Abstract
Summary
We measured bone mineral densities in 28 intracranial germ cell tumor long-term survivors. There was the high prevalence of osteoporosis and osteopenia, 25.0% and 42.9%, respectively, and three additional risk factors, male sex, a low lean mass, and adult growth hormone replacement, were identified.
Introduction
Intracranial germ cell tumor long-term survivors (iGCTLS) have many risk factors for osteoporosis, including irradiation from cancer therapy and multiple hormone deficiencies. However, no study of bone mineral density (BMD) has been conducted in iGCTLS because these tumors are rare. The aims of this study were to evaluate the prevalence of osteoporosis and to identify risk factors associated with reduced bone mass in iGCTLS.
Methods
We evaluated BMD and body composition of 28 iGCTLS (10.9 ± 5.2 years after cancer treatment; 13 males) using dual-energy X-ray absorptiometry. To determine risk factors, we analyzed the medical history, including the nature of the tumor, treatment modality, endocrine status, hormone replacement therapy, lifestyle, and biochemical parameters.
Results
Twenty-five percent of iGCTLS were diagnosed with osteoporosis and 42.9% with osteopenia. Most males (92.3%) had low BMD. Lean mass (LM) was positively correlated with BMD in all regions of interest, and the starting age of adult growth hormone (GH) replacement was negatively correlated with the BMD Z-score at the femur neck. In logistic regression analysis, male sex and low LM were related to low BMD.
Conclusions
The iGCTLS had a high prevalence of low BMD. We found that male sex, low LM, and delayed start of adult GH replacement were risk factors for osteoporosis. Therefore, the BMD of all iGCTLS should be evaluated, and if it is low, proper management should be started early.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intracranial germ cell tumors (iGCTs) are rare neoplasms that occur mostly in the suprasellar and pineal regions [1–3]. Although germ cell tumors (GCTs) comprise a heterogeneous group that includes germinomas, embryonal carcinomas, yolk-sac tumors, choriocarcinomas, teratomas, and mixed germ cell tumors, most are radiosensitive, and standard therapy for iGCTs includes cranial or craniospinal irradiation [1–5]. The event-free survival rates of germinomas and other iGCTs were reported to be up to 100% and 80%, respectively, in recently published studies [1, 4, 5].
Osteoporosis was previously considered to be a disease of the elderly, but it is now universally agreed that osteoporosis has a pediatric origin [6]. Individuals who fail to achieve optimal peak bone mass (PBM) and strength during childhood and adolescence are more likely to develop osteoporosis later in life [7, 8]. In particular, childhood cancer survivors, especially brain tumor survivors, are known to have a high prevalence of osteoporosis or low bone mineral density (BMD) in early adulthood [9, 10]. Radiotherapy, chemotherapy, nutritional deficits, low physical activity levels, and hormone deficiencies are frequently cited as risk factors for osteoporosis and fracture in childhood cancer survivors [11].
The incidence of iGCTs peaks in the second decade of life [1–5], the period during which nearly 50% of maximal adult BMD increase occurs [1]. In addition, cranial irradiation is well known to decrease bone mass through direct and indirect effects [12]. One of the probable mechanisms is through growth hormone deficiency (GHD) [13]. Growth hormone (GH) and insulin-like growth factor-1 (IGF-1) greatly influence bone homeostasis and remodeling by stimulating the recruitment of osteoblast precursors and osteoclast bone resorption [13, 14].
However, there have been no studies of bone health and osteoporosis in intracranial germ cell tumor long-term survivors (iGCTLS), to our knowledge. Although GCTs are rare, they account for approximately 3–11% of all tumors arising within the central nervous system in the pediatric age group, and the survival rate is high with proper hormone replacement. The Children's Oncology Group Long-Term Follow-Up Guidelines recommend the evaluation of BMD for survivors who are predisposed to BMD deficits [15]. Therefore, the aims of this study were to evaluate the prevalence of osteoporosis or low BMD and to identify risk factors associated with reduced bone mass in iGCTLS.
Subjects and methods
Subjects
Between October 2010 and March 2011, iGCTLS were evaluated for BMD and body composition to assess the prevalence of osteoporosis/osteopenia and its risk factors. Twenty-eight subjects (13 males and 15 females) who met the following criteria were recruited: (1) diagnosed and treated for iGCT at Seoul National University Hospital; (2) no history of relapse for at least 5 years of follow-up; (3) proper replacement of deficient hormones during growth; and (4) completion of physical growth (epiphyses closed on the left hand and no growth for 3 years).
The clinical characteristics of the subjects are summarized in Table 1. Their mean age at evaluation was 23.1 ± 4.4 years. The mean interval from diagnosis to evaluation was 11.6 ± 5.0 years. The follow-up duration from the time of tumor treatment completion was 10.9 ± 5.2 years. Of the 28 subjects, 25 were diagnosed by histology (20 germinomas, 5 mixed germ cell tumors) and 3 by brain MRI and laboratory findings. At the time of diagnosis, the tumor was found in the suprasellar region in 18 subjects and in the pineal gland in 8 subjects. In the other 2 subjects, the tumor was located in the basal ganglia and frontal lobe region. At the time of diagnosis, 15 subjects were in the prepubertal period and 13 were in the pubertal period. Of the 27 subjects who had undergone radiotherapy, 19 had received both cranial and spinal radiation therapy. The mean cumulative dose of irradiation on the tumor was 50.7 ± 5.1 Gy (cranial 29.0 ± 8.8 Gy; spinal 21.0 ± 10.4 Gy). Of the 19 subjects who had undergone chemotherapy, 16 received “cisplatin/carboplatin + etoposide”-based chemotherapy and 3 received “8 drugs in 1 day” chemotherapy. There was no clinical difference between male and female subjects except in the hormone deficiencies. The hormone deficiencies were detected with regular surveillance and replaced properly by the pediatric endocrinologist, in accordance with the Korean National Health Insurance (KNHI) guidelines.
Methods
Anthropometric measurements were obtained for each subject. Height was measured twice to the first decimal place with a Harpenden stadiometer (Haltain Ltd., Crymmych, Wales, UK), and weight to the first decimal place with a digital scale (150 A; Cas Co. Ltd., Seoul, Korea). Body mass indices (BMI) were calculated by dividing weight by height squared (kilograms per square meter). All participants were assessed for cessation of growth using a growth chart and left-hand X-ray, and epiphyseal closure was confirmed by the pediatric endocrinologist. Corrected final adult height was adult height corrected for genetic potential [final adult height standard deviation score (SDS) minus mid-parental height SDS].
Dual-energy X-ray absorptiometry (DXA) was performed for evaluation of osteoporosis or osteopenia. The BMDs of the lumbar spines (L1–L4), femur neck, and total body were measured serially by Lunar Prodigy Advance DXA bone densitometry (Lunar Corporation, General Electric, Madison, WI, USA) with pediatric and adult software (ver. enCore 2005 9.15.010, GE Lunar Corporation, Madison, WI, USA). Total body DXA scans provided total body BMD and body composition details such as bone mineral content (BMC), lean mass (LM), and fat mass (FM) [16].
Osteoporosis was diagnosed when BMD at the spine, hip, or total body had a T-score < −2.5, and osteopenia was diagnosed when −2.5 ≤ T-score < −1.0. For subjects who were less than 20 years of age, Z-scores of <−2.0 for osteoporosis and −2.0 ≤ Z-score < −1.0 for osteopenia were used [17]. We defined low BMD as a Z-score < −1.0 in risk factor analysis. For body composition analysis, Z-scores from age- and sex-specific reference data from our previous report with the same DXA device were used [16].
The serum levels of calcium, phosphate, and alkaline phosphatase activity (ALP) were measured to check problems related to bone health. Sex hormones such as estradiol and testosterone, IGF-1, and thyroid function were also evaluated to check the current status of hormone replacement. The serum IGF-1 level was considered low when it was more than 2 standard deviations (SD) below the mean (Endocrinology Reference Laboratory; male 490 ± 120 ng/mL; female 460 ± 113 ng/mL). History related to bone health, such as previous fracture, bone pain, sun exposure time, and exercise frequency, was sought and reviewed using medical records.
Statistical analysis
Normally distributed data were presented as means ± SD, and median and ranges were used for skewed data. Student's t tests or chi-square tests were used to examine differences between the two groups, classified according to sex and low BMD. To adjust for sex differences, BMD, BMC, LM, and FM data were transformed to Z-scores using population reference data [16, 18]. Spearman's correlation coefficient was calculated to test the association. Univariate logistic regression analysis was used to determine the risk factors for decreased BMD. P values < 0.05 were considered significant. All statistical analyses were performed using SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Clinical characteristics
Ninety-six percent of subjects (n = 27) had received radiotherapy and 67.9% of subjects had received chemotherapy. Most of them had multiple hormone deficiencies. The prevalences of GH, gonadotrophic hormone, adrenocorticotropic hormone, thyroid hormone, and antidiuretic hormone (ADH) deficiency were 82.1%, 67.9%, 64.3%, 75.0%, and 67.9%, respectively. Female subjects had a higher rate of GH, gonadotrophic hormone, thyroid hormone, and ADH deficiency (all P < 0.05). Hormone replacements were carried out properly when detected with regular surveillance. Twenty of the GHD subjects started adult GH replacement at a mean age of 19.5 ± 3.9 years in accordance with the KNHI guidelines (0.042–0.083 mg/kg/week). The mean age of GH replacement for adult GHD was higher in male subjects than in female subjects (males 23.0 ± 4.9 years; females 18.0 ± 2.2 years; P = 0.057). The mean age of sex hormone replacement did not differ significantly between the sexes (males 17.5 ± 1.7 years; females 17.0 ± 2.5 years; P = 0.608). The serum levels of testosterone (for males), estradiol (for females), follicle-stimulating hormone, luteinizing hormone, thyroid-stimulating hormone, and free thyroxine were all within the normal range. A low serum IGF-1 level was found in 14 subjects, most of whom were female. Four subjects (all female) had an ALP level over 115 IU/L, the upper normal limit in our institute. Average sun exposure time of subjects was 3 h per day and exercise frequency was 2 days per week (Table 1).
BMD results for each region of interest in iGCTLS
The BMD results for the lumbar spine, femur neck, and total body for all subjects are summarized in Table 2. The mean BMD Z-scores were −1.1 ± 1.3, −0.4 ± 1.4, and −0.8 ± 1.6 (T-scores of age > 20 years were −0.9 ± 1.2, 0.0 ± 1.3, and −0.5 ± 1.6) at the spine, femur neck, and total body, respectively. Male subjects had lower BMD Z-scores than female subjects at the lumbar spine (males −1.8 ± 0.9; females −0.5 ± 1.2; P = 0.003) and femur neck (males −0.9 ± 1.4; females 0.1 ± 1.3; P = 0.079).
Prevalence of osteoporosis and fractures in iGCTLS
Seven (25.0%) subjects were diagnosed with osteoporosis and 12 (42.9%) were diagnosed with osteopenia in all regions of interest (ROI) (Fig. 1). Six (21.4%) had total body osteoporosis, 6 (21.4%) had osteoporosis in the lumbar spine, and 2 (7.1%) had osteoporosis in the femur neck. Four subjects (14.3%) had fracture history, all of whom showed low BMD (spine BMD Z-scores of the four subjects −2.0, −2.4, −1.2, and −1.5).
The effects of body composition on low BMD
The body composition measures of BMC, LM, and FM are also summarized in Table 2. The mean Z-scores of FM, LM, and BMC were 2.5 ± 2.1, −1.9 ± 2.2, and −1.0 ± 2.1, respectively. The FM was increased while the LM and BMC were decreased in iGCTLS. The LM Z-scores were correlated with the BMD of the lumbar spine (r = 0.584, P < 0.001), femur neck (r = 0.568, P = 0.002), and total body (r = 0.569, P = 0.002) in the total subject group. In female subjects, both LM and FM Z-scores were correlated with the BMD of the femur neck (LM, r = 0.700, P = 0.004; FM, r = 0.811, P < 0.001) and total body (LM, r = 0.677, P = 0.006; FM, r = 0.799, P < 0.001). Only the FM Z-score was correlated with the lumbar spine BMD Z-score (r = 0.765, P = 0.001) in female subjects (Fig. 2). Males showed no correlation between body composition and BMD of each ROI. However, males showed greater increase in the FM Z-score (males 3.3 ± 2.2; females 1.8 ± 1.9) and decrease in the LM Z-score (males −3.3 ± 1.0; females −0.7 ± 2.3) and BMC Z-score (males −1.9 ± 1.4; females −0.3 ± 2.3) than females (Table 2).
Risk factors for low BMD: clinicopathological variables related to BMD
The clinical and laboratory values, including body composition, for low-BMD subjects (Z-score < −1.0) and normal-BMD subjects are compared in Table 3.
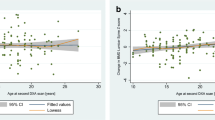
The corrected final adult height SDS was positively correlated with the BMD Z-score at the femur neck (r = 0.481, P = 0.010) and total body (r = 0.517, P = 0.005). The BMI SDS was also positively correlated with the BMD Z-score at the spine (r = 0.427, P = 0.024) and total body (r = 0.552, P = 0.002). The starting age of adult GH replacement was negatively correlated with the BMD Z-score at the femur neck (r = −0.484, P = 0.031) (Fig. 3). Tumor location and histology, treatment modalities such as surgery, radiotherapy, and chemotherapy, hormone-deficient states such as GHD, low IGF-1, antiepileptic medications, sun exposure time, and exercise frequency were not related to low BMD.
In univariate logistic regression analysis, male sex (OR = 13.7, P = 0.024), low BMI (OR = 6.0, P = 0.055), and low LM (OR = 6.0, P = 0.055) were related to low BMD. The starting age of adult GH replacement showed marginal significance (P = 0.083).
Discussion
To our knowledge, the present study is the first to determine the BMDs of each ROI including the lumbar spine, femur neck, and total body in iGCTLS. In this study, we identified a high prevalence of osteoporosis and osteopenia in young adulthood of iGCTLS. We also identified three additional risk factors for osteoporosis in iGCTLS with proper management: male sex, low LM, and adult GH replacement.
In this study, 25.0% of the iGCTLS had osteoporosis, and 42.9% had osteopenia in all ROI. Previous studies have reported a high prevalence of BMD deficits in survivors of childhood malignancy, including brain tumors [10, 13, 19–21]. Both the tumor itself and its treatment potentially encompass risk factors for BMD deficits such as high cumulative doses of steroids, methotrexate, cranial radiation, and testicular radiation. In brain tumor survivors, radiation is the most important risk factor for osteoporosis [13, 20, 21]. Radiation might directly affect BMD by damaging the bone marrow stroma [22]. Radiation-induced fractures have been described in survivors treated with radiation doses of ≥40 Gy [23, 24]. Radiation to the neuroendocrine axis after cranial and total body irradiation fields can lead to GHD and central hypogonadism, both of which are associated with deficits in BMD [15, 25]. In our subjects, the cumulative dose of irradiation on the tumor was 50.7 ± 5.1 Gy (cranial 29.0 ± 8.8 Gy; spinal 21.0 ± 10.4 Gy). Eighty-two percent of subjects had GHD and 75% had hypogonadism, which is consistent with previous studies [10, 26, 27].
In the present study, male sex was also found to be a significant predictor of future osteoporosis in iGCTLS. The prevalence of osteoporosis and osteopenia was higher in males than females. Males were found to have lower BMD Z-scores in the lumbar spine and in the femur neck than females. In adults, females achieve a lower PBM than males and experience more rapid bone loss, as evidenced by a higher incidence of osteoporosis. However, males are disproportionately affected in secondary osteoporosis related to cancer, especially childhood cancer [19, 28]. The precise reason for this is not known. One possible explanation is that peak BM acquisition in males might be inadequate compared with female iGCTLS. In our study subjects, mean age at diagnosis was 11.6 years in males and 11.3 years in females. At that age, the BMDs of the lumbar spine were 81.5% of the adult level for females but only 67.4% of the adult level for males in our previous study [18]. Because of cancer itself and its treatment, childhood cancer survivors suffer nutritional deficits, inadequate physical activity, and hormonal deficiencies [11]. The duration of LM and bone acquisition is longer for males than females [7, 8, 16, 29]. The other explanation is that the effect of FM on BMD differs between males and females: FM has a protective effect on BMD in females, but not in males [28]. In a previous study, we found that FM was closely related to BMD in females only [16]. Garnett et al. [30] reported that FM is mediated by fat-related hormones, such as leptin and estrogens, which are also involved in critical bone metabolism and maturation pathways.
In the present study, decreased LM was also found to be a significant predictor of osteoporosis in iGCTLS. Low-BMD subjects showed a low LM Z-score of −2.7 ± 1.4, while normal-BMD subjects showed an LM Z-score of −0.2 ± 2.6. LM was found to be closely related to total body BMD in our iGCTLS, which concurs with a previous report [31]. Koskelo et al. [32] reported alterations in LM and, in particular, wasting of skeletal muscle in pediatric cancer subjects. The reason for decreased LM might be lower LM acquisition during growth. Not only BM but also LM accumulates during childhood and puberty along with PBM [16, 29, 31]. In our previous study, in females, LM increased steadily until the age of 13 years and thereafter increased only slightly. However, in males, LM increased abruptly after the age of 12 years until 17 years of age, when peak LM was attained [16].
Interesting findings of this study are that the starting age of adult GH replacement was lower in the normal-BMD group than in the low-BMD group, and that the BMD Z-score in the femur neck was negatively correlated with the timing of adult GH replacement age. It is well known that GHD is related to low BMD in both adults and children [14, 33, 34]. Baroncelli et al. [35] reported that lumbar BMD declined rapidly after discontinuation of GH in GHD compared with controls. Replacement of GH improves both LM and BMD [14]. GH directly causes bone formation and resorption, which results in a net accumulation of bone mass [14]. In this study, those who had adult GH replacement at an older age showed decreased BMD. Thus, early replacement of adult GH is important for the prevention not only of cardiovascular disease but also of osteoporosis.
The results of the current study should be interpreted in the light of potential study limitations. The first limitation is the small size of the study population. However, considering the rarity of iGCTs, it was enough to show tendency of bone deficit in iGCTs. Further study with a larger population, such as a multicenter study, is needed. The second limitation is that we did not consider correction of height in the BMD analysis. In subjects who are very short, measurements may not be reliable [36]. Webber et al. [37] also suggested to make an adjustment base on body weight or height, which exerted a considerable impact on measured BMD especially in children. However, only 2 (7%) of the iGCTLS in this study were short according to Korean standards [38]. The last limitation is that eight of our subjects were less than 20 years old. However, we confirmed that all patient bone ages were above 18 years, the age at which the BMDs of each ROI are compatible with those of 20–30-year-old Koreans [18].
In conclusion, we found that 67.9% of the iGCTLS had a BMD less than −1 SD, which is a higher percentage than expected, based on the World Health Organization's report [39] and survivors of other types of brain tumor [9, 10, 13, 20, 21]. The main reason might be radiation therapy and the high prevalence of GHD. We also found that other risk factors for low BMD in iGCTLS were male sex, low LM, and, possibly, timing of adult GH replacement. Therefore, initial evaluation and regular follow-up of bone health should be performed in all iGCTLS just after the discontinuation of the tumor therapy schedule, especially in males and those with low LM. Further longitudinal studies will need to be conducted to confirm these findings.
References
Kaltsas T, Pontikides N, Tsiotsia E et al (2004) Consequences of an intracranial germ cell-tumor in a young adolescent. Pediatr Endocrinol Rev 1:501–504
Packer RJ, Cohen BH, Cooney K (2000) Intracranial germ cell tumors. Oncologist 5:312–320
Ho DM, Liu HC (1992) Primary intracranial germ cell tumor. Pathologic study of 51 patients. Cancer 70:1577–1584
Bamberg M, Kortmann RD, Calaminus G et al (1999) Radiation therapy for intracranial germinoma: results of the German cooperative prospective trials MAKEI 83/86/89. J Clin Oncol 17:2585–2592
Buckner JC, Peethambaram PP, Smithson WA et al (1999) Phase II trial of primary chemotherapy followed by reduced-dose radiation for CNS germ cell tumors. J Clin Oncol 17:933–940
Cooper C, Westlake S, Harvey N, Javaid K, Dennison E, Hanson M (2006) Review: developmental origins of osteoporotic fracture. Osteoporos Int 17:337–347
Mora S, Gilsanz V (2003) Establishment of peak bone mass. Endocrinol Metab Clin North Am 32:39–63
Heaney RP, Abrams S, Dawson-Hughes B et al (2000) Peak bone mass. Osteoporos Int 11:985–1009
Gurney JG, Kadan-Lottick NS, Packer RJ et al (2003) Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors: Childhood Cancer Survivor Study. Cancer 97:663–673
Barr RD, Simpson T, Webber CE et al (1998) Osteopenia in children surviving brain tumours. Eur J Cancer 34:873–877
Sala A, Pencharz P, Barr RD (2004) Children, cancer, and nutrition—–a dynamic triangle in review. Cancer 100:677–687
Gilsanz V, Carlson ME, Roe TF, Ortega JA (1990) Osteoporosis after cranial irradiation for acute lymphoblastic leukemia. J Pediatr 117:238–244
Petraroli M, D'Alessio E, Ausili E (2007) Bone mineral density in survivors of childhood brain tumours. Child Nerv Syst 23:59–65
Carroll PV, Christ ER, Bengtsson BA et al (1998) Growth hormone deficiency in adulthood and the effects of growth hormone replacement: a review. Growth Hormone Research Society Scientific Committee. J Clin Endocrinol Metab 83:382–395
Children's Oncology Group (2007) Establishing and enhancing services for childhood cancer survivors: long-term follow-up program resource guide. Available at http://www.survivorshipguidelines.org/
Lim JS, Hwang JS, Cheon GJ et al (2009) Gender differences in total and regional body composition changes as measured by dual-energy X-ray absorptiometry in Korean children and adolescents. J Clin Densitom 12:229–237
Lewiecki EM, Gordon CM, Baim S et al (2008) International Society for Clinical Densitometry 2007 adult and pediatric official positions. Bone 43:1115–1121
Lim JS, Hwang JS, Lee JA et al (2010) Bone mineral density according to age, bone age, and pubertal stages in Korean children and adolescents. J Clin Densitom 13:68–76
Tillmann V, Darlington AS, Eiser C, Bishop NJ, Davies HA (2002) Male sex and low physical activity are associated with reduced spine bone mineral density in survivors of childhood acute lymphoblastic leukemia. J Bone Miner Res 17:1073–1080
Pietila S, Sievanen H, Ala-Houhala M, Koivisto AM, Liisa Lenko H, Makipernaa A (2006) Bone mineral density is reduced in brain tumour patients treated in childhood. Acta Paediatr 95:1291–1297
Odame I, Duckworth J, Talsma D et al (2006) Osteopenia, physical activity and health-related quality of life in survivors of brain tumors treated in childhood. Pediatr Blood Cancer 46:357–362
Banfi A, Bianchi G, Galotto M, Cancedda R, Quarto R (2001) Bone marrow stromal damage after chemo/radiotherapy: occurrence, consequences and possibilities of treatment. Leuk Lymphoma 42:863–870
Wagner LM, Neel MD, Pappo AS et al (2001) Fractures in pediatric Ewing sarcoma. J Pediatr Hematol Oncol 23:568–571
Paulino AC (2004) Late effects of radiotherapy for pediatric extremity sarcomas. Int J Radiat Oncol Biol Phys 60:265–274
Meacham L (2003) Endocrine late effects of childhood cancer therapy. Curr Probl Pediatr Adolesc Health Care 33:217–242
Lackner H, Benesch M, Schagerl S, Kerbl R, Schwinger W, Urban C (2000) Prospective evaluation of late effects after childhood cancer therapy with a follow-up over 9 years. Eur J Pediatr 159:750–758
Benesch M, Lackner H, Schagerl S, Gallistl S, Frey EM, Urban C (2001) Tumor- and treatment-related side effects after multimodal therapy of childhood intracranial germ cell tumors. Acta Paediatr 90:264–270
Muller HL, Schneider P, Bueb K et al (2003) Volumetric bone mineral density in patients with childhood craniopharyngioma. Exp Clin Endocrinol Diabetes 111:168–173
Loomba-Albrecht LA, Styne DM (2009) Effect of puberty on body composition. Curr Opin Endocrinol Diabetes Obes 16:10–15
Garnett SP, Hogler W, Blades B et al (2004) Relation between hormones and body composition, including bone, in prepubertal children. Am J Clin Nutr 80:966–972
Muszynska-Roslan K, Konstantynowicz J, Krawczuk-Rybak M, Protas P (2007) Body composition and bone mass in survivors of childhood cancer. Pediatr Blood Cancer 48:200–204
Koskelo EK, Saarinen UM, Siimes MA (1990) Skeletal muscle wasting and protein-energy malnutrition in children with a newly diagnosed acute leukemia. Cancer 66:373–376
Saggese G, Baroncelli GI, Bertelloni S, Cinquanta L, Di Nero G (1993) Effects of long-term treatment with growth hormone on bone and mineral metabolism in children with growth hormone deficiency. J Pediatr 122:37–45
van der Sluis IM, Boot AM, Hop WC, De Rijke YB, Krenning EP, de Muinck Keizer-Schrama SM (2002) Long-term effects of growth hormone therapy on bone mineral density, body composition, and serum lipid levels in growth hormone deficient children: a 6-year follow-up study. Horm Res 58:207–214
Baroncelli GI, Bertelloni S, Sodini F, Saggese G (2004) Longitudinal changes of lumbar bone mineral density (BMD) in patients with GH deficiency after discontinuation of treatment at final height; timing and peak values for lumbar BMD. Clin Endocrinol Oxf 60:175–184
Leonard MB, Propert KJ, Zemel BS, Stallings VA, Feldman HI (1999) Discrepancies in pediatric bone mineral density reference data: potential for misdiagnosis of osteopenia. J Pediatr 135:182–188
Webber CE, Sala A, Barr RD (2009) Accounting for body size deviations when reporting bone mineral density variables in children. Osteoporos Int 20:113–121
Korea Centers for Disease Control and Prevention (2007) 2007 Korean national growth charts. Available at http://www.cdc.go.kr
Kanis JA, Johnell O, Oden A, Jonsson B, De Laet C, Dawson A (2000) Risk of hip fracture according to the World Health Organization criteria for osteopenia and osteoporosis. Bone 27:585–590
Acknowledgments
The authors wish to express their gratitude to all the volunteers. This study was supported by the Pediatric Research Fund of Seoul National University Hospital (grant no. 50240-2010). We also thank the 21-year-old female iGCT patient who inspired us to conduct this study after longitudinal follow-up of BMD for 6 years.
Conflicts of interest
The authors declare that they have no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements) that might be considered to represent a conflict of interest in connection with this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, M.J., Kim, S.M., Lee, Y.A. et al. Risk factors for osteoporosis in long-term survivors of intracranial germ cell tumors. Osteoporos Int 23, 1921–1929 (2012). https://doi.org/10.1007/s00198-011-1821-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-011-1821-9