Abstract
Hypermethioninemia is a condition defined as elevated plasma methionine levels and may be a consequence of different conditions that include non-genetic and genetic causes. In severe cases, hypermethioninemia may lead to development of neurological and hepatic impairments, but mechanisms are still not well elucidated. Therefore, this review aims to reunite the knowledge acquired about the methionine-induced brain and liver toxicity focusing on the results obtained by studies from patients, in vitro experiments, and in vivo animal models. In general, some studies have shown that methionine decreases Na+,K+-ATPase activity, induces oxidative stress, increases acetylcholinesterase activity, and leads to dendritic spine downregulation in brain. Concerning to liver, hypermethioninemia seems to provoke changes in cell morphology, lipid accumulation, oxidative stress, inflammation, and ATP depletion. It is possible to infer that oxidative damage is one of the most important mechanisms responsible for methionine toxicity, since different studies showed that this amino acid induces oxidative stress in brain and liver tissues. Besides, reactive oxygen species may mediate other alterations induced by methionine, such as the reduction in brain Na+,K+-ATPase activity, and liver inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Roles of methionine
Methionine (Met) is an essential sulfur-containing amino acid obtained from diet or degradation of endogenous proteins. Some of the main functions of Met in organism include: production of its derivative molecules cysteine, glutathione, carnitine, taurine, and creatine (Wesseling et al. 2009; Wyss and Kaddurah-Daouk 2000; Crill and Helms 2007), protein synthesis since Met composes proteins and peptides and is the only natural initiating amino acid in the eukaryotic translation (Lucas-Lenard 1971), as well as donation of its methyl group to a variety of molecules such as nucleic acids, histones, amino acids, and lipid-derivatives (Chiang et al. 1996).
Besides, it has been reported that Met residues in proteins also provide antioxidant protection since they are often positioned so that they establish an interaction, through hydrophobic bond, between their sulfur atoms and the rings of aromatic amino acids (Valley et al. 2012), which are much susceptible to oxidation by reactive species (El Refaey et al. 2015). Furthermore, the oxidation of surface exposed Met protects the other residues because reactive species may oxidize Met to Met sulfoxide, which may be reduced back by the enzyme Met sulfoxide reductase (Brot et al. 1981).
Metabolism of methionine
Met is mainly metabolized in the liver by the enzyme Met adenosyltransferase (MAT, EC 2.5.1.6), which is present in three isoforms. MAT I and III are encoded by the same gene MAT1A and predominate in adult liver. MAT II activity is present at smaller amount in adult liver and its activity is predominant in non-hepatic tissues, fetal liver, and hepatocellular carcinoma (Frago et al. 1998; Horikawa et al. 1990, 1993; Okada et al. 1981; Gil et al. 1996; Cai et al. 1996). This enzyme transfers the adenosyl group from ATP to Met, forming S-adenosylmethionine (AdoMet) and tripolyphosphate. AdoMet is reacquired as a methyl donor in reactions that include methylation of nucleic acids, proteins, and lipids. The product of AdoMet transmethylation is the S-adenosylhomocysteine (AdoHcy), which is hydrolyzed by AdoHcy hydrolase (AHCY, EC 3.3.1.1), resulting in homocysteine (Hcy) formation (Mudd 1962; Cantoni 1953; Finkelstein 1990; de la Haba and Cantoni 1959).
Hcy can be metabolized by two different pathways: remethylation or transsulfuration. Remethylation is catalyzed by Met synthase (MS, EC 2.1.1.13), a vitamin B12-dependent enzyme that regenerates Met by transferring a methyl group to Hcy. The methyl group is derived from the endogenous 5-methyltetrahydrofolate (5-methyl-THF), which is formed during the metabolism of folic acid. Additionally, betaine-Hcy-methyltransferase (BHMT) uses betaine derived from choline as a methyl donor for Hcy remethylation, which is considered a salvage pathway when toxins compromise the action of MS. BHMT transfers the methyl group from betaine to Hcy, forming Met and N,N-dimethylglycine (DMG). Transsulfuration pathway catalyzes the condensation of Hcy with serine to form cystathionine through the action of a vitamin B6-dependent enzyme named cystathionine β-synthase (CBS, EC 4.2.1.22). Cystathionine is then converted to α-ketobutyrate and cysteine by the enzyme γ-cystathionase, which is also dependent of vitamin B6. Therefore, transsulfuration pathway is a very important source of non-enzymatic antioxidant protection to the liver, since it forms cysteine, the precursor of glutathione (Finkelstein 2000; Selhub 1999; Beatty and Reed 1980; Mosharov et al. 2000). The Met/Hcy cycle is shown in Fig. 1.
Pathways of Met metabolism in mammals. MAT Methionine adenosyltransferase, AdoMet S-adenosylmethionine, AdoHcy S-adenosylhomocysteine, AHCY S-adenosylhomocysteine hydrolase, CBS cystathionine β-synthase, 5,10-methylene-THF 5,10-methylenetetrahydrofolate, 5-methyl-THF 5-methyltetrahydrofolate, THF tetrahydrofolate, BHMT betaine-homocysteine-methyltransferase, DMG N,N-dimethylglycine
In cerebral tissue, Met is primarily metabolized through remethylation pathway. Some years ago, data published in literature indicated that the transsulfuration was incomplete in the brain due to absence of the enzyme γ-cystathionase, leading to cystathionine accumulation in this organ (Finkelstein 1998). However, Vitvitsky et al. (2006) have demonstrated the existence of a functional transsulfuration pathway in human neurons and astrocytes and in mouse brain, suggesting that this may contribute to the protection under oxidative stress conditions through brain glutathione synthesis.
Hypermethioninemia
Normal plasma concentration of Met range from 13 to 45 µM (Stabler et al. 2002). Hypermethioninemia occurs when Met levels increase in blood, which may be a consequence of different conditions. Non-genetic causes for hypermethioninemia include liver disease, premature birth (frequently transient), and diet rich in proteins, which may increase plasma Met levels to 1206 µM when protein intake achieves 7 g/kg/day. On the other hand, hypermethioninemia from genetic causes (hereditary conditions) includes: MAT I/III deficiency, classical homocystinuria (due to CBS deficiency), deficiencies of glycine N-methyltransferase (GNMT, EC 2.1.1.49), AHCY, citrin, and fumarylacetoacetate hydrolase (tyrosinemia type I) (Mudd 2011; Levy et al. 1969).
A characteristic that distinguishes MAT I/III deficiency from GNMT, AHCY, and CBS deficiencies is that the first one leads to isolated hypermethioninemia, with plasma Met reaching levels from 600 to 2541 µM in patients with homozygous mutations (Mudd et al. 1995; Chamberlin et al. 1996; Nagao and Oyanagi 1997). The term isolated hypermethioninemia designates elevated plasma Met levels which are not associated with the increase in Met metabolites, including AdoMet, AdoHcy, Hcy, and cystathionine. As exception, patients with severe MAT I/III deficiency may have plasma Hcy slightly elevated, but the mechanisms involving this effect are still not well understood (Stabler et al. 2002; Lagler et al. 2000). Besides, MAT I/III deficiency may lead to decreased AdoMet, while the other causes of hypermethioninemia often enhance AdoMet levels (Mudd 2011). Therefore, the reader should be clarified that the effects of hypermethioninemia may differ depending on the cause, since AdoMet may be involved in the pathological effects either when increased or decreased.
Pathological effects of hypermethioninemia
Met is crucial for normal growth and development, but when this amino acid and/or its metabolites are present at abnormally elevated plasma levels, potentially toxic events may occur. Although it may be asymptomatic, hypermethioninemia can cause the following pathological effects: myopathy, hypotonia, altered erythrocyte morphology with consequent splenic hemosiderosis, facial dysmorphia associated to abnormal teeth and hair, anorexia and digestive disturbances, development of neurological problems (tremor, dystonia, and cognitive deficit), and/or liver diseases (Chamberlin et al. 1996; Gaull et al. 1981a; Guízar Vázquez et al. 1980; Benevenga and Steele 1984; Higashi 1982; Lynch and Strain 1989; Labrune et al. 1990; Gout et al. 1977; Chamberlin et al. 1997; Harvey Mudd et al. 2003; Mudd et al. 2001). In view of severity of the symptoms, this review will empathize the neurological and hepatic effects of hypermethioninemia.
Neurological effects
The increase in Met levels can be toxic to the brain regardless of the cause. In general, patients with severe hypermethioninemia may present neurological dysfunction, including mental retardation and cognitive deficit. It has been also reported that cerebral edema may be observed during CBS and MAT I/III deficiencies and during excessive Met diet when plasma Met achieves levels extremely elevated (Harvey Mudd et al. 2003; Mudd et al. 2001; Braverman et al. 2005). However, the mechanisms involved in these alterations are still not well elucidated. In the attempt to understand such mechanisms, some studies have been developed.
Na+,K+-ATPase activity and oxidative stress
Na+,K+-ATPase plays a crucial role in maintaining the ionic gradient required for neuronal excitability and regulation of neuronal cell volume through the transport of Na+ and K+ ions in the nervous system (Glynn 1985). Inhibition of this enzyme may induce brain edema, neuronal death, and impairment of learning and memory (Wyse et al. 2004; de Lores Arnaiz and Ordieres 2014). In this context, the decrease in brain Na+,K+-ATPase activity seems to be involved in neurological diseases, such as dystonia (Cannon 2004), Alzheimer disease (Zhang et al. 2013), bipolar affective disorder (Mynett-Johnson et al. 1998), ischemia (de Souza Wyse et al. 2000), epilepsy (Grisar et al. 1992), depressive disorders in rats (Gamaro et al. 2003; Acker et al. 2009), hyperprolinemia (Ferreira et al. 2011), and phenylketonuria (Wyse et al. 1999).
Oxidative stress is characterized by an imbalance between reactive oxygen species (ROS) and the cellular antioxidant defenses that include non-enzymatic protection, such as vitamins C and E and reduced glutathione, and enzymatic protection, such as glutathione peroxidase, superoxide dismutase (SOD), and catalase (CAT) (Apel and Hirt 2004). Increased ROS production can directly cause tissue damage and lead to inflammation process (Geronikaki and Gavalas 2006). Besides, Na+,K+-ATPase activity may be affected by ROS through lipid peroxidation and sulfhydryl groups oxidation.
In this context, an in vitro study showed that Met inhibits Na+,K+-ATPase in synaptic plasma membrane from hippocampus of rats (Streck et al. 2002a). Posteriorly, Stefanello et al. (2005) verified that the preincubation of hippocampal homogenates with antioxidants (glutathione and tocopherol) prevented the inhibitory action of Met on Na+,K+-ATPase. In the same work, the evaluation about the in vitro effects of Met on some parameters of oxidative stress demonstrated that this amino acid caused lipoperoxidation and reduced non-enzymatic antioxidant capacity in rat hippocampus. Together, these results suggest that Met-induced Na+,K+-ATPase inhibition is possibly mediated by free radical formation.
Therefore, Stefanello et al. (2007a) extended the investigations and developed an in vivo model for hypermethioninemia in which developing Wistar rats receive injections of Met leading to concentrations approximately 30-fold the control levels. Using this experimental model, it was demonstrated that both chronic and acute administration of Met lead to lipoperoxidation and decreased Na+,K+-ATPase activity in Wistar rat hippocampus. Since Na+,K+-ATPase is embedded in cellular membrane, it is possible that peroxidative process could provoke changes of fluidity or other membrane properties, prejudicing the enzyme functioning and decreasing its activity (Stefanello et al. 2007b).
In a further study, Stefanello et al. (2007c) also demonstrated that chronic injections of Met significantly reduced Na+,K+-ATPase activity in rat cerebral cortex accompanied by reduced amount of gangliosides (GM1, GD1a, GD1b, and GT1b), phospholipids (sphingomyelin, phosphatidylcholine, and phosphatidylethanolamine) and cholesterol. Lipoperoxidative process was also observed, strengthening the hypothesis that oxidative damage of the cellular membrane lipids could provoke changes in lateral assembly of glycosphingolipids, unsaturated glycerophospholipids and cholesterol, leading to alteration in Na+,K+-ATPase activity.
The neurotoxic effects of Met were also demonstrated in Sprague–Dawley rats submitted to a Met-enriched diet during 8 weeks. The results from this study showed an enhance in the activity of the antioxidant enzyme SOD in cerebral cortex of the rats fed on 1 and 5 % Met, suggesting a metabolic adjustment to combat a possible augment in ROS production. This alteration was accompanied by apparent impairment of locomotor skills and synaptic plasticity in rats fed on 5 % Met (Viggiano et al. 2012).
More recently, an animal model for maternal hypermethioninemia was developed. In this study, pregnant Wistar rats received injections of Met during gestational period. The administration of 2.68 μmol Met/g body weight increased encephalon Met levels (without Hcy elevation) in the offspring. Decrease in the activities of Na+,K+-ATPase, Mg2+-ATPase, and CAT, as well as in total sulfhydryl content was also found. However, cerebral lipoperoxidation was not observed and in this case, the reduction in Na+,K+-ATPase activity may be associated to attack of reactive species to the sulfhydryl groups present in the enzyme (Schweinberger et al. 2014).
Acetylcholinesterase activity
Schulpis et al. (2006) published data showing that Met is able to increase hippocampal acetylcholinesterase (AChE) activity in vitro. At the following year, Stefanello et al. (2007d) showed that chronic subcutaneous injections of Met in developing Wistar rats increased AChE activity in cerebral cortex associated to an impaired working memory performance. Since AChE acts into the synapse by rapid hydrolysis of the acetylcholine (Ach), a neurotransmitter whose adequate maintenance has been associated with cognitive manifestations (learning and memory) (Bartus et al. 1982), the stimulation of this enzyme activity could lead to a decrease in cerebral Ach levels and provide an explanation for the memory deficit found in the hypermethioninemic rats. In agreement, studies showed that long-term Met exposure caused an important increase in brain AChE activity and memory deficit in zebrafish (Vuaden et al. 2012). Since Ach has a role as an anti-inflammatory molecule, some studies have correlated increased AChE activity with neuroinflammation (Scherer et al. 2014), what could be also related to the pathogenic effects found in hypermethioninemia.
Dendritic spine downregulation
In 1952, Osmond and Smythies (1952) proposed the ‘‘transmethylation theory’’ of schizophrenia, suggesting that this psychotic disease is a result of a disturbance in methylation. In 2009, Grayson et al. also reported that Met treatment could worsen schizophrenia symptoms, possibly because it increases brain levels of AdoMet. More specifically, excessive AdoMet could provoke hypermethylation of Reelin gene promoter. Since Reelin is a glycoprotein secreted by GABAergic neurons that stimulates dendritic spines development, this process could be impaired by Met (Levenson et al. 2008).
Indeed, it has been demonstrated that the treatment with Met causes a decrease in dendritic spine density of layer III pyramidal neurons in frontal cortex of mice, a pathological alteration similar to the dendritic spine downregulation found in brain during schizophrenia (Tueting et al. 2010). In agreement, clinical studies have demonstrated that patients with psychotic disorders present increased Met levels in cerebrospinal fluid (Regland et al. 2004).
Besides, it should be noted that that learning and novel sensory experiences lead to spine formation and the new spines that are preserved seem to provide a structural basis for memory retention (Yang et al. 2009). Thus, when hypermethioninemia is associated with enhanced AdoMet levels, the reduction in dendritic spine density may occur and cause lifelong memory impairment.
Hepatic effects
Since Met is primarily metabolized in the liver (Finkelstein 1990), it has been suggested that excess of Met may cause liver injury, but mechanisms are still not well elucidated. In this context, several studies have been performed to figure it out.
Liver cell alterations
In humans, electron microscopy revealed augmented smooth endoplasmic reticulum, reduced rough endoplasmic reticulum, enhanced lysosomes, and short breaks in the outer membranes of liver from patients with persistent hypermethioninemia (MAT activity ranged from 7.8 to 17.5 %) and with no abnormalities in other sulfur amino acid concentrations (Gaull et al. 1981b). In rats, excess dietary Met (10–12.4 % dl-Met) caused atrophy of liver cells and changes in the distribution of the chromatin, which was condensed and deposited at the periphery of the nucleus (Earle et al. 1942).
Hepatic lipid accumulation
Whereas the liver is the organ directly related to lipid metabolism, fatty accumulation (steatosis) may be observed during some pathological conditions. Steatosis is associated with hepatocyte damage and consequently can cause cirrhosis, inflammation, and liver failure leading to end-stage disease (Angulo 2010). In this context, histological examinations of liver tissues from patients with persistent and transient hypermethioninemia showed moderate fatty degeneration, wherein the condition improved after low Met diet (Tsuchiyama et al. 1982).
Furthermore, Lu et al. (2001) evaluated the effect of MAT1A knockout in mice and observed, at 3 months, an increase of 776 % in plasma Met levels and reduction of liver AdoMet content. At 8 months, development of spontaneous macrovesicular steatosis and predominantly periportal mononuclear cell infiltration occurred. These changes were accompanied by augmented expression of acute phase-response/inflammatory markers (orosomucoid, amyloid, metallothionein, Fas antigen) and growth-related genes (early growth response 1 and proliferating cell nuclear antigen), as well as increased liver weights. Posteriorly, Martínez-Chantar et al. (2002) also demonstrated that knockout in MAT1A gene leads to abnormal expression of genes involved in the metabolism of lipids and carbohydrates associated with hyperglycemia and increased hepatic triglyceride levels in mice.
Met diet supplementation was also able to induce hepatic damage by stimulating cholesterol synthesis in liver cells (probably through increased hepatic expression of 3-hydroxy-3-methylglutaryl coenzyme A reductase) (Hirche et al. 2006), augmenting accumulation of hepatic total lipids and phospholipids (Yang and Kadowaki 2011), and inducing microvesicular steatosis, hepatocyte degeneration, and inflammatory reactions in liver of rats (Yalçinkaya et al. 2009). Met diet restriction, on the other hand, seems to be advantageous as described in a previous study, which demonstrated that rats submitted to restrictive Met intake presented reduced visceral fat associated to a decrease in basal insulin, glucose, and leptin, and increased adiponectin and triiodothyronine. Besides, Met restriction prevented age-associated increase in serum lipids (Malloy et al. 2006). In 2013, Malloy et al. also demonstrated that Met restriction was able to reverse the severity of steatosis in obese mice accompanied by reduced hepatic triglycerides levels, increased VLDL secretion, and increased mRNA levels of apolipoprotein B and microsomal triglyceride transfer protein. The expression of inflammatory markers (Tnf-α and Ccr2) was also attenuated by Met restriction in this study.
It is important to note that excessive lipids in liver may cause lipid peroxidation, which can increase the production of pro-inflammatory cytokines (Bradbury 2006). Besides, the increase in lipids can exceed mitochondrial beta-oxidation further enhancing oxidative stress and inflammation (Schreuder et al. 2008). On this basis, Met-induced lipid accumulation in liver could lead to oxidative stress, which may have a role in hepatic damage during hypermethioninemia.
Oxidative stress
The role of oxidative stress on the hepatic toxicity caused by Met has been shown in different animal studies: enriched Met diet increased lipid peroxidation in liver of rats and rabbits, as well as, altered antioxidant enzyme activities and induced inflammatory infiltration of portal triads in liver of rabbits (Lynch and Strain 1989; Mori and Hirayama 2000; Toborek et al. 1996); high Met diet also increased hepatotoxicity and oxidative stress in the liver of chronically ethanol-treated rats (Yalçinkaya et al. 2007); MAT1A knockout increased susceptibility to oxidative stress and reduced glutathione content in mice liver (Lu et al. 2001; Martínez-Chantar et al. 2002).
To further the knowledge about these mechanisms, Stefanello et al. (2009) evaluated the toxic effects of chronic Met injections in rats. The treatment decreased non-enzymatic antioxidant defenses, increased protein carbonylation, and altered the activities of the antioxidant enzymes glutathione peroxidase and CAT in the liver, indicating oxidative stress. These alterations were accompanied by morphological alterations in liver.
In addition, rats fed with a high Met diet (2 %, w/w) during 6 months presented hepatic oxidative and nitrosative stress characterized by increased lipid peroxide and nitrotyrosine levels, as well as decreased non-enzymatic and enzymatic antioxidant defenses in liver. Increased levels of alanine transaminase and aspartate transaminase in blood and altered apoptotic parameters in liver indicated that the hepatic tissue was disrupted. These alterations were accompanied by enhanced Hcy levels in blood (Yalçinkaya et al. 2009).
Gomez et al. (2009) also demonstrated that Wistar rats fed a Met supplemented diet (2.5 g/100 g) for 7 weeks had increased mitochondrial ROS generation and oxidative damage to mitochondrial DNA in liver. In agreement, Caro et al. (2008) showed that lowered Met ingestion has the exactly opposite effects, decreasing mitochondrial ROS production and DNA oxidative damage in liver of rats. More recently, a swine model was used to determine if a methionine-restricted diet for 2 weeks could reduce oxidative stress in hepatic mitochondria. The results showed that methionine restriction decreased markers of oxidative damage to DNA and proteins in liver mitochondria of pigs, being that effects probably were consequence of attenuated ROS production since a reduction in H2O2 generation and in free radical leak was also observed. The authors suggest that the decrease in ROS generation possibly occurred due to reduced complex I activity, which was associated with decreased levels of the apoptosis inducing factor, a protein related to complex I function (Ying et al. 2015).
Besides, excessive Met intake by γ-cystathionase-deficient mice led to the development of acute hepatitis attended by serum and hepatic lipoperoxidation (Yamada et al. 2012). It has been previously described that peroxidized fatty acids (arachidonic and linolenic) stimulate interleukin-8 production by peripheral blood monocytes in liver (Jayatilleke and Shaw 1998). Interleukin-8, in turn, has been associated with hepatic neutrophil infiltration and to activation of hepatic profibrogenic cells (Bird 1994; Zimmermann et al. 2011; Taïeb et al. 2000; Dong and Zheng 2015; Tachibana et al. 2007).
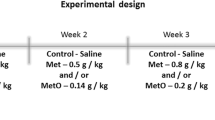
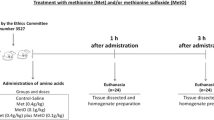
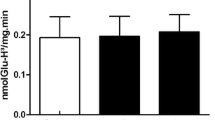
More recently, Costa et al. (2013) performed in vitro and in vivo studies about the toxic effects of Met in liver. For in vitro studies, liver homogenates were incubated with Met and results showed changes in CAT and SOD activities, as well as in ROS production. For in vivo studies, the animals received injections of Met (0.4 g/kg) and were euthanized after 1 and 3 h. Results showed that Met enhanced carbonyl content at 1 h, as well as decreased CAT activity 1 and 3 h after administration. Data indicated that Met modifies liver homeostasis by altering the redox cellular state both in vivo and in vitro.
Cholestasis
Cholestasis is a pathological condition defined as an impairment of bile flow that causes the accumulation of toxic compounds, which induce liver damage, biliary fibrosis, cirrhosis, and finally end-stage liver disease. Studies performed in rabbits by Moss et al. (1999) showed that intravenous administration of Met (121 mg kg−1 d−1) leads to decreased bile flow. The excretion of a bilirubin analog (bromosulfophthalein) tended to be delayed by Met treatment. It was also verified histological liver injury, balloon degeneration, and inflammation characterized by infiltration of the portal triads with eosinophils. Therefore, these results suggest that excessive Met may lead to cholestasis.
In addition, four cases of human neonates positive for hypermethioninemia and two for both hypermethioninemia and hypergalactosemia have been described, which presented severe intrahepatic cholestasis of unknown origin (Ohura et al. 2003). Cholestasis induced by hypermethioninemia may be a consequence of the inflammatory process induced by Met since the cytokines produced under this condition may impair the hepatocellular transport systems that mediate biliary excretion of bile salts and non-bile salt organic anions (Trauner et al. 1999).
ATP depletion
Since Met transmethylation initiates through the ATP-dependent conversion of Met to AdoMet (Finkelstein 1990), ATP depletion from excessive AdoMet formation may induce or augment hepatotoxicity during hypermethioninemia (Hardwick et al. 1970). In accordance with this hypothesis, injections of Met in guinea pigs led to accumulation of AdoMet with concomitant ATP deficiency and nucleolar disaggregation in liver (Shinozuka et al. 1971). Besides, Regina et al. (1993) performed an experiment in which the feeding of toxic levels of Met led to a pronounced accumulation of AdoMet in liver of rats.
Met transamination
Met transamination consists of an alternative pathway for Met metabolism and results in the formation of 2-keto-4-methylthiobutyric acid, which is oxidatively decarboxylated to form 3-methylthiopropionic acid (3-MTP) (Cooper 1989; Scislowski and Pickard 1993; Steele and Benevenga 1978). 3-MTP is then metabolized to highly toxic molecules, including methanethiol, a compound that inhibits enzymes involved in protection against peroxidative damage (Finkelstein and Benevenga 1986).
In this context, Dever and Elfarra (2008) demonstrated that Met is hepatotoxic through an experiment in which freshly isolated male mouse hepatocytes were incubated with different doses of this amino acid, leading to cell disruption and glutathione depletion. The exposure of hepatocytes to 3-MTP resulted in similar effects. Besides, the addition of aminooxyacetic acid, an inhibitor of Met transamination, partially blocked Met-induced cytotoxicity, indicating that the toxicity was at least partially mediated by Met transamination.
Final considerations
Based on the information presented above, it is possible to infer that oxidative damage is one of the main mechanisms responsible for toxicity caused by Met, since oxidative stress was induced in brain and liver tissues in different studies that includes in vitro experiments or in vivo animal models by injecting Met, enriching Met in diet and/or knocking MAT1A gene. Besides, oxidative stress seems to mediate, at least partially, other alterations induced by Met, such as the reduction of brain Na+,K+-ATPase activity and liver inflammation.
Some Met metabolites, such as Hcy, may induce oxidative stress and alter AChE and Na+,K+-ATPase activities in brain and liver, contributing to the toxic effects of Met in some cases (Streck et al. 2002b; Scherer et al. 2011, 2013, 2014; Machado et al. 2011; Matté et al. 2004; 2009a, b). However, this review described different in vitro studies and animal models that induced isolated hypermethioninemia, which caused pathological effects, suggesting that Met per se is able to elicit important hepatic and neurological toxicity.
In conclusion, Met may be extremely toxic to brain by inducing oxidative stress, decreasing Na+,K+-ATPase activity and dendritic spine density, as well as increasing AChE activity. In liver, hypermethioninemia seems to induce histological changes, liver lipid accumulation, oxidative stress, inflammation, and ATP depletion. Schematic representations of Met effects in brain and liver are shown in Fig. 2.
Schematic representation of Met effects reported in the literature up to now. In brain, hypermethioninemia increases ROS production and decreases antioxidant defenses, leading to oxidative stress, which in turn may reduce Na+,K+-ATPase activity. Na+,K+-ATPase inhibition is related to cerebral edema and memory deficit. Increased AChE activity and dendritic spine downregulation (induced by decreased Reelin levels) may also impair memory during hypermethioninemia. In liver, hypermethioninemia induces steatosis that increases mitochondrial beta-oxidation, leading to increased ROS production. Hypermethioninemia also induces 3-MTP formation which reduces antioxidant defenses. This imbalance between ROS and antioxidants induces oxidative stress. Inflammation is both consequence and cause of oxidative stress and is able to lead to cholestasis. Inflammation and Met-induced ATP depletion causes cell death
Dedication
This review is dedicated to the memory of Dr. S. Harvey Mudd, who developed a superb work on diseases involving disturbances of sulfur amino acid metabolism The studies performed by Dr. Mudd motivated us to develop experimental models of hypermethioninemia and hyperhomocysteinemia in the attempt to better understand the underlying mechanisms involved in the pathophysiology of these conditions We express our gratitude to this eminent scientist for his scientific contribution and for the opportunity to have exchanged ideas about our research.
References
Acker CI, Luchese C, Prigol M, Nogueira CW (2009) Antidepressant-like effect of diphenyl diselenide on rats exposed to malathion: involvement of Na+, K+-ATPase activity. Neurosci Lett 455:168–172
Angulo P (2010) Long-term mortality in nonalcoholic fatty liver disease: is liver histology of any prognostic significance? Hepatology 51:373–375
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Bartus RT, Dean RL, Beer B, Lippa AS (1982) The cholinergic hypothesis of geriatric memory dysfunction. Science 217:408–414
Beatty PW, Reed DJ (1980) Involvement of the cystathionine pathway in the biosynthesis of glutathione by isolated rat hepatocytes. Arch Biochem Biophys 204:80–87
Benevenga NJ, Steele RD (1984) Adverse effects of excessive consumption of amino acids. Annu Rev Nutr 4:157–181
Bird G (1994) Interleukin-8 in alcoholic liver disease. Acta Gastroenterol Belg 57:255–259
Bradbury MW (2006) Lipid Metabolism and Liver Inflammation. I. Hepatic fatty acid uptake: possible role in steatosis. Am J Physiol Gastrointest Liver Physiol 290:194–198
Braverman NE, Mudd SH, Barker PB, Pomper MG (2005) Characteristic MRI changes in severe hypermethioninemic states. Am J Neuroradiol 26:2705–2706
Brot N, Weissbach L, Werth J, Weissbach H (1981) Enzymatic reduction of protein-bound methionine sulfoxide. Proc Natl Acad Sci USA 78:2155–2158
Cai J, Sun WM, Hwang JJ, Stain SC, Lu SC (1996) Changes in S-Adenosylmethionine synthetase in human liver cancer: molecular characterization and significance. Hepatology 24:1090–1097
Cannon SC (2004) Paying the price at the pump: dystonia from mutations in a Na+, K+-ATPase. Neuron 43:153–154
Cantoni GL (1953) S-Adenosylmethionine; a new intermediate formed enzymatically from l-methionine and adenosinetriphosphate. J Biol Chem 204:403–416
Caro P, Gómez J, López-Torres M, Sánchez I, Naudí A, Jove M, Pamplona R, Barja G (2008) Forty percent and eighty percent methionine restriction decrease mitochondrial ROS generation and oxidative stress in rat liver. Biogerontology 9:183–196
Chamberlin ME, Ubagai T, Mudd SH, Wilson WG, Leonard JV, Chou JY (1996) Demyelination of the brain is associated with methionine adenosyltransferase I/III deficiency. J Clin Invest 98:1021–1027
Chamberlin ME, Ubagai T, Mudd SH, Levy HL, Chou JY (1997) Dominant inheritance of isolated hypermethioninemia is associated with a mutation in the human methionine adenosyltransferase 1A gene. Am J Hum Genet 60:540–546
Chiang PK, Gordon RK, Tal J, Zeng GC, Doctor BP, Pardhasaradhi K, McCann PP (1996) S-Adenosylmethionine and methylation. FASEB J 10:471–480
Cooper AJ (1989) Methionine transamination in vivo. Biochem J 262:689–690
Costa MZ, da Silva TM, Flores NP, Schmitz F, da Silva Scherer EB, Viau CM, Saffi J, Barschak AG, de Souza Wyse AT, Spanevello RM, Stefanello FM (2013) Methionine and methionine sulfoxide alter parameters of oxidative stress in the liver of young rats: in vitro and in vivo studies. Mol Cell Biochem 384:21–28
Crill CM, Helms RA (2007) The use of carnitine in pediatric nutrition. Nutr Clin Pract 22:204–213
de la Haba G, Cantoni GL (1959) The enzymatic synthesis of S-adenosyl-L-homocysteine from adenosine and homocysteine. J Biol Chem 234:603–608
de Lores Arnaiz GR, Ordieres MG (2014) Brain Na+, K+-ATPase activity in aging and disease. Int J Biomed Sci 10:85–102
de Souza Wyse AT, Streck EL, Worm P, Wajner A, Ritter F, Netto CA (2000) Preconditioning prevents the inhibition of Na+, K+-ATPase activity after brain ischemia. Neurochem Res 25:971–975
Dever JT, Elfarra AA (2008) l-methionine toxicity in freshly isolated mouse hepatocytes is gender-dependent and mediated in part by transamination. J Pharmacol Exp Ther 326:809–817
Dong R, Zheng S (2015) Interleukin-8: aA critical chemokine in biliary atresia. J Gastroenterol Hepatol 30:970–976
Earle DP, Smull K, Victor J (1942) Effects of excess dietary cysteic acid, dl-methionine, and taurine on the rat liver. J Exp Med 76:317–324
El Refaey M, Watkins CP, Kennedy EJ, Chang A, Zhong Q, Ding KH, Shi XM, Xu J, Bollag WB, Hill WD, Johnson M, Hunter M, Hamrick MW, Isales CM (2015) Oxidation of the aromatic amino acids tryptophan and tyrosine disrupts their anabolic effects on bone marrow mesenchymal stem cells. Mol Cell Endocrinol 410:87–96
Ferreira AG, Stefanello FM, Cunha AA, da Cunha MJ, Pereira TC, Bonan CD, Bogo MR, Netto CA, Wyse AT (2011) Role of antioxidants on Na+, K+-ATPase activity and gene expression in cerebral cortex of hyperprolinemic rats. Metab Brain Dis 26:141–147
Finkelstein JD (1990) Methionine metabolism in mammals. J Nutr Biochem 1:228–237
Finkelstein JD (1998) Methionine-sparing effect of cystine in human subjects. Am J Clin Nutr 68:224–225
Finkelstein JD (2000) Pathways and regulation of homocysteine metabolism in mammals. Semin Thromb Hemost 26:219–225
Finkelstein A, Benevenga NJ (1986) The effect of methanethiol and methionine toxicity on the activities of cytochrome c oxidase and enzymes involved in protection from peroxidative damage. J Nutr 116:204–215
Frago LM, Giménez A, Rodriguez EN, Varela-Nieto I (1998) Pattern of methionine adenosyltransferase isoenzyme expression during rat liver regeneration after partial hepatectomy. FEBS Lett 426:305–308
Gamaro GD, Streck EL, Matté C, Prediger ME, Wyse AT, Dalmaz C (2003) Reduction of hippocampal Na+, K+ATPase activity in rats subjected to an experimental model of depression. Neurochem Res 28:1339–1344
Gaull GE, Bender AN, Vulovic D, Tallan HH, Schaffner F (1981a) Methioninemia and myopathy: a new disorder. Ann Neurol 9:423–432
Gaull GE, Tallan HH, Lonsdale D, Przyrembel H, Schaffner F, von Bassewitz DB (1981b) Hypermethioninemia associated with methionine adenosyltransferase deficiency: clinical, morphologic, and biochemical observations on four patients. J Pediatr 98:734–741
Geronikaki AA, Gavalas AM (2006) Antioxidants and inflammatory disease: Synthetic and natural antioxidants with anti-inflammatory activity. Comb Chem High Throughput Screen 9:425–442
Gil B, Casado M, Pajares MA, Bosca L, Mato JM, Martin-Sanz P, Alvarez L (1996) Differential expression pattern of S-adenosylmethionine synthetase isozymes during rat liver development. Hepatology 24:876–881
Glynn IM (1985) The Na+, K+-transporting adenosine triphosphatase. In: Martonosi AN (ed) The enzymes of biological membranes. Plenum, New York, pp 35–114
Gomez J, Caro P, Sanchez I, Naudi A, Jove M, Portero-Otin M, Lopez-Torres M, Pamplona R, Barja G (2009) Effect of methionine dietary supplementation on mitochondrial oxygen radical generation and oxidative DNA damage in rat liver and heart. J Bioenerg Biomembr 41:309–321
Gout JP, Serre JC, Dieterlen M, Antener I, Frappat P, Bost M, Beaudoing A (1977) Still another cause of hypermethioninemia in children: S-adenosylmethionine synthetase deficiency. Arch Fr Pediatr 34:416–423
Grayson DR, Chen Y, Dong E, Kundakovic M, Guidotti A (2009) From trans-methylation to cyotsine methylation evolution of the methylation hypothesis of schizophrenia. Epigenetics 4:144–149
Grisar T, Guillaume D, Delgado-Escueta AV (1992) Contribution of Na+, K+-ATPase to focal epilepsy: a brief review. Epilepsy Res 12:141–149
Guízar Vázquez J, Sánchez Aguilar G, Velázquez A, Fragoso R, Rostenberg I, Alejandre I (1980) Hypermethioninemia. Apropos of a case in a consanguineous couple. Bol Med Hosp Infant Mex 37:1237–1244
Hardwick DF, Applegarth DA, Cockcroft DM, Ross PM, Cder RJ (1970) Pathogenesis of methionine-induced toxicity. Metabolism 19:381–391
Harvey Mudd S, Braverman N, Pomper M, Tezcan K, Kronick J, Jayakar P, Garganta C, Ampola MG, Levy HL, McCandless SE, Wiltse H, Stabler SP, Allen RH, Wagner C, Borschel MW (2003) Infantile hypermethioninemia and hyperhomocysteinemia due to high methionine intake: a diagnostic trap. Mol Genet Metab 79:6–16
Higashi T (1982) Impaired metabolism of methionine in severe liver diseases. II. Clinical and experimental studies on role of impaired methionine metabolism in pathogenesis of hepatic encephalopathy. J Gastroenterol 17:125–134
Hirche F, Schröder A, Knoth B, Stangl GI, Eder K (2006) Effect of dietary methionine on plasma and liver cholesterol concentrations in rats and expression of hepatic genes involved in cholesterol metabolism. Br J Nutr 95:879–888
Horikawa S, Sasuga J, Shimizu K, Ozasa H, Tsukada K (1990) Molecular cloning and nucleotide sequence of cDNA encoding the rat kidney S-adenosylmethionine synthetase. J Biol Chem 265:13683–13686
Horikawa S, Ozasa H, Ota K, Tsukada K (1993) Immunohistochemical analysis of rat Sadenosylmethionine synthetase isozymes in developmental liver. FEBS Lett 330:307–311
Jayatilleke A, Shaw S (1998) Stimulation of monocyte interleukin-8 by lipid peroxidation products: a mechanism for alcohol-induced liver injury. Alcohol 16:119–123
Labrune P, Perignon JL, Rault M, Brunet C, Lutun H, Charpentier C, Saudubray JM, Odievre M (1990) Familial hypermethioninemia partially responsive to dietary restriction. J Pediatr 117:220–226
Lagler F, Muntau AC, Beblo S, Röschinger W, Linnebank M, Fowler B, Koch HG, Roscher AA (2000) Hypermethioninemia and hyperhomocysteinemia in methionine adenosyltransferase I/III deficiency. J Inherit Metab Dis 23:68
Levenson JM, Qiu S, Weeber EJ (2008) The role of reelin in adult synaptic function and the genetic and epigenetic regulation of the reelin gene. Biochem Biophys Acta 1779:422–431
Levy HL, Shih VE, Madigan PM, Karolkewicz V, Carr JR, Lum A, Richards AA, Crawford JD, Maccready RA (1969) Hypermethioninemia with other hyperaminoacidemias. Studies in infants on high-protein diets. Am J Dis Child 117:96–103
Lu SC, Alvarez L, Huang ZZ, Chen L, An W, Corrales FJ, Avila MA, Kanel G, Mato JM (2001) Methionine adenosyltransferase 1A knockout mice are predisposed to liver injury and exhibit increased expression of genes involved in proliferation. Proc Natl Acad Sci USA 98:5560–5565
Lucas-Lenard J (1971) Protein biosynthesis. Annu Rev Biochem 40:409–448
Lynch SM, Strain J (1989) Increased hepatic lipid peroxidation with methionine toxicity in the rat. Free Radic Res Commun 5:221–226
Machado FR, Ferreira AG, da Cunha AA, Tagliari B, Mussulini BH, Wofchuk S, Wyse AT (2011) Homocysteine alters glutamate uptake and Na+, K+-ATPase activity and oxidative status in rats hippocampus: protection by vitamin C. Metab Brain Dis 26:61–67
Malloy V, Krajcik R, Bailey S, Hristopoulos G, Plummer J, Orentreich N (2006) Methionine restriction decreases visceral fat mass and preserves insulin action in aging male Fischer 344 rats independent of energy restriction. Aging Cell 5:305–314
Malloy VL, Perrone CE, Mattocks DA, Ables GP, Caliendo NS, Orentreich DS, Orentreich N (2013) Methionine restriction prevents the progression of hepatic steatosis in leptin-deficient obese mice. Metabolism 62:1651–1661
Martínez-Chantar ML, Corrales FJ, Martínez-Cruz LA, García-Trevijano ER, Huang ZZ, Chen L, Kanel G, Avila MA, Mato JM, Lu SC (2002) Spontaneous oxidative stress and liver tumors in mice lacking methionine adenosyltransferase 1A. FASEB J 16:1292–1294
Matté C, Monteiro SC, Calcagnotto T, Bavaresco CS, Netto CA, Wyse AT (2004) In vivo and in vitro effects of homocysteine on Na+, K+-ATPase activity in parietal, prefrontal and cingulate cortex of young rats. Int J Dev Neurosci 22:185–190
Matté C, Mackedanz V, Stefanello FM, Scherer EB, Andreazza AC, Zanotto C, Moro AM, Garcia SC, Gonçalves CA, Erdtmann B, Salvador M, Wyse AT (2009a) Chronic hyperhomocysteinemia alters antioxidant defenses and increases DNA damage in brain and blood of rats: protective effect of folic acid. Neurochem Int 54:7–13
Matté C, Stefanello FM, Mackedanz V, Pederzolli CD, Lamers ML, Dutra-Filho CS, Dos Santos MF, Wyse AT (2009b) Homocysteine induces oxidative stress, inflammatory infiltration, fibrosis and reduces glycogen/glycoprotein content in liver of rats. Int J Dev Neurosci 27:337–344
Mori N, Hirayama K (2000) Long-term consumption of methionine-supplemented diet increases iron and lipid peroxide levels in rat liver. J Nutr 130:2349–2355
Mosharov E, Cranford MR, Benerjee R (2000) The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry 39:13005–13011
Moss RL, Haynes AL, Pastuszyn A, Glew RH (1999) Methionine infusion reproduces liver injury of parenteral nutrition cholestasis. Pediatr Res 45:664–668
Mudd SH (1962) Activation of methionine for transmethylation. V. The mechanism of action of the methionine-activating enzyme. J Biol Chem 237:1372–1375
Mudd SH (2011) Hypermethioninemias of genetic and non-genetic origin: a review. Am J Med Genet C Semin Med Genet 157:3–32
Mudd SH, Levy HL, Tangerman A, Boujet C, Buist N, Davidson-Mundt A, Hudgins L, Oyanagi K, Nagao M, Wilson WG (1995) Isolated persistent hypermethioninemia. Am J Hum Genet 57:882–892
Mudd SH, Levy HL, Kraus JP (2001) Disorders of transsulfuration. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill, New York, pp 2007–2056
Mynett-Johnson L, Murphy V, McCormack J, Shields DC, Claffey E, Manley P, McKeon P (1998) Evidence for an allelic association between bipolar disorder and a Na+, K+ adenosine triphosphatase alpha subunit gene (ATP1A3). Biol Psych 44:47–51
Nagao M, Oyanagi K (1997) Genetic analysis of isolated persistent hypermethioninemia with dominant inheritance. Acta Paediatr Jpn 39:601–606
Ohura T, Kobayashi K, Abukawa D, Tazawa Y, Aikawa J, Sakamoto O, Saheki T, Iinuma K (2003) A novel inborn error of metabolism detected by elevated methionine and/or galactose in newborn screening: neonatal intrahepatic cholestasis caused by citrin deficiency. Eur J Pediatr 162:317–322
Okada G, Teraoka H, Tsukada K (1981) Multiple species of mammalian S-adenosylmethionine synthetase. Partial purification and characterization. Biochemistry 20:934–940
Osmond H, Smythies J (1952) Schizophrenia: a new approach. J Ment Sci 98:309–315
Regina M, Korhonen VP, Smith TK, Alakuijala L, Eloranta TO (1993) Methionine toxicity in the rat in relation to hepatic accumulation of S-adenosylmethionine: prevention by dietary stimulation of the hepatic transsulfuration pathway. Arch Biochem Biophys 300:598–607
Regland B, Abrahamsson L, Blennow K, Grenfeldt B, Gottfries CG (2004) CSF-methionine is elevated in psychotic patients. J Neural Transm 111:631–640
Scherer EB, da Cunha AA, Kolling J, da Cunha MJ, Schmitz F, Sitta A, Lima DD, Delwing D, Vargas CR, Wyse AT (2011) Development of an animal model for chronic mild hyperhomocysteinemia and its response to oxidative damage. Int J Dev Neurosci 29:693–699
Scherer EB, Loureiro SO, Vuaden FC, Schmitz F, Kolling J, Siebert C, Savio LE, Schweinberger BM, Bogo MR, Bonan CD, Wyse AT (2013) Mild hyperhomocysteinemia reduces the activity and immunocontent, but does not alter the gene expression, of catalytic α subunits of cerebral Na+, K+-ATPase. Mol Cell Biochem 378:91–97
Scherer EB, Loureiro SO, Vuaden FC, da Cunha AA, Schmitz F, Kolling J, Savio LE, Bogo MR, Bonan CD, Netto CA, Wyse AT (2014) Mild hyperhomocysteinemia increases brain acetylcholinesterase and proinflammatory cytokine levels in different tissues. Mol Neurobiol 50:589–596
Schreuder TC, Verwer BJ, van Nieuwkerk CM, Mulder CJ (2008) Nonalcoholic fatty liver disease: an overview of current insights in pathogenesis, diagnosis and treatment. World J Gastroenterol 14:2474–2486
Schulpis KH, Kalimeris K, Bakogiannis C, Tsakiris T, Tsakiris S (2006) The effect of in vitro homocystinuria on the suckling rat hippocampal acetylcholinesterase. Metab Brain Dis 21:21–28
Schweinberger BM, Schwieder L, Scherer E, Sitta A, Vargas CR, Wyse AT (2014) Development of an animal model for gestational hypermethioninemia in rat and its effect on brain Na+, K+-ATPase/Mg2+-ATPase activity and oxidative status of the offspring. Metab Brain Dis 29:153–160
Scislowski PW, Pickard K (1993) Methionine transamination–metabolic function and subcellular compartmentation. Mol Cell Biochem 129:39–45
Selhub J (1999) Homocysteine metabolism. Annu Rev Nutr 19:217–246
Shinozuka H, Estes LW, Farber E (1971) Studies on acute methionine toxicity. I. Nucleolar disaggregation in guinea pig hepatic cells with methionine or ethionine and its reversal with adenine. Am J Pathol 64:241–256
Stabler SP, Steegborn C, Wahl MC, Oliveriusova J, Kraus JP, Allen RH, Wagner C, Mudd SH (2002) Elevated plasma total homocysteine in severe methionine adenosyltransferase I/III deficiency. Metabolism 51:981–988
Steele RD, Benevenga NJ (1978) Identification of 3-methylthiopropionic acid as an intermediate in mammalian methionine metabolism in vitro. J Biol Chem 253:7844–7850
Stefanello FM, Chiarani F, Kurek AG, Wannmacher CM, Wajner M, Wyse AT (2005) Methionine alters Na+, K+-ATPase activity, lipid peroxidation and nonenzymatic antioxidant defenses in rat hippocampus. Int J Dev Neurosci 23:651–656
Stefanello FM, Matté C, Scherer EB, Wannmacher CM, Wajner M, Wyse AT (2007a) Chemically induced model of hypermethioninemia in rats. J Neurosci Methods 160:1–4
Stefanello FM, Scherer EB, Kurek AG, Mattos CB, Wyse AT (2007b) Effect of hypermethioninemia on some parameters of oxidative stress and on Na+, K+-ATPase activity in hippocampus of rats. Metab Brain Dis 22:172–182
Stefanello FM, Kreutz F, Scherer EB, Breier AC, Vianna LP, Trindade VM, Wyse AT (2007c) Reduction of gangliosides, phospholipids and cholesterol content in cerebral cortex of rats caused by chronic hypermethioninemia. Int J Dev Neurosci 25:473–477
Stefanello FM, Monteiro SC, Matté C, Scherer EB, Netto CA, Wyse AT (2007d) Hypermethioninemia increases cerebral acetylcholinesterase activity and impairs memory in rats. Neurochem Res 32:1868–1874
Stefanello FM, Matté C, Pederzolli CD, Kolling J, Mescka CP, Lamers ML, de Assis AM, Perry ML, dos Santos MF, Dutra-Filho CS, Wyse AT (2009) Hypermethioninemia provokes oxidative damage and histological changes in liver of rats. Biochimie 91:961–968
Streck EL, Zugno AI, Tagliari B, Wannmacher CMD, Wajner M, Wyse ATS (2002a) Inhibition of Na+, K+-ATPase activity by the metabolites accumulating in homocystinuria. Metab Brain Dis 17:83–91
Streck EL, Matte C, Vieira PS, Rombaldi F, Wannmacher CM, Wajner M, Wyse AT (2002b) Reduction of Na+, K+-ATPase activity in hippocampus of rats subjected to chemically induced hyperhomocysteinemia. Neurochem Res 27:1593–1598
Tachibana Y, Nakamoto Y, Mukaida N, Kaneko S (2007) Intrahepatic interleukin-8 production during disease progression of chronic hepatitis C. Cancer Lett 251:36–42
Taïeb J, Mathurin P, Elbim C, Cluzel P, Arce-Vicioso M, Bernard B, Opolon P, Gougerot-Pocidalo MA, Poynard T, Chollet-Martin S (2000) Blood neutrophil functions and cytokine release in severe alcoholic hepatitis: effect of corticosteroids. J Hepatol 32:579–586
Toborek M, Kopieczna-Grzebieniak E, Drózdz M, Wieczorek M (1996) Increased lipid peroxidation and antioxidant activity in methionine-induced hepatitis in rabbits. Nutrition 12:534–537
Trauner M, Fickert P, Stauber RE (1999) Inflammation-induced cholestasis. J Gastroenterol Hepatol 14:946–959
Tsuchiyama A, Oyanagi K, Nakata F, Uetsuji N, Tsugawa S, Nakao T, Mori M (1982) A new type of hypermethioninemia in neonates. Tohoku J Exp Med 138:281–288
Tueting P, Davis JM, Veldic M, Pibiri F, Kadriu B, Guidotti A, Costa E (2010) L-methionine decreases dendritic spine density in mouse frontal cortex. NeuroReport 21:543–548
Valley CC, Cembran A, Perlmutter JD, Lewis AK, Labello NP, Gao J, Sachs JN (2012) The methionine-aromatic motif plays a unique role in stabilizing protein structure. J Biol Chem 287:34979–34991
Viggiano A, Viggiano E, Monda M, Ingrosso D, Perna AF, De Luca B (2012) Methionine-enriched diet decreases hippocampal antioxidant defences and impairs spontaneous behaviour and long-term potentiation in rats. Brain Res 1471:66–74
Vitvitsky V, Thomas M, Ghorpade A, Gendelman HE, Banerjee R (2006) A functional transsulfuration pathway in the brain links to glutathione homeostasis. J Biol Chem 281:35785–35793
Vuaden FC, Savio LE, Piato AL, Pereira TC, Vianna MR, Bogo MR, Bonan CD, Wyse AT (2012) Long-term methionine exposure induces memory impairment on inhibitory avoidance task and alters acetylcholinesterase activity and expression in zebrafish (Danio rerio). Neurochem Res 37:1545–1553
Wesseling S, Koeners MP, Joles JA (2009) Taurine: red bull or red herring? Hypertension 53:909–911
Wyse AT, Noriler ME, Borges LF, Floriano PJ, Silva CG, Wajner M, Wannmacher CM (1999) Alanine prevents the decrease of Na+, K+-ATPase activity in experimental phenylketonuria. Metab Brain Dis 14:95–101
Wyse AT, Bavaresco CS, Reis EA, Zugno AI, Tagliari B, Calcagnotto T, Netto CA (2004) Training in inhibitory avoidance causes a reduction of Na+, K+-ATPase activity in rat hippocampus. Physiol Behav 80:475–479
Wyss M, Kaddurah-Daouk R (2000) Creatine and creatinine metabolism. Physiol Rev 80:1107–1213
Yalçinkaya S, Unlüçerçi Y, Uysal M (2007) Methionine-supplemented diet augments hepatotoxicity and prooxidant status in chronically ethanol-treated rats. Exp Toxicol Pathol 58:455–459
Yalçinkaya S, Unlüçerçi Y, Giris M, Olgac V, Dogru-Abbasoglu S, Uysal M (2009) Oxidative and nitrosative stress and apoptosis in the liver of rats fed on high methionine diet: protective effect of taurine. Nutrition 25:436–444
Yamada H, Akahoshi N, Kamata S, Hagiya Y, Hishiki T, Nagahata Y, Matsuura T, Takano N, Mori M, Ishizaki Y, Izumi T, Kumagai Y, Kasahara T, Suematsu M, Ishii I (2012) Methionine excess in diet induces acute lethal hepatitis in mice lacking cystathionine γ-lyase, an animal model of cystathioninuria. Free Radic Biol Med 52:1716–1726
Yang L, Kadowaki M (2011) Addition of methionine to rice protein affects hepatic cholesterol output inducing hypocholesterolemia in rats fed cholesterol-free diets. J Med Food 14:445–453
Yang G, Pan F, Gan WB (2009) Stably maintained dendritic spines are associated with lifelong memories. Nature 462:920–924
Ying Y, Yun J, Guoyao W, Kaiji S, Zhaolai D, Zhenlong W (2015) Dietary L-methionine restriction decreases oxidative stress in porcine liver mitochondria. Exp Gerontol 65:35–41
Zhang LN, Sun YJ, Pan S, Li JX, Qu YE, Li Y, Wang YL, Gao ZB (2013) Na+, K+-ATPase, a potent neuroprotective modulator against Alzheimer disease. Fundam Clin Pharmacol 27:96–103
Zimmermann HW, Seidler S, Gassler N, Nattermann J, Luedde T, Trautwein C, Tacke F (2011) Interleukin-8 is activated in patients with chronic liver diseases and associated with hepatic macrophage accumulation in human liver fibrosis. PLoS One 6:e21381
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: C.-A. A. Hu.
Rights and permissions
About this article
Cite this article
Schweinberger, B.M., Wyse, A.T.S. Mechanistic basis of hypermethioninemia. Amino Acids 48, 2479–2489 (2016). https://doi.org/10.1007/s00726-016-2302-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-016-2302-4