Abstract
Protein/peptide hormones are the largest group of endogenous signaling molecules and exert various biological functions by binding to specific cell membrane receptors. To study the interactions between these hormones and their receptors, quantitative ligand–receptor binding assays have been widely used for decades. However, the assays conventionally relied on the use of radioligands, which have some major drawbacks and can only be used in laboratories with a radioactive material license. We recently developed novel bioluminescent binding assays for several protein/peptide hormones using the brightest bioluminescent reporter known to date, nanoluciferase (NanoLuc). The NanoLuc reporter can be either chemically conjugated to an appropriate position, or genetically fused at one terminus, of protein/peptide hormones. Compared to conventional radioligands, these bioluminescent ligands have higher sensitivity, better safety, and longer shelf lives, and thus, represent a novel class of non-radioactive tracers for quantitative receptor binding assays. In the present review, we provide some general considerations and specific examples for setting up the bioluminescent binding assays. Such techniques can be applied to other protein/peptide hormones in future to facilitate their interaction studies with their receptors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protein/peptide hormones include all secretory proteins and peptides with signal transduction functions, such as classical protein/peptide hormones, cytokines, chemokines, and neuropeptides. Thus, protein/peptide hormones are the largest group of endogenous signaling molecules known to date. These hormones exert a variety of biological functions by binding to specific cell membrane receptors, including G protein-coupled receptors, kinase receptors, and ion channels.
The interaction between protein/peptide hormones and their receptors has three major characteristics. First, it shows high diversity. To date, hundreds of secretory proteins and peptides have been identified as protein/peptide hormones with known or as yet unknown receptors, and it is likely that even more protein/peptide hormones will be uncovered in the future. Second, the interaction is highly specific, with each protein/peptide hormone binding a unique or very limited number of receptor(s) to initiate highly specific downstream signaling. Third, as their in vivo concentration is very low, the protein/peptide hormones bind to their receptors with high affinity, i.e., dissociation constants (K d) are typically in the nanomolar to picomolar range.
To study the interactions between protein/peptide hormones and their receptors, quantitative ligand–receptor binding assays, known as radioligand binding assays, have been used for decades (Bonini et al. 1991; Bylund and Toews 2011; de Jong et al. 2005; Hulme and Trevethick 2010; Maguire et al. 2012; McKinney and Raddatz 2006; Sykes et al. 2010). Receptor binding affinity (dissociation constant, K d) of the ligand and quantity of the receptor (maximal binding capacity, B max) can be accurately measured using saturation binding assays. Competition binding assays can be used to quantify and compare receptor binding potencies (IC50 or K i) of various natural or designed ligands. In addition, kinetic binding assays can determine association and dissociation velocity of a ligand with its receptor. However, a major hurdle is that cell membrane receptors are difficult to purify in most cases, and thus, the quantities available for binding assays are very limited (typically in the femtomole range). Therefore, to detect the receptors on living cells or crude cell membrane fractions, binding assays typically utilize highly sensitive tracers (ligands labeled by an appropriate probe).
Tracers for receptor binding assays need to fulfill the following criteria: (1) high binding affinity with their receptors, and therefore, the disturbance of the labeled probe needs to be sufficiently low; (2) high sensitivity with a detection limit typically in the attomole range; and (3) low background (i.e., other components of the assays, such as whole cells or cell membrane fractions, should have no interference) for accurate quantification of the tracer. Conventionally, radioisotope-labeled ligands, termed radioligands, are used as tracers in ligand–receptor binding assays because they can fulfill these criteria (Bonini et al. 1991; Bylund and Toews 2011; de Jong et al. 2005; Hulme and Trevethick 2010; Maguire et al. 2012; McKinney and Raddatz 2006; Sykes et al. 2010). As listed in Table 1, the radioisotopes commonly used for ligand labeling can be conveniently quantified according to their irradiation, with the detection limit in the attomole range, and without interference from other components of the assays. For example, protein/peptide hormones are typically labeled by iodine-125 (125I) that has a specific radioactivity of 2175 C i/mmol (or 4830 dpm/fmol). The detection limit of the γ counter or liquid scintillator is typically 60–100 dpm, and thus, 125I has a detection limit of ~20 amol. For the 125I-labeled proteins/peptides, their specific radioactivity depends upon the labeled 125I number, typically 1–10 125I-moieties per protein/peptide molecule.
Although radioligands have been used for decades in ligand–receptor binding assays, they have drawbacks. In particular, radioligands are radioactive hazards to both the operators and the environments, and thus they can only be used in laboratories with a radioactive material license. In addition, the shelf lives of the radioligands are short due to radioactive decay of the radioisotopes and their destruction by irradiation (e.g., shelf lives of 125I-labeled proteins/peptides are typically within 2 months). Moreover, radioligands undergo constant decomposition due to radioisotope decay and irradiation damage, and therefore, cannot be accurately quantified after storage. To overcome these drawbacks, non-radioactive ligand–receptor binding assays have recently been developed, such as lanthanide-based time-resolved fluorescent assays (Handl and Gillies 2005; Selvin 2002; Shabanpoor et al. 2008, 2011, 2012; Zhang et al. 2012a, b, c, 2013b). However, detection sensitivity of the lanthanide-based ligands is not sufficiently high. For example, the specific activity of europium (Eu3+) is approximately 2000 RFU/fmol when measured on a SpectraMax M5 plate reader using a white opaque 384-well plate. Detection limit of the plate reader is typically 100–200 RFU, and thus, Eu3+ has a detection limit of ~100 amol on the SpectraMax M5 plate reader. If a more sensitive plate reader is used, the detection sensitivity of Eu3+ will be about tenfold higher. Thus, Eu3+ has a detection limit of approximately 10–100 amol nowadays depending upon the plate readers (Table 1). Moreover, preparation of the lanthanide-based non-radioactive tracers is difficult and expensive, thus other non-radioactive binding assays are urgently needed. In this review, we outline a newly developed bioluminescent ligand–receptor binding assay and describe specific examples of how to prepare and utilize this assay for characterizing the interactions between protein/peptide hormones and their receptors.
Bioluminescence and the newly developed NanoLuc reporter
Bioluminescence occurs widely in marine vertebrates and invertebrates, as well as in some fungi and bacteria. Some enzymes catalyzing bioluminescence reactions have been developed as reporters (e.g., firefly luciferase, Renilla luciferase, and Gaussia luciferase) for use in gene reporter assays, in vivo imaging, and some other quantitative assays (Badr 2014; Frank and Krasitskaya 2014; Scott et al. 2011). However, these conventional luciferase reporters also have some disadvantages, such as low specific activity, low stability, and large size. Therefore, a new generation of luciferase reporter, termed nanoluciferase (NanoLuc®, Promega Incorporation) was developed in 2012, offering several advantages over the conventional luciferase reporters (Hall et al. 2012).
The advantages of NanoLuc include: (1) higher sensitivity (its specific activity is ~150-fold higher than that of the firefly luciferase and Renilla luciferase, making it the brightest luciferase reported to date); (2) smaller size (171 amino acids or 19 kDa in size without any posttranslational modifications, which makes it the smallest luciferase reporter known to date); (3) higher stability; (4) and highly reproducible and convenient measurement (emits a glow-type bioluminescence independent of ATP with a long emission half life). Due to these advantages, NanoLuc was nominated as the Top 10 Innovations of 2012 by the journal Scientists. Since then, it has been used in many assays, such as high throughput screening (Ho et al. 2013), in vivo imaging (Karlsson et al. 2015; Stacer et al. 2013; Sun et al. 2014; Tran et al. 2013), BRET assays (Machleidt et al. 2015; Mo et al. 2016; Robers et al. 2015b; Stoddart et al. 2015), and membrane receptor internalization assays (Liu et al. 2015b; Robers et al. 2015a; Song et al. 2013).
Novel bioluminescent receptor binding assays for protein/peptide hormones
Based on its abovementioned properties, NanoLuc has potential as a probe for developing novel non-radioactive receptor binding assays for protein/peptide hormones. Indeed, NanoLuc is highly sensitive and its activity can be conveniently measured on various luminometers that are available in most laboratories. For example, the measured specific activity of NanoLuc is 1.5 × 105 RLU/fmol on a SpectraMax M5 plate reader and 3 × 106 RLU/fmol on a Lumat LB9507 luminometer. The detection limit of these luminometers is typically 100–200 RLU, and thus, NanoLuc has a detection limit of approximately 1–0.1 amol (Table 1), which is 10–100-fold lower than that of 125I. Moreover, if more sensitive luminometers are used, the detection sensitivity of NanoLuc can be further increased. NanoLuc is also the smallest luciferase reporter developed to date, with high physical stability, and thus, the NanoLuc-based ligands likely retain high receptor binding affinity and can be stored for a long time without activity loss. Finally, bioluminescence of NanoLuc has low background and broad linear range; it can be accurately quantified with a linear range of five orders of magnitude on most luminometers, and shows no interference from other components of the assays (e.g., whole cells or cell membrane fractions) because they have no endogenous NanoLuc activity.
To validate the bioluminescent binding assay, we tested it on several protein/peptide hormones with various sizes and distinct receptors in our recent studies (He et al. 2014; Liu et al. 2015a; Song et al. 2015; Wu et al. 2016; Zhang et al. 2013a). So far, the bioluminescent ligand–receptor binding assay has been shown to work well on relaxin (receptor RXFP1), INSL3 (receptor RXFP2), chimeric relaxin family peptide R3/I5 (receptor RXFP3 and RXFP4), ghrelin (receptor GHSR1a), leukemia inhibitory factor (receptor LIFR/gp130), erythropoietin (receptor EPOR), and fibroblast growth factor 2 (receptor FGFR). Thus, it seems that this assay can be applied to a variety of protein/peptide hormones.
Compared with conventional radioligands, the novel bioluminescent ligands have several advantages, including: (1) higher sensitivity, the detection sensitivity of the bioluminescent ligands is at least tenfold higher than that of the 125I-labeled radioligands; (2) improved safety, the use of the bioluminescent ligands has no safety concerns and can be carried out in every laboratory; (3) longer shelf lives, the bioluminescent ligands can be stored at −80 °C for several months or even longer; and (4) more convenience, as the luminescence measurement is quick and luminometers are available in most laboratories. Thus, the novel bioluminescent binding assays can facilitate interaction studies of protein/peptide hormones with their receptors, such as characterization of natural or designed ligands, screening of novel ligands, or identification of unknown receptors.
General considerations for preparation of the NanoLuc-based ligands
For setting up these bioluminescent binding assays, preparation of bioluminescent ligands is a key but challenging step. First of all, the bioluminescent ligands should retain high binding affinity with their receptors, and therefore, the NanoLuc moiety should be attached to an appropriate position using a suitable method to minimize its disturbance to receptor binding. Considering the high diversity of protein/peptide hormones, we previously established two methods to prepare NanoLuc-based bioluminescent ligands: chemical conjugation and genetic fusion (He et al. 2014; Liu et al. 2015a; Song et al. 2015; Wu et al. 2016; Zhang et al. 2013a). For the chemical conjugation method, rationally designed recombinant or synthetic protein/peptide hormones are chemically conjugated with an engineered NanoLuc in a site-specific manner. For the genetic fusion method, the NanoLuc reporter is fused either at the N terminus or at the C terminus of the protein/peptide hormones and the fusion proteins are overexpressed in suitable host cells. Each method has its own advantages and disadvantages, and can be applied to different protein/peptide hormones. For example, chemical conjugation offers more choice for the conjugation site, but it is more complex than the genetic fusion method. In contrast, the genetic fusion method is simpler, but NanoLuc has to be fused at one terminus of the polypeptide chain and this may disturb receptor binding and/or folding of the protein/peptide hormones.
The chemical conjugation method
For site-specific conjugation of a protein/peptide hormone with the NanoLuc reporter, a unique reactive moiety should be generated on both the hormone and the reporter. We previously introduced a unique exposed Cys residue at either the N terminus or the C terminus of the NanoLuc reporter, and showed that these constructs retained full enzymatic activity (Liu et al. 2015a; Zhang et al. 2013a). Moreover, the constructs could be conveniently prepared through overexpression in E. coli. We also determined that the 6 × His-NanoLuc-Cys reporter is better than the 6× His-Cys-NanoLuc because it contains a long flexible arm between the exposed C terminal Cys and the NanoLuc molecule.
For the protein/peptide hormones, a unique reactive moiety should also be generated at an appropriate position to minimize disturbance of the conjugated NanoLuc. Considering the high diversity of protein/peptide hormones, one of the following reactive moieties can be generated for conjugation, according to the property of each protein/peptide hormone. For protein/peptide hormones without free Cys residues, a unique exposed primary amine moiety can be generated and used for conjugation with the NanoLuc reporter. Primary amine moieties are present at the N terminus of the polypeptide chain and at the side-chain of Lys residues, and they can be specifically modified by a variety of commercially available chemical reagents. A unique exposed primary amine moiety can be generated at an appropriate position of the protein/peptide hormones using one or more of the following approaches: (1) blocking the unnecessary N terminal primary amine moiety by introducing a Gln residue that can be chemically or enzymatically converted to a pyroglutamate residue without a primary amine moiety; (2) replacing the unnecessary Lys residue(s) with other residues, typically by Arg (as both Lys and Arg carry a positive charge); or (3) introducing a Lys residue at an appropriate position. Once a unique and appropriate primary amine moiety is generated, the protein/peptide hormone is modified by a bifunctional reagent carrying a primary amine-specific moiety (e.g., N-hydroxysuccinimidyl ester) and a sulfhydryl-specific moiety (e.g., pyridyldithiol). Once a sulfhydryl-specific moiety is introduced, the protein/peptide hormone can be conjugated with the engineered NanoLuc that carries a unique exposed Cys residue.
For protein/peptide hormones without disulfides or essential free Cys residues, a unique exposed Cys residue can also be generated, and then its side-chain sulfhydryl moiety can be specifically modified by a variety of commercially available reagents. A unique exposed Cys residue can be generated at an appropriate position of the protein/peptide hormones using one or more of the following approaches: (1) replacing the original exposed Cys residue(s) with other residues, typically by Ser or Ala; (2) introducing a Cys residue at an appropriate position if necessary. Once a unique exposed Cys residue is generated, the protein/peptide hormone can be directly conjugated with the activated NanoLuc reporter whose exposed Cys was activated by a pyridyldithiol moiety.
For some protein/peptide hormones, other reactive moieties can also be generated and used for conjugation with the NanoLuc reporter, such as azido moiety, alkyne moiety, or a formylglycine residue. Once a unique reactive moiety is generated, the protein/peptide hormone reacts with a suitable bifunctional reagent for introduction of a sulfhydryl-specific moiety, which then reacts with the unique exposed Cys residue of the engineered NanoLuc reporter and forms an intermolecular crosslinkage.
The NanoLuc-conjugated proteins/peptides can be purified by ion-exchange chromatography, confirmed by non-reducing SDS-PAGE, and quantified by bioluminescence measurement. For receptor binding assays, reducing reagents (such as dithiothreitol or 2-mercaptoethanol) cannot be included in the binding and washing steps if the conjugates have an intermolecular disulfide linkage (as these agents can break the disulfide bonds), but they can be included in the step of bioluminescence measurement. Occasionally, conjugation of a protein/peptide is detrimental to the NanoLuc activity and reducing reagents may be required to release the NanoLuc reporter from the conjugate during the bioluminescence measurement for accurate quantification, provided a reversible disulfide linkage is used in the conjugate.
The genetic fusion method
The genetic fusion method can also be used for preparation of the bioluminescent tracers for some protein/peptide hormones. However, as the NanoLuc reporter is fused at one terminus of the protein/peptide hormones, it can disturb receptor binding and/or folding in some cases. For fusion with the NanoLuc reporter, one terminus of the protein/peptide hormones should be distanced from the receptor binding site, to ensure that the fused NanoLuc reporter does not disturb receptor-binding. Meanwhile, a flexible linker peptide should be introduced between the NanoLuc reporter and the target protein/peptide hormone to lower their interference with each other. The NanoLuc-fusion proteins can be overexpressed in suitable host cells, such as E. coli, yeast, or cultured mammalian cells, according to property of each protein/peptide hormone. These fusion proteins can be purified using conventional approaches or even directly used without purification. If the overexpressed NanoLuc-fusion proteins retain considerable binding affinity with their receptor, they can be used as non-radioactive tracers in subsequent ligand–receptor binding assays.
Examples of bioluminescent ligand–receptor binding assays
In the present section, we provide details for setting up NanoLuc-based binding assays using four protein/peptide hormones as examples. In future, more bioluminescent binding assays may be established for other protein/peptide hormones using similar procedures.
Recombinant INSL3 as an example of the chemical conjugation method
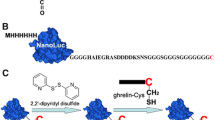
The relaxin family peptides play a variety of biological functions, and so far, four G protein-coupled receptors (RXFP1–4) have been identified as their receptors (Bathgate et al. 2013; Halls et al. 2015). The mature relaxin family peptides are typically composed of two polypeptide chains, and they can be efficiently prepared through overexpression of designed single-chain precursors in E. coli or Pichia pastoris, and subsequent in vitro maturation (Guo et al. 2015; Luo et al. 2010; Zhang et al. 2012a, b, c, 2013b). Recently, we generated several bioluminescent relaxin family peptides to study their interactions with their receptors. Here, we use INSL3 as an example of recombinant proteins to describe how to prepare bioluminescent tracers using the chemical conjugation method (Zhang et al. 2013a). The following three steps were used to generate the bioluminescent INSL3 tracer. (1) Selection of an appropriate conjugation site. The A-chain N terminus of INSL3 is suitable for conjugation because it is located at the opposite side of the receptor binding surface. (2) Generation of a unique reactive moiety. While the primary amine moiety of the A-chain N terminus can be used for conjugation, other primary amine moieties of INSL3 must be eliminated for site-specific conjugation with NanoLuc (Zhang et al. 2012a, 2013a). Therefore, to eliminate the B-chain N terminal primary amine moiety, we introduced a Gln residue to the B-chain N terminus and converted it to a primary amine-less pyroglutamate residue with papaya glutaminyl cyclase. To eliminate internal side-chain primary amine moieties, we replaced two Lys residues of INSL3 with Arg residue. The resultant easily-labeled INSL3 is fully active and suitable for site-specific conjugation with the engineered NanoLuc reporter. (3) Using a practical conjugation procedure. We introduced a sulfhydryl-specific moiety into the A-chain N terminus of the easily-labeled INSL3, using N-succinimidyl 3-(2-pyridyldithio)propionate (SPDP) as a bifunctional crosslinker because its pyridyldithiol moiety is stable under non-reducing conditions, and thus, the modified product can be conveniently purified. The introduced pyridyldithiol moiety at the A-chain N terminus of INSL3 can react with the unique exposed Cys of the engineered NanoLuc reporter to form an intermolecular disulfide linkage, as shown in Fig. 1a.
Application of the chemical conjugation method to INSL3 and ghrelin for novel bioluminescent receptor-binding assays (Liu et al. 2015a; Zhang et al. 2013a). a Schematic presentation of the NanoLuc-conjugated recombinant INSL3 (INSL3-Luc). b Saturation binding of INSL3-Luc with living HEK293T cells overexpressing receptor RXFP2. Nonspecific binding data were obtained by competition with 1.0 μM of INSL3. Left inner panel measured total binding versus nonspecific binding of INSL3-Luc. Right inner panel scatchard plot of the specific binding data. c Competition binding of wild-type and mutant INSL3s with the overexpressed RXFP2 using INSL3-Luc as a tracer. d Schematic presentation of the NanoLuc-conjugated synthetic ghrelin (ghrelin-Luc). e Saturation binding of ghrelin-Luc with living HEK293T cells overexpressing receptor GHSR1a. Nonspecific binding data were obtained by competition with 1.0 μM of ghrelin. f Competition binding of various ligands with the overexpressed GHSR1a using ghrelin-Luc as a tracer
NanoLuc-conjugated protein/peptide hormones must retain high receptor binding affinity. Saturation binding studies (Fig. 1b) showed that the NanoLuc-conjugated INSL3 (INSL3-Luc) bound living human embryonic kidney (HEK) 293T cells overexpressing receptor RXFP2 with a calculated dissociation constant (K d) of 2.0 ± 0.1 nM (n = 3). Thus, INSL3-Luc retained high receptor binding affinity, suggesting that the NanoLuc moiety has no serious detriments to receptor binding of INSL3. Moreover, nonspecific binding of INSL3-Luc was quite low due to the hydrophilic nature of the NanoLuc moiety. Since INSL3-Luc retained high receptor binding affinity, we used it as a novel bioluminescent tracer in competition binding assays to measure the receptor binding potencies of various ligands. As shown Fig. 1c, INSL3-Luc can discriminate the receptor binding potencies of different ligands by giving different IC50 values. In summary, a novel bioluminescent INSL3 tracer can be prepared using the chemical conjugation method and used for ligand–receptor interaction studies.
Synthetic ghrelin as an example of the chemical conjugation method
Ghrelin is a 28 amino acid peptide hormone that has a special n-octanoyl moiety on the side-chain of Ser3 (Kojima et al. 1999). Ghrelin exerts its biological functions through the receptor GSHR1a, or through other as yet unknown receptors. Here, we use ghrelin as an example of synthetic peptides to describe how to prepare bioluminescent tracers using the chemical conjugation method (Liu et al. 2015a). The following three steps were used to generate the bioluminescent ghrelin tracer. (1) Selection of an appropriate conjugation site. It is known that the N terminal part of ghrelin is essential while the C terminal part is unnecessary for receptor binding. Therefore, conjugation of the NanoLuc reporter at the C terminus is reasonable. (2) Generation of a suitable unique reactive moiety. As the primary amine moiety cannot be used for ghrelin conjugation because its N terminus is essential for activity, and considering that this peptide has no free Cys residues or disulfide bonds, we introduced a free Cys at its C terminus as a unique conjugation site. (3) Using a practical conjugation procedure, the unique exposed Cys of the engineered 6 × His-NanoLuc-Cys was first activated by a pyridyldithiol moiety through treatment with 2,2′-dipyridyl disulfide, and then reacted with ghrelin-Cys to form an intermolecular disulfide linkage (Fig. 1d). Alternatively, ghrelin-Cys could also be activated by 2,2′-dipyridyl disulfide treatment, and then reacted with 6 × His-NanoLuc-Cys to form an intermolecular disulfide linkage.
As tested on living HEK293T cells overexpressing the GHSR1a receptor (Fig. 1e), the NanoLuc-conjugated ghrelin (ghrelin-Luc) retained high binding affinity with GHSR1a, with a calculated K d of 1.14 ± 0.13 nM (n = 3). When ghrelin-Luc was used as a tracer in competition binding assays (Fig. 1f), typical sigmoidal competition curves were obtained for different ligands, from which the receptor binding potencies of these ligands could be calculated and compared. In summary, a novel bioluminescent ghrelin tracer could be prepared through the chemical conjugation method and used for ligand–receptor interaction studies.
LIF as an example of the genetic fusion method
Leukemia inhibitory factor (LIF) is an interleukin-6 family cytokine with pleiotropic effects on a diverse range of cells. LIF activates a heterodimeric cell membrane receptor composed of a LIFR subunit (gp190) and a gp130 subunit (Heinrich et al. 2003). The mature human LIF protein contains 180 amino acids and forms a four-helix bundle structure with three conserved disulfide bonds. Significant quantities of biologically active LIF protein are required for stem cell research, as it can promote long-term maintenance of mouse embryonic stem cells by suppressing spontaneous differentiation. Unfortunately, recombinant expression of LIF in E. coli is very difficult due to its high aggregation propensity. However, after the NanoLuc reporter was fused at its N terminus, a soluble 6 × His-NanoLuc-LIF fusion protein (Fig. 2a) could be efficiently overexpressed in E. coli and conveniently purified to homogeneity (He et al. 2014).
Application of the genetic fusion method to LIF and EPO for novel bioluminescent receptor-binding assays (He et al. 2014; Song et al. 2015). a Schematic presentation of the NanoLuc-fused LIF (6 × His-NanoLuc-LIF) overexpressed in E. coli. b Saturation binding of 6 × His-NanoLuc-LIF with murine leukemia M1 cells. Nonspecific binding data were obtained by competition with 30 nM of mature LIF. c Scatchard plot of the specific binding data. d Competition binding of recombinant LIF with the endogenously expressed receptor LIFR/gp130 using 6 × His-NanoLuc-LIF as a tracer. e Schematic presentation of the NanoLuc-fused EPO (EPO-Luc) overexpressed in HEK293T cells. f Bioluminescence measurement of the secreted EPO-Luc in the culture medium of the transiently transfected HEK293T cells. g Western blotting of the secreted EPO-Luc in the culture medium by polyclonal antibodies against NanoLuc. h Saturation binding of EPO-Luc with living HEK293T cells overexpressing receptor EPOR. Nonspecific binding data were obtained by competition with 100 nM of purified EPO protein. Left inner panel scatchard plot of the specific binding data. Right inner panel measured total and nonspecific binding of NanoLuc and EPO-Luc with the transfected or non-transfected HEK293T cells
Saturation binding studies (Fig. 2b) showed that the 6 × His-NanoLuc-LIF fusion protein bound the living murine leukemia M1 cells with a calculated K d of 33.1 ± 3.2 pM and a calculated B max of 8915 ± 225 RLU/105 cells (equal to ~350 receptors/cell). In contrast, NanoLuc itself had no detectable specific binding with M1 cells. Thus, 6 × His-NanoLuc-LIF retained high receptor binding affinity, even though a large NanoLuc moiety was fused at the N terminus. The Scatchard plot of the specific binding data was linear (Fig. 2c), suggesting that only the high affinity binding site is present on M1 cells. Finally, when 6 × His-NanoLuc-LIF was used as a tracer in a competition binding assay, a typical sigmoidal curve was obtained for mature LIF (Fig. 2d). Thus, the fusion protein 6 × His-NanoLuc-LIF produced in E. coli is a novel bioluminescent tracer for non-radioactive receptor binding assays.
EPO as an example of the genetic fusion method
Erythropoietin (EPO) is a glycosylated cytokine that promotes erythropoiesis by binding and activating the cell membrane receptor, EPOR (Chateauvieux et al. 2011; Jelkmann 2007). The mature human Epo has 166 amino acids with two disulfide bonds, as well as three N-linked and one O-linked carbohydrates. To apply the genetic fusion method to EPO, we developed a quick approach through secretory overexpression of the NanoLuc-fused EPO (EPO-Luc) in transiently transfected HEK293T cells (Song et al. 2015). The NanoLuc reporter was fused at the C terminus of the EPO precursor that contains an N terminal signal peptide for secretion (Fig. 2e). After this fusion protein was overexpressed in the transiently transfected HEK293T cells, high NanoLuc activity could be detected by bioluminescence measurement in the culture medium after transfection (Fig. 2f), suggesting secretory expression of the EPO-Luc fusion protein. Identity of the fusion protein was confirmed by SDS-PAGE and Western blotting using the antibodies against the NanoLuc reporter (Fig. 2g).
Saturation binding studies (Fig. 2h) indicated that the secreted EPO-Luc fusion protein bound the overexpressed EPOR in a typical saturation manner, with a calculated K d value of 0.83 ± 0.11 nM (n = 3). In summary, the fusion protein EPO-Luc produced in HEK293T cells is a novel bioluminescent tracer for interaction studies of EPO with its receptor.
Future work for the bioluminescent binding assay
In our recent studies, we validated the NanoLuc-based bioluminescent ligand–receptor binding assay using several protein/peptide hormones with different sized and distinct receptors. However, possible influence of the NanoLuc reporter to receptor binding is still a major concern for this novel assay, since the NanoLuc reporter is much large than the conventional radioisotopes. In future, more protein/peptide hormones should be tested for the NanoLuc-based binding assay.
For the tested protein/peptide hormones, the NanoLuc-based tracers retained similar or slightly decreased receptor-binding affinity compared to the previously reported radioactive tracers. However, there are no direct head-to-head comparison of binding of the NanoLuc-based tracers and the conventional radioligands. Moreover, the impact of NanoLuc labeling on signaling bias of receptors is also remain unknown so far. These questions will be addressed in future work as the novel bioluminescent binding assay is widely applied to a variety of protein/peptide hormones.
References
Badr CE (2014) Bioluminescence imaging: basics and practical limitations. Methods Mol Biol 1098:1–18
Bathgate RA, Halls ML, van der Westhuizen ET, Callander GE, Kocan M, Summers RJ (2013) Relaxin family peptides and their receptors. Physiol Rev 93:405–480
Bonini PA, Banfi G, Pazzagli M, Messeri G, Roda A (1991) Detection, signal processing, and calibration in immunoassay systems. J Autom Chem 13:45–47
Bylund DB, Toews ML (2011) Radioligand binding methods for membrane preparations and intact cells. Methods Mol Biol 746:135–164
Chateauvieux S, Grigorakaki C, Morceau F, Dicato M, Diederich M (2011) Erythropoietin, erythropoiesis and beyond. Biochem Pharmacol 82:1291–1303
de Jong LA, Uges DR, Franke JP, Bischoff R (2005) Receptor-ligand binding assays: technologies and applications. J Chromatogr B Anal Technol Biomed Life Sci 829:1–25
Frank LA, Krasitskaya VV (2014) Application of enzyme bioluminescence for medical diagnostics. Adv Biochem Eng Biotechnol 144:175–197
Guo YQ, Wu QP, Shao XX, Shen T, Liu YL, Xu ZG, Guo ZY (2015) Secretory overexpression and isotopic labeling of the chimeric relaxin family peptide R3/I5 in Pichia pastoris. Amino Acids 47:1117–1125
Hall MP, Unch J, Binkowski BF, Valley MP, Butler BL, Wood MG, Otto P, Zimmerman K, Vidugiris G, Machleidt T, Robers MB, Benink HA, Eggers CT, Slater MR, Meisenheimer PL, Klaubert DH, Fan F, Encell LP, Wood KV (2012) Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem Biol 7:1848–1857
Halls ML, Bathgate RA, Sutton SW, Dschietzig TB, Summers RJ (2015) International Union of Basic and Clinical Pharmacology. XCV. Recent advances in the understanding of the pharmacology and biological roles of relaxin family peptide receptors 1–4, the receptors for relaxin family peptides. Pharmacol Rev 67:389–440
Handl HL, Gillies RJ (2005) Lanthanide-based luminescent assays for ligand-receptor interactions. Life Sci 77:361–371
He SX, Song G, Shi JP, Guo YQ, Guo ZY (2014) Nanoluciferase as a novel quantitative protein fusion tag: application for overexpression and bioluminescent receptor-binding assays of human leukemia inhibitory factor. Biochimie 106:140–148
Heinrich PC, Behrmann I, Haan S, Hermanns HM, Uller-Newen GM, Schaper F (2003) Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 374:1–20
Ho PI, Yue K, Pandey P, Breault L, Harbinski F, McBride AJ, Webb B, Narahari J, Karassina N, Wood KV, Hill A, Auld DS (2013) Reporter enzyme inhibitor study to aid assembly of orthogonal reporter gene assays. ACS Chem Biol 8:1009–1017
Hulme EC, Trevethick MA (2010) Ligand binding assays at equilibrium: validation and interpretation. Br J Pharmacol 161:1219–1237
Jelkmann W (2007) Erythropoietin after a century of research: younger than ever. Eur J Haematol 78:183–205
Karlsson EA, Meliopoulos VA, Savage C, Livingston B, Mehle A, Schultz-Cherry S (2015) Visualizing real-time influenza virus infection, transmission and protection in ferrets. Nat Commun 6:6378
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660
Liu Y, Shao XX, Zhang L, Song G, Liu YL, Xu ZG, Guo ZY (2015a) Novel bioluminescent receptor-binding assays for peptide hormones: using ghrelin as a model. Amino Acids 47:2237–2243
Liu Y, Song G, Shao XX, Liu YL, Guo ZY (2015b) Quantitative measurement of cell membrane receptor internalization by the nanoluciferase reporter: using the G protein-coupled receptor RXFP3 as a model. Biochim Biophys Acta 1848:688–694
Luo X, Bathgate RA, Zhang WJ, Liu YL, Shao XX, Wade JD, Guo ZY (2010) Design and recombinant expression of insulin-like peptide 5 precursors and the preparation of mature human INSL5. Amino Acids 39:1343–1352
Machleidt T, Woodroofe CC, Schwinn MK, Méndez J, Robers MB, Zimmerman K, Otto P, Daniels DL, Kirkland TA, Wood KV (2015) NanoBRET—a novel bret platform for the analysis of protein–protein interactions. ACS Chem Biol 10:1797–1804
Maguire JJ, Kuc RE, Davenport AP (2012) Radioligand binding assays and their analysis. Methods Mol Biol 897:31–77
McKinney M, Raddatz R (2006) Practical aspects of radioligand binding. Curr Protoc Pharmacol Chapter 1:Unit 1.3
Mo XL, Luo Y, Ivanov AA, Su R, Havel JJ, Li Z, Khuri FR, Du Y, Fu H (2016) Enabling systematic interrogation of protein-protein interactions in live cells with a versatile ultra-high-throughput biosensor platform. J Mol Cell Biol. doi:10.1093/jmcb/mjv064
Robers MB, Binkowski BF, Cong M, Zimprich C, Corona C, McDougall M, Otto G, Eggers CT, Hartnett J, Machleidt T, Fan F, Wood KV (2015a) A luminescent assay for real-time measurements of receptor endocytosis in living cells. Anal Biochem 489:1–8
Robers MB, Dart ML, Woodroofe CC, Zimprich CA, Kirkland TA, Machleidt T, Kupcho KR, Levin S, Hartnett JR, Zimmerman K, Niles AL, Ohana RF, Daniels DL, Slater M, Wood MG, Cong M, Cheng YQ, Wood KV (2015b) Target engagement and drug residence time can be observed in living cells with BRET. Nat Commun 6:10091
Scott D, Dikici E, Ensor M, Daunert S (2011) Bioluminescence and its impact on bioanalysis. Annu Rev Anal Chem (Palo Alto Calif) 4:297–319
Selvin PR (2002) Principles and biophysical applications of lanthanide-based probes. Annu Rev Biophys Biomol Struct 31:275–302
Shabanpoor F, Hughes RA, Bathgate RA, Zhang S, Scanlon DB, Lin F, Hossain MA, Separovic F, Wade JD (2008) Solid-phase synthesis of europium-labeled human INSL3 as a novel probe for the study of ligand-receptor interactions. Bioconjug Chem 19:1456–1463
Shabanpoor F, Separovic F, Wade JD (2011) General method for selective labelling of double-chain cysteine-rich peptides with a lanthanide chelate via solid-phase synthesis. J Pept Sci 17:169–173
Shabanpoor F, Bathgate RA, Belgi A, Chan LJ, Nair VB, Wade JD, Hossain MA (2012) Site-specific conjugation of a lanthanide chelator and its effects on the chemical synthesis and receptor binding affinity of human relaxin-2 hormone. Biochem Biophys Res Commun 420:253–256
Song G, Jiang Q, Xu T, Liu YL, Xu ZG, Guo ZY (2013) A convenient luminescence assay of ferroportin internalization to study its interaction with hepcidin. FEBS J 280:1773–1781
Song G, Wu QP, Xu T, Liu YL, Xu ZG, Zhang SF, Guo ZY (2015) Quick preparation of nanoluciferase-based tracers for novel bioluminescent receptor-binding assays of protein hormones: using erythropoietin as a model. J Photochem Photobiol B 153:311–316
Stacer AC, Nyati S, Moudgil P, Iyengar R, Luker KE, Rehemtulla A, Luker GD (2013) NanoLuc reporter for dual luciferase imaging in living animals. Mol Imaging 12:1–13
Stoddart LA, Johnstone EK, Wheal AJ, Goulding J, Robers MB, Machleidt T, Wood KV, Hill SJ, Pfleger KD (2015) Application of BRET to monitor ligand binding to GPCRs. Nat Methods 12:661–663
Sun C, Gardner CL, Watson AM, Ryman KD, Klimstra WB (2014) Stable, high-level expression of reporter proteins from improved alphavirus expression vectors to track replication and dissemination during encephalitic and arthritogenic disease. J Virol 88:2035–2046
Sykes DA, Dowling MR, Charlton SJ (2010) Measuring receptor target coverage: a radioligand competition binding protocol for assessing the association and dissociation rates of unlabeled compounds. Curr Protoc Pharmacol Chapter 9:Unit 9.14
Tran V, Moser LA, Poole DS, Mehle A (2013) Highly sensitive real-time in vivo imaging of an influenza reporter virus reveals dynamics of replication and spread. J Virol 87:13321–13329
Wu QP, Zhang L, Shao XX, Wang JH, Gao Y, Xu ZG, Liu YL, Guo ZY (2016) Application of the novel bioluminescent ligand-receptor binding assay to relaxin-RXFP1 system for interaction studies. Amino Acids 48:1099–1107
Zhang WJ, Gao XJ, Liu YL, Shao XX, Guo ZY (2012a) Design, recombinant preparation and europium-labeling of a fully active easily-labeled INSL3 analogue for receptor-binding assays. Process Biochem 47:1856–1860
Zhang WJ, Luo X, Liu YL, Shao XX, Wade JD, Bathgate RA, Guo ZY (2012b) Site-specific DOTA/europium-labeling of recombinant human relaxin-3 for receptor-ligand interaction studies. Amino Acids 43:983–992
Zhang WJ, Luo X, Song G, Wang XY, Shao XX, Guo ZY (2012c) Design, recombinant expression and convenient A-chain N-terminal europium-labelling of a fully active human relaxin-3 analogue. FEBS J 279:1505–1512
Zhang L, Song G, Xu T, Wu QP, Shao XX, Liu YL, Xu ZG, Guo ZY (2013a) A novel ultrasensitive bioluminescent receptor-binding assay of INSL3 through chemical conjugation with nanoluciferase. Biochimie 95:2454–2459
Zhang WJ, Jiang Q, Wang XY, Song G, Shao XX, Guo ZY (2013b) A convenient method for europium-labeling of a recombinant chimeric relaxin family peptide R3/I5 for receptor-binding assays. J Pept Sci 19:350–354
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31470767, 31270824), and the Fundamental Research Funds for the Central Universities (2000219098).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Handling Editor: J. D. Wade.
Rights and permissions
About this article
Cite this article
Liu, YL., Guo, ZY. Novel bioluminescent binding assays for interaction studies of protein/peptide hormones with their receptors. Amino Acids 48, 1151–1160 (2016). https://doi.org/10.1007/s00726-016-2220-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-016-2220-5