Abstract
Bone acquisition failure during growth or low bone mineral density (BMD) in childhood and adolescence might increase future osteoporosis risk. To identify these children and adolescents, appropriate reference values are necessary. The robust reference values must be community based as well as sex-, age-, and ethnicity specific. In addition, body size adjustment is necessary because individuals demonstrate different body sizes and different tempos of growth, which affect measured BMD. We aimed to provide reference data with body size adjustment of Korean children and adolescents. We used dual-energy X-ray absorptiometry data of 1,650 subjects (aged 10–20 years; 788 female) from the Korea National Health and Nutrition Examination Survey (2009–2010). The BMD of each region of interest (ROI), including the lumbar spine, total body less head, total body, and femoral neck, were obtained. We calculated the mean and percentiles for each ROI. Because height and weight variations were high and correlated independently with BMD within the same age group, we developed equations to calculate the “predicted BMD Z score.” Although 12.8–17.9 % of subjects with short stature showed a low measured BMD Z score depending on the measured site, only 2.6 % of those of short stature had a low adjusted BMD Z score after applying the predicted BMD Z score. We also compared the BMD of children and adolescents of other ethnicities using the same device. This study provided robust reference values for the assessment and monitoring of bone health in Korean children and adolescents. Additionally, it extended the knowledge of bone acquisition in Asian children and adolescents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis previously was considered a disease of the elderly; however, it is now universally agreed that it has a pediatric origin [1, 2]. Individuals who fail to achieve optimal peak bone mass (PBM) and strength during childhood and adolescence are more likely to develop osteoporosis later in life [3]. Children with a chronic disease, such as cancer, have lower bone mineral density (BMD) than do their healthy counterparts [4, 5]. Some of these children who are treated with specific medications, such as corticosteroids, anticonvulsants, or chemotherapeutics, fail to acquire adequate BMD and, thus, have increased risk of fracture even in young adulthood [4, 6–9]. Therefore, many expert groups, including the International Society of Clinical Densitometry (ISCD), recommend evaluating BMD in individuals predisposed to BMD deficits [2, 10].
Dual-energy X-ray absorptiometry (DXA) is the gold standard for the noninvasive measurement of BMD and is used to diagnose osteoporosis in both children and adults [2]. DXA is not only safe and easy to perform but is also a good surrogate measure of bone health and associated fracture risk even in children [11, 12]. Therefore, many pediatric references have been reported, including our own [13–16]. However, a child who is short for the associated chronological age might have a measured BMD that is less than the expected-for-age BMD, even though the child’s bone strength is adequate to sustain average mechanical burdens [17]. Because some children and adolescents are small because of delayed maturation, some investigators have suggested making an adjustment based on body weight and/or height [17–19]. However, to our knowledge, there is no Asian, community-based, pediatric, DXA reference adjusted for body size.
The aims of this study were as follows: (1) to provide pediatric reference data of the BMD of multiple skeletal sites by using DXA, (2) to provide a weight- and height-adjusted BMD equation, and (3) to determine the BMD characteristics of Korean children and adolescents by comparing our DXA results with those of other ethnicities.
Materials and methods
Study population
The data source was the Korea National Health and Nutrition Examination Survey (KNHANES, 2009–2010) [20]. Briefly, the KNHANES has been conducted periodically since 1998 to assess the health and nutritional status of the noninstitutionalized civilian population of Korea. The KNHANES is a cross-sectional, nationally representative survey conducted by the Division of Chronic Disease Surveillance of the Korea Centers for Disease Control (KCDC). A total of 1,981 subjects aged 10–20 years were identified as potential subjects for this study. Of the 1,981 subjects, those who had chronic disease, took medication that affects bone growth or metabolism, or did not complete all region of interest (ROI) measurements were excluded. Therefore, the final analytical sample consisted of 1,650 subjects (862 males, 788 females; weighted number, 23,388). The Ethics Committee of the KCDC approved the study protocol, and written informed consent was obtained from all participants or their parents.
Anthropometry and DXA analysis
Weight was determined to the nearest 0.1 kg on a medical balance (GL-6000-20; CAS, Seoul, Korea). Height was measured to the nearest 0.1 cm with a wall-mounted stadiometer (Seca 220; Seca, Hamburg, Germany). All anthropometry data were calculated to Z score based on Korean reference data [21]. The BMD of each ROI, including the lumbar spine (BMDLS), total body less head (BMDTBLH), total body (BMDTB), and left femoral neck (BMDFN), was measured serially, using a bone densitometer (Discovery-W; QDR 4500, Hologic, Denver, CO, USA) located in a mobile examination center, according to manufacturer guidelines as described previously [15]. All scans were analyzed centrally by the DXA Core Laboratory (Yensei University College of Medicine, Seoul, Korea) using Hologic software release 12.3.
Statistical analyses
Statistical analysis was performed using SPSS 17.0 for Windows (SPSS, Chicago, IL, USA). We calculated the mean ± standard deviation (SD) and percentiles for BMDLS, BMDTBLH, BMDTB, and BMDFN according to age group and sex. Each age group included subjects of a specific age range; e.g., age group 11 contained subjects of ages 11.0–11.9 years. The difference between age groups was tested using analysis of variance to find the time of plateau of the BMD of each ROI. The difference in BMD between sexes in each age group was tested using the independent t test. The error bar of the figure (Fig. 1) shows the mean ± 2 SEM.
Because the BMD of each ROI closely correlated with chronological age, body weight, and height, multiple regression analyses were performed for the BMD of each ROI using the independent variables of chronological age, weight, and height. Even in the same age group, BMD variation was too high according to weight and height status. Therefore, we determined the “predicted BMD Z score” in each age group. Each predicted BMD Z score, R 2 value (explanatory value of the predicted BMD Z score), and standard error of estimate (SEE) was calculated using multiple regression analysis with “measured BMD Z score for age group” as the dependent variable and weight Z score (WZ) and height Z score (HZ) as the independent variables, as derived from Korean reference data. In all analyses, a P value < 0.01 was considered significant.
Results
Clinical characteristics and regression equations for BMD of each ROI
The final analytical sample consisted of 1,650 subjects (862 males, 788 females), which represented 23,388 Korean subjects. The mean age, height, HZ, weight, WZ, and body mass index (BMI) of the subjects according to sex are described in Table 1.
Chronological age-based BMD
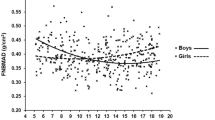
BMD increased with regard to chronological age in both sexes. Generally, females showed the highest BMD increase and plateau in each ROI 1 or 2 years earlier than did males (Fig. 1). BMDLS showed the highest increase between age groups 11 and 13 in girls and between age groups 12 and 14 in boys (Fig. 1a). BMDTBLH and BMDTB showed continuous increase between age groups 10 and 15 in girls and between age groups 10 and 18 in boys (Fig. 1b, c). BMDFN showed continuous increase between age groups 10 and 15 in girls and between age groups 10 and 16 in boys (Fig. 1d). The plateau in BMDLS, BMDTBLH, BMDTB, and BMDFN in girls occurred after age groups 14, 15, 15, and 14, respectively (after this age, the BMD difference among age groups, all P > 0.05). The plateau in the BMD of all ROIs occurred after age group 16 in boys. The difference in the BMD of each ROI between Korean female and male children and adolescents is depicted in Fig. 1.
Comparison of the bone mineral density (BMD) of each region of interest (ROI) between Korean female and male subjects. a BMDLS: females had higher BMD of the lumbar spine between 10 and 13 years of age. After 14 years of age, no significant difference in BMD was observed between females and males. b BMDTBLH: no significant difference in BMD of the total body less head was observed between females and males until 13 years of age. Females had lower BMD after 14 years of age. c BMDTB: a significant difference in BMD of the total body was observed between females and males after 17 years of age. d BMDFN: females had lower BMD of the femoral neck in all age groups except 12 and 13 years. Filled red circles, females; blue squares, males. The error bar of each curve shows the mean ± 2 SEM. FN femoral neck, LS lumbar spine (L1–L4), TB total body, TBLH total body less head
Age- and sex-specific reference percentiles for BMD of each ROI
The mean, SD, and reference percentiles for BMDLS, BMDTBLH, BMDTB, and BMDFN according to age group and sex are noted in Tables 2, 3, 4, 5. The BMD of each ROI closely correlated not only with chronological age but also with weight and height after controlling for other variables (all P < 0.001). Therefore, multiple regression equations were developed for the BMD of each ROI using the independent variables of chronological age, weight, and height (Table 6). In these equations, weight was the most important independent variable, after age, that influenced BMD.
Equations for calculating predicted BMD Z score adjusted for WZ and HZ in each age group
The BMD of subjects varied within the same age group. For example, BMDLS varied as much as 0.567 g/cm2 (range, 0.440–1.007 g/cm2) in girls in age group 11. This group showed height and weight variations up to 37 cm and 48 kg, respectively. Even in the same sex and age group, the BMD of each ROI closely correlated with weight and height (R = 0.47–0.72; all P < 0.001). Thus, linear regression equations were developed to calculate the predicted BMD Z score for each ROI in each age group using the independent variables of WZ and HZ. The equations for calculating the predicted BMD Z score are provided in Tables 2, 3, 4, 5.
Adjusted BMD Z score using predicted BMD Z score
The body size adjustment of the measured BMD consisted of three steps: (1) calculation of the “measured BMD Z score for age” based on mean and SD or percentiles in Tables 2, 3, 4, 5; (2) calculation of the predicted BMD Z score based on age, WZ, and HZ; and (3) calculation of the “adjusted BMD Z score” by subtracting the predicted BMD Z score from the measured BMD Z score.
The following is an example for a girl aged 11.7 years with height of 137.1 cm, weight of 35.0 kg, and BMDLS of 0.490 g/cm2. Her rounded-off age is 11 years, the mean BMD of 11-year-old girls is 0.707 g/cm2, and the SD from Table 2 is 0.116 g/cm2. Thus, her measured BMDLS Z score for age is (0.490 − 0.707)/0.116 = −1.87 Z. Her WZ and HZ are −1.1 and −2.0 SD, respectively. The predicted BMDLS Z score of an 11-year-old female with −1.1 WZ and −2.0 HZ is calculated using the adjustment equation in Table 2: −0.023 + (−1.1 × 0.472) + (−2.0 × 0.370)] = −1.28 Z. Therefore, her adjusted BMDLS Z score is −1.87 Z − (−1.28 Z) = −0.59 Z.
Adjusted BMD Z score in short stature
Among 1,650 subjects, 39 (2.4 %) had short stature (height Z score < −2.0). Among 39 short children, 5–7 had low measured BMD that depended on the measured site (5 at BMDLS, 7 at BMDTBLH, 5 at BMDTB, and 5 at BMDFN). However, applying the predicted BMD Z score, only 1 subject (2.6 % of short stature) showed low adjusted BMDLS, BMDTBLH, and BMDTB (Z score < −2.0). Thus, only 14.3–20.0 % of short children who had low measured BMD Z scores truly had low BMD.
Discussion
This study provides sex- and age-specific reference values for BMD with body size adjustment equations in healthy Korean children and adolescents. Even in the same age group, children and adolescents have a different rate of maturation, which results in variations in height and weight. Some children and adolescents are mistakenly diagnosed with low BMD because of delayed maturation. These reference values might help clinicians to identify children who have impaired bone mineral accrual and who might fall into osteoporosis. To our knowledge, these are the first Asian ethnicity-specific reference values for BMD derived from a large, nationally representative, healthy sample.
Osteoporosis and its associated fractures are a significant cause of morbidity and mortality [1, 22]. Recently, the prevalence of osteoporosis in young adulthood has increased, especially in survivors of childhood chronic disease, such as cancer [5–9]. Furthermore, many of these fail to acquire optimal PBM as a consequence of the disease itself, malnutrition, or decreased physical activity during treatment [1, 5, 23]. Even healthy children with low BMD have a high risk of fracture, similar to adults with osteoporosis [11, 12]. Additionally, it is known that the majority of children in the lowest tertile of BMD at prepuberty remain in the same tertile in adolescence [24, 25]. Fujita et al. [25] reported that most boys and girls in the lowest quartile of BMD still showed the lowest mean BMD after a 6-year follow-up from prepuberty, and that sports activities increased BMD in Japanese boys. Thus, the ISCD recommends periodic DXA assessment in children and adolescents who have a disease or take medication that might affect the skeleton, such as osteogenesis imperfecta, inflammatory bowel disease, cerebral palsy, acute lymphocytic leukemia, or glucocorticoid therapy, to minimize their risk of developing osteoporosis and fragility fractures later in life [5]. To find and follow up with children and adolescents at high risk of low BMD or osteoporosis in childhood or adulthood, robust community-based, pediatric, reference data are vital, because differences in ethnicity or race with respect to BMD acquisition have been noted previously [26, 27].
Koreans achieve PBM at 19 and 21 years of age in females and males, respectively. Subsequently, BMD decreases by 0.49–1.29 %, depending on the ROI, annually [28]. We found that Korean children and adolescents had earlier BMD acquisition, compared with other ethnicities, using the same DXA device and similar software [13, 15, 16]. Korean children showed the highest increase in BMDLS between age groups 11 and 13 in girls and between age groups 12 and 14 in boys, similar to the results of our previous study [14]. BMDTBLH showed a relatively modest increase between age groups 10 and 15 in girls and between age groups 10 and 18 in boys. White and Hispanic children showed the highest increase in BMDLS a year later [15, 16]. Therefore, Koreans showed higher BMD until 15 years of age, but lower BMD after 15 years of age, compared with whites and Hispanics. One reasonable explanation is that Koreans might experience puberty earlier than other races. In our previous study, approximately 32.3 % of Korean girls and 33.2 % of boys had an advanced bone age of more than 1 year [14]. Differences in sexual maturation time have been reported in different races, with Asians said to undergo puberty earlier than other races [26, 27]. Korean females reach peak BMD by 19 years of age, whereas white females gain 5–12 % more BMD, depending on the ROI, during the third decade of life [29].
The ISCD recommends that BMD results of the spine and TBLH should be adjusted for body size, such as height age or height-specific Z scores [30]. Children who are short for their chronological age or who experience delayed puberty are particularly prone to being mistakenly diagnosed with low BMD or osteoporosis [31]. The timing of individual development of height, weight, and Tanner stage are different, even in the same ethnicity [32]. We previously found that approximately half of normal children and adolescents showed delayed or advanced bone age of more than 1 year, compared with chronological age [14]. In this study, subjects’ height and weight variations were high, as much as 37 cm and 48 kg, respectively, within the same age group, which also might be true for other community-based studies [13, 15, 16]. Height and weight are highly correlated to BMD, along with age, as previously mentioned [14, 17, 18]. To solve this problem, some investigators have suggested making an adjustment based on body weight and/or height [17–19]. Mølgaard et al. [18] proposed a three-stage approach using height-for-age, bone area-for-height, and bone mineral content (BMC)-for-bone area to correspond to short bones, narrow bones, and light bones. Zemel et al. [16, 19] proposed the height-for-age Z score (HAZ) adjustment method, and developed reference data for American children and adolescents based on HAZ adjustment equations. We first adopted a height adjustment method, but the R 2 of each ROI was low, especially in BMDLS. Furthermore, the BMD of each ROI was influenced more by weight than by height in the total subjects (and in the same age group). El Hage et al. [33] reported that weight is correlated more to the BMD of the weight-bearing bones (L2–L4 and femoral neck) than is height in Lebanese adolescent boys. They also showed that weight and BMI, but not height, are positively associated with the bone mineral apparent density of the weight-bearing bones. Thus, we employed Webber’s proposal of a height-, age-, and weight-adjusted (HAW) score, and determined a predicted BMD Z score, which had a higher R 2 in each ROI up to 0.56. Using these equations, only 2.6 % of children with short stature showed an adjusted BMDTBLH Z score ≤2.0, whereas 17.9 % had a low measured BMD Z score.
The R 2 value of the prediction equation varied from 0.00 to 0.56 depending on age group and ROI (measured site). The R 2 value was high in the 10- to 12-year-old age group in girls and the 11- to 13-year-old age group in boys. In these ages is the time of puberty. Timing of puberty varies in each individual. Some females enter puberty at the age of 10 years whereas others enter at the age of 12 years. Height and weight also showed a significant difference depending on pubertal stage. Thus, the explained variances (the R 2 values) of the prediction equation, composed of height Z score and weight Z score, in these age groups were high. However, when subjects had almost finished their growth in height or weight, the variance of height and weight is small. Thus, the R 2 value of the prediction equation after 17 years of age is low. The R 2 value of the prediction equation also differs depending on other variables including ROI. Zemel et al. [19] reported the R 2 value of their prediction models was greater for BMC than for BMD measures, greater for females than males, greater for non-black than black children, and greater for the spine than other regions such as hip and forearm.
We acknowledge some limitations in this study. The primary limitation is that we did not evaluate this adjustment method in children with chronic diseases that affect bone acquisition. However, Webber et al. [17] demonstrated the HAW score validity in a child with acute lymphoblastic leukemia. Future studies are needed to evaluate the validity of our adjustment method in identifying individual children at risk for fracture. The second limitation is that we could not create a reference curve applying the LMS approach because of the small sample size. The final limitation is that we could not acquire the Tanner stage of the subjects. Tanner stage is known to closely correlate with the BMD of each ROI.
In conclusion, this study provides sex- and age-specific, DXA-based, pediatric BMD reference values with body size adjustment equations in healthy Korean children and adolescents. Along with our previously reported pediatric DXA reference values, these robust reference values might help clinicians in assessing and monitoring bone health in Korean children and adolescents. Body size deviations exert a considerable impact on measured BMD, as shown in this study; therefore, height and weight adjustment, along with age adjustment, is important to prevent the misdiagnosis of low BMD or osteoporosis, especially in children with short stature, as recommended by the ISCD.
References
Cooper C, Westlake S, Harvey N, Javaid K, Dennison E, Hanson M (2006) Review: developmental origins of osteoporotic fracture. Osteoporos Int 17:337–347
Baim S, Leonard MB, Bianchi ML, Baim S, Leonard MB, Bianchi ML, Hans DB, Kalkwarf HJ, Langman CB, Rauch F (2008) Official Positions of the International Society for Clinical Densitometry and executive summary of the 2007 ISCD Pediatric Position Development Conference. J Clin Densitom 11:6–21
Mora S, Gilsanz V (2003) Establishment of peak bone mass. Endocrinol Metab Clin N Am 32:39–63
van der Sluis IM, van den Heuvel-Eibrink MM, Hählen K, Krenning EP, de Muinck Keizer-Schrama SM (2002) Altered bone mineral density and body composition, and increased fracture risk in childhood acute lymphoblastic leukemia. J Pediatr 141:204–210
Bishop N, Braillon P, Burnham J, Cimaz R, Davies J, Fewtrell M, Hogler W, Kennedy K, Mäkitie O, Mughal Z, Shaw N, Vogiatzi M, Ward K, Bianchi ML (2008) Dual-energy X-ray absorptiometry assessment in children and adolescents with diseases that may affect the skeleton: the 2007 ISCD Pediatric Official Positions. J Clin Densitom 11:29–42
Allen DB, Mullen M, Mullen B (1994) A meta-analysis of the effect of oral and inhaled corticosteroids on growth. J Allergy Clin Immunol 93:967–976
Sheth RD, Gidal BE, Hermann BP (2006) Pathological fractures in epilepsy. Epilepsy Behav 9:601–605
Kang MJ, Kim SM, Lee YA, Shin CH, Yang SW, Lim JS (2012) Risk factors for osteoporosis in long-term survivors of intracranial germ cell tumors. Osteoporos Int 23:1921–1929
Lim JS, Kim DH, Lee JA, Kim DH, Cho J, Cho WH, Lee SY, Jeon DG (2013) Young age at diagnosis, male sex, and decreased lean mass are risk factors of osteoporosis in long-term survivors of osteosarcoma. J Pediatr Hematol Oncol 35:54–60
Children’s Oncology Group (2007) Establishing and enhancing services for childhood cancer survivors: long-term follow-up program resource guide. http://www.survivorshipguidelines.org/. Accessed 15 Feb 2012
Ferrari SL, Chevalley T, Bonjour JP, Rizzoli R (2006) Childhood fractures are associated with decreased bone mass gain during puberty: an early marker of persistent bone fragility? J Bone Miner Res 21:501–507
Clark EM, Ness AR, Bishop NJ, Tobias JH (2006) Association between bone mass and fractures in children: a prospective cohort study. J Bone Miner Res 21:1489–1495
Lazcano-Ponce E, Tamayo J, Cruz-Valdez A, Díaz R, Hernández B, Del Cueto R, Hernández-Avila M (2003) Peak bone mineral area density and determinants among females aged 9 to 24 years in Mexico. Osteoporos Int 14:539–547
Lim JS, Hwang JS, Lee JA, Kim DH, Park KD, Cheon GJ, Shin CH, Yang SW (2010) Bone mineral density according to age, bone age, and pubertal stages in Korean children and adolescents. J Clin Densitom 13:68–76
Kelly TL, Wilson KE, Heymsfield SB (2009) Dual energy X-ray absorptiometry body composition reference values from NHANES. PLoS One 4:e7038
Zemel BS, Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, Frederick MM, Huang X, Lu M, Mahboubi S, Hangartner T, Winer KK (2011) Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab 96:3160–3169
Webber CE, Sala A, Barr RD (2009) Accounting for body size deviations when reporting bone mineral density variables in children. Osteoporos Int 20:113–121
Mølgaard C, Thomsen BL, Prentice A, Cole TJ, Michaelsen KF (1997) Whole body bone mineral content in healthy children and adolescents. Arch Dis Child 76:9–15
Zemel BS, Leonard MB, Kelly A, Lappe JM, Gilsanz V, Oberfield S, Mahboubi S, Shepherd JA, Hangartner TN, Frederick MM, Winer KK, Kalkwarf HJ (2010) Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab 95:1265–1273
Korea Centers for Disease Control and Prevention. The Korea National Health and Nutrition Examination Survey. http://knhanes.cdc.go.kr/knhanes/index.do Accessed 12 Jan 2012
Moon JS, Lee SY, Nam CM, Choi JM, Choe BK, Seo JW, Oh K, Jang MJ, Hwang SS, Yoo MH, Kim YT, Lee CG (2008) 2007 Korean National Growth Charts: review of developmental process and an outlook. Korean J Pediatr 51:1–25
Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17:1726–1733
NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285:785–795
Foley S, Quinn S, Jones G (2009) Tracking of bone mass from childhood to adolescence and factors that predict deviation from tracking. Bone (NY) 44:752–757
Fujita Y, Iki M, Ikeda Y, Morita A, Matsukura T, Nishino H, Yamagami T, Kagamimori S, Kagawa Y, Yoneshima H (2011) Tracking of appendicular bone mineral density for 6 years including the pubertal growth spurt: Japanese Population-based Osteoporosis kids cohort study. J Bone Miner Metab 29:208–216
Wu T, Mendola P, Buck GM (2002) Ethnic differences in the presence of secondary sex characteristics and menarche among US girls: the Third National Health and Nutrition Examination Survey, 1988–1994. Pediatrics 110:752–757
Bachrach LK, Hastie T, Wang MC, Narasimhan B, Marcus R (1999) Bone mineral acquisition in healthy Asian, Hispanic, black, and Caucasian youth: a longitudinal study. J Clin Endocrinol Metab 84:4702–4712
Lee EY, Kim D, Kim KM, Kim KJ, Choi HS, Rhee Y, Lim SK (2012) Age-related bone mineral density patterns in Koreans (KNHANES IV). J Clin Endocrinol Metab 97:3310–3318
Recker RR, Davies KM, Hinders SM, Heaney RP, Stegman MR, Kimmel DB (1992) Bone gain in young adult women. JAMA 4:2403–2408
Gordon CM, Bachrach LK, Carpenter TO, Crabtree N, El-Hajj Fuleihan G, Kutilek S, Lorenc RS, Tosi LL, Ward KA, Ward LM, Kalkwarf HJ (2008) Dual energy X-ray absorptiometry interpretation and reporting in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom 11:43–58
Gafni RI, Baron J (2004) Overdiagnosis of osteoporosis in children due to misinterpretation of dual-energy X-ray absorptiometry (DEXA). J Pediatr 144:253–257
Ranjitkar S, Lin NH, Macdonald R, Taylor JA, Townsend GC (2006) Stature and skeletal maturation of two cohorts of Australian children and young adults over the past two decades. Aust Orthod J 22:47–58
El Hage R, Moussa E, El Hage Z, Theunynck D, Jacob C (2011) Influence of age and morphological characteristics on whole body, lumbar spine, femoral neck and 1/3 radius bone mineral apparent density in a group of Lebanese adolescent boys. J Bone Miner Metab 29:477–483
Acknowledgments
We thank Sanghui Kweon from the Division of Health and Nutrition Survey for her technical support in analyzing the DXA results.
Conflict of interest
The authors declare that we have no commercial associations that might be considered to represent a conflict of interest in connection with this study.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Yi, K.H., Hwang, J.S., Kim, E.Y. et al. Reference values for bone mineral density according to age with body size adjustment in Korean children and adolescents. J Bone Miner Metab 32, 281–289 (2014). https://doi.org/10.1007/s00774-013-0488-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-013-0488-z