:10.1136/ard.2007.076604
Kuriyama K, Higuchi C, Tanaka K, Yoshikawa H, Itoh K (2002) A novel anti-rheumatic drug, T-614, stimulates osteoblastic differentiation in vitro and bone morphogenetic protein-2-induced bone formation in vivo. Biochem Bioph Res Co 299(5):903–909
Gan K, Yang L, Xu L, Feng X, Zhang Q, Wang F, Tan W, Zhang M (2016) Iguratimod (T-614) suppresses RANKL-induced osteoclast differentiation and migration in RAW264.7 cells via NF-kappaB and MAPK pathways. Int Immunopharmacol 35:294–300. doi:10.1016/j.intimp.2016.03.038
Luo Q, Sun Y, Liu W, Qian C, Jin B, Tao F, Gu Y, Wu X, Shen Y, Xu Q (2013) A novel disease-modifying antirheumatic drug, iguratimod, ameliorates murine arthritis by blocking IL-17 signaling, distinct from methotrexate and leflunomide. J Immunol 191(10):4969–4978. doi:10.4049/jimmunol.1300832
Wei Y, Sun X, Hua M, Tan W, Wang F, Zhang M (2015) Inhibitory effect of a novel antirheumatic drug T-614 on the IL-6-induced RANKL/OPG, IL-17, and MMP-3 expression in synovial fibroblasts from rheumatoid arthritis patients. Biomed Res Int 2015:214683. doi:10.1155/2015/214683
Hara M, Abe T, Sugawara S, Mizushima Y, Hoshi K, Irimajiri S, Hashimoto H, Yoshino S, Matsui N, Nobunaga M, Nakano S (2007) Efficacy and safety of iguratimod compared with placebo and salazosulfapyridine in active rheumatoid arthritis: a controlled, multicenter, double-blind, parallel-group study. Mod Rheumatol 17(1):1–9. doi:10.1007/s10165-006-0542-y
Ishiguro N, Yamamoto K, Katayama K, Kondo M, Sumida T, Mimori T, Soen S, Nagai K, Yamaguchi T, Hara M, Iguratimod-Clinical Study G (2013) Concomitant iguratimod therapy in patients with active rheumatoid arthritis despite stable doses of methotrexate: a randomized, double-blind, placebo-controlled trial. Mod Rheumatol 23(3):430–439. doi:10.1007/s10165-012-0724-8
Duan XW, Zhang XL, Mao SY, Shang JJ, Shi XD (2015) Efficacy and safety evaluation of a combination of iguratimod and methotrexate therapy for active rheumatoid arthritis patients: a randomized controlled trial. Clin Rheumatol 34(9):1513–1519. doi:10.1007/s10067-015-2999-6
Xia Z, Lyu J, Hou N, Song L, Li X, Liu H (2015) Iguratimod in combination with methotrexate in active rheumatoid arthritis : therapeutic effects. Z Rheumatol. doi:10.1007/s00393-015-1641-y
Xu S, Wang Y, Lu J, Xu J (2012) Osteoprotegerin and RANKL in the pathogenesis of rheumatoid arthritis-induced osteoporosis. Rheumatol Int 32(11):3397–3403. doi:10.1007/s00296-011-2175-5
Ellabban AS, Kamel SR, Ahmed SS, Osman AM (2012) Receptor activator of nuclear factor kappa B ligand serum and synovial fluid level. A comparative study between rheumatoid arthritis and osteoarthritis. Rheumatol Int 32(6):1589–1596. doi:10.1007/s00296-011-1831-0
Swierkot J, Gruszecka K, Matuszewska A, Wiland P (2015) Assessment of the effect of methotrexate therapy on bone metabolism in patients with rheumatoid arthritis. Arch Immunol Ther Ex 63(5):397–404. doi:10.1007/s00005-015-0338-x
Hensvold AH, Joshua V, Li W, Larkin M, Qureshi F, Israelsson L, Padyukov L, Lundberg K, Defranoux N, Saevarsdottir S, Catrina AI (2015) Serum RANKL levels associate with anti- citrullinated protein antibodies in early untreated rheumatoid arthritis and are modulated following methotrexate. Arthritis Res Ther 17:239. doi:10.1186/s13075-015-0760-9
Boumans MJ, Thurlings RM, Yeo L, Scheel-Toellner D, Vos K, Gerlag DM, Tak PP (2012) Rituximab abrogates joint destruction in rheumatoid arthritis by inhibiting osteoclastogenesis. Ann Rheum Dis 71(1):108–113. doi:10.1136/annrheumdis-2011-200198
Seror R, Boudaoud S, Pavy S, Nocturne G, Schaeverbeke T, Saraux A, Chanson P, Gottenberg JE, Devauchelle-Pensec V, Tobon GJ, Mariette X, Miceli-Richard C (2016) Increased Dickkopf-1 in recent-onset rheumatoid arthritis is a new biomarker of structural severity. Data from the ESPOIR cohort. Scientific reports 6:18421. doi:10.1038/srep18421
Glantschnig H, Hampton RA, Lu P, Zhao JZ, Vitelli S, Huang L, Haytko P et al (2010) Generation and selection of novel fully human monoclonal antibodies that neutralize Dickkopf-1 (DKK-1) inhibitory function in vitro and increase bone mass in vivo. J Biol Chem 285(51):40135–40147. doi:10.1074/jbc.M110.166892
Briot K, Rouanet S, Schaeverbeke T, Etchepare F, Gaudin P, Perdriger A, Vray M, Steinberg G, Roux C (2015) The effect of tocilizumab on bone mineral density, serum levels of Dickkopf-1 and bone remodeling markers in patients with rheumatoid arthritis. Joint Bone Spine 82(2):109–115. doi:10.1016/j.jbspin.2014.10.015
Garnero P (2014) New developments in biological markers of bone metabolism in osteoporosis. Bone 66:46–55. doi:10.1016/j.bone.2014.05.016
Acknowledgements
This study was supported by grants from the Science and Technology Ministry “12th Five-Year Plan” to support science and technology of China (No. 2014BAI07B00). We thank Professor Bo Li and Professor Pinghui Sun from the Department of Epidemiology and Biostatistics, Jilin University School of Public Health for assistance with statistical analysis. We also appreciate the anonymous referees for their valuable suggestions and questions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Rights and permissions
About this article
Cite this article
Wang, X., Ma, C., Li, P. et al. Effects of iguratimod on the levels of circulating regulators of bone remodeling and bone remodeling markers in patients with rheumatoid arthritis. Clin Rheumatol 36, 1369–1377 (2017). https://doi.org/10.1007/s10067-017-3668-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-017-3668-8