Abstract
This study was conducted to test the hypothesis that preweaning glycine supplementation to breast-fed piglets alleviated the post-weaning apoptosis of jejunal epithelium through CHOP signaling. Seven-day-old sow-reared piglets were orally administrated with 0, 50, 100, or 200% of glycine intake from sow’s milk twice daily for 14 days and then were weaned at 21 days of age. Tissue samples were collected at 28 days of age for determining intestinal morphology, serum diamine oxidase (DAO) activity, abundances of proteins involved in ER stress and apoptosis. Glycine (100–200%) administration increased villus height, the ratio of villus height to crypt depth in the jejunum. Glycine supplementation (200%) enhanced average daily weight gain during the first 2 weeks post-weaning. Serum DAO activity and jejunal epithelium apoptosis were decreased, but the number of goblet cells in the jejunum was increased. Western blot analysis showed that 100–200% glycine enhanced the protein levels of occludin, claudin-1, and zonula occludens (ZO)-1 without affecting those of claudin-3, ZO-2, and ZO-3. Further studies showed that protein abundances of glucose-regulated protein 78 (BiP/GRP78) and p-IRE1α, instead of ATF6α, were reduced by glycine. Among the proteins related to apoptosis, abundances of CHOP and p53 were reduced, whereas those of Bcl-2 and Bcl-xL were enhanced in the jejunum of 100–200% glycine-supplemented piglets. Collectively, our results indicated that preweaning glycine supplementation improved the intestinal development of post-weaning piglets. The beneficial effect of glycine was associated with improved intestinal mucosal barrier and reduced apoptosis of enterocytes through CHOP signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The small intestine is the site for the terminal digestion and absorption of nutrients (Wu 2018). Intestinal mucosal barrier is critical for preventing harmful antigens, pathogens and toxins in the intestinal lumen from entering the systemic circulation (Moens and Veldhoen 2012; Turner 2009). To maintain intracellular homeostasis, intestinal epithelial cells are tightly bound together by junctional complexes, which are essential for the epithelium to maintain its physiological function (Turner 2009; Marchiando et al. 2010). Disruption of tight junction proteins (TJPs) and increased intestinal permeability have been reported to be associated with the development of numerous gastrointestinal diseases, such as inflammatory bowel disease, infectious enterocolitis, irritable bowel syndrome, and celiac disease (Odenwald and Turner 2013; Arrieta et al. 2006). Weaning stress is one of the most stressful events that involved multiple factors (Wijtten et al. 2011). It is well known that weanling piglets have a decreased feed intake, reduced villous height, increased apoptosis of epithelial cells, and dysfunction of intestinal mucosal barrier during the first week post-weaning (Yang et al. 2016; Bauer et al. 2011; Wijtten et al. 2011; Wu et al. 1996).
The endoplasmic reticulum (ER) is the intracellular organelle in which proteins are synthesized, folded, and secreted (Ron and Walter 2007). A variety of conditions, such as hypoxia, starvation, and infections, cause the accumulation of misfolded proteins within the ER and result in the endoplasmic reticulum stress (Iurlaro and Munoz-Pinedo 2016). Eukaryotic cells have evolved a protective strategy, collectively known as the unfolded protein response (UPR) to restore ER homeostasis by reducing protein synthesis, promoting proper protein folding, or enhancing the degradation of unfolded proteins (Ron and Walter 2007; Hetz et al. 2015). The UPR is mainly mediated by three ER membrane-associated proteins, PKR-like eukaryotic initiation factor 2α kinase (PERK), inositol requiring enzyme 1 alpha (IRE1α), and activating transcription factor-6 alpha (ATF6α) (Hetz et al. 2015). Under normal conditions, the transmembrane proteins are bound to the chaperone BiP/GRP78 in the intralumenal domain and, therefore, are maintained in an inactive state (Hotamisligil 2010). In response to ER stress, the sensor proteins (including PERK, IRE1α, and ATF6α) are released from BiP, thus activating downstream signaling cascades to reduce the deleterious effect of unfolded protein accumulation (Bertolotti et al. 2000; Hotamisligil 2010). UPR can activate downstream pro-apoptotic proteins, such as CHOP (also known as growth arrest and DNA damage-inducible gene 153, GADD153), Noxa, Bim, or repress anti-apoptotic proteins such as Bcl-2 and Bcl-xL, ultimately leading to cell death (Iurlaro and Munoz-Pinedo 2016).
Glycine has traditionally been categorized as a nutritionally nonessential amino acid because it is synthesized in the body (Wang et al. 2013). However, glycine is severely deficient in milk and plant proteins (Birchenough et al. 2015; Wu et al. 2014). More and more evidence shows that the amount of glycine synthesized de novo is insufficient to meet maximal growth or optimal health of piglets (Melendez-Hevia et al. 2009; Wang et al. 2014a). Based on glycine content in sow’s milk and its accretion in the whole body, it is estimated that sow’s milk meets at most only 23% of daily glycine needs for protein synthesis in piglets (Wu 2010). In addition, dietary glycine supplementation improves the abundances of intestinal epithelial TJPs and improves intestinal health in piglets (Wang et al. 2014a). Moreover, glycine administration is associated with reduced apoptosis in cultured cells (Bhattacharyya et al. 2012; Weinberg et al. 2016). However, the underlying mechanisms are largely unknown.
Based on the foregoing, this study was conducted to test the hypothesis that preweaning glycine supplementation to suckling piglets during 7–21 days of age may prevent post-weaning intestinal epithelial apoptosis in the small intestine, in which ER stress signaling is involved.
Materials and methods
Piglets and experimental design
Experiment 1: Piglets were the offspring of Yorkshire × Landrace and maintained at the Yinfa Animal Husbandry Co. farm (Henan, China). The average birth weight of the piglets used for this study was 1.55 kg. Multiparous sows were fed a corn- and soybean meal-based diet (containing 18.1% crude protein and 3160 kcal metabolizable energy/kg diet) during lactation (Supplemental Table 1). At 7 days of age, 64 piglets from 8 litters (8 piglets per litter) with the body weight of 3.1 ± 0.07 kg were allotted randomly into one of the four groups based on body weight and litter origin. The piglets were orally administered with 0, 50, 100, or 200% of glycine intake from sow’s milk daily until 21 days of age. l-alanine was used as the isonitrogenous control (Table 1). Supplemented glycine or l-alanine was dissolved in 10-mL saline and supplied twice daily at 0800 and 1400 h. The amount of glycine given to piglets was calculated based on milk intake as previously described (Kim and Wu 2004; Wu et al. 2004; Sun et al. 2015). All piglets had free access to sow’s milk and water throughout days 1–21 of age. Piglets were weaned at 21 days of age to a corn- and soybean meal-based diet, and transferred to a nursery room. There were 8 pens (2 piglets/pen) per treatment group. All piglets had free access to drinking water and the post-weaning basal diet was formulated to meet nutritional requirements [National research council (NRC), 2012] of piglets (Table 2) throughout days 21–42 of age. The body weights of preweaning piglets, as well as the feed intake and body weights of post-weaning piglets, were recorded weekly.
Experiment 2: To investigate the molecular mechanisms for glycine to alleviate the weaning stress-induced apoptosis of the small intestine, Experiment 2 was conducted as in Experiment 1, except that each treatment group of Experiment 2 had 3 pens (2 piglets/pen). Blood samples were collected 1 h after feeding at 28 days of age (7 days post-weaning). Thereafter, all piglets were euthanized by intravenous administration of sodium pentobarbital (50 mg/kg BW) (Schering-Plough, Canada). The jejunal tissues were collected and frozen in liquid nitrogen for later analysis.
Histologic analyses of the jejunum and goblet cell staining
The segments (1 cm) of the jejunal tissues fixed in 4% paraformaldehyde were embedded in paraffin and were sectioned (5 μm of thickness), stained with hematoxylin–eosin for histological analysis (Wu et al. 1996). The goblet cells were stained with Alcian Blue and periodic acid-Schiff reagent (Beijing ZSGB-BIO Co. Ltd), according to the protocol provided by the manufacturer. Briefly, the tissue sections were incubated with Alcian Blue solution for 30 min, and then were washed with water for 5 min. After incubation with periodic acid for 10 min, the sections were incubated with Schiff reagent for 10 min. The goblet cells were observed under a microscopy (Axio Vert.A1; Zeiss).
Analyses of diamine oxidase (DAO) activity in serum
The DAO activity in serum was determined by the enzymatic assay using glutamate dehydrogenase and NADH in the indicator system. The ammonia formation per min was determined by the decrease in extinction due to an equimolar oxidation of NADH, which was a measure of DAO activity as previously described (Kusche et al. 1974). The kit used for DAO analysis was obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
TUNEL assay
Jejunal tissues fixed with optimal cutting media (O.C.T., Tissue-Tek) were sectioned (8 μm of thickness). Apoptosis was determined by the TUNEL assay (Beyotime Biotechnology, China) following the manufacturer’s instruction. The nuclei were stained with the Hoechst 33258 (1 mg/mL) for 1 min at 25 °C and then were observed under a fluorescence microscope (Axio Vert.A1; Zeiss).
Western blot analysis
Jejunal tissues were homogenized and lysed in ice-cold lysis buffer containing 50 mmol/L Tris HCl (pH 7.4), 150 mmol/L NaCl, 1% nonidet P (NP)-40, 0.1% SDS, 1.0 mmol/L PMSF, 1.0 mmol/L Na3VO4, 1.0 mmol/L sodium fluoride (NaF), and protease inhibitor cocktail (Roche, Indianapolis, IN). Equal amounts of protein (50 μg) were separated on SDS-PAGE gels and then transferred to polyvinylidene difluoride membranes (Millipore). The membranes were blocked in a 5% skimmed-milk solution at 25 °C for 1 h. The blots were incubated with a primary antibody overnight at 4 °C and then incubated with an appropriate secondary antibody at 25 °C for 1 h. The blots were detected with the ImageQuant LAS 4000 mini system (GE Healthcare BioSciences) after reaction with ECL Plus detection reagents (Amersham Biosciences). The chemiluminescence signal was determined and band density was quantified with the One Quantity software (Bio-Rad Laboratories). Anti-occludin (1:2000, 40–4700), anti-claudin-1 (1:2000, 51–9000), anti-claudin-3 (1:2000, 34-1700), anti-ZO-1 (1:2000, 61–7300), anti-ZO-2 (1:2000, 38–9100), and anti-ZO-3 (1:2000, 36–4100) polyclonal antibodies were obtained from Invitrogen (California, USA). Anti-GRP78/BiP (1:2000, 3183, polyclonal antibody) and anti-p53 (1:2000, 2524, monoclonal antibody) were purchased from Cell Signaling Technology (Massachusetts, USA). Anti-p-IRE1α (1:2000, phosphorylated serine 724, ab48187) polyclonal antibody was procured from Abcam (Cambridge, UK). Anti-ATF6α (1:1000, sc-22799), Anti-Bcl-2 (1:1000, sc-492), Anti-Bcl-xL (1:1000, sc-634), Anti-Bax (1:1000, sc-493), and Anti-CHOP (1:1000, sc-575) polyclonal antibodies were obtained from Santa Cruz (California, USA). All results were normalized to GAPDH (1:2000, sc-59540, Santa Cruz) and expressed as the relative values to the control group.
Statistical analysis
All data are presented as mean ± SEMs and were analyzed by one-way ANOVA. Differences between means were determined by the Duncan multiple comparison test. All statistical analyses were performed by the SPSS statistical software (SPSS for Windows, version 17.0). P < 0.05 was considered significant.
Results
Growth performance and intestinal morphology of piglets
As shown in Table 3, oral administration of glycine to preweaning piglets did not affect their growth performance before weaning (P > 0.05), as compared with the control group. Interestingly, preweaning supplementation of 200% glycine (days 7–21 of age) enhanced (P < 0.05) daily body weight gain and decreased feed/gain ratio in post-weaning piglets between 21 and 28 days of age (the first week post-weaning) when compared with alanine-supplemented piglets. The beneficial effect of preweaning glycine supplementation lasted until 35 days of age (2 weeks post-weaning). No effect on weight gain was observed (P > 0.05) between 35 and 42 days of age (P > 0.05). Compared with the control group, preweaning supplementation of 200% glycine enhanced (P < 0.05) the post-weaning daily BW gain between 21 and 42 days of age by 14%. Data on villus height, crypt depth, and villus height–crypt depth ratio in the jejunum of 28-day-old pigs (1 week post-weaning) are summarized in Table 4. Preweaning glycine supplementation increased villus height and reduced crypt depth (P < 0.05) in the jejunum of weanling piglets. The ratio of villus height to crypt depth in the jejunum was higher (P < 0.05) in glycine-supplemented piglets, compared with the controls.
The activity of DAO in serum
An increase in the amount of DAO in serum is considered as an indicator of damage in intestinal mucosa integrity (Yi et al. 2017; Wu 2018). As shown in Fig. 1, preweaning glycine administration reduced serum DAO activity in a dose-dependent manner (P < 0.05), as compared to controls, indicating a beneficial effect of glycine on the integrity of intestinal mucosa.
Serum activity of DAO in 28-day-old piglets that were orally administered with glycine or alanine (the isonitrogenous control) between 7 and 21 days of age before weaning. Piglets were weaned at 21 days of age, and serum was obtained 1 week post-weaning. Values are mean ± SEM, n = 6 piglets/group. Means without a common letter differ, P < 0.05
Tight junction protein expression in jejunum
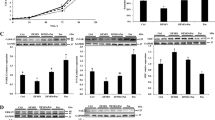
Western blot analysis revealed that preweaning supplementation of 100% and 200% glycine enhanced (P < 0.05) protein levels of occludin, claudin-1 and ZO-1 (Fig. 2a, b, d) in jejunal tissues of piglets at day 7 post-weaning, as compared with the controls. The protein abundances of jejunal ZO-2, ZO-3, and claudin-3 (Fig. 2c, e, f) were not affected (P > 0.05) by glycine supplementation.
Protein abundances of occludin (a), claudin-1 (b), claunin-3 (c), ZO-1 (d), ZO-2 (e), and ZO-3 (f) in the jejunum of 28-day-old piglets that were orally administered with glycine or alanine (the isonitrogenous control) between 7 and 21 days of age before weaning. Piglets were weaned at 21 days of age, and jejunum was obtained one week post-weaning. Representative results are protein abundance from two individual piglets. Values are mean ± SEM, n = 6 piglets/group. Means without a common letter differ, P < 0.05
Goblet cell morphology and apoptosis
Goblet cells can synthesize and secret mucins, which form a viscoelastic gel and protect the intestinal epithelium against external insult (Wu et al. 2018). To assess a functional role of glycine on goblet cells and the production of mucins, alcian Blue and periodic acid-Schiff staining were performed. We found that preweaning supplementation of 100% glycine, instead of 50% and 200% glycine, increased (P < 0.05) the number of goblet cells in post-weaning piglets (Fig. 3), as compared with the control group. Apoptosis of enterocytes as showed by TUNEL-positive cells in the jejunal tissues was detected (Fig. 4). Interestingly, glycine administration markedly decreased (P < 0.05) the number of apoptotic cells in a dose-dependent manner in the jejunal epithelium of post-weaning piglets, indicating a inhibitory effect of glycine on cell death in the small intestine.
Goblet cells staining with Alcian Blue and periodic acid-Schiff assay in jejunum of 28-day-old piglets that were orally administered with glycine or alanine (the isonitrogenous control) between 7 and 21 days of age before weaning. Piglets were weaned at 21 days of age, and jejunum was obtained one week post-weaning
Apoptotic cells in the jejunum of 28-day-old piglets that were orally administered with glycine or alanine (the isonitrogenous control) between 7 and 21 days of age before weaning. Piglets were weaned at 21 days of age, and jejunum was obtained one week post-weaning. a Apoptotic cells were stained with an in situ TUNEL staining reagent (green, arrow). Nuclei are stained with DAPI (blue). b The percentage of apoptosis was counted from three random areas of individual sections. Values are mean ± SEM, n = 6 piglets/group. Means without a common letter differ, P < 0.05
ER stress and apoptosis-related protein expression
As shown in Fig. 5, dietary supplementation of 200% glycine decreased (P < 0.05) the protein abundance of BiP in the jejunum of post-weaning piglets (Fig. 5a), and supplementation of 50–200% glycine decreased (P < 0.05) the abundances of p-IRE1α, CHOP, and p53 proteins (Fig. 5c–e) in the jejunum, as compared to the controls. In addition, supplementation of 50% glycine, instead of 100% and 200% glycine, decreased (P < 0.05) jejunal protein expression of Bax (Fig. 5f) as compared with the control group. The protein abundances of jejunal Bcl-2 and Bcl-xL were markedly augmented (P < 0.05) by preweaning supplementation of 100 and 200% glycine (Fig. 5g, h). However, glycine had no effect (P > 0.05) on the expression of jejunal ATF6α (Fig. 5b).
Protein abundances for BiP (a), ATF6α (b), IRE1α (c), CHOP (d), p53 (e), Bax (f), Bcl-2 (g), and Bcl-xL (h) in the jejunum of 28-day-old piglets that were orally administered with glycine or alanine (the isonitrogenous control) between 7 and 21 days of age before weaning. Piglets were weaned at 21 days of age, and jejunum was obtained one week post-weaning. Representative results are protein abundance from 2 individual piglets. Values are mean ± SEM, n = 6 piglets/group. Means without a common letter differ, P < 0.05
Discussion
In the present study, preweaning administration of glycine to sow-reared piglets improved intestinal mucosal barrier function at day 7 post-weaning, as shown by increases in jejunal villus height, goblet cells, protein abundances of TJPs, as well as decreases in serum DAO activity and jejunal epithelium apoptosis. This effect of glycine was associated with downregulation of the expression of proteins implicated in ER stress and apoptosis signaling.
The small intestine of weanling piglets is subjected to dramatic changes due to exposure to various factors, including pathogens and toxins present in the intestinal lumen (Campbell et al. 2013; Yi et al. 2018). Previous study has reported that weaning stress results in reduced growth performance, which is associated with decreased TJPs and increased intestinal permeability (Hu et al. 2013). Hence, nutritional strategies to alleviate the damaging effects of weaning stress on intestinal–mucosal barrier are of significance for the health, growth and development of neonates. Growing evidence indicates that the traditionally classified nutritionally non-essential amino acids, such as arginine, glycine, glutamine, and glutamate, promote intestinal development and regulate nutrient metabolism in intestinal tissues through various mechanisms (Li and Wu 2018; Rhoads and Wu 2009; Wu 2009).
Glycine is the simplest amino acid in nature and a major constituent in extracellular structural proteins in animals (Wu 2009). Extensive studies have shown that glycine plays an important role in modulating animal behavior, food intake, DNA synthesis, cell proliferation, immune response, and whole-body homeostasis (Zhong et al. 2003; Hall 1998; Amelio et al. 2014). We and others have reported that glycine supplementation enhances growth performance (Powell et al. 2011; Wang et al. 2014a), and regulates the expression of TJPs in the intestinal cells of pigs (Li et al. 2016). However, it is unknown whether glycine can alleviate weaning stress-induced intestinal mucosal barrier disruption, thereby improving the growth performance of post-weaning piglets. In the present study, we found that preweaning glycine supplementation during 7–21 days of age increased the growth of 21- to 35-day-old post-weaning pigs (2 weeks post-weaning).
DAO is an enzyme that is widely distributed in intestinal villus cells (Wu et al. 2018). Intestinal mucosal damage leads to an increase of serum DAO activity (Tossou et al. 2016; Yi et al. 2018). Therefore, it is regarded as an indicator of intestinal epithelial integrity and permeability (Miyoshi et al. 2015). A novel finding of the present study is that oral administration of glycine to piglets during the suckling period decreased serum DAO activity. The intestinal mucosal barrier is mainly formed by the epithelial cells and paracellular TJPs, which prevent bacteria, endotoxins, and other lumen substance from entering the blood circulation (Jacobi and Odle 2012). Breakdown of TJPs augments intestinal permeability and serum DAO activity, while decreasing the intestinal absorption of nutrients in humans and animals (Gilani et al. 2017). Interestingly, weaning stress impaired the expression of TJPs and promoted apoptosis in the small intestine, which was attenuated by preweaning supplementation of glycine to neonates. This indicates that milk-born glycine is insufficient for: (1) the preweaning development of the small intestine in piglets; and (2) the post-weaning health of the piglet intestine or the post-weaning growth performance of piglets.
Goblet cells are specialized for the synthesis and secretion of mucins, critical components of the mucus layer (Birchenough et al. 2015; Hou and Wu 2018). The gastrointestinal mucus systems separate the luminal content (especially bacteria) from direct contact with the epithelial cells. In our study, the jejunal mucins were more abundant in glycine-supplemented pigs compared to the control group, which is consistent with a previous report (Ospina-Rojas et al. 2013), indicating that glycine supplementation increases the mucus layer and protects the integrity of the intestinal epithelium. Oral administration of glycine may promote the biosynthesis of mucin, which is then secreted to protect epithelial cells from insults by various pathogens in the intestinal lumen. Moreover, it has been reported that the enterocyte apical glycocalyx (also known as the pericellular matrix) mainly consists of transmembrane mucins and the tight junctions (Sheng et al. 2013; Turner 2009). Enhanced production of mucins may improve intestinal permeability through an enhanced interaction between mucin and TJPs, ultimately contributing to intestinal integrity and growth performance.
Glycine exerts anti-inflammatory, immunomodulatory, and anti-apoptotic effects in various tissues (Fuchs et al. 2012; de Aguiar Picanco et al. 2011; Amin et al. 2003; Jacob et al. 2003). In our previous study, we found that glycine attenuated 4-hydroxynonenal (an end product of lipid oxidation)-induced apoptosis in enterocytes (Wang et al. 2014b). However, in vivo data on the anti-apoptotic effect are not available. Using the piglet as an animal model, we found that glycine supplementation prevented cell death in the jejunum of weanling piglets (Fig. 4), validating an in vivo effect of glycine on apoptosis. Mechanistically, this effect of glycine is associated with up-regulation of anti-apoptotic proteins of Bcl-2 family, including Bcl-2 and Bcl-xL, which has been reported in various cells (Lu et al. 2012). It has also been demonstrated that Bax, a pro-apoptotic protein, can be induced by p53 and contribute to stress-induced cell death (Wawryk-Gawda et al. 2014). Results of the present study indicated that preweaning glycine supplementation reduced the protein levels of p53 and Bax in the small intestine of post-weaning pigs, indicating a functional role of p53 on the apoptosis of intestinal epithelial cells by regulating the expression of Bcl-2 family proteins. ER stress has been reported to stimulate p53 expression through NF-κB signaling, causing ER stress-related cell death (Lin et al. 2012). In a recent study, Yi et al. reported that phosphorylation of NF-κB was correlated with enhanced IL-6 expression in the jejunum of weanling piglets (Yi et al. 2016). It is possible that the elevation of p53 in the jejunum of weanling piglets might be due to ER stress and/or NF-κB activation.
Another novel and important finding from the present study is that preweaning glycine supplementation to piglets attenuated the expression of jejunal CHOP (an apoptotic protein) in response to ER stress signaling during the weaning period. Induction of CHOP has been reported to promote cell death via upregulating the expression of several apoptotic genes, including the death receptor 5 and Bcl-2 family proteins (Szegezdi et al. 2006). In our study, we observed significant downregulation of Bcl-2 in post-weanling piglets supplemented with glycine prior to weaning. Nevertheless, it remains unknown whether other pro-apoptotic proteins may also be implicated in the protective effect of glycine on the small intestine. More studies are required to address this question.
In response to ER stress, PERK, IRE1α, and ATF6α are released from BiP, thus activating downstream signaling cascades to restore homeostasis (Bertolotti et al. 2000). The enhanced protein abundances of ATF6α and phosphorylation of IRE1α in the small intestine of piglets indicated a disruption of cellular homeostasis (Petrat et al. 2011; Stoffels et al. 2011; Diestel et al. 2007). Both PERK and IRE1α activate CHOP and contribute to cell death. We found that the downregulation of CHOP by glycine supplementation was accompanied by a decrease in jejunal protein levels of IRE1α, suggesting an inhibition of IRE1α by glycine in the small intestine of weanling piglets. Considering that the CHOP can also be regulated via post-translational modification by p38 MAPK, which, in turn, interacts with the IRE1α-TRAF2 complex on ER (Szegezdi et al. 2006), further studies are needed to explore the underlying mechanisms responsible for CHOP repression in the jejunal tissues of piglets receiving preweaning administration of glycine.
It should be noted that we did not detect the protein of PERK in the piglet jejunum, due to no cross reaction between the antibody used in our study and porcine PERK protein. It was not conclusive whether PERK is involved in or regulates jejunal CHOP expression in young pigs. Milk is the main nutrient required for neonatal growth and intestinal health (Wu 2018). Considering a limited availability of glycine in the human milk and the sow’s milk (Hou et al. 2016), as well its beneficial effect on the intestine, skeletal muscle cells, adipocytes, and whole body metabolism (Chen et al. 2018; Li and Wu 2018; Sun et al. 2016), dietary supplementation of glycine to formulas may improve the growth, development and health of infants, particularly in preterm or low-birth weight neonates nursed by women or other mammals (Wang et al. 2012; Dai et al. 2015; Wu 2013; Wu et al. 2014).
In conclusion, oral administration of glycine to suckling piglets improved intestinal mucosal barrier function during the post-weaning period. The protective effect of glycine was associated with reduced apoptosis and increased protein levels of tight junction proteins in the small intestine. We suggest that repression of ER stress-induced CHOP induction is critical for the anti-apoptotic effect of glycine in the small intestine of piglets. More studies are needed to understand how the intestine is reprogrammed by glycine during the suckling period for improved health, growth and development in later life.
References
Amelio I, Cutruzzola F, Antonov A, Agostini M, Melino G (2014) Serine and glycine metabolism in cancer. Trends Biochem Sci 39(4):191–198
Amin K, Li J, Chao WR, Dewhirst MW, Haroon ZA (2003) Dietary glycine inhibits angiogenesis during wound healing and tumor growth. Cancer Biol Ther 2(2):173–178
Arrieta MC, Bistritz L, Meddings JB (2006) Alterations in intestinal permeability. Gut 55(10):1512–1520
Bauer E, Metzler-Zebeli BU, Verstegen MW, Mosenthin R (2011) Intestinal gene expression in pigs: effects of reduced feed intake during weaning and potential impact of dietary components. Nutr Res Rev 24(2):155–175
Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D (2000) Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol 2(6):326–332
Bhattacharyya S, Ghosh J, Sil PC (2012) Iron induces hepatocyte death via MAPK activation and mitochondria-dependent apoptotic pathway: beneficial role of glycine. Free Radic Res 46(10):1296–1307
Birchenough GM, Johansson ME, Gustafsson JK, Bergstrom JH, Hansson GC (2015) New developments in goblet cell mucus secretion and function. Mucosal Immunol 8(4):712–719
Campbell JM, Crenshaw JD, Polo J (2013) The biological stress of early weaned piglets. J Anim Sci Biotechno 4:19
Chen JQ, Ma XS, Yang Y, Dai ZL, Wu ZL, Wu G (2018) Glycine enhances expression of adiponectin and IL-10 in 3T3-L1 adipocytes without affecting adipogenesis and lipolysis. Amino Acids 50:629–640
Dai Z, Wu Z, Hang S, Zhu W, Wu G (2015) Amino acid metabolism in intestinal bacteria and its potential implications for mammalian reproduction. Mol Hum Reprod 21(5):389–409
de Aguiar Picanco E, Lopes-Paulo F, Marques RG, Diestel CF, Caetano CE, de Souza MV, Moscoso GM, Pazos HM (2011) L-arginine and glycine supplementation in the repair of the irradiated colonic wall of rats. Int J Colorectal Dis 26(5):561–568
Diestel CF, Marques RG, Lopes-Paulo F, Paiva D, Horst NL, Caetano CE, Portela MC (2007) Role of l-glutamine and glycine supplementation on irradiated colonic wall. Int J Colorectal Dis 22(12):1523–1529
Fuchs SA, Peeters-Scholte CM, de Barse MM, Roeleveld MW, Klomp LW, Berger R, de Koning TJ (2012) Increased concentrations of both NMDA receptor co-agonists d-serine and glycine in global ischemia: a potential novel treatment target for perinatal asphyxia. Amino Acids 43(1):355–363
Gilani S, Howarth GS, Kitessa SM, Tran CD, Forder REA, Hughes RJ (2017) New biomarkers for increased intestinal permeability induced by dextran sodium sulphate and fasting in chickens. J Anim Physiol Anim Nutr (Berl) 101(5):e237–e245
Hall JC (1998) Glycine. J Parenter Enteral Nutr 22(6):393–398
Hetz C, Chevet E, Oakes SA (2015) Proteostasis control by the unfolded protein response. Nat Cell Biol 17(7):829–838
Hotamisligil GS (2010) Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140(6):900–917
Hou Y, Wu G (2018) L-Glutamate nutrition and metabolism in swine. Amino Acids 50(11):1497–1510
Hou Y, Yao K, Yin Y, Wu G (2016) Endogenous synthesis of amino acids limits growth, lactation, and reproduction in animals. Adv Nutr 7(2):331–342
Hu CH, Xiao K, Luan ZS, Song J (2013) Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J Anim Sci 91(3):1094–1101
Iurlaro R, Munoz-Pinedo C (2016) Cell death induced by endoplasmic reticulum stress. FEBS J 283(14):2640–2652
Jacob T, Ascher E, Hingorani A, Kallakuri S (2003) Glycine prevents the induction of apoptosis attributed to mesenteric ischemia/reperfusion injury in a rat model. Surgery 134(3):457–466
Jacobi SK, Odle J (2012) Nutritional factors influencing intestinal health of the neonate. Adv Nutr 3(5):687–696
Kim SW, Wu G (2004) Dietary arginine supplementation enhances the growth of milk-fed young pigs. J Nutr 134(3):625–630
Kusche J, van Trotha U, Muhlberger G, Lorenz W (1974) The clinical-chemical application of the NADH test for the determination of diamine oxidase activity in human pregnancy. Agents Actions 4(3):188–189
Li P, Wu G (2018) Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids 50:29–38
Li W, Sun K, Ji Y, Wu Z, Wang W, Dai Z, Wu G (2016) Glycine regulates expression and distribution of claudin-7 and ZO-3 Proteins in intestinal porcine epithelial cells. J Nutr 146(5):964–969
Lin WC, Chuang YC, Chang YS, Lai MD, Teng YN, Su IJ, Wang CC, Lee KH, Hung JH (2012) Endoplasmic reticulum stress stimulates p53 expression through NF-kappaB activation. PLoS One 7(7):e39120
Lu Y, Zhang J, Ma B, Li K, Li X, Bai H, Yang Q, Zhu X, Ben J, Chen Q (2012) Glycine attenuates cerebral ischemia/reperfusion injury by inhibiting neuronal apoptosis in mice. Neurochem Int 61(5):649–658
Marchiando AM, Graham WV, Turner JR (2010) Epithelial barriers in homeostasis and disease. Annu Rev Pathol 5:119–144
Melendez-Hevia E, De Paz-Lugo P, Cornish-Bowden A, Cardenas ML (2009) A weak link in metabolism: the metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis. J Biosci 34(6):853–872
Miyoshi J, Miyamoto H, Goji T, Taniguchi T, Tomonari T, Sogabe M, Kimura T, Kitamura S, Okamoto K, Fujino Y, Muguruma N, Okahisa T, Takayama T (2015) Serum diamine oxidase activity as a predictor of gastrointestinal toxicity and malnutrition due to anticancer drugs. J Gastroen Hepatol 30(11):1582–1590
Moens E, Veldhoen M (2012) Epithelial barrier biology: good fences make good neighbours. Immunology 135(1):1–8
Odenwald MA, Turner JR (2013) Intestinal permeability defects: is it time to treat? Clin Gastroenterol H 11(9):1075–1083
Ospina-Rojas IC, Murakami AE, Oliveira CA, Guerra AF (2013) Supplemental glycine and threonine effects on performance, intestinal mucosa development, and nutrient utilization of growing broiler chickens. Poult Sci 92(10):2724–2731
Petrat F, Drowatzky J, Boengler K, Finckh B, Schmitz KJ, Schulz R, de Groot H (2011) Protection from glycine at low doses in ischemia-reperfusion injury of the rat small intestine. Eur Surg Res 46(4):180–187
Powell S, Bidner TD, Payne RL, Southern LL (2011) Growth performance of 20- to 50-kg pigs fed low-crude-protein diets supplemented with histidine, cystine, glycine, glutamic acid, or arginine. J Anim Sci 89(11):3643–3650
Rhoads JM, Wu GY (2009) Glutamine, arginine, and leucine signaling in the intestine. Amino Acids 37(1):111–122
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8(7):519–529
Sheng YH, Triyana S, Wang R, Das I, Gerloff K, Florin TH, Sutton P, McGuckin MA (2013) MUC1 and MUC13 differentially regulate epithelial inflammation in response to inflammatory and infectious stimuli. Mucosal Immunol 6(3):557–568
Stoffels B, Turler A, Schmidt J, Nazir A, Tsukamoto T, Moore BA, Schnurr C, Kalff JC, Bauer AJ (2011) Anti-inflammatory role of glycine in reducing rodent postoperative inflammatory ileus. Neurogastroenterol Motil 23(1):76–78
Sun Y, Wu Z, Li W, Zhang C, Sun K, Ji Y, Wang B, Jiao N, He B, Wang W, Dai Z, Wu G (2015) Dietary l-leucine supplementation enhances intestinal development in suckling piglets. Amino Acids 47(8):1517–1525
Sun KJ, Wu ZL, Ji Y, Wu G (2016) Glycine regulates protein turnover by activating protein kinase B/Mammalian target of rapamycin and by inhibiting MuRF1 and atrogin-1 gene expression in C2C12 myoblasts. J Nut 146:2461–2467
Szegezdi E, Logue SE, Gorman AM, Samali A (2006) Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep 7(9):880–885
Tossou MC, Liu H, Bai M, Chen S, Cai Y, Duraipandiyan V, Liu H, Adebowale TO, Al-Dhabi NA, Long L, Tarique H, Oso AO, Liu G, Yin Y (2016) Effect of high dietary tryptophan on intestinal morphology and tight junction protein of weaned pig. Biomed Res Int 2016:2912418
Turner JR (2009) Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9(11):799–809
Wang J, Wu Z, Li D, Li N, Dindot SV, Satterfield MC, Bazer FW, Wu G (2012) Nutrition, epigenetics, and metabolic syndrome. Antioxid Redox Signal 17(2):282–301
Wang W, Wu Z, Dai Z, Yang Y, Wang J, Wu G (2013) Glycine metabolism in animals and humans: implications for nutrition and health. Amino Acids 45(3):463–477
Wang W, Dai Z, Wu Z, Lin G, Jia S, Hu S, Dahanayaka S, Wu G (2014a) Glycine is a nutritionally essential amino acid for maximal growth of milk-fed young pigs. Amino Acids 46(8):2037–2045
Wang W, Wu Z, Lin G, Hu S, Wang B, Dai Z, Wu G (2014b) Glycine stimulates protein synthesis and inhibits oxidative stress in pig small intestinal epithelial cells. J Nutr 144(10):1540–1548
Wawryk-Gawda E, Chylinska-Wrzos P, Lis-Sochocka M, Chlapek K, Bulak K, Jedrych M, Jodlowska-Jedrych B (2014) P53 protein in proliferation, repair and apoptosis of cells. Protoplasma 251(3):525–533
Weinberg JM, Bienholz A, Venkatachalam MA (2016) The role of glycine in regulated cell death. Cell Mol Life Sci 73(11–12):2285–2308
Wijtten PJ, van der Meulen J, Verstegen MW (2011) Intestinal barrier function and absorption in pigs after weaning: a review. Br J Nutr 105(7):967–981
Wu G (2009) Amino acids: metabolism, functions, and nutrition. Amino Acids 37(1):1–17
Wu G (2010) Functional amino acids in growth, reproduction, and health. Adv Nutr 1(1):31–37
Wu G (2013) Functional amino acids in nutrition and health. Amino Acids 45(3):407–411
Wu G (2018) Principles of animal nutrition. CRC Press, Poca Raton
Wu G, Meier SA, Knabe DA (1996) Dietary glutamine supplementation prevents jejunal atrophy in weaned pigs. J Nutr 126(10):2578–2584
Wu G, Knabe DA, Kim SW (2004) Arginine nutrition in neonatal pigs. J Nutr 134(10 Suppl):2783S–2790S
Wu G, Bazer FW, Dai Z, Li D, Wang J, Wu Z (2014) Amino acid nutrition in animals: protein synthesis and beyond. Annu Rev Anim Biosci 2:387–417
Wu T, Lv Y, Li X, Zhao D, Yi D, Wang L, Li P, Chen H, Hou Y, Gong J, Wu G (2018) Establishment of a recombinant Escherichia coli-induced piglet diarrhea model. Front Biosci (Landmark Ed) 23:1517–1534
Yang H, Xiong X, Wang X, Tan B, Li T, Yin Y (2016) Effects of weaning on intestinal upper villus epithelial cells of piglets. PLoS One 11(3):e0150216
Yi H, Jiang D, Zhang L, Xiong H, Han F, Wang Y (2016) Developmental expression of STATs, nuclear factor-kappaB and inflammatory genes in the jejunum of piglets during weaning. Int Immunopharmacol 36:199–204
Yi D, Hou Y, Xiao H, Wang L, Zhang Y, Chen H, Wu T, Ding B, Hu CA, Wu G (2017) N-Acetylcysteine improves intestinal function in lipopolysaccharides-challenged piglets through multiple signaling pathways. Amino Acids 49(12):1915–1929
Yi D, Li BC, Hou YQ, Wang L, Zhao D, Chen HB, Wu T, Zhou Y, Ding BY, Wu G (2018) Dietary supplementation with an amino acid blend enhances intestinal function in piglets. Amino Acids 50:1089–1100
Zhong Z, Wheeler MD, Li X, Froh M, Schemmer P, Yin M, Bunzendaul H, Bradford B, Lemasters JJ (2003) L-Glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr Opin Clin Nutr Metab Care 6(2):229–240
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 31572412, 31572410, 31625025, 31272451 and 31272450), the Zhengzhou 1125 Talent Program, Agriculture and Food Research Initiative Competitive Grants (2014-67015-21770, 2015-67015-23276 and 2016-67015-24958) from the USDA National Institute of Food and Agriculture, and Texas A&M AgriLife Research (H-8200).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics statement
The studies were approved by China Agricultural University Institutional Animal Science and Technology College and conducted according to the Guidelines for Experimental Animal Research of the Ministry of Science and Technology (Beijing, China).
Informed consent
All authors have read and approved the final manuscript.
Additional information
Handling Editor: E. Closs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fan, X., Li, S., Wu, Z. et al. Glycine supplementation to breast-fed piglets attenuates post-weaning jejunal epithelial apoptosis: a functional role of CHOP signaling. Amino Acids 51, 463–473 (2019). https://doi.org/10.1007/s00726-018-2681-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-018-2681-9