Abstract
Vitamin D deficiency is associated with an increased risk of many diseases (skeletal and nonskeletal). Emerging data also associate high concentrations of serum parathyroid hormone (PTH) with morbidity and increased mortality in patients both with and without known chronic kidney disease (CKD). Understanding the relationship between vitamin D and PTH and the determinants of PTH is therefore important. We performed a cross-sectional study of 203 patients with varying stages of CKD randomly recruited from the Renal Unit database at our institution. Detailed case review was performed, and samples of fasting blood were taken for biochemical analyses. We measured standard biochemistry, 25-hydroxyvitamin D (25-OHD), 1,25-OHD, and three PTH measurements [1–84 PTH, total PTH, and derived N-terminal truncated, 7–84 PTH (cPTH)]. Vitamin D deficiency was high, with 86% of patients having 25-OHD levels below 30 ng/ml. Estimated glomerular filtration rate (eGFR) was not associated with 25-OHD levels, whereas 1,25-OHD was lower in those with CKD stage 5 versus stage 4, who were not treated with vitamin D metabolites (18 vs. 65 pg/ml, respectively; P < 0.05). All three PTH measurements increased with worsening eGFR, with this finding being more pronounced in those patients who were not treated with vitamin D metabolites. The slope of the regression line of cPTH on eGFR tended to be steeper, –0.90, compared to –0.81 for total PTH and –0.80 for 1–84 PTH (P = 0.06). The ratio of total PTH to cPTH did decrease significantly through the range of CKD stages (P = 0.03). The determinants of PTH were similar for all three PTH measurements, with eGFR having a strong inverse relationship, with weaker relationships for 25-OHD and ionized calcium on multivariate analyses. We confirm that there is a complex relationship between 25-OHD, eGFR, and PTH. Total PTH, 1–84 PTH, and cPTH increase with increasing CKD stages, with a relatively greater increase in cPTH, although the clinical significance of this finding remains uncertain. The three PTH measurements had similar correlations with the biochemical and clinical variables studied, suggesting that either total PTH or 1–84 PTH can be used in clinical practice when evaluating vitamin D and PTH status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin D deficiency, in association with secondary increases in parathyroid hormone (PTH), is common in the population, and is present to an even greater extent in patients with chronic kidney disease (CKD). Although the importance of vitamin D and PTH to bone health is well recognized, recent epidemiological studies show that vitamin D deficiency is also associated with increased risk and severity of many nonskeletal diseases such as cancer, autoimmune, and cardiovascular diseases [1–3]. What is not commonly appreciated is that increased PTH is also associated with morbidity and mortality that are independent of bone disease. Thus, increased PTH has been shown to be associated with increased morbidity and mortality independent of age and known comorbidities in individuals both without [4, 5] and with [6–8] CKD.
Understanding the relationship between vitamin D and PTH and the determinants of PTH concentration is therefore important. In a previous study [9], we have shown that the PTH response for a given 25-OHD level is variable and dependent on many factors, of which glomerular filtration rate (GFR) was the strongest. These patients were studied retrospectively and included all patients who had 25-OHD level measured over a period of 12 months at our hospital. The nature of the study design meant that there were relatively few clinical details available for these patients. Additionally, we used an intact PTH assay (PTH 1–84) in our previous study and therefore could not be certain as to whether the relationships with PTH were to some extent influenced by the measurement of fragments that cross-react with the intact PTH assay (PTH 7–84). In the present study, we recruited a well-characterized group of patients with a wide range of renal function to (1) evaluate measurements of mineral metabolism across the range of stages of chronic kidney disease (CKD), (2) compare the performance of three PTH measurements, and (3) determine whether our previous findings of the determinants of PTH can be reproduced in a better defined and well-studied population.
Materials and methods
Following approval from the local ethics committee, we recruited patients registered with the South West Thames Renal Unit based at our institution. At the start of the study (January 2007), there were 1,740 registered patients who lived within 25 miles, aged between 18 and 80 years, who had CKD but had not received a transplant and were not treated with bisphosphonate, strontium ranelate, estrogens, selective estrogen modulators, cinacalcet, or paricalcitol. They did not have other diseases known to affect bone metabolism such myeloma or primary hyperparathyroidism. Using a random number generator, over the course of the study, 1,000 patients were invited by letter and then by telephone to participate in this study, resulting in 203 patients who were recruited with complete data.
Participating patients attended our Clinical Trials Unit where a clinical trials trained nurse obtained informed consent for the study, then took a detailed history, performed height and weight measurements, and took a fasting blood sample that was immediately processed in the laboratory (described below). Patients also filled in a questionnaire to establish dietary calcium intake [10].
Biochemical measurements
Fasting blood samples, taken between 0800 and 1100, were obtained for all patients and were pre-dialysis for those on hemodialysis. Samples were analyzed for biochemistry including serum sodium, potassium, urea, total calcium, albumin (Alb), magnesium (Mg), phosphate (PO4), total protein, and total alkaline phosphatase (total ALP), measured by standard kits on an Advia 2400 analyzer (Siemans Healthcare, Frimley, UK). Albumin-adjusted calcium was obtained using the formula ACa = TCa + [(40 – Alb) × 0.02]. Using heparinized blood, ionized calcium (iCa) was measured by ISE (ion selective electrode) on an ABL 800 analyser (Radiometer, Copenhagen, Denmark). Serum creatinine was determined by the O’Leary modified Jaffe method (Sinemed Europe, Burgess Hill, UK). Estimated glomerular filtration rate (eGFR) was calculated using the method-adjusted MDRD formula [eGFR = 186 × {[(serum creatinine/0.85)] × 0.011312}–1.154 × [age]–0.203] [11]. Patients were classified according to K/DOQI guidelines [12]. Samples were separated into aliquots and frozen at –80ºC for subsequent batched assays. Ethylenediaminetetraacetic acid (EDTA) plasma was used to measure 1–84 PTH (which only measures the 1–84 fragment) and total PTH (which measures both 1–84 PTH and 7–84 PTH) by immunoassay (Scantibodies, Santee, CA, USA). N-terminal truncated 7–84 PTH (cPTH) levels were calculated by subtracting 1–84 PTH from total PTH. Serum was used to measure 25-OHD by HPLC-MSMS (Liverpool University); 25-OHD2 and 25-OHD3 and deuterated internal standard were extracted from serum samples, following protein precipitation, using Isolute C18 solid-phase extraction cartridges. Potential interfering compounds were removed by initial elution with 50% methanol followed by elution of the vitamins using 10% tetrahydrofuran in acetonitrile. Dried extracts were reconstituted before injection into a HPLC tandem mass spectrometer in the multiple reaction mode (MRM). The MRM transitions (m/z) used were 413.2 > 395.3, 401.1 > 383.3, and 407.5 > 107.2 for 25-OHD2, 25-OHD3, and hexa deuterated (OH) vitamin D3, respectively. Coefficients of variations (CVs) for the assay were <10% across a working range of 0.1–250 ng/ml for both D2 and D3. 1,25-OHD was measured as previously described [13].
Statistical analysis
Clinical data are presented as mean (SD) unless data were skewed, where median (IQR) is given. We used the Kruskal–Wallis test to determine whether there were significant differences between multiple CKD groups. If this was significant, the Mann–Whitney U test was used to determine when differences were significant between groups. Thus, the Mann–Whitney U test was used to compare CKD stages in pairs (CKD2 to CKD1; CKD3 to CKD2; CKD4 to CKD3; CKD5 to CKD4; and CDK5 to CKD5D). The Bonferroni correction was used to maintain the type 1 error rate to <0.05 within each set of comparisons. The slope of the linear regression lines for comparison of the relationship between the PTH measurements and eGFR was assessed for statistical difference using Prism for Windows 4.0 (GraphPad Software, La Jolla, CA, USA). All other statistical analyses were performed using SPSS version 15.0 for Windows (SPSS, Chicago, IL, USA). All tests of significance were two tailed and considered significant at the 5% level.
Results
We recruited 203 patients, 130 men and 73 women; their characteristics are shown in Tables 1 and 2.
Demographics, comorbidities, physical activity, dietary calcium intake, and medication use
Patient mean age was 64 years (SD, 13; range, 23–80). Their ethnicity was Caucasian, 179; African, 5; Oriental, 2; Asian, 12; other, 5. With respect to comorbidities, 41 (20%) patients had a diagnosis of diabetes mellitus, 36 (18%) patients had known coronary artery disease, 16 (8%) patients had stroke, and 49 (24%) patients had peripheral vascular disease. A history of low trauma fracture was given by 29 (14%) patients.
With respect to physical activity (see Table 1), the mean number of hours that patients reported as being on their feet per day was 5.7 (SD, 3.2; range, 0–16). With deteriorating CKD, patients spent fewer hours on their feet, although this improved when on dialysis; 3 (2%) patients left their home less than twice per week, 14 (7%) patients left their home 2–3 times per week, 97 (48%) patients left their home 4–6 times per week, and 88 (43%) patients left their home daily. Dietary calcium intake was significantly lower in the CKD 5 dialysis group compared to the CKD 5 nondialysis group. With respect to drug therapy, 30 (15%) patients were taking calcium supplements, 43 (21%) were taking 1-α-calcidol, and 144 (71%) patients were being treated with neither; 13 (6%) patients were being treated with glucocorticoids. As would be expected, more patients with CKD stage 5 and dialysis were being treated with calcium supplements and 1-α-calcidol compared to other stages.
Calcium, phosphate, and magnesium
For all 203 patients, ionized Ca and adjusted Ca remained stable across the CKD stages (see Table 2); however, serum PO4 and Ca × PO4 product increased with lower GFR (especially CKD4 and CKD5). Similar changes were seen in those 144 patients not taking calcium supplements or 1-α-calcidol (Table 3). No significant differences were found between CKD stages 5 and 5D.
Vitamin D metabolites
The prevalence of vitamin D deficiency was high, with 17 of 203 (86%) patients having a total 25-OHD level of <30 ng/ml, 126 of 203 (62%) <20 ng/ml, and 26 of 203 (13%) <10 ng/ml.
Renal function did not influence 25-OHD, with levels remaining stable through the range of CKD stages (other than in CKD 3) for all 203 patients (Table 2) and the 144 patients not taking calcium supplements or 1-α-calcidol (Table 3). As would be expected, 1,25-OHD was significantly lower in CKD 5 and 5D only for patients not taking 1-α-calcidol (Table 3), with a nonsignificant reduction in CKD 5D for all 203 patients. We looked for a “substrate effect” of 25-OHD on 1,25-OHD and found no association of 25-OHD with 1,25-OHD at r = –0.01 (P = 0.89) for all 203 patients and r = 0.06 (P = 0.50) for the 144 patients not taking calcium or 1-α-calcidol). This lack of association persisted even when a subset of patients with low 25-OHD (<20 ng/ml) was examined.
Parathyroid hormone measurements
Changes in PTH (total PTH, 1–84 PTH ,and cPTH) with CKD stage were examined in three ways.
We determined the median PTH for each CKD group. The three PTH measurements showed significant and similar increases in concentrations with higher CKD stages (CKD 3–5D) for all 203 patients (see Table 2), with greater increases seen in those patients not taking calcium supplements or 1-α-calcidol (see Table 3), although this did not reach significance in view of the small numbers of subjects.
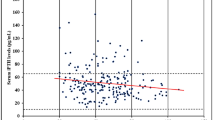
Second, we then examined the slope of the regression of PTH on eGFR data for all 203 patients (after log transformation) (Fig. 1). For all 203 patients, r 2 was 0.54 for total PTH (P < 0.0001), 0.56 for 1–84 PTH (P < 0.0001), and 0.56 for cPTH (P < 0.0001). The slope was –0.81 [95% confidence interval (CI), –0.91 to –0.70] for total PTH, –0.80 (95% CI, –0.87 to –0.68) for 1–84 PTH, and –0.90 (95% CI, –1.01 to –0.80) for cPTH. The slope for cPTH tended to be steeper compared to 1–84 PTH, but this difference was not statistically significant (P = 0.06). Similarly, for the 144 patients who were not taking calcium and 1-α-calcidol, r 2 was 0.52 for total PTH (P < 0.0001), 0.51 for 1–84 PTH (P < 0.0001), and 0.49 for cPTH (P < 0.0001). The slope was –0.83 (95% CI, –0.96 to –0.70) for total PTH, –0.89 (95% CI, –1.03 to –0.75) for 1–84 PTH, and –1.00 (95% CI, –1.16 to –0.83) for cPTH. Again, the slopes were not statistically different between 1–84 PTH and cPTH (P = 0.26).
Third, we determined the ratio of 1–84 PTH to cPTH. The ratio decreased significantly with increasing CKD stages (from CKD stage 3) for both patient groups (see Tables 2, 3) suggesting that there were relatively greater increases in cPTH compared to 1–84 PTH with increasing CKD stages.
Determinants of PTH
Correlations for the potential determinants of PTH are shown for all three PTH measurements in Table 4 for all 203 patients. eGFR had the largest correlation coefficient with PTH (showing a strong inverse relationship), with weaker inverse relationships for calcium intake, ionized calcium, total 25-OHD, 1,25-OHD, and albumin. A strong positive relationship was seen with serum phosphate, which was strongly inversely related to eGFR (r = –0.55; P < 0.0001) and a weaker relationship with serum magnesium.
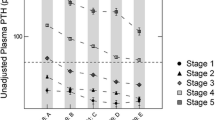
Multiple stepwise linear regression analysis with PTH as a dependent variable was performed for each of the PTH measurements, with eGFR being the strongest determinant, with 25-OHD and ionized calcium being weaker determinants (Table 5). The relationship between PTH, eGFR, and 25-OHD is shown in Fig. 2 for all nondialysis patients. PTH was significantly higher for any tertile of 25-OHD with higher CKD stages. The effect of eGFR on PTH was most pronounced with lower 25-OHD (particularly in CKD stages 3, 4, and 5, where PTH was higher in tertile 1 compared to tertile 3 of 25-OHD). This finding was seen for all three PTH measurements.
Discussion
Vitamin D deficiency with secondary hyperparathyroidism is common. Although it is recognised that vitamin D deficiency is associated with an increased risk of many diseases (skeletal and nonskeletal), there are also emerging data that associate high concentrations of serum PTH with morbidity and increased mortality in patients both with and without known CKD [4–7]. Understanding the relationship between vitamin D and PTH and the determinants of the PTH response for a given level of vitamin D is therefore important. In the present study, we have extended our previous work [9], by studying total PTH, 1–84 PTH, and cPTH in a group of well-defined patients with a broad range of GFR. We also examined changes in mineral metabolism with differing stages of CKD in a UK-based population as there are differences in social, ethnic, nutritional, and health system characteristics among countries.
Changes in bone chemistry and vitamin D with CKD stages
We found that ionized calcium (iCa) was preserved within a tight range, which is expected given the essential role of calcium in homeostasis, and that serum PO4 (and calcium phosphate product) as well as serum Mg increased with deteriorating renal function. As expected, 25-OHD deficiency was common. 25-OHD was lower in patients with CKD 4, 5, and 5D, which has been noted in some [14] but not all previous studies [15, 16]. This variation in findings is likely to be related to differences in the populations studied (such as skin type, latitude, sunlight exposure, diet, and comorbidities). The potential clinical relevance of these differences is that it cannot be assumed that deteriorating CKD will always be associated with reduced 25-OHD; therefore, patients should be individually assessed. In contrast to 25-OHD, 1,25-OHD concentrations are consistently reduced with reduced GFR in the current and in previous studies [14–16], which is likely to be caused by reduction in the activity of the 1-α-converting enzyme with reduced kidney function. We investigated whether substrate deficiency of 25-OHD would result in low 1,25-OHD levels and found no association between 25-OHD and 1,25-OHD even in a subset of patients with low 25-OHD (<20 ng/ml). There are conflicting findings in patients with known CKD, with some studies showing no significant association between 25-OHD and 1,25-OHD [14] or positive associations [15, 17]. Similarly, previous studies of patients without CKD have showed variable associations [18]. This variance is likely to be caused by different population characteristics. Thus, 25-OHD levels will be affected by factors that are not kidney specific, such as skin type, latitude, and diet, whereas 1,25-OHD will be influenced by GFR and PTH (which stimulates the 1-α-converting enzyme) and, in some populations, possibly circulating 25-OHD, which is the substrate for conversion to 1,25-OHD.
PTH and GFR
In this study we have examined and described changes in total PTH, 1–84 PTH, and derived cPTH. All three PTH measurements increased with higher CKD stages from CKD stage 3, with, as expected, greater fold increases in patients who were not treated with calcium and 1-α-calcidol (Table 3).
We compared the changes with eGFR between the PTH measurements in two ways. First, we examined the slope of the regression equations of the three PTH measurements against eGFR. This approach has the advantage that the analysis is not affected by differing baseline values (as would be the case if absolute or percentage changes were examined). The steeper the slope, the greater the rate of change in PTH with declining eGFR. Second, similar to previous studies [19], we determined the 1–84 PTH/cPTH ratio for each stage of CKD. We conclude that cPTH does increase more than 1–84 PTH with reducing eGFR, which has been noted previously [19]. Thus, cPTH did tend to have a steeper slope compared to 1-84 PTH, and the ratio of 1-84PTH/cPTH decreased with lower GFR (see Tables 2, 3), which was true for all 203 patients and in the 144 patients who were not taking calcium or vitamin D analogues. This finding is consistent with previous work showing that cPTH undergoes renal clearance and accumulates in renal failure [20, 21].
Whether the relatively greater increase in cPTH is of any clinical value or concern remains contentious. The ratio of 1–84 PTH whole/cPTH has been shown in some [22] but not all studies [23–25] to be related to bone turnover and bone histology. In particular, it has been suggested that a lower ratio is associated with low bone turnover/adynamic bone disease [26]. More recently, Kurajoh et al. [27] suggested that active PTH fragments increase with worsening renal failure from their study of pre-dialysis patients (mean GFR, 22 ml/min). They examined the 1–84 PTH (whole)/intact PTH (1–84 and N-truncated fragments 7–84) ratio, finding that this ratio is inversely related to GFR. They did not determine, as we have done in the present study, the 1–84/cPTH ratio, and so direct comparison is not possible, partly because of this difference and partly because they have restricted their study to pre-dialysis patients whereas our data were more inclusive of a full range of CKD/GFR. Prospective studies that correlate baseline PTH measurements (and ratios) with clinical outcomes, such as fracture, will be needed to fully define the putative role of these derived indices. In the meantime, this area remains contentious.
Determinants of PTH
Although it is common to presuppose that 25-OHD deficiency will result in secondary hyperparathyroidism, this is clearly not always the case, with data from our previous study and others demonstrating that the relationship is more complex. That is, not all patients with a low 25-OHD will have higher PTH levels as there are other variables determining whether PTH will be increased [9, 28–32]. For example, in our previous work [9], we found that only 24% of patients with a 25-D of less than 50 nmol/l had increased PTH levels.
The current study examines potential determinants more exhaustively, in that we have measured dietary calcium intake, plasma ionized calcium (iCa), serum Mg, and 1,25-OHD in addition to other commonly assessed variables. We overcame a major limitation of our previous study, and that of other authors [33], by measuring total (intact) PTH, 1–84 PTH (whole), and cPTH (N-terminally truncated) so that we could see if our previous finding that GFR has a major role in the determinant of the PTH response for a given 25-OHD level was the result of accumulation of the biologically inactive PTH fragment, which has been previously noted with CKD.
Univariate analysis for all three PTH measurements showed similar strengths of relationships to the parameters examined (Table 4). Inverse relationships with PTH were seen for dietary calcium and plasma iCa. Serum Mg had a positive relationship with PTH, although the significance of this is likely to be small, as serum Mg increases with worsening eGFR (r = −0.30; P < 0.001), similar to PTH.
Multiple regression analyses also showed the same findings for all three PTH measurements (Table 5). With each PTH measurement as the independent variable, we found that eGFR was significantly associated with the three PTH measurements, but 25-OHD was only included in the models for total PTH and 1–84 PTH, with iCa replacing 25-OHD for cPTH. Serum 25-OHD has a direct inhibitory influence on the synthesis of PTH, as represented by both total and 1–84 PTH, whereas cPTH is a product of the metabolism of secreted PTH and will be affected more by renal excretion, on which 25-OHD does not have a major effect. cPTH increases with decreasing GFR because it is usually cleared by glomerular filtration. With decreasing eGFR, ionized Ca influences PTH secretion; therefore, this finding suggests that cPTH is more influenced by ionized Ca than 25-OHD. Those patients who were not treated with calcium or 1-α-calcidol also had higher levels of PTH measurements (see Table 3). Our interpretation of these data are that all three PTH measurements essentially behave in a similar way and for clinical use either total (intact) PTH or 1–84 PTH (whole) would be appropriate.
We recognize a number of strengths and limitations of this study. We randomly selected patients from a database of patients with CKD who were then well characterized and for whom comprehensive biochemical analyses were performed including PTH measurements, vitamin D metabolites, and potential determinants including calcium intake, ionized calcium, and Mg that have been omitted in previous studies. Limitations include the cross-sectional study design and the relatively low recruitment rate, which reflects the geographical spread of the patients in relationship to our hospital. Additionally, the cross-sectional study design limits the ability to draw firm causal conclusions, and further longitudinal studies will be required to explore some of the findings.
Conclusion and future recommendations
In conclusion, we have studied changes in bone mineral parameters and examined the relationship between 25-OHD and PTH in a well-characterized group of patients with varying stages of CKD. We have shown that although 1,25-OHD levels do decrease with decreasing GFR, 25-OHD levels remain relatively stable. We confirm that there is a complex relationship between 25-OHD, GFR, and PTH. Total PTH, 1–84 PTH, and cPTH increase as eGFR decreases with a relatively greater increase in cPTH, although the clinical significance of this finding remains uncertain. The three PTH measurements had similar correlations with the biochemical and clinical variables examined in the study, with the highest correlation being with eGFR followed by 25-OHD, suggesting that either total PTH (intact) or 1–84 PTH (whole) can be used in clinical practice when evaluating vitamin D and bone mineral status in patients with CKD. However, there remain considerable issues with measuring and interpreting PTH measurements, particularly in patients with CKD, and further refinement of assays and clinical outcome studies are needed.
References
Hayes CE, Nashold FE, Space KM, Pedersen LB (2003) The immunological function of the vitamin D endocrine system. Cell Mol Biol 49:277–300
Garland CF, Garland FC, Gorham ED et al (2006) The role of vitamin D in cancer prevention. Am J Public Health 96:252–261
Wang TJ, Pecan MJ, Booth SL et al (2008) Vitamin D deficiency and risk of cardiovascular disease. Circulation 117:503–511
Sambrook PN, Chen JS, March LM et al (2004) Serum parathyroid hormone is associated with increased mortality independent of 25-hydroxy vitamin D status bone mass and renal function in the frail and very old. J Clin Endocrinol Metab 89:5469–5476
Sambrook PN, Chen C, March LM et al (2004) Serum parathyroid hormone predicts time to first fall independent of vitamin D status in a frail elderly population. J Clin Endocrinol Metab 89:1572–1576
Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K (2008) Secondary hyperparathyroidism is associated with higher mortality in men with moderate to severe chronic kidney disease. Kidney Int 73:1296–1302
Tentori F, Blayney MJ, Albert JM et al (2008) Mortality risk for dialysis patients with different levels of serum calcium, phosphorus and PTH: the dialysis outcomes and practice patterns study (DOPPS). Am J Kidney Dis 52:519–530
Smith DH, Johnson ES, Thorp ML, Yang X, Neil N (2009) Hyperparathyroidism in chronic kidney disease: a retrospective cohort study of costs and outcomes. J Bone Mineral Metabol 27:287–294
Patel S, Hyer S, Barron J (2007) Glomerular filtration rate is as major determinant of the relationship between 25-hydroxyvitamin D and parathyroid hormone. Calcif Tissue Int 80:221–226
Selby P, Weaver SM, Welch G, Davies M (1995) Evaluation of a new means of assessing calcium intake. Bone (NY) 16:690
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 130:461–470
National Kidney Foundation: K/DOQI (2002) Clinical practice guidelines for chronic kidney disease. Am J Kidney Dis 39:S17–S92
Fraser WD, Durham BH, Berry JL, Mawer EC (1997) Measurement of plasma 1,25-dihyroxyvitamin D using a novel immunoextraction technique and immunoassay with iodine labelled vitamin D tracer. Ann Clin Biochem 34:632–637
Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL (2007) Prevalence of abnormal serum vitamin D PTH calcium and phosphorus in patients with chronic kidney disease. Kidney Int 71:31–38
Carter JL, O’Riordan SE, Eaglestone GL, Delaney MP, Lamb EJ (2008) Bone mineral metabolism and its relationship to kidney disease in a residential care home population: a cross-sectional study. Nephrol Dial Transplant 23:3554–3565
Craver L, Marco MP, Martinez I, Rue M, Borras M, Martin ML, Sarro F, Valdivielso JM, Fernandez E (2007) Mineral metabolism parameters throughout chronic kidney disease stages 1–5: achievement of K/DOQI target stages. Nephrol Dial Transplant 22:1171–1176
Taskapan H, Ersoy FF, Passadakis PS, Tam P, Memmos DE, Katopodis KP et al (2006) Severe vitamin D deficiency in chronic renal failure patients on peritoneal dialysis. Clin Nephrol 4:247–255
Rejnmark L, Vestergaard P, Heickendorff L, Mosekilde L (2008) Plasma 1,25(OH)2D levels decrease in postmenopausal women with hypovitaminosis D. Eur J Endocrinol 158:571–576
Herberth J, Fahrleitner A, Obermayer-Pietsch B, Krisper P, Holzer H, Malluche HH, Dobnig H (2006) Changes in total parathyroid hormone (PTH), PTH-(1–84) and large C-PTH fragments in different stages of chronic kidney disease. Clin Nephrol 65:328–334
D’Amour P (2006) Circulating PTH molecular forms: what we know and what we don’t. Kidney Int 70:S29–S33
Souberbielle J-CP, Roth H, Fouque DP (2010) Parathyroid measurement in CKD. Kidney Int 77:93–100
Monier-Faugere MC, Geng Z, Mawad H, Friedler RM et al (2001) Improving assessment of bone turnover by the PTH-(1–84)/large C-PTH fragments ratio in ESRD patients. Kidney Int 60:1460–1468
Godber IM, Parker CR, Lawson N et al (2002) Comparison of intact and “whole molecule” parathyroid hormone assays in patients with histologically confirmed post-renal transplant osteodystrophy. Ann Clin Biochem 39:314–317
Coen G, Bonucci E, Ballanti P et al (2002) PTH 1–84 and PTH 7–84 in the noninvasive diagnosis of renal bone disease. Am J Kidney Dis 40:348–354
Lehman G, Stein G, Huller M et al (2005) Specific measurement of PTH (1–84) in various forms of renal osteodystrophy (ROD) as assessed by bone histomorphometry. Kidney Int 68:1206–1214
Donadio C, Ardini M, Lucchesi A, Donadio E, Cantor T (2007) Parathyroid hormone and large related C-terminal fragments increase at different rates with worsening of renal function in chronic kidney disease patients. A possible indicator of bone turnover status? Clin Nephrol 67:131–139
Kurajoh M, Inaba M, Yamada S, Imanishi Y, Tsuchida T, Ishimura E, Nishizawa Y (2008) Association of increased active PTH (1–84) fraction with decreased GFR and serum Ca in predialysis CFR patients: modulation by serum 25-OH-D. Osteoporos Int 19:70–716
Tanpricha V, Pearce EN, Chen TC, Holick MF (2002) Vitamin D insufficiency among free-living healthy young adults. Am J Med 112:659–662
Isaia G, Giorgino R, Rini GB, Bevilacqua M, Maugeri D, Adami S (2003) Prevalence of hypovitaminosis D in elderly women in Italy: clinical consequences and risk factors. Osteoporosis Int 14:577–582
Vieth R, Ladak Y, Walfish PG (2003) Age related changes in the 25-hydroxyvitamin D versus parathyroid hormone relationship suggests a different reason why older adults require more vitamin D. J Clin Endocrinol Metab 88:185–191
Sahota O, Mundey MK, San P et al (2004) The relationship between vitamin D and parathyroid hormone: calcium homeostasis, bone turnover and bone mineral density in postmenopausal women with established osteoporosis. Bone 35:312–319
Pepe J, Romagnoli E, Nofroni I et al (2005) Vitamin D status as the major factor determining the circulating levels of parathyroid hormone: a study in normal subjects. Osteoporos Int 16:805–812
Gunnarsson O, Indidason OS, Franzon L, Sigurdsson G (2008) Factors associated with elevated or blunted PTH response in vitamin D insufficient adults. J Intern Med 265:189–488
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Patel, S., Barron, J.L., Mirzazedeh, M. et al. Changes in bone mineral parameters, vitamin D metabolites, and PTH measurements with varying chronic kidney disease stages. J Bone Miner Metab 29, 71–79 (2011). https://doi.org/10.1007/s00774-010-0192-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-010-0192-1