Abstract
In the present study we developed a chemically induced experimental model for gestational hypermethioninemia in rats and evaluated in the offspring the activities of Na+,K+-ATPase and Mg2+-ATPase, as well as oxidative stress parameters, namely sulfhydryl content, thiobarbituric acid-reactive substances and the antioxidant enzymes superoxide dismutase and catalase in encephalon. Serum and encephalon levels of methionine and total homocysteine were also evaluated in mother rats and in the offspring. Pregnant Wistar rats received two daily subcutaneous injections of methionine throughout the gestational period (21 days). During the treatment, a group of pregnant rats received dose 1 (1.34 μmol methionine/g body weight) and the other one received dose 2 (2.68 μmol methionine/g body weight). Control group received saline. After the rats give birth, a first group of pups was killed at the 7th day of life and the second group at the 21th day of life for removal of serum and encephalon. Mother rats were killed at the 21th day postpartum for removal of serum and encephalon. Both doses 1 and 2 increased methionine levels in encephalon of the mother rats and dose 2 increased methionine levels in encephalon of the offspring. Maternal hypermethioninemia also decreased the activities of Na+,K+-ATPase, Mg2+-ATPase and catalase, as well as reduced total sulfhydryl content in the encephalon of the pups. This chemical model seems to be appropriate for studies aiming to investigate the effect of maternal hypermethioninemia on the developing brain during gestation in order to clarify possible neurochemical changes in the offspring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypermethioninemia is a condition characterized by elevated plasma Methionine (Met) levels and may occur in a variety of metabolic disorders. The most common genetic cause for isolated hypermethioninemia is the deficiency of Met adenosyltransferase (MAT) I/III, an enzyme that catalyzes the synthesis of S-adenosylmethionine (AdoMet) from Met and ATP. MAT I and MATIII are expressed predominantly in liver and are encoded by the MAT1A gene (Mudd 2011). MAT1A R264H in heterozygosis has been shown to be one of the most frequent mutations and may lead to mild hypermethioninemia (Couce et al. 2013). Other causes for hypermethioninemia include classical homocystinuria (due to cystathionine beta-synthase deficiency), deficiencies of citrin, glycine N-methyltransferase, S-adenosylhomocysteine hydrolase, and fumarylacetoacetate hydrolase (tyrosinemia type I) (Mudd et al. 2001).
The clinical consequences of MAT I/III deficiency may include neurological disorders, such as cognitive deficits, cerebral edema and demyelination. However, despite a great deal of works on the neurotoxic effects of Met, the mechanisms involved in these alterations are still not well-understood (Chamberlin et al. 1996; Mudd et al. 2000, 2001).
In a previous study, a chronic experimental model of hypermethioninemia was induced in developing rats (6th to the 28th postpartum day). The results of such study suggest that the brain toxicity mediated by Met may be a consequence of a reduction in Na+,K+-ATPase activity (Stefanello et al. 2011), an integral membrane protein responsible for the maintenance of intra and extracellular electrolyte balance (Lees 1991). Studies show that Na+,K+-ATPase can be inhibited by reactive oxygen species (ROS) (Lees 1993), lipid peroxidation (Mishra et al. 1989; Viani et al. 1991) and oxidation of the sulfhydryl (SH) group (Yufu et al. 1993). Evidences also show that administration of antioxidants were able to partially prevent the induced Met-inhibition of this enzyme in rat hippocampus (Stefanello et al. 2011), suggesting that oxidative stress is involved in the inhibition of Na+,K+-ATPase during hypermethioninemia.
Although it is known that elevated blood levels of certain amino acids can cause severe neuronal damage to the fetus during pregnancy (Mabry et al. 1963; Huether et al. 1992; de Franceschi et al. 2013), the effect of maternal hypermethioninemia on the developing brain during intrauterine life is poor studied. Therefore, the objective of this study was to develop a chemically induced experimental model for gestational hypermethioninemia. The serum and encephalon levels of Met and its metabolite homocysteine (Hcy) were evaluated in the offspring of rats exposed to Met during pregnancy. We also evaluated the activities of Na+,K+-ATPase and Mg2+-ATPase, as well as oxidative stress parameters, namely sulfhydryl content, thiobarbituric acid-reactive substances (TBARS) and the antioxidant enzymes superoxide dismutase (SOD) and catalase (CAT) in encephalon.
Materials and methods
Animals and reagents
Female Wistar rats were obtained from the Central Animal House of the Departamento de Bioquímica, Instituto de Ciências Básicas da Saúde, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil. Animals were maintained on a 12/12 h light/dark cycle in an air-conditioned constant temperature (22 ± 1 °C) colony room. Rats had free access to a 20 % (w/w) protein commercial chow and water. The NIH “Guide for the Care and Use of Laboratory Animals” (NIH publication No. 80–23, revised 1996) and the official governmental guidelines in compliance with the Federação das Sociedades Brasileiras de Biologia Experimental were followed in all experiments. All chemicals were obtained from Sigma Chemical Co., St. Louis, MO, USA.
Chronic methionine treatment
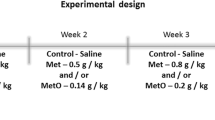
After mating the female rats with males of the same strain, pregnancy was confirmed by the presence of sperm in the vaginal smear. The pregnant rats (70–90 days of age) received two daily subcutaneous injections of Met (at intervals of 12 h) throughout the gestational period (21 days). During the treatment, a group of pregnant rats received 1.34 μmol Met/g body weight and the other one received 2.68 μmol Met/g body weight. These doses were calculated based on a previous work that induced elevated concentrations of Met in the blood by injecting subcutaneously Met (1.34–2.68 μmol/g of body weight) to developing animals of various ages (Stefanello et al. 2006). Control rats received saline. After birth, a first group of pups was killed at the 7th day of life and the second group at the 21th day of life. Mother rats were killed 21 days after the last injection.
Tissue preparation and serum obtainment
Animals were killed by decapitation without anesthesia followed by the removal of encephalon and blood. Encephalon was divided into two parts. The first part was homogenized in 10 volumes (1:10, w/v) of Medium buffer for determining the activities of Na+,K+-ATPase and Mg2+-ATPase. The second part was homogenized in 10 volumes (1:10, w/v) of buffer solution (sodium phosphate 20 mM, KCl 140 mM, pH 7.4) for determining oxidative stress parameters. To obtain serum, blood was collected and centrifuged at 1000xg (3,000 rpm) for 10 min at 4 °C. After, serum was removed by suction and stored at −80 °C for subsequent determination of serum Met and total Hcy (tHcy) levels.
Methionine levels determination
The concentrations of Met in serum and encephalon were determined by high-performance liquid chromatography (HPLC) according to Joseph and Marsden (1986). The analysis was performed using a reverse phase column (ODS 25 cm × 4.6 mm × 5 μm) and fluorescent detection after precolumn derivatization with OPAplus mercaptoethanol. The flow rate was adjusted to 1.4 mL/min in a gradient of the mobile phase of methanol and 0.5 M sodium phosphate buffer pH 5.5 (buffer A, 80 % methanol; buffer B, 20 % methanol). Each sample run lasts 45 min. Met was identified by its retention time and was quantitatively determined by using its chromatographic peak area and correlating with the internal standard peak area (homocysteic acid).
Total homocysteine levels determination
tHcy levels in serum and encephalon were determined as described by Magera et al. (1999), using liquid chromatography electrospray tandem mass spectrometry (LC–MS/MS). After samples reduction and deproteinization, the tHcy concentration was detected through the transition from the precursor to the product ion (m/z 136 to m/z 90). Homocysteine-d (8) was added as an internal standard.
Na+,K+-ATPase activity assay
The reaction mixture for Na+,K+-ATPase activity assay contained 5.0 mM MgCl2, 80.0 mM NaCl, 20.0 mM KCl and 40.0 mM Tris–HCl, pH 7.4, in a final volume of 170 μL. The reaction was initiated by the addition of ATP. Controls were carried out under the same conditions with the addition of 1.0 mM ouabain. The activity was calculated by the difference between the two assays, as previously described (Wyse et al. 2000). Released inorganic phosphate (Pi) was measured by the method of Chan et al. (1986). Specific activity of the enzyme was expressed as nmol Pi released per min per mg of protein. All samples were run in duplicate.
Mg2+-ATPase activity assay
Total ATPase activity was assayed by the addition of ATP at the mixture containing 5.0 mM MgCl2, 80.0 mM NaCl, 20.0 mM KCl and 40.0 mM Tris–HCl, pH 7.4. Pi released was then measured. The activity of Mg2+-ATPase was calculated by the difference between the total ATPase activity and Na+,K+-ATPase activity. Specific activity of the enzyme was expressed as nmol Pi released per min per mg of protein. All samples were run in duplicate.
Thiobarbituric acid-reactive substances
TBARS were measured according to Ohkawa et al. (1979). Briefly, the following reagents were added (in this order) to glass tubes: 200 μL of tissue supernatant; 20 μL of sodium dodecyl sulfate (SDS) 8.1 %; 600 μL of 20 % acetic acid in aqueous solution (v/v) pH 3.5; 600 μL of 0.8 % thiobarbituric acid. The mixture was vortexed and the reaction was carried out in a boiling water bath for 1 h. The tube was then allowed to cool on water for 5 min, and was centrifuged at 1,000 g for 10 min. The resulting pink stained TBARS were determined spectrophotometrically at 535 nm in a Beckman DU® 800 (Beckman Coulter, Inc., Fullerton, CA, USA). A calibration curve was generated using 1,1,3,3-tetramethoxypropane as a standard. TBARS were calculated as nmol TBARS/mg protein.
Sulfhydryl content
This assay is based on the reduction of 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) by thiols, which in turn become oxidized (disulfide), generating the yellow derivative thionitrobenzoic acid (TNB) whose absorption is measured spectrophotometrically at 412 nm (Aksenov and Markesbery 2001). Briefly, 50 μL of homogenate were added to 1 mL of PBS buffer pH 7.4 containing 1 mM EDTA. Then 30 μL of 10 mM DTNB, prepared in a 0.2 M potassium phosphate solution pH 8.0, were added. Subsequently, 30 min incubation at room temperature in a dark room was performed. Absorption was measured at 412 nm using a Beckman DU1 640 spectrophotometer. The sulfhydryl content is inversely correlated to oxidative damage to proteins. Results were reported as nmol TNB/mg protein.
Superoxide dismutase assay
SOD activity assay is based on the capacity of pyrogallol to autoxidize, a process highly dependent on superoxide, which is the substrate for SOD. The inhibition of the autoxidation of this compound occurs in the presence of SOD, whose activity can be then indirectly assayed at 420 nm using the SpectraMax M5/M5 Microplate Reader (Molecular Devices, MDS Analytical Technologies, Sunnyvale, California, USA) (Marklund 1985). A calibration curve was performed with purified SOD as standard, in order to calculate the activity of SOD present in the samples. The results are reported as units/mg protein.
Catalase assay
CAT activity was assayed using SpectraMax M5/M5 Microplate Reader (Molecular Devices, MDS Analytical Technologies, Sunnyvale, California, USA). The method used is based on the disappearance of hydrogen peroxide (H2O2) at 240 nm in a reaction medium containing 20 mM H2O2, 0.1 % Triton X-100, 10 mM potassium phosphate buffer pH 7.0, and 0.1–0.3 mg protein/mL (Aebi 1984). One CAT unit is defined as 1 μmol of H2O2 consumed per minute and the specific activity is calculated as pmol/mg protein.
Protein determination
Protein concentration was measured by the method of Lowry et al. (1951) and Bradford (1976) using bovine serum albumin as standard.
Statistical determination
Data were analyzed by One-way ANOVA followed by the Tukey test, when F-test was significant. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) software in a PC-compatible computer. Differences were considered statistically significant if p < 0.05.
Results
Methionine and total homocysteine levels in serum and encephalon of the mother rats
At the 21th day after giving birth, mother rats were decapitated followed by the removal of serum and encephalon for evaluation of Met levels. Since Hcy is formed during Met metabolism, tHcy levels were also evaluated. As can be observed in Table 1, results showed that female rats that received Met during pregnancy, had no difference in serum Met [F(2,9) = 0.10; p > 0.05] and tHcy levels [F(2,9) = 0.52; p > 0.05] when compared to the control group. On the other hand, encephalon Met levels were significantly increased in female rats treated with dose 1 (~45 %) [F(2,9) = 11.61; p < 0.05] and dose 2 (~59 %) [F(2,9) = 11.61; p < 0.01]. Encephalon tHcy levels of treated-rats were not different from the control [F(2,9) = 5.12; p > 0.05].
Methionine and total homocysteine levels in serum of the offspring
Table 2 shows that serum Met levels of 21 days-of-age pups from Met-treated mothers did not differ from pups whose mothers were treated with saline [F(2,9) = 0.27; p > 0.05]. Our findings also demonstrated that animals submitted to the model had no difference in tHcy serum levels when compared to the control [F(2,9) = 1.14; p > 0.05]. Met and tHcy serum levels were not evaluated in 7 days-of-age pups due to the low volume of samples.
Methionine and total homocysteine levels in encephalon of the offspring
Table 3 shows that encephalon Met levels were significantly higher in 21 days-of-age pups whose mothers were treated with dose 2 (~230 %) [F(2,9) = 11.65; p < 0.01] but not with dose 1 [F(2,9) = 11.65; p > 0.05]. Dose 2 also increased Met levels in encephalon of 7 days-of age pups (~129 %) [F(2,9) = 3.77; p < 0.01], while dose 1 did not alter Met levels [F(2,9) = 3.77; p > 0.05]. Encephalon tHcy levels were also evaluated and it was observed no difference between the groups in pups of 21 [F(2,9) = 1.27; p > 0.05] and 7 days of age [F(2,9): 7.80 = p > 0.05].
Effect of gestational hypermethioninemia on Na+K+-ATPase activity in encephalon of the offspring
Figure 1 shows that maternal hypermethioninemia significantly decreased Na+K+-ATPase activity in encephalon of 21 days-of-age pups whose mothers where treated with dose 1 [F(2,15) = p < 0.001] and dose 2 [F(2,15) = p < 0.001]. This parameter was not altered in 7 days-of-age pups (control: 24.20 ± 8.16; dose 1: 19.48 ± 5.87; dose 2: 23.69 ± 6.57; p > 0.05) (data not shown).
Effect of gestational hypermethioninemia on Mg2+-ATPase activity in encephalon of the offspring
Figure 2 indicates that gestational hypermethioninemia significantly reduced Mg2+-ATPase activity in encephalon of 21 days-of-age pups whose mothers where treated with dose 1 [F(2,15) = p < 0.001] and dose 2 [F(2,15) = p < 0.01]. This parameter was not altered in 7 days-of-age pups (control: 285.88 ± 3.14; dose 1: 275.19 ± 38.65; dose 2: 241.61 ± 30.71; p > 0.05) (data not shown).
Effect of gestational hypermethioninemia on parameters of oxidative stress in the encephalon of the offspring
Encephalon lipid damage was measured by TBARS levels and we observed that gestational hypermethioninemia did not change this parameter neither in 7 (control: 6.34 ± 1.2; dose 1: 7.50 ± 1.27; dose 2: 7.20 ± 2.41; p > 0.05) and 21 days-of-age pups (control: 4.97 ± 0.66; dose 1: 4.38 ± 0.31; dose 2: 5.77 ± 0.57; p > 0.05) (data not shown). On the other hand, we observed that proteins were affected by the Met treatment since SH content was significantly decreased in encephalon of 21 days-of-age pups whose mothers were treated with dose 1 [F(2,15) = 5.76; p < 0.05] and dose 2 [F(2,15) = 5.76; p < 0.05] (Fig. 3). Met treatment did not alter SH content in 7 days-of-age pups (control: 67.09 ± 1.05; dose 1: 65.29 ± 10.87; dose 2: 52.92 ± 8.99; p > 0.05) (data not shown).
Antioxidant enzymes were also evaluated, and we observed that the treatment did not change SOD activity in pups of both 7 (control: 5.46 ± 1.17; dose 1: 4.18 ± 1.10; dose 2: 4.46 ± 1.69; p > 0.05) and 21 days of age (control: 3.26 ± 0,40; dose 1: 3.20 ± 0.49; dose 2: 3.12 ± 0.46; p > 0.05) (data not shown). Aversely, CAT activity was significantly reduced in 21 days-of-age pups whose mothers were treated with dose 2 [F(2,15) = 7.63; p < 0.05], but not with dose 1 [F(2,15) = 7.63; p > 0.05] (Fig. 4). Pups of 7 days of age did not present changes in CAT activity in encephalon (control: 2.12 ± 0.75; dose 1: 2.86 ± 0.50; dose 2: 2.40 ± 0.79; p > 0.05) (data not shown).
Discussion
In face of the importance of identifying factors that may cause damage to the structures and functions of the developing brain during the prenatal period and since hypermethioninemia may be associated with neurological disorders (Mudd et al. 2000, 2001), the main objective of the present study was to develop an experimental model for gestational hypermethioninemia in rats.
In our study, Wistar rats received daily subcutaneous injection of Met in two different doses (1.34 or 2.68 μmol Met/g body weight) during all gestational period. Serum Met and tHcy levels of the treated-mother rats and their pups demonstrated no significant difference when compared to the control, probably because Met levels return back to the control values 12 h after the injection of this amino acid (Stefanello et al. 2006). Enhanced Met levels in encephalon, on the other hand, persisted 21 days after the interruption of the treatment in mother rats treated with doses 1 and 2, as well as in pups whose mothers were treated with dose 2.
Since cerebral dysfunction may be observed in patients with hypermethioninemia and changes in the activity of the enzyme Na+,K+-ATPase seem to be associated with neurological diseases (Cannon 2004; de Carvalho et al. 2004; Zhang et al. 2013; Banerjee et al. 2012), the next step of this study was to investigate the effect of maternal hypermethioninemia on encephalon Na+,K+-ATPase activity of the offspring. The results demonstrated a significant decrease in the activity of this enzyme in 21 days-of-age pups, corroborating with other work described in literature which shows that acute and chronic hypermethioninemia reduce Na+,K+-ATPase activity in rat hippocampus (Stefanello et al. 2011). Such inhibition may lead to an impairment of sodium and potassium membrane transport with a consequent intracellular accumulation of sodium and water, which could explain the cerebral edema sometimes observed during hypermethioninemia (Mudd et al. 2003). Besides, it has been reported that administration of Na+,K+-ATPase inhibitors alters neuronal firing (Johnson et al. 1992; Vaillend et al. 2002) and impairs learning process (Mizumori et al. 1987; Sato et al. 2004; Zhan et al. 2004).
Mg2+-ATPase is the main enzyme in maintenance of high brain intracellular Mg2+ concentrations, which is involved in controlling protein synthesis and cell growth (Sanui and Rubin 1982). In the present study, Mg2+-ATPase activity was analyzed and it was found a decrease in the encephalon activity of this enzyme in 21 days-of-age pups. Reduced Mg2+-ATPase activity has been correlated with reduced learning performance (Carageorgiou et al. 2008), what could elucidate, at least partially, the cognitive deficits found in some patients with hypermethioninemia.
Given that previous studies suggest a link between hypermethioninemia and the induction of oxidative stress in hippocampus of rats (Stefanello et al. 2007) and that SH groups of Na,+K+-ATPase and Mg2+-ATPase are susceptible to oxidative damage, we also evaluated SH content in the encephalon of the offspring. Met treatment significantly reduced this parameter in 21 days-of-age pups, what may possibly indicate that hypermethioninemia leads to an increased superoxide radical production, which can combine with nitric oxide (NO) to form ONOO− or can be dismutated to H2O2, being that both may oxidize proteins bound SH (Winterbourn and Hampton 2008) and might explain the reduced activities of the ATPase enzymes observed in this study.
In addition, because the enzymes Na,+K+-ATPase and Mg2+-ATPase are embedded in cellular membrane and reactive species may lead to peroxidation of membrane lipids, TBARS levels were measured to identify lipid damage. Maternal hypermethioninemia did not alter this parameter in the encephalon of the offspring, suggesting that lipoperoxidation is not involved in the alterations of ATPase enzymes activities. In accordance, a recent study demonstrated that chronic administration of Met does not change TBARS levels in liver of rats (Stefanello et al. 2009).
In order to evaluate whether Met induces alterations in the behavior of antioxidant enzymes, we studied the effect of this amino acid on the activities of SOD and CAT, which represent an efficient system responsible for removing ROS (Halliwell 2001; Halliwell and Gutteridge 2007). Results showed that maternal hypermethioninemia did not alter cerebral SOD activity; however CAT activity was significantly reduced in encephalon of 21 days-of-age pups whose mothers were treated with dose 2. These findings are in agreement with a previous work, which shows that hypermethioninemia provokes a significant decrease in CAT activity in liver of rats, but does not affect SOD activity (Stefanello et al. 2009). This condition can make the cellular environment more susceptible to the formation of H2O2 and consequently could lead to oxidative stress generation.
However, it should be emphasized that Hcy is formed from Met metabolism. Hyperhomocysteinemia has been reported to inhibit Na+,K+-ATPase in brain, to decrease CAT activity in brain, lung and heart and to reduce total thiol content in liver of rats (Streck et al. 2002; da Cunha et al. 2011; Kolling et al. 2011). Although we did not observe increased tHcy levels in serum and encephalon of the pups, we cannot discard the possibility that Hcy is involved in the changes occurred in the encephalon of the offspring observed in the present study. It is also important to note that interestingly only 21 days-of-age pups presented alterations on the parameters evaluated. During the gestational period, placenta exerts a maternal-fetal transference of antioxidants, such as vitamin A, maintaining adequate supply to the fetus (Underwood 1994; Dimenstein et al. 1996). On this basis, it is possible that younger pups have a more efficient antioxidant protection, which is derived from their mothers.
In summary, our data show that gestational Met-treatment promotes, in the encephalon of the offspring, a reduction in the activities of Na+,K+-ATPase and Mg2+-ATPase as well as alters the oxidative status, reducing CAT activity and total SH content. In the present study, the largest number of altered parameters occurred in 21-days-of-age pups whose mothers were treated with dose 2. Therefore, this chemical model seems to be appropriate for futures studies aiming to investigate the effect of maternal hypermethioninemia on the developing brain during gestation in order to clarify possible neurochemical and/or behavioral changes in the offspring.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Aksenov MY, Markesbery WR (2001) Change in thiol content and expression of glutathione redox system gene in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett 302:141–145
Banerjee U, Dasgupta A, Rout JK, Singh OP (2012) Effects of lithium therapy on Na+-K+-ATPase activity and lipid peroxidation in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatr 37:56–61
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cannon SC (2004) Paying the price at the pump: dystonia from mutations in a Na+/K+-ATPase. Neuron 43:153–154
Carageorgiou H, Sideris AC, Messari I, Liakou CI, Tsakiris S (2008) The effects of rivastigmine plus selegiline on brain acetylcholinesterase, (Na, K)-, Mg-ATPase activities, antioxidant status, and learning performance of aged rats. Neuropsychiatr Dis Treat 4:687–699
Chamberlin ME, Ubagai T, Mudd SH, Wilson WG, Leonard JV, Chou JY (1996) Demyelination of the brain is associated with methionine adenosyltransferase I/III deficiency. J Clin Invest 98:1021–1027
Chan KM, Delfert D, Junger JK (1986) A direct colorimetric assay for Ca2+-stimulated ATPase activity. Anal Biochem 157:375–380
Couce ML, Bóveda MD, García-Jimémez C, Balmaseda E, Vives I, Castiñeiras DE, Fernández-Marmiesse A, Fraga JM, Mudd SH, Corrales FJ (2013) Clinical and metabolic findings in patients with methionine adenosyltransferase I/III deficiency detected by newborn screening. Mol Genet Metab 110:218–221
da Cunha AA, Ferreira AG, da Cunha MJ, Pederzolli CD, Becker DL, Coelho JG, Dutra-Filho CS, Wyse AT (2011) Chronic hyperhomocysteinemia induces oxidative damage in the rat lung. Mol Cell Biochem 358:153–160
de Carvalho AP, Sweadner KJ, Penniston JT, Zaremba J, Liu L, Caton M, Linazasoro G, Borg M, Tijssen MA, Bressman SB, Dobyns WB, Brashear A, Ozelius LJ (2004) Mutations in the Na+/K+-ATPase alpha3 gene ATP1A3 are associated with rapid-onset dystonia parkinsonism. Neuron 43:169–175
de Franceschi ID, Rieger E, Vargas AP, Rojas DB, Campos AG, Rech VC, Feksa LR, Wannmacher CM (2013) Effect of leucine administration to female rats during pregnancy and lactation on oxidative stress and enzymes activities of phosphoryltransfer network in cerebral cortex and hippocampus of the offspring. Neurochem Res 38:632–643
Dimenstein R, Trugo NMF, Donangelo CM, Trugo LC, Anastácio AS (1996) Effest of subadequate maternal vitamin A status on placental transfer of retinol and beta-carotene to the human fetus. Biol Neonate 69:230–234
Halliwell B (2001) Role of free radicals in the neurodegenerative diseases. Therapeutic implications for antioxidant treatment. Drugs Aging 18:685–716
Halliwell B, Gutteridge JMC (2007) Free radicals in biology and medicine. Oxford University Press, New York
Huether G, Thömke F, Adler L (1992) Administration of tryptophan-enriched diets to pregnant rats retards the development of the serotonergic system in their offspring. Brain Res Dev Brain Res 68:175–181
Johnson SW, Seutin V, North RA (1992) Burst firing in dopamine neurons induced by N-methyl-D-aspartate: role of electrogenic sodium pump. Science 258:665–667
Joseph MH, Marsden CA (1986) Amino acids and small peptides. In: Lim CK (ed) HPLC of small peptides, 1st edn. IRL Press, Oxford, pp 13–27
Kolling J, Scherer EB, da Cunha AA, da Cunha MJ, Wyse AT (2011) Homocysteine induces oxidative-nitrative stress in heart of rats: prevention by folic acid. Cardiovasc Toxicol 11:67–73
Lees GJ (1991) Inhibition of sodium-potassium-ATPase: a potentially ubiquitous mechanism contributing to central nervous system neuropathology. Brain Res 16:283–300
Lees GJ (1993) Contributory mechanisms in the causation of neurodegenerative disorders. Neuroscience 54:287–322
Lowry OH, Rosebrough NJ, Farr AL, Randal RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Mabry CC, Denniston JC, Nelson TL, Son CD (1963) Maternal phenylketonuria. A cause of mental retardation in children without the metabolic defect. N Engl J Med 269:1404–1408
Magera MJ, Lacey JM, Casetta B, Rinaldo P (1999) Method for the determination of total homocysteine in plasma and urine by stable isotope dilution and electrospray tandem mass spectrometry. Clin Chem 45:1517–1522
Marklund SL (1985) Pyrogallol Autoxidation. In: Greenwald RA (ed) Handbook of methods for oxygen radical research, 4th edn. CRC Press, Boca Raton, pp 243–247
Mishra OP, Delivoria-Papadopoulos M, Cahillane G, Wagerle LC (1989) Lipid peroxidation as the mechanism of modification of the affinity of Na+, K+-ATPase active sites for ATP, K+, Na+, and strophanthidin in vitro. Neurochem Res 14:845–851
Mizumori SJ, Sakai DH, Rosenzweig MR, Bennett EL, Wittreich P (1987) Investigations into the neuropharmacological basis of temporal stages of memory formation in mice trained in an active avoidance task. Behav Brain Res 23:239–250
Mudd SH (2011) Hypermethioninemias of genetic and non-genetic origin: a review. Am J Med Genet C: Semin Med Genet 157:3–32
Mudd SH, Jenden DJ, Capdevila A, Roch M, Levy HL, Wagner C (2000) Isolated hypermethioninemia: measurements of S-adenosylmethionine and choline. Metabolism 49:1542–1547
Mudd SH, Levy HL, Kraus JP (2001) Disorders of transsulfuration. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease, 8th edn. McGraw-Hill, New York, pp 2007–2056
Mudd SH, Braverman N, Pomper M, Tezcan K, Kronick J, Jayakar P, Garganta C, Ampola MG, Levy HL, McCandless SE, Wiltse H, Stabler SP, Allen RH, Wagner C, Borschel MW (2003) Infantile hypermethioninemia and hyperhomocysteinemia due to high methionine intake: a diagnostic trap. Mol Genet Metab 79:6–16
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Sanui H, Rubin H (1982) The role of magnesium in cell proliferation and transformation. In: Boynton AL, McKeehan WL, Whitfield JP (eds) Ions, cell proliferation and cancer. Academic Pr, New York, pp 517–537
Sato T, Tanaka K, Ohnishi Y, Teramoto T, Irifune M, Nishikawa T (2004) Effects of steroid hormones on (Na+, K+)-ATPase activity inhibition-induced amnesia on the step-through passive avoidance task in gonadectomized mice. Pharmacol Res 49:151–159
Stefanello FM, Matté C, Scherer EB, Wannmacher CM, Wajner M, Wyse AT (2006) Chemically induced model of hypermethioninemia in rats. J Neurosci Methods 160:1–4
Stefanello FM, Scherer EB, Kurek AG, Mattos CB, Wyse AT (2007) Effect of hypermethioninemia on some parameters of oxidative stress and on Na+, K+-ATPase activity in hippocampus of rats. Metab Brain Dis 22:172–182
Stefanello FM, Matté C, Pederzolli CD, Kolling J, Mescka CP, Lamers ML, de Assis AM, Perry ML, dos Santos MF, Dutra-filho CS, Wyse AT (2009) Hypermethioninemia provokes oxidative damage and histological changes in liver of rats. Biochimie 91:961–968
Stefanello FM, Ferreira AG, Pereira TC, da Cunha MJ, Bonan CD, Bogo MR, Wyse AT (2011) Acute and chronic hypermethioninemia alter Na+K+-ATPase activity in rat hippocampus: prevention by antioxidants. Int J Dev Neurosci 29:483–488
Streck EL, Matté C, Vieira PS, Rombaldi F, Wannmacher CM, Wajner M, Wyse AT (2002) Reduction of Na+, K+-ATPase activity in hippocampus of rats subjected to chemically induced hyperhomocysteinemia. Neurochem Res 27:1593–1598
Underwood BA (1994) Maternal vitamin A status and its importance in infancy an early childhood. Am J Clin Nutr 59:517S–524S
Vaillend C, Mason SE, Cuttle MF, Alger BE (2002) Mechanisms of neuronal hyperexcitability caused by partial inhibition of Na+-K+-ATPases in the rat CA1 hippocampal region. J Neurophysiol 88:2963–2978
Viani P, Cervato G, Fiorilli A, Cestaro B (1991) Age-related differences in synaptosomal peroxidative damage and membrane properties. J Neurochem 56:253–258
Winterbourn CC, Hampton MB (2008) Thiol chemistry and specificity in redox signaling. Free Radic Biol Med 45:549–561
Wyse AT, Streck EL, Worm P, Wajner A, Ritter F, Netto CA (2000) Preconditioning prevents the inhibition of Na+, K+-ATPase activity after brain ischemia. Neurochem Res 25:971–975
Yufu K, Itho T, Edamatsu R, Mori A, Hirakawa M (1993) Effect of hyperbaric oxygenation on the Na+, K+-ATPase and membrane fluidity of cerebrocortical membranes after experimental subarachnoid hemorrhage. Neurochem Res 16:1033–1039
Zhan H, Tada T, Nakazato F, Tanaka Y, Hongo K (2004) Spatial learning transiently disturbed by intraventricular administration of ouabain. Neurol Res 26:35–40
Zhang LN, Sun YJ, Pan S, Li JX, Qu YE, Li Y, Wang YL, Gao ZB (2013) Na+ -K+ -ATPase, a potent neuroprotective modulator against Alzheimer disease. Fundam Clin Pharmacol 27:96–103
Acknowledgments
This work was supported in part by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Brazil) and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS, RS, Brazil).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schweinberger, B.M., Schwieder, L., Scherer, E. et al. Development of an animal model for gestational hypermethioninemia in rat and its effect on brain Na+,K+-ATPase/Mg2+-ATPase activity and oxidative status of the offspring. Metab Brain Dis 29, 153–160 (2014). https://doi.org/10.1007/s11011-013-9451-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-013-9451-x