Abstract
Purpose
Radiotherapy is widely used for cancer treatment but has harmful effects. This study aimed to assess the effects of L-arginine and glycine supplementation on the colon wall of rats submitted to abdominal irradiation.
Methods
Forty male Wistar rats were randomly divided into four groups: I—healthy, II—irradiated with no amino acid supplementation, III—irradiated and supplemented with L-arginine, and IV—irradiated and supplemented with glycine. The animals received supplementation for 14 days, with irradiation being applied on the eighth day of the experiment. All animals underwent laparotomy on the 15th day for resection of a colonic segment for stereologic analysis. Parametric and nonparametric tests were used for statistical analysis, with the level of significance set at p ≤0.05.
Results
Stereologic analysis showed that irradiation induced a reduction of the total volume of the colon wall of group II and III animals compared to healthy controls, but not of group IV animals supplemented with glycine. The mucosal layer of the irradiated animals of all groups was reduced compared to healthy group I animals, but supplementation with L-arginine and glycine was effective in maintaining the epithelial surface of the mucosal layer.
Conclusion
The present results suggest that glycine supplementation had a superior effect on the irradiated colon wall compared to L-arginine supplementation since it was able to maintain the thickness of the wall and the epithelial surface of the mucosa, whereas L-arginine maintained the partial volume of the epithelium and the epithelial surface, but not the total volume of the intestinal wall.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pelvic and abdominal radiotherapy is a widely used therapeutic modality for the treatment of cancers of the bowel, bladder, and rectum [1]. Several factors are involved in tissue response such as tumor sensitivity, localization, and oxygenation, as well as kind, quantity, and time of radiation exposure [2]. However, radiotherapy of the abdomen and pelvis generates inevitable damage to peritumoral normal tissues [2] and an increased risk of radiation enteritis and/or proctitis [3], evidenced by the destruction of crypt cells [4], reduction of villus height, epithelial ulceration, and necrosis [2, 5].
Tissues containing rapid renewal cells, such as those of the gastrointestinal mucosa require fast cell proliferation, are more susceptible to the acute toxic effects of radiotherapy [6] and can limit the response to treatment because of symptoms like diarrhea, abdominal pain, nausea, and vomiting [7, 8]. Another complication of radiotherapy is bacterial translocation caused by physical disruption of the intestinal mucosal barrier, increased intestinal permeability, formation of bacterial overgrowth, and breakdown of peristaltic clearance [9], which contributes to intestinal mucosa ulceration and sepsis [10].
Many kinds of diets have been tested to minimize the adverse effects of radiation by stimulating the immune response and tissue repair [9, 11]. Of these, nutrition supplements are the more extensively investigated [2, 9].
Arginine is a nonessential amino acid under normal circumstances [2]. It has various metabolic and immunologic effects and has been considered to be conditionally essential particularly under trauma and stress [9, 12, 13]. It has been suggested that its oral and enteral supplementation could have protective effects on the intestinal mucosa during the post-irradiation period, improving the healing and preventing bacterial translocation and weight loss, thus minimizing radiation enteritis [2, 9].
Glycine is the smallest amino acid and is classified as a nonessential amino acid [14, 15]. Its protective effect has been demonstrated in some experimental models such as alcoholic hepatitis [16], arthritis [17], ischemia [15, 18], hepatic transplantation, and general tumors [19]. The exact mechanism of action of glycine is not completely understood, but the inhibition of the activation of inflammatory and immune cells [20, 21], the reduction of the formation of free radicals and other toxic metabolites, and the prevention of mucosal permeability are some possibilities that can explain its benefits [15].
Ersin et al. [9] showed that arginine-enriched diets have protective effects on the gut mucosa of rats in the post-irradiation state. Diestel et al. [22] observed that glycine supplementation is relevant in maintaining the total volume of the colonic wall in rats submitted to abdominal radiation. However, Hwang et al. [2] and Hall [14] observed fewer benefits with arginine and glycine supplementation, respectively. Thus, the data about the action of L-arginine and glycine in the protection and repair of acute actinic enteritis are insufficient and inconclusive, especially regarding the repair of the colonic wall after radiotherapy.
Methods
The study was approved by the Ethics Committee on Animal Research of the Biology Institute Roberto Alcântara Gomes, Rio de Janeiro State University, Brazil. All procedures rigorously followed current guidelines for animal experimentation [23].
Animals
Forty adult male Wistar rats weighing 270–330 g were randomly divided into four groups of ten animals each: I (healthy)—animals not submitted to abdominal radiation, II (control)—irradiated rats receiving no amino acid supplementation, III—irradiated rats receiving L-arginine supplementation throughout the study, and IV—irradiated rats receiving glycine supplementation throughout the study.
All rats were housed in cages with five animals each, in a room with controlled temperature and humidity, and exposed to 12-h day/night cycles. The animals were allowed to feed on standard commercial rat chow, with free access to water. Chow intake and body weight were controlled daily, always at the same time of day.
Irradiation
Group I healthy animals were maintained under standard laboratory conditions, while animals belonging to the other groups were submitted to abdominal irradiation on the eighth day of the experiment. Animals were irradiated with a single dose of 1,164 cGy applied to the abdomen, with the thorax, head, and extremities being shielded off. A 6 × 4-cm X-ray field, source-to-skin distance of 100 cm, was centered on the abdomen from the xyphoid process to the pubis. The dose was estimated to deliver 1,164 cGy at 240 cGy/min (total thickness = 3 cm).
Amino acid supplementation
Group III animals received supplementation with L-arginine (L-arginine®—Sigma Aldrich) and group IV received glycine (Glicina®, Vetec Química Fina Ltda) once a day for the 14 days of the study at a dose of 0.65 g/kg per day. The dose was prepared in an aqueous solution at 4% concentration in a volume of 5 ml and was administered intragastrically as a bolus with an orogastric catheter. Group I and II animals received 5 ml of water similarly administered once a day for the 14 days of the study.
Resection of colonic segments
On the 15th day of the experiment, the animals were anesthetized with an intraperitoneal injection of sodium thiopental (50 mg/kg). A centimeter-graduated catheter was introduced through the anal orifice as far as the exact site for resection of a circumferential colonic segment located between 6 and 8 cm from the anal sphincter. Next, the animals underwent abdominal trichotomy, antisepsis with iodopovidine, and placement of surgical fields, followed by laparotomy with resection of the previously demarcated colonic segment. Laparorrhaphy was carried out on two planes (peritoneal–aponeurotic and skin) using continuous 3-0 polyglecaprone 25 sutures. At the end of the procedure, the animals were killed with an anesthetic overdose.
Histological processing
The circumferential colonic segments were opened longitudinally, washed in physiological solution to remove fecal remains, and extended over a cork. The tissue was fixed in 10% buffered formalin and subsequently processed with increasing alcohol and xylol concentrations, embedded in paraffin, and cut into 4-μm-thick slices. Slides prepared from these slices (eight from each animal) were stained with Gomori trichrome and analyzed by light microscopy.
Stereological analysis
A cycloid test system with 35 cycloid arcs and 70 points was used for sampling [24]. In the colonic segment, the horizontal plane was considered as the limit of the mucosal and submucosal layers to the anisotropy compensation. Briefly, the total volume of the colonic wall (V) and partial volumes of the mucosa (Vvm), muscularis mucosae (Vvmm), submucosa (Vvsm), and muscularis propria (Vvmp) were studied at 100× magnification. The volume of the colonic wall (V) corresponded to the number of test system points hitting the structure, and the densities (Vv) were obtained as the ratio between the points hitting the structures (Pp) and the number of points on the total colon wall (V).
The partial volumes of the epithelium and lamina propria were studied at 400× magnification, and the volume densities (Vv) were obtained as the ratio between the points hitting the structures (Pp) and the total number of points in the test system (P T = 70). The surface density of the epithelium was estimated as Sv = 2I/L T, where I is the number of intersections of the Lieberkühn crypts and the cycloid arcs, and L T is the total test-line length [25].
Statistical analysis
Data regarding percent body weight variation and chow intake were analyzed statistically by analysis of variance (ANOVA) and by Tukey’s post test. Stereological evaluation data were analyzed by the Kruskal–Wallis test and by Dunn’s post test. The level of significance was set at p ≤0.05 in all analyses. Statistical analyses were performed with the GraphPad Prism 4®—2003 software.
Results
In group III, one animal died during the post-irradiation period. In this group, nine animals were used for analysis.
During the experiment, percent body weight loss did not differ significantly between the animal groups submitted to abdominal irradiation, but was significantly reduced in these groups compared to healthy animals (Table 1). After irradiation, a significant decrease in chow intake was observed, resulting in a significantly lower total chow intake by the rats of the same groups compared to healthy animals (Table 1). Although percent body weight loss was similar between these groups, animals of group IV (irradiated and supplemented with L-arginine) showed a significantly lower intake compared to group II (irradiated and not supplemented) (Table 1).
The total volume of the colonic wall was significantly decreased in animals of groups II (irradiated and not supplemented) and III (irradiated and supplemented with L-arginine) when compared to healthy animals (p < 0.01 and p < 0.001, respectively). The rats of group IV (irradiated and supplemented with glycine) showed an increase in total volume of the colonic wall when compared to animals of group III (p < 0.05), but with no difference when compared to healthy animals of group I (Figs. 1 and 2).
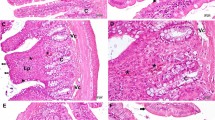
Photomicrographs of the colonic wall of the different study groups. Control (a), Irradiated (Irr) (b), I + L-arginine (c), and I + Glycine (d). Bar = 200 μm (×100). V total volume, Vvm partial volume of mucosa, Vvmm partial volume of muscularis mucosae, Vvsm partial volume of submucosa, Vvmp partial volume of muscularis propria
Analysis of the partial volumes of the histological layers of the colonic wall revealed a reduction of the mucosal layer in animals of the irradiated groups (II, III, and IV) compared to healthy group I rats (p < 0.05, p < 0.01, and p < 0.001, respectively) (Fig. 3a). Regarding the partial volume of the muscularis mucosae layer, a significant decrease was observed in animals of group II when compared to the healthy animals (p < 0.001) and to group IV (p < 0.01) (Fig. 3b). The partial volume of the submucosal layer of group IV animals was increased when compared to groups I (p < 0.01) and II (p < 0.05) (Fig. 3c). The volume of the muscularis propria layer was significantly decreased in group III rats compared to groups I and IV (p < 0.001 and p < 0.01) (Fig. 3d).
Partial epithelial volume was reduced in group II and IV animals compared to group I (p < 0.001 and p < 0.01), and this reduction was also observed in group II compared to group III (p < 0.05) (Figs. 4a and 5).
Partial lamina propria volume was increased in groups II and IV compared to group I (p < 0.001 and p < 0.01, respectively). An increase in lamina propria volume was also observed in group II compared to group III (p < 0.05) (Figs. 4b and 5). The epithelial surface of the colonic mucosa was significantly decreased in group II compared to groups I and III (p < 0.01). No other differences were observed between the other groups (Fig 6).
Discussion
Radiotherapy can cause numerous acute or chronic side effects in healthy tissues adjacent to the irradiated area, such as the intestine [26, 27]. In this context, some alternatives have been investigated to minimize these effects, preventing and/or repairing the structural and histological alterations [2, 8, 28].
L-arginine stimulates growth hormone secretion and collagen synthesis [29], characterizing its participation in the healing process, and acts in the modulation of the immune system by stimulating the function of macrophages and neutrophils [30].
Glycine has been used as a control amino acid in isonitrogenous experimental models in studies mainly focusing on supplementation with another amino acids [20, 31, 32]. However, some authors have recently described some benefits that can be obtained by glycine supplementation [14, 15, 33], in addition to its well-known role in inhibiting free-radical formation [14] that could possibly prevent damage due to radiotherapy.
There is still no consensus about the ideal dose for dietary supplementation with these amino acids in animals. L-arginine was used at different doses in similar studies with rats submitted to abdominal irradiation [2, 8]. Gurbuz et al. used it at doses of 2–4% of the total energetic value for 7 days after irradiation with a single dose of 1,100 cGy [12]. Hwang et al. used it at doses of 2% of water intake by animals irradiated with the same radiation dose as cited above [2], and Ersin et al. studied animals supplemented 7 days before and 7 days after irradiation with amino acids and irradiation doses similar to those used by Gurbuz et al. [8]. In humans, the recommended dose of supplementation is about 4–6% of the total energetic value, and an increase in arginine intake from 5% to 7% of total amino acids may result in immunomodulatory effects [34].
There is no standardization of glycine dose in human or experimental trials, but glycine has been continuously used at different doses and many times without being the main study subject [12, 21]. Jacob et al. and Kallakuri et al. used this amino acid at a dose of 0.5–1.0 g/kg weight in a study about damage caused by ischemia and reperfusion [18]. Glycine was also used in another study on the same subject at a dose of 5% of the diet offered [35]. In our laboratory, we have been using these and other amino acids at a dose of 0.65 g/kg/per day in several studies.
Here, we used a single irradiation dose of 1,164 cGy, already described by various authors as being able to cause actinic enteritis and bacterial translocation in rats [2, 8, 12]. Animals began to have diarrhea around the third day after irradiation, as also reported by other authors [21, 36, 37]. We may suggest that this effect can be due to the loss of epithelium integrity associated with changes in motility and with increased intestinal secretions [36]. Furthermore, around the third day after irradiation, inflammatory signs and intestinal mucosal ulceration became evident [12], events that can also contribute to diarrhea.
All animals submitted to abdominal irradiation, with or without amino acid supplementation, ingested less chow during the study when compared to healthy animals, as also observed by Klimberg et al. and Diestel et al. [22, 31]. This decrease can be attributed to anorexia and to other side effects (diarrhea and abdominal pain) commonly observed in actinic enteritis [6]. As observed by Diestel et al. [22], the percent weight loss of all irradiated groups was similar and significant compared to the control (healthy) group, which showed weight gain.
We observed a significant decrease in total volume of the colonic wall in irradiated (group II) but not supplemented (groups III and IV) animals compared to healthy animals, corroborating similar studies [2, 22]. Also in humans, Luccichenti et al. observed a decrease in colonic wall thickness by a computed tomography study after radiotherapy for colon cancer treatment [38]. This event can be the result of an increase of cell apoptosis and of the free-radical effect induced by irradiation [38].
When we separately assessed each layer in the irradiated group without amino acid supplementation, we observed that mucosa and muscular layer volume showed a significant decrease compared to the healthy group, probably due to the acute toxic effects of radiotherapy that are observed more often in tissues with rapid turnover such as the gastrointestinal mucosa [6]. The decrease of the colonic mucosa layer contributes to atrophy of the intestinal epithelium [39], which was observed in this study as a partial decrease of mucosal epithelium volume, but not of lamina propria volume. The latter was actually increased, probably due to the acute inflammation caused by radiotherapy.
L-arginine supplementation was inefficient in maintaining the total volume of the colonic wall after irradiation in group III, which behaved similarly to group II (irradiated without amino acid supplementation). Regarding the colonic wall layers, a decrease in mucosal layer volume was observed in this same group compared to the healthy group, similar to group II. Also, this supplementation was not effective in maintaining mucosal layer thickness, possibly because L-arginine is not the major substrate for the intestinal epithelium, confirming the results of Ersin et al. [9].
Many of the functions of L-arginine are based on the action of its metabolites, mainly nitric oxide (NO), produced by nitric oxide synthase (NOS) that exists as inductive (iNOS) and constitutive forms (cNOS) [40]. The inductive form, as the name implies, should be induced and is usually activated by cytokines and endotoxins under stress such as that induced, for example, by radiotherapy, and produces large quantities of NO for extended periods. This can lead to intestinal mucosal disorders, inhibition of cell proliferation [41], and increased apoptosis [42]. iNOS also increases the expression of tumor necrosis factor-alpha (TNF-α), which contributes to apoptosis [43].
Nitric oxide synthase also works by regulating the action of arginase since they compete for the same substrate, L-arginine [44]. This enzyme metabolizes L-arginine to ornithine [45], which is the forerunner of proline, an important collagen amino acid.
Arginase acts on muscle cells inducing collagen production. We may speculate that the increase in NO production induced by iNOS led to NO inhibition, which in turn led to a significant decrease in muscle layer volume in group III rats compared to healthy rats.
The mucosal layer is the most affected by radiation [46], as shown by the partial volume of the epithelium and of the lamina propria. Due to the action of NO, L-arginine in particular plays an essential role in inflammation and in the regulation of immunity [45].
Supplementation with L-arginine induces an increase in monocyte and lymphocyte proliferation and improves T-helper cell formation and natural killer cell activity, as well as macrophage cytotoxicity, phagocytic activity, and cytokine production [41]. These cell defense properties have led to the investigation of L- in many studies involving immunomodulation [2, 8, 29, 41] and contributed to the understanding of the reason why the partial volume of the epithelium and lamina propria of L-arginine-supplemented animals (group III) was similar to that of healthy animals (group I), with maintenance of the epithelial surface. Nevertheless, when these animals were compared to group II (irradiated without supplementation), a significant increase in the partial volume of the epithelium and of the epithelial surface was observed, as well as a decrease in the lamina propria, possibly caused by the anti-inflammatory effect of amino acid supplementation.
Glycine was efficient in maintaining the total volume of the irradiated colonic wall, in agreement with a previous study [22]. As observed in the other irradiated groups (II and III), glycine supplementation was also unable to maintain the mucosal layer volume, which is due to this higher systemic action [14, 15] and to the fact that the intestinal epithelium is not the predominant substrate of glycine (similar to L-arginine).
Another factor that influenced this result was the supplementation dose. Diestel et al. used a higher dose of this amino acid (1 g/kg/day) in this same irradiated model and observed the maintenance of this parameter compared to healthy rats [22].
Glycine is a component of collagen structure [14] and is synthesized by smooth muscle cells which deposit this protein in the submucosa [47]. With this increased availability due to supplementation, a probable increase in collagen production occurred after irradiation. Possibly this was the reason for the maintenance of muscle layer thickness (group III) and for the significant increase of the submucosal layer compared to group I [48].
In the mucosal layer, the epithelial volume was decreased and the lamina propria volume was increased compared to non-irradiated rats (group I), corroborating other results, although the epithelial surface was maintained [22]. Despite previous literature reports, the immunomodulation properties of glycine [14] did not show such benefits in this experiment. Due to the scarcity of experimental studies and models using this amino acid as the main research subject, the doses and time necessary for this full performance have not been established. The architecture of the mucosal layer was maintained in the supplemented groups (glycine and L-arginine), even with less partial volume, in comparison to healthy animals. Our results suggest that glycine supplementation had greater effects on the structure of the colonic wall compared to L-arginine supplementation since it was able to maintain the thickness of the wall and the epithelial surface of the mucosa, whereas L-arginine maintained the partial volume of the epithelium and the epithelial surface, but not the total volume of the intestinal wall.
Additional studies are needed to determine the efficacy of different amino acid doses over different periods of time, and the use of higher and fractionated irradiation doses, which may eventually provide data regarding the clinical applicability of supplementation with glycine and other amino acids for the repair of irradiated colonic mucosa and for the treatment of other colonic diseases.
References
Carroll MP, Zera RT, Roberts JC, Schlafmann SE, Feeney DA, Johnston GR et al (1995) Efficacy of radioprotective agents in preventing small and large bowel radiation injury. Dis Colon Rectum 38:716–722
Hwang JM, Chan DC, Chang TM, Tsao TY, Tsou SS, Lu RH, Tsai LM (2003) Effects of oral arginine and glutamine on radiation-induced injury in the rat. J Surg Res 109:149–154
Bismar MM, Sinicrope FA (2002) Radiation enteritis. Curr Gastroenterol Rep 4:361–365
Diestel CF, Lopes-Paulo F, Marques RG, Horst NL, Caetano CE (2005) Effect of oral supplement of L-glutamine in colonic wall of rats subjected to abdominal irradiation. Acta Cir Bras 20(1):S2005–S2095
Marks G, Mohiudden M (1983) The surgical management of the radiation-injured intestine. Surg Clin North Am 63:81–96
Guzman-Stein G, Bonsack M, Liberty J, Delaney JP (1989) Abdominal radiation causes bacterial translocation. J Surg Res 46:104–107
Andreyev HJ (2007) Gastrointestinal problems after pelvic radiotherapy: the past, the present and the future. Clin Oncol 19:790–799
Linard C, Grémy O, Benderitter M (2008) Reduction of peroxisome proliferation activated receptor gamma expression by gamma-irradiation as a mechanism contributing to inflammatory response in rat colon: modulation by the 5-aminosalicylic acid agonist. J Pharmacol Exp Ther 324:911–920
Ersin S, Tuncyurek P, Esassolak M, Alkanat M, Buke C, Yilmaz M et al (2000) The prophylactic and therapeutic effects of glutamine and arginine enriched diets on radiation-induced enteritis in rats. J Surg Res 89:121–125
Xavier RJ, Podolsky DK (2007) Unravelling the pathogenesis of inflammatory bowel disease. Nature 448:427–434
Barbul A, Lazarou SA, Efron DT, Wasserkrug HL, Efron G (1990) Arginine enhances wound healing and lymphocyte immune responses in humans. Surgery 108:331–336
Gurbuz AT, Kunzelman J, Ratze EE (1998) Supplemental dietary arginine accelerates intestinal mucosal regeneration and enhances bacterial clearance following radiation enteritis in rats. J Surg Res 74:149–154
Morris SM (2004) Enzymes of arginine metabolism. J Nutr 134(10 Suppl):2743S–2747S
Hall JC (1998) Glycine. J Parenter Enteral Nutr 22:393–398
Zhong Z, Wheeler MD, Li X, Froh M, Schemmer P, Yin M et al (2003) L-glycine: a novel anti-inflammatory, immunomodulatory, and cytoprotective agent. Curr Opin Clin Nutr Metab Care 6:229–240
Limuro Y, Bradford BU, Yamashina S, Rusyn I, Nakagamy M, Enomoto N et al (2000) The glutathione precursor L-2-oxothiazolidine-4-carboxylic acid protects against liver injury due to chronic enteral ethanol exposure in the rat. Hepatology 31:391–398
Li X, Bradford BU, Wheeler MD, Stimpson SA, Pink HM, Brodie TA et al (2001) Dietary glycine prevents peptidoglycan polysaccharide-induced reactive arthritis in the rat: role for glycine-gated chloride channel. Infect Immun 69:5883–5891
Jacob T, Ascher E, Hingorani A, Kallakuri S (2003) Glycine prevents the induction of apoptosis attributed to mesenteric ischemia/reperfusion injury in a rat model. Surgery 134:457–466
Yamashina S, Konno A, Wheeler MD, Rusyn I, Rusyn EV, Cox AD et al (2001) Endothelial cells contain a glycine-gated chloride channel. Nutr Cancer 40:197–204
Ikejima K, Iimuro Y, Forman DT, Thurman RG (1996) A diet containing glycine improves survival in endotoxin shock in the rat. Am J Physiol 271:G97–G103
Stachlewitz RF, Li X, Smith S, Bunzendahl H, Graves LM, Thurman RG (2000) Glycine inhibits growth of T lymphocytes by an IL-2-independent mechanism. J Immunol 164:176–182
Diestel CF, Marques RG, Lopes-Paulo F, Paiva D, Horst NL, Caetano CE, Portela MC (2007) Role of L-glutamine and glycine supplementation on irradiated colonic wall. Int J Colorectal Dis 22:1523–1529
Marques RG, Morales MM, Petroianu A (2009) Brazilian law for scientific use of animals. Acta Cir Bras 24:69–74
Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A et al (1988) Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. Acta Pathol Microbiol Immunol Scand 96:379–394
Mandarim-de-Lacerda CA (2003) Stereological tools in biomedical research. An Acad Bras Cienc 75:469–486
Breiter N, Trott KR (1986) The pathogenesis of the chronic radiation ulcer of the large bowel in rats. Br J Cancer Suppl 7:29–30
Nielsen OH, Vainer B, Rask-Madsen J (2008) Non-IBD and noninfectious colitis. Nat Clin Pract Gastroenterol Hepatol 5:28–39
Shukla J, Chatterjee S, Thakur VS, Premachandran S, Checker R, Poduval TB (2009) L-arginine reverses radiation-induced immune dysfunction: the need for optimum treatment window. Radiat Res 171:180–187
Shang HF, Wang YY, Lai YN, Chiu WC, Yeh SL (2004) Effects of arginine supplementation on mucosal immunity in rats with septic peritonitis. Clin Nutr 23:561–569
Moinard C, Caldefie-Chezet F, Walrand S, Vasson MP, Cynober L (2002) Evidence that glutamine modulates respiratory burst in stressed rat polymorphonuclear cells through its metabolism into arginine. Br J Nutr 88:689–695
Klimberg VS, Salloum RM, Kasper M, Plumley DA, Dolson DJ, Hautamaki RD et al (1990) Oral glutamine accelerates healing of the small intestine and improves outcome after whole abdominal radiation. Arch Surg 125:1040–1045
Souba WW, Klimberg VS, Hautamaki RD, Mendenhall WH, Bova FC, Howard RJ et al (1990) Oral glutamine reduces bacterial translocation following abdominal radiation. J Surg Res 48:1–5
Tsune I, Ikejima K, Hirose M, Yoshikawa M (2003) Dietary glycine prevents chemical-induced experimental colitis in rat. Gastroenterology 125:775–785
Stein J, Boehles HJ, Blumenstein I, Goeters C, Schulz R (2009) Amino acids—guidelines on parenteral nutrition, chapter 4. Ger Med Sci 18:7, Doc24
Lee MA, McCauley RD, Kong SE, Hall JC (2002) Influence of glycine on intestinal ischemia–reperfusion injury. J Parenter Enteral Nutr 26:130–135
Picard C, Wysocki J, Fioramonti J, Griffiths NM (2001) Intestinal and colonic motor alterations associated with irradiation-induced diarrhoea in rats. Neurogastroenterol Motil 13:19–26
Huang EY, Wang CJ, Hsu HC, Sun LM (2006) Characteristics and predictive factors of early-onset diarrhoea during pelvic irradiation. Br J Radiol 79:419–424
Luccichenti G, Cademartiri F, Sianesi M, Roncoroni L, Pavone P, Krestin GP (2005) Radiologic assessment of rectosigmoid cancer before and after neoadjuvant radiation therapy: comparison between quantitation techniques. Am J Roentgenol 184:526–530
Fajardo LF (2005) The pathology of ionizing radiation as defined by morphologic patterns. Acta Oncol 44:13–22
Salzman AL (1995) Nitric oxide in the gut. New Horiz 3:352–364
Suchner U, Heyland DK, Peter K (2002) Immune-modulatory actions of arginine in the critically ill. Br J Nutr 87(Suppl 1):S121–S132
Liu XM, Chapman GB, Peyton KJ, Schafer AI, Durante W (2002) Carbon monoxide inhibits apoptosis in vascular smooth muscle cells. Cardiovasc Res 55:396–405
Grémy O, Benderitter M, Linard C (2008) Acute and persisting Th2-like immune response after fractionated colorectal γ-irradiation. World J Gastroenteol 14:7075–7085
Durante W, Johnson FK, Johnson RA (2007) Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol 34:906–911
Albina JE, Abate JA, Mastrofrancesco B (1993) Role of ornithine as a praline precursor in healing wounds. J Surg Res 55:97–102
Igaki H, Nakagawa K, Uozaki H, Akahane M, Hosoi Y, Fukayama M et al (2008) Pathological changes in the gastrointestinal tract of a heavily radiation-exposed worker at the Tokai-mura criticality accident. J Radiat Res 49:55–62
Matthes H, Herbst H, Schuppan D, Stallmach A, Milani S, Stein H et al (1992) Cellular localization of procollagen gene transcripts in inflammatory bowel diseases. Gastroenterology 102:431–442
Wang J, Zheng H, Hauer-Jensen M (2001) Influence of short-term octreotide administration on chronic tissue injury, transforming growth factor beta (TGF-beta) overexpression, and collagen accumulation in irradiated rat intestine. J Pharmacol Exp Ther 297:35–42
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was partially supported by a grant from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Brazil.
Rights and permissions
About this article
Cite this article
de Aguiar Picanço, E., Lopes-Paulo, F., Marques, R.G. et al. L-arginine and glycine supplementation in the repair of the irradiated colonic wall of rats. Int J Colorectal Dis 26, 561–568 (2011). https://doi.org/10.1007/s00384-011-1154-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-011-1154-3