Abstract
Creatine (Cr) is a natural compound that plays an important role in cellular energy homeostasis. In addition, it ameliorates oxidative stress, glutamatergic excitotoxicity, and apoptosis in vitro as well as in vivo. Since these pathomechanisms are implicated to play a role in several neurodegenerative diseases, Cr supplementation as a neuroprotective strategy has received a lot of attention with several positive animal studies in models of Parkinson’s disease (PD), Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS). This has led to a number of randomized clinical trials (RCT) with oral Cr supplementation, with durations up to 5 years. In this paper, we review the evidence and consequences stemming from these trials. In the case of PD, the initial phase II RCT was promising and led to a large and well-designed phase III trial, which, however, turned out to be negative for all outcome measures. None of the RCTs that have examined effects of Cr in ALS patients showed any clinical benefit. In HD, Cr in high doses (up to 30 g/day) was shown to slow down brain atrophy in premanifest Huntingtin mutation carriers. In spite of this, proof is still lacking that Cr can also have beneficial clinical effects in this group of patients, who will go on to develop HD symptoms. Taken together, the use of Cr supplementation has so far proved disappointing in clinical studies with a number of symptomatic neurodegenerative diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Rationale of creatine for neuroprotection
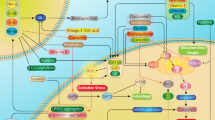
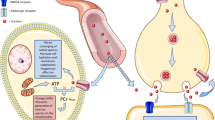
Creatine (Cr) is a natural compound that plays an important role in mitochondrial energy metabolism. In combination with phosphocreatine (PCr) and the creatine kinases (CK), it serves as an efficient energy buffering and energy transportation system (Hemmer and Wallimann 1993; Wallimann et al. 2011). It is widely used by athletes as a nutritional supplement to enhance muscular performance (Tarnopolsky 2010). It also has anti-apoptotic, anti-excitotoxic, and direct anti-oxidative properties, both in vitro and in vivo (Bender et al. 2005; Genius et al. 2012; Lawler et al. 2002; O’Gorman et al. 1997). These properties make it an attractive neuroprotective candidate, because oxidative stress, excitotoxicity, apoptosis, and mitochondrial dysfunction are all suggested to contribute to neurodegeneration, such as in Parkinson disease (PD) and amyotrophic lateral sclerosis (ALS) (Olanow 2007; Cleveland and Rothstein 2001). As Cr has a very good safety profile even in aged individuals with chronic diseases, is readily available as a supplement or naturally occurring in fish and meat, and has demonstrated a wide spectrum of supposedly positive effects, it is suggested to be beneficial in several neurological and non-neurological human diseases (Bender et al. 2008; Gualano et al. 2012). In fact, Cr meets all requirements proposed by the Committee to identify Neuroprotective Agents in Parkinson’s disease (CINAPS): (1) scientific rationale, (2) evidence of blood–brain barrier penetration, (3) adequate safety data, and (4) efficacy in animal models (NINDS NET-PD Investigators 2006). Several reviews have already highlighted the neuroprotective effect of Cr in neurodegenerative disease and have expressed high hopes for its evaluation in clinical phase II and III studies (Beal 2011; Klopstock et al. 2011; Smith et al. 2014). In recent years, substantial efforts have been made to translate the encouraging neuroprotective effects of Cr in in vitro and in animal disease models to human disease. This review summarizes and discusses the results of these efforts.
Methods
Both authors have independently conducted a literature search, using PubMed (http://www.ncbi.nlm.nih.gov/pubmed), the Cochrane library (http://www.cochranelibrary.com), and the NIH clinicaltrials.gov database (https://clinicaltrials.gov) to identify randomized controlled clinical trials, which have tested a neuroprotective effect of oral Cr supplementation on disease progression in neurodegenerative disease. Key words were: Cr, randomized controlled trial, neuroprotection, neurodegenerative disease. The search was conducted in September 2015 and was not limited to specific time periods. For animal studies, only the PubMed resource was used with the following key words: Cr [title word], supplementation, neuroprotection, neurodegenerative disease. In vitro studies were not included.
Parkinson disease
Data from animal models
Even though its pathogenesis is far from understood, mitochondrial dysfunction and oxidative damage have been suggested to play a key role in PD (Schapira 2008). The continuous evaluation of Cr as a neuroprotective agent in PD started in 1999 when it was shown to be strongly protective in a toxic mouse model for PD, targeting mitochondrial function (Matthews et al. 1999). This model is based on the i.p. injection of the neurotoxin 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP), which blocks complex I of the mitochondrial electron transport chain, leading to depleted striatal ATP concentrations. Intoxicated mice quickly develop dopamine depletion and substantial neuronal loss in the substantia nigra, mainly affecting dopaminergic neurons. Compared to a control diet, pre-treatment of mice with 1 % Cr in the chow for 2 weeks prior to MPTP injections almost completely abolished its deleterious effects on dopaminergic neurons. The same group replicated this result in 2003 and reported additive neuroprotective effects, when Cr was combined with the cyclooxygenase 2 (COX-2) inhibitor rofecoxib (Klivenyi et al. 2003). Later, in a mouse model of chronic s.c. MPTP intoxication, they found that the combination of Cr with coenzyme Q10 yielded even more neuroprotection, including less lipid peroxidation damage and less alpha-synuclein accumulation within dopaminergic substantia nigra neurons (Yang et al. 2009).
Randomized controlled human trials
Encouraged by the impressive neuroprotective capacity of Cr in animal models, we conducted a randomized placebo-controlled clinical trial (RCT) of oral Cr supplementation (up to 4 g per day) over 2 years in 60 PD patients (Bender et al. 2006). The trial was negative for the primary outcomes, i.e. there was no effect on SPECT variables of disease progression and no effect on overall scores of the UPDRS clinical rating scale. Yet, Cr-treated patients scored slightly higher on measures for mood and behaviour and they had to increase their dopamine dose for symptomatic PD treatment less than the control group over the 2-year study course. Importantly, Cr was well tolerated and did not have negative effects on renal function (Bender et al. 2008).
In the same year, a so called futility RCT with 10 g of Cr per day in 200 early PD patients (n = 67 patients received Cr) for 12 months was published and showed that Cr could not be rejected as futile with regard to slowing clinical disease progression (NINDS NET-PD Investigators 2006). Cr was retained by 91 % of patients throughout the study and was altogether well tolerated at this higher dosage of 10 g per day. Even though this trial was not designed and powered to show actual slowing of disease progression, the authors concluded that Cr showed potential for success for long-term Phase III neuroprotection trials.
A meta-analysis of the 194 patients of the two pilot or Phase II trials revealed no effect on either motor function or activities of daily living (ADL) but concluded that data was insufficient to draw a firm conclusion (Xiao et al. 2014).
In 2007, the National Institute of Neurological Disorders and Stroke (NINDS) launched a multicentre Phase III double-blind neuroprotection RCT of Cr within the Exploratory Trials of Parkinson Disease (NET-PD) program. Given that Cr is an over-the-counter food supplement without major pharmaceutical companies having strategic interest in it, this so called Long-Term Study 1 (LS-1) was an extraordinary effort and enroled 1741 patients in 45 sites with early (patients on dopaminergic drugs for at least three to a maximum of 24 months) and stable PD, who received 10 g of Cr per day or placebo (Kieburtz et al. 2015). The study was designed and powered to detect a slowing of disease progression of 1 year over a treatment period of 5 years, using an aggregate outcome measure, consisting of five tests and scores (for ADL, ambulatory capacity, disease-specific health problems, cognitive functioning, overall outcome). The study was stopped for futility after the second interim analysis in 2013 and a total of 955 patients, who were available for the 5-year follow-up assessments. There were no differences in any of the five individual outcome measures nor in the aggregate measure (p = 0.45). If anything, Cr patients had higher values in every one of the five individual outcome measures, indicating more disease progression. There were also no differences in secondary outcome measures, such as levodopa equivalent daily dose, depression, cognition, or quality of life. There were no differences in adverse or serious adverse events (SAE) between study groups. With regards to adherence to study medication, significantly more patients stopped study medication in the Cr group than in the placebo group (34 vs. 26 %).
Interesting data stem from a recent clinical trial, investigating the effect of a combination therapy of Cr (5 g b.i.d) and coenzyme Q10 (100 mg t.i.d) compared to placebo on motor as well as cognitive functioning in 75 PD patients with mild cognitive impairment (MCI) (Li et al. 2015). 38 patients were randomized to the active combination therapy group and 37 to the placebo control group and received study medication for 18 months. At 12 and 18 months, the cognitive decline determined by the Montreal Cognitive Assessment (MoCA) was less pronounced in the combination therapy group with Cr (drop in MoCA scores from 20.15 ± 3.11 to 18.55 ± 4.11 points after 18 months) than in the placebo group (drop in MoCA scores from 19.63 ± 4.12 to 13.33 ± 3.58 points; p < 0.01). There was no significant treatment effect on motor symptoms quantified with the UPDRS, though.
The overall available clinical evidence for a neuroprotective effect of Cr in PD is summarized in Table 1.
Huntington’s disease
Huntington’s disease (HD) is a neurogenetic trinucleotide repeat disease with full penetrance. The mechanisms by which mutant huntingtin is believed to exert its neurotoxic effects suggest a mitochondrial dysfunction with perturbation of energy homoeostasis, oxidative stress, and excitotoxicity (Lodi et al. 2000; Tabrizi et al. 1999; Kim et al. 2010).
Data from animal models
Cr was tested both in toxic as well as in genetic HD models. Systemic administration of the respiratory chain complex II inhibitors 3-nitropropionic acid or malonate to rats leads to a behavioural and neuropathological phenotype resembling human HD (Beal et al. 1993). In this model, oral Cr was associated with lower lesion volume and higher brain ATP and PCr levels (Matthews et al. 1998). The transgenic mouse models of HD are obviously even more appropriate models to study human disease. There, Cr also provided significant neuroprotection, improvement in motor performance, and higher overall survival in two different transgenic mouse models, when oral supplementation started prior to the development of the phenotype (Ferrante et al. 2000; Andreassen et al. 2001a). Even more relevant to application in human disease, the same effect was obtained when Cr was commenced after symptom onset (Dedeoglu et al. 2003).
Randomized controlled human trials
So far, two phase II RCTs of Cr in human manifest and one in premanifest HD have been published (Table 2). In the first, 5 g of Cr for 1 year had no effect on clinical outcome measures in 41 HD patients (Verbessem et al. 2003). A slightly higher dose of 8 g of Cr per day was given to 64 HD patients for 4 months. Under this setting, Cr seemed to cross the blood–brain barrier, and was well tolerated (Hersch et al. 2006). There was no effect on clinical outcomes, however, but Cr was able to decrease elevated serum levels of 8-hydroxy-2’-deoxyguanosine (8OH2’dG), a marker of oxidative stress. The following PRECREST trial enrolled 64 patients in a premanifest state or at 50 % genetic risk of HD to receive either up to 30 g of Cr per day (n = 32) or placebo with a double-blind protocol for 6 months (Rosas et al. 2014). Primary endpoints of this high-dose trial were tolerability and safety. Diarrhoea and nausea occurred more often under this high-dose Cr regime, but Cr was otherwise well tolerated and proved to be safe. There was no effect on secondary clinical outcome measures, although Cr slowed the rate of region specific brain atrophy in repeated MRI scans.
The Cr Safety, Tolerability, and Efficacy in HD (CREST-E) phase III trial then set out to enrol 650 early symptomatic HD patients to receive up to 40 g of Cr per day for up to 4 years. According to http://www.clinicaltrials.gov (Study identifier NCT00712426) and the webpage of the study consortium (http://huntingtonstudygroup.org) CREST-E was discontinued in early 2015 after 551 patients had enrolled in the study and after interim analysis showed that Cr was unlikely to be effective in slowing functional loss in HD patients, even at this very high Cr dosage. To the best of the author’s knowledge, so far, further data from this trial have not yet been published.
Amyotrophic lateral sclerosis (motor neuron disease)
Mitochondrial dysfunction, glutamate-mediated excitotoxicity, and oxidative stress have been implicated as pathomechanisms in ALS, again rendering it a potential target for Cr-mediated neuroprotection (Cleveland and Rothstein 2001). About 10 % of the cases are caused by pathogenic gene mutations, one of the most common ones being in copper/zinc superoxide dismutase (SOD1), a cytosolic enzyme responsible for scavenging oxidative stress in the cell (Peters et al. 2015).
Data from animal models
Transgenic mice carrying 23 copies of the human SOD1G93A gene have become the standard rodent ALS model. These mice show early mitochondrial swelling and vacuolization as well as altered electron transport chain enzyme activities, corresponding to similar mitochondrial abnormalities observed in post-mortem spinal cords of ALS patients (Gurney et al. 1994; Wong et al. 1995; Hervias et al. 2006). Two Cr supplementation studies by the same group reported a dose-dependent increase in survival compared to placebo, reduction of neuronal cell death, as well as a delay of the motor phenotype, with 1–2 % of Cr, started within 1–2 months of animal age (Klivenyi et al. 1999; Andreassen et al. 2001b). Cr was further evaluated in the same animal model in combination with other potentially neuroprotective agents. Here, the neuroprotective Cr effect was comparable but not additive to the standard clinical ALS drug, riluzole (Snow et al. 2003). In contrast, the combination of minocycline (a tetracycline antibiotic with neuroprotective properties) and Cr as well as the combination of Cr with the COX-2 inhibitors celecoxib and/or rofecoxib produced additive neuroprotective effects (Zhang et al. 2003; Klivenyi et al. 2004). It is noteworthy and deserves scientific credit, that one group undertook validation studies of some of the previously published successful murine SOD-trials. They controlled for common methodological issues and confounders in animal studies, such as small animal numbers per group, lack of control for low transgene copy number, and deaths unrelated to ALS (Scott et al. 2008). Using 77 transgenic mice, they could not replicate the positive results of the originally reported life-span extensions between 17.8 % (with 13 mice) and 19.8 % (with 24 mice) by Cr but they instead found only a non-significant life-span extension of less than 1 %.
Randomized controlled human trials
So far, three human randomized placebo-controlled trials of Cr in ALS with doses of 5 g or 10 g have been published (Table 3). In the first, 175 patients received either 10 g of Cr or placebo for up to 16 months (Groeneveld et al. 2003). Outcome was measured across different domains, such as tracheostomy-free survival time, overall survival, lung capacity, motor function, and a disease-specific functional rating scale. The trial was terminated, when it became clear, that there was no effect by Cr on either one of those outcomes. The same was true for a study on 104 ALS patients with 5 g of Cr over a time period of 6 months and yet another multicentre trial with 5 g in 107 patients over 9 months (Shefner et al. 2004; Rosenfeld et al. 2008).
Not surprising, a systematic Cochrane collaboration meta-analysis covering these three individual RCTs with a total of 386 patients found no benefit of Cr in any of the chosen outcome domains (Pastula et al. 2012). At the time of the preparation of this manuscript, no on-going clinical Cr trials could be identified at http://www.clinicaltrials.gov. One phase II trial randomising 60 ALS patients to receive either 30 g of Cr or one of two different tamoxifen doses is marked completed on this NIH webpage (identifier NCT01257581), but results have not yet been published.
Safety and side effects of Cr in clinical trials
Cr is in wide and relatively uncontrolled use by athletes (Tarnopolsky 2010). In addition, it is being tested for several clinical indications outside the neurodegeneration field, such as in myopathies, psychiatric, or cardiac disease (Gualano et al. 2012). Possible safety concerns regarding alleged deleterious effects of Cr on renal function have repeatedly been raised in the scientific field, as well as by the lay press (Thorsteinsdottir et al. 2006; Poortmans and Francaux 2000). Even though the results of the Cr neuroprotection trials for symptomatic neurodegenerative disease are disappointing, it is nevertheless of interest to look at the safety and side effect profile of Cr in these studies, to learn something for potential usage of Cr in other fields of application.
In the RCTs, 1687 patients or healthy controls have received a daily average of 9.5 g of Cr for a total of 5480 patient years (figures calculated from Tables 1–3). Cr was safe in all of these trials and there was no statistical difference in any of the documented SAE. Rates of discontinuation of Cr study medication in these trials ranged between 7 and 34 % (NINDS NET-PD Investigators 2006; Kieburtz et al. 2015). In the most comprehensive study so far, 5 % of patients receiving Cr discontinued study medication by protocol because their serum creatinine (Crn) levels or estimated glomerular filtration rates crossed pre-specified safety limits (Kieburtz et al. 2015). This may not necessarily reflect true impairment of renal function since oral Cr intake leads to a significant elevation of the total Cr body pool (mostly in muscles and brain). Since the conversion of Cr to Crn is determined by a chemical equilibrium with a fixed time constant, an elevation of the total Cr pool in the body will automatically result in a higher production of Crn leading to elevated serum Crn levels and renal Crn excretion. The Crn clearances in such subjects are hard to interpret. In patients with decreased Crn clearance, cystatin c clearance as another marker for glomerular filtration remained normal (Bender et al. 2008).
In the trials summarized in Tables 1–3, the only side effects that occurred with higher frequency in the Cr group than in the placebo group were nausea, diarrhoea, and overall gastrointestinal symptoms (Bender et al. 2008; Rosas et al. 2014).
Taken together, these randomized controlled trials show that Cr is safe and well tolerated even in considerable doses of up to 30 g per day for many months in aged patient populations.
Discussion
The published evidence from randomized clinical trials argues against major neuroprotective effects of Cr in human studies with patients presenting with neurodegenerative diseases. This seems especially true for Cr use in PD, where a large multicentre phase III trial (LS-1) showed no effects on any of the chosen clinical markers for disease progression (Kieburtz et al. 2015). The usual criticism of failed neuroprotection trials is that study medication was given too late in the course of the disease, that trials are not long enough, that study medication may have been underdosed, or that the wrong outcome measures were chosen (Ahlskog 2007; Brew 2007). In the case of Cr in PD, it is worth looking at the whole storyline, beginning at the initial rodent disease models. First, it is arguable whether toxic animal models based on mitochondrial complex I inhibition are really suitable to draw firm conclusions for human PD, even if they show typical clinical and neuropathological hallmarks of the disease. It would have been interesting to use another disease model, such as one of the genetic mouse models. Second, in these models, Cr was administered even before the intoxication started, which is of course far from the actual clinical setting. Then, the phase II pilot trial by the NINDS NET-PD investigators was designed as a futility trial that was not tailored to show significant treatment effects (NINDS NET-PD Investigators 2006). Still, this was a very practical approach but it differed from the following negative phase III trial because patients in phase II were de novo, i.e. had not yet received dopaminergic therapy and were thus earlier in the disease than in the phase III trial, where all patients were actually required to take dopaminergic therapy. Indeed, the start of Cr administration might therefore have been too late in the disease. Still, with 5 years, the duration of the trial was exceptionally long for an investigator- or academia-driven trial. If Cr were neuroprotective in PD, one would have expected to at least see trends after 5 years in some of the outcome measures. But in fact, the opposite was true. Looking at the raw data, the Cr group had non-significant worse absolute values in all of the five chosen outcome domains than the placebo group. This observation of no positive trend whatsoever in the Cr group also argues against the fact that Cr might just have been underdosed with 10 g daily intake. Even though 20 or even 30 g lead to stronger proton MRS Cr brain signals than 10 g a day, it has been shown that such lower doses exert biologically measurable effects in the brain, for example on the glutamate system (Atassi et al. 2010; Bender et al. 2005). Assuming a dose–response curve for neuroprotective Cr effects, it seems unlikely that there is not even a trend in a single outcome measure towards a slight improvement in the treatment group, even if 10 g of Cr were underdosed for neuroprotection in PD. Yet, a recent in vitro study showed, that there may be a rather complex U-shaped dose–response curve for a neuroprotective Cr effect (Stevens et al. 2014).
It would have been desirable to have more information on further subgroup or responder analyses of the large LS-1 phase III trial, but this has so far not been published. For example, it would be of special interest, if women with PD were more likely to profit, given that rodent models of depression suggest a therapeutic Cr gender effect, favouring female animals (Allen et al. 2012). Also, comparing vegetarian to non-vegetarian patients in the PD phase III trial would be an interesting subgroup analysis, if this information were part of the study protocol. It was suggested that healthy vegetarian women had improved memory functions compared to omnivores upon supplementation of 20 g of Cr for 5 days (Benton and Donohoe 2011). On the other hand, a recent proton MRS study showed that brain Cr content was not related to dietary Cr intake (Yazigi Solis et al. 2014).
So in our opinion, the most reasonable explanation for this negative trial is that Cr does not provide neuroprotection in PD. This is not only scientifically disappointing but may also be a major setback for academia-driven neuroprotection trials, given that the LS-1 phase III trial was a multimillion dollar venture within the $60 million effort by NINDS to find treatment strategies in PD (Couzin 2007).
Only the recent 18 months randomized controlled trial with Cr and coenzyme Q10 provided evidence for a positive effect of this combination therapy on the rate of cognitive decline (Li et al. 2015). This may be interpreted as a neuroprotective effect, even though it is unclear, which role Cr has in it, because there were no treatment arms applying only one of the two agents. Also, this phase II trial had only included 75 patients, while the much larger LS-1 phase III trial with almost 1000 patients showed no benefit of Cr alone on measures for cognitive functioning (Kieburtz et al. 2015).
Interesting data come from a study looking at the immunohistochemical distribution of the Cr transporter (CrT) in human brain (Lowe et al. 2015). This transporter is necessary for the uptake of Cr into neuronal cells (Braissant et al. 2001). While there was abundant expression in certain neuronal populations, such as the pyramidal cortical neurons or the ventral horn spinal neurons, the CrT could hardly be identified in the striatum and the dopaminergic substantia nigra neurons, the latter being of course the cell population, which predominantly dies in human PD. So, while it is clear that there is a sustained (at least for months) Cr increase in the human brain with the typical Cr dosing regimens used in the clinical trials, it may be that its biological effects are heterogeneous, depending on brain area and cell type (Atassi et al. 2010; Lyoo et al. 2003; Hersch et al. 2006). There are no data implying that the distribution of the CreaT would be different in rodents compared to humans (Mak et al. 2009). This does therefore not explain the discrepancy between the promising preclinical data and the overall disappointing results of randomized controlled human trials.
The investigators of the PRECREST trial of Cr in premanifest HD have chosen a very interesting and promising study design in terms of trying to start neuroprotection treatment as early as possible in neurodegenerative disease (Rosas et al. 2014). They administered up to 30 g of Cr for 6 months to genetically diagnosed premanifest HD patients, as well as to patients with a 50 % risk of HD, ending up with 47 genetically proven HD patients. Even though clinical markers of disease progression were similar between the Cr and the placebo group at the end of the trial, MRI showed marked slowing of brain atrophy in distinct brain areas in the Cr group (p < 0.0001). This is indeed promising and warrants a phase III trial in premanifest mutation carriers. Of course, the fact that a current phase III trial of Cr in early symptomatic HD seems to have been terminated due to futility argues against strong neuroprotective Cr effects (http://www.clinicaltrials.gov: identifier NCT00712426; CREST-E).
For the case of Cr in ALS, data are also not encouraging. So far, 191 patients have been treated with doses between 5 and 10 g of Cr and none of the outcomes or endpoints have been positive after 6 to 16 months of treatment (Table 3). On enrolment, patients were already limited in their pulmonal vital capacity (76–91 % of predicted normal), i.e. they were well into the pathophysiological process. Therefore, the start of Cr treatment might have been too late and Cr was possibly underdosed. Yet, again the most likely explanation seems to be that Cr is not neuroprotective in human ALS, as well.
It remains unclear why there seems to be a species barrier between man and mice with regard to Cr-mediated neuroprotection in neurodegenerative disease, but this phenomenon does not appear to be specific to failed Cr trials [for review: (Ergorul and Levin 2013)]. The fact that no neuroprotective Cr effect is found in genetic ALS mouse models, if a rigorous methodical approach is applied, is disturbing but emphasizes the need for animal studies to adopt control of confounders similar to human RCTs (Scott et al. 2008; National Academies of Sciences, Engineering, and Medicine 2015).
In the case of Cr, dosing between mice and humans may have also been an issue. It can be roughly estimated that human trials with 10 g of Cr per day represent still only 10 % of the dose in rodent models, if calculated to g per kg bodyweight, even though this estimation may be too simplistic (Bender et al. 2006). This probable underdosing even in the relatively high-dose human trials with 30 g of Cr/day may also be reflected by the fact that in the typical mouse studies, brain Cr increased by 21 ± 3.8 % while in the human randomized trials, only increases between 7.5 and 13 % could be obtained (Ferrante et al. 2000; Atassi et al. 2010; Hersch et al. 2006; Lyoo et al. 2003). Considering the relatively low expression of the CrT in human and rat basal ganglia and substantia nigra, the possibly much higher Cr dose in the animal studies might partially account for the lack of translation to the human disease situation, because neurons with low CrT expression might just need higher Cr plasma concentrations for significant uptake (Mak et al. 2009; Lowe et al. 2015). Interestingly, cortical neurons show abundant CrT immunoreactivity. This differential expression might account for the differential clinical effects, observed in a clinical trial of a combination therapy with Cr and coenzyme Q10 in PD patients (Li et al. 2015). While there was no positive effect on motor function—possibly corresponding to the low abundance of CrT within substantia nigra and basal ganglia—the rate of cognitive decline was markedly slowed down. Yet, the inability of Cr to protect motor neurons in ALS argues strongly against a strong effect of the regional distribution of the CrT on the clinical response rates to Cr therapy, because pyramidal neurons and ventral horn spinal cord neurons also show strong CrT signals, i.e. they should be susceptible to Cr neuroprotection (Lowe et al. 2015).
Despite the possible dosing issues, Cr has the same biological effect for example on reduction of glutamatergic signals in human MR spectroscopy studies as in in vitro studies (Atassi et al. 2010; Bender et al. 2005; Genius et al. 2012). So lack of clinical efficacy of Cr cannot solely be attributed to its low capacity to be transported into the brain, because there are measurable effects.
One apparent difference between some of the animal studies, especially the neurotoxic models, and the human disease environment is the timing of Cr administration. Neuroprotective Cr effects were demonstrated in rodent models, where Cr administration was begun weeks prior to initiation of the neurotoxin (Matthews et al. 1998, 1998; Yang et al. 2009). This prophylactic Cr loading seems unfeasible in the case of human neurodegenerative disease, where at symptom onset and time of diagnosis the neuropathological processes are believed to have been active for a long time (Braak et al. 2004). Yet, time of diagnosis would be the earliest possible time for the commencement of Cr supplementation in sporadic neurodegenerative disease, such as PD or ALS and this may just be too late for Cr to exert significant neuroprotective effects. This is why the recent publication by Rosas and colleagues is so noteworthy, because they found a way to give Cr to premanifest HD patients, i.e. much earlier in the disease process than after establishment of the clinical diagnosis (Rosas et al. 2014). Indeed, they reported decreased brain atrophy rates in the Cr group compared to the placebo group. If this were to translate into clinical patient benefit in the long-term, it would be important for other neurodegenerative diseases, such as PD or ALS, to develop early biomarkers or genetic testing for high disease risk or early disease activity, so that Cr might indeed be started as early as possible. On the other hand, in genetic HD animal models, Cr was still neuroprotective, even when commenced after the onset of disease-related symptoms (Dedeoglu et al. 2003). At least in animals, it seems not a prerequisite for Cr to be given prior to the initiation of the pathophysiological processes. Also, if Cr indeed were to be neuroprotective in human neurodegenerative disease, why should it not be able to at least reduce the rate of clinical decline and neurodegeneration, even when given well after the beginning of the pathophysiological process, where all the alleged mechanisms, such as oxidative stress, mitochondrial dysfunction, or neuroinflammation are very likely to still be active? Indeed, the large Cr phase III trial in PD should have been able to show a disease modifying effect due to the long duration of the trial with several years of Cr intake (Kieburtz et al. 2015).
Cr does not readily cross the blood–brain barrier so that its neuronal uptake relies on the CrT, which seems to be differentially expressed in the brain with low transport efficiency in disease-critical brain areas, such as the basal ganglia in PD (Lowe et al. 2015; Perasso et al. 2003). It is therefore worth contemplating different routes of application, chemical forms, and analogues compounds, in order to overcome the issue of low neuronal uptake (reviewed in Perasso et al. 2013). All of the human trials referenced in this review have used Cr monohydrate as the active study medication. Cr-derived compounds with increased blood–brain barrier penetrance and increased neuronal uptake, such as cyclocreatine or PCr-magnesium-complex-acetate have been shown to be neuroprotective in vitro and in animal studies (Matthews et al. 1999; Perasso et al. 2008, 2009). Yet, these compounds have not yet been tested in human controlled trials. In theory, if bioavailability of Cr within critical populations of neurons were the limiting factor for successful human neuroprotection trials, then intrathecal administration would be an interesting strategy. This is far from feasibility in the context of neurodegenerative disease, though but would possibly be an alternative in states of critical acute brain energy failure, such as in global cerebral ischaemia or traumatic brain injury, where often external ventricular catheters provide access to the cerebrospinal fluid compartment (CSF).
In summary, Cr has failed as a neuroprotective strategy in neurodegenerative disease with the exception that it may be beneficial in premanifest HD when given in high doses, which will have to be further examined in a phase III trial. Cr is ergogenic and can stabilize cellular and neuronal energy homoeostasis, serving as an energy buffer in times of energy crisis (Balestrino et al. 2002; Andres et al. 2008). In theory, it should therefore be neuroprotective as a prophylactic strategy in conditions with imminent neurological damage, such as off-pump cardiothoracic surgery or carotid artery repair and there are data supporting this view (Perasso et al. 2013). In fact, Cr was recently shown to prevent neuropsychological deficits in healthy volunteers under experimental oxygen deprivation (Turner et al. 2015). While Cr has been disappointing in the field of neurodegeneration, the obvious advantages of such a prophylactic use for neuroprotection in at-risk populations or patients would be that Cr as an energy buffer could be given before the actual insult and therefore be able to prevent the initiation of the pathophysiological cascades leading to brain damage. This discussion though has been the focus of another recent review (Perasso et al. 2013).
References
Ahlskog JE (2007) I can’t get no satisfaction: still no neuroprotection for Parkinson disease. Neurology 69(15):1476–1477. doi:10.1212/01.wnl.0000277645.60799.0e
Allen PJ, D’Anci KE, Kanarek RB, Renshaw PF (2012) Sex-specific antidepressant effects of dietary creatine with and without sub-acute fluoxetine in rats. Pharmacol Biochem Behav 101(4):588–601. doi:10.1016/j.pbb.2012.03.005
Andreassen OA, Dedeoglu A, Ferrante RJ, Jenkins BG, Ferrante KL, Thomas M, Friedlich A, Browne SE, Schilling G, Borchelt DR, Hersch SM, Ross CA, Beal MF (2001a) Creatine increase survival and delays motor symptoms in a transgenic animal model of Huntington’s disease. Neurobiol Dis 8(3):479–491
Andreassen OA, Jenkins BG, Dedeoglu A, Ferrante KL, Bogdanov MB, Kaddurah-Daouk R, Beal MF (2001b) Increases in cortical glutamate concentrations in transgenic amyotrophic lateral sclerosis mice are attenuated by creatine supplementation. J Neurochem 77(2):383–390
Andres RH, Ducray AD, Schlattner U, Wallimann T, Widmer HR (2008) Functions and effects of creatine in the central nervous system. Brain Res Bull 76(4):329–343. doi:10.1016/j.brainresbull.2008.02.035
Atassi N, Ratai EM, Greenblatt DJ, Pulley D, Zhao Y, Bombardier J, Wallace S, Eckenrode J, Cudkowicz M, Dibernardo A (2010) A phase I, pharmacokinetic, dosage escalation study of creatine monohydrate in subjects with amyotrophic lateral sclerosis. Amyotroph Lateral Scler 11(6):508–513. doi:10.3109/17482961003797130
Balestrino M, Lensman M, Parodi M, Perasso L, Rebaudo R, Melani R, Polenov S, Cupello A (2002) Role of creatine and phosphocreatine in neuronal protection from anoxic and ischemic damage. Amino Acids 23(1–3):221–229. doi:10.1007/s00726-001-0133-3
Beal MF (2011) Neuroprotective effects of creatine. Amino Acids 40(5):1305–1313. doi:10.1007/s00726-011-0851-0
Beal MF, Brouillet E, Jenkins BG, Ferrante RJ, Kowall NW, Miller JM, Storey E, Srivastava R, Rosen BR, Hyman BT (1993) Neurochemical and histologic characterization of striatal excitotoxic lesions produced by the mitochondrial toxin 3-nitropropionic acid. J Neurosci 13(10):4181–4192
Bender A, Auer DP, Merl T, Reilmann R, Saemann P, Yassouridis A, Bender J, Weindl A, Dose M, Gasser T, Klopstock T (2005) Creatine supplementation lowers brain glutamate levels in Huntington’s disease. J Neurol 252(1):36–41. doi:10.1007/s00415-005-0595-4
Bender A, Koch W, Elstner M, Schombacher Y, Bender J, Moeschl M, Gekeler F, Muller-Myhsok B, Gasser T, Tatsch K, Klopstock T (2006) Creatine supplementation in Parkinson disease: a placebo-controlled randomized pilot trial. Neurology 67(7):1262–1264
Bender A, Samtleben W, Elstner M, Klopstock T (2008) Long-term creatine supplementation is safe in aged patients with Parkinson disease. Nutr Res 28(3):172–178. doi:10.1016/j.nutres.2008.01.001
Benton D, Donohoe R (2011) The influence of creatine supplementation on the cognitive functioning of vegetarians and omnivores. Br J Nutr 105(7):1100–1105. doi:10.1017/S0007114510004733
Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K (2004) Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 318(1):121–134
Braissant O, Henry H, Loup M, Eilers B, Bachmann C (2001) Endogenous synthesis and transport of creatine in the rat brain: an in situ hybridization study. Brain Res Mol Brain Res 86(1–2):193–201
Brew BJ (2007) Lost in translation: again, another failed neuroprotection trial. Neurology 69(13):1308–1309. doi:10.1212/01.wnl.0000277530.05450.ff
Cleveland DW, Rothstein JD (2001) From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci 2(11):806–819. doi:10.1038/35097565
Couzin J (2007) Clinical research. Testing a novel strategy against Parkinson’s disease. Science 315(5820):1778
Dedeoglu A, Kubilus JK, Yang L, Ferrante KL, Hersch SM, Beal MF, Ferrante RJ (2003) Creatine therapy provides neuroprotection after onset of clinical symptoms in Huntington’s disease transgenic mice. J Neurochem 85(6):1359–1367
Ergorul C, Levin LA (2013) Solving the lost in translation problem: improving the effectiveness of translational research. Curr Opin Pharmacol 13(1):108–114. doi:10.1016/j.coph.2012.08.005
Ferrante RJ, Andreassen OA, Jenkins BG, Dedeoglu A, Kuemmerle S, Kubilus JK, Kaddurah-Daouk R, Hersch SM, Beal MF (2000) Neuroprotective effects of creatine in a transgenic mouse model of Huntington’s disease. J Neurosci 20(12):4389–4397
Genius J, Geiger J, Bender A, Moller HJ, Klopstock T, Rujescu D (2012) Creatine protects against excitoxicity in an in vitro model of neurodegeneration. PLoS One 7(2):e30554. doi:10.1371/journal.pone.0030554
Groeneveld GJ, Veldink JH, van der Tweel I, Kalmijn S, Beijer C, de Visser M, Wokke JH, Franssen H, van den Berg LH (2003) A randomized sequential trial of creatine in amyotrophic lateral sclerosis. Ann Neurol 53(4):437–445. doi:10.1002/ana.10554
Gualano B, Roschel H, Lancha-Jr AH, Brightbill CE, Rawson ES (2012) In sickness and in health: the widespread application of creatine supplementation. Amino Acids 43(2):519–529. doi:10.1007/s00726-011-1132-7
Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX et al (1994) Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science 264(5166):1772–1775
Hemmer W, Wallimann T (1993) Functional aspects of creatine kinase in brain. Dev Neurosci 15(3–5):249–260
Hersch SM, Gevorkian S, Marder K, Moskowitz C, Feigin A, Cox M, Como P, Zimmerman C, Lin M, Zhang L, Ulug AM, Beal MF, Matson W, Bogdanov M, Ebbel E, Zaleta A, Kaneko Y, Jenkins B, Hevelone N, Zhang H, Yu H, Schoenfeld D, Ferrante R, Rosas HD (2006) Creatine in Huntington disease is safe, tolerable, bioavailable in brain and reduces serum 8OH2′dG. Neurology 66(2):250–252. doi:10.1212/01.wnl.0000194318.74946.b6
Hervias I, Beal MF, Manfredi G (2006) Mitochondrial dysfunction and amyotrophic lateral sclerosis. Muscle Nerve 33(5):598–608. doi:10.1002/mus.20489
Kieburtz K, Tilley BC, Elm JJ, Babcock D, Hauser R, Ross GW, Augustine AH, Augustine EU, Aminoff MJ, Bodis-Wollner IG, Boyd J, Cambi F, Chou K, Christine CW, Cines M, Dahodwala N, Derwent L, Dewey RB Jr, Hawthorne K, Houghton DJ, Kamp C, Leehey M, Lew MF, Liang GS, Luo ST, Mari Z, Morgan JC, Parashos S, Perez A, Petrovitch H, Rajan S, Reichwein S, Roth JT, Schneider JS, Shannon KM, Simon DK, Simuni T, Singer C, Sudarsky L, Tanner CM, Umeh CC, Williams K, Wills AM (2015) Effect of creatine monohydrate on clinical progression in patients with Parkinson disease: a randomized clinical trial. JAMA 313(6):584–593. doi:10.1001/jama.2015.120
Kim J, Moody JP, Edgerly CK, Bordiuk OL, Cormier K, Smith K, Beal MF, Ferrante RJ (2010) Mitochondrial loss, dysfunction and altered dynamics in Huntington′s disease. Hum Mol Genet 19(20):3919–3935. doi:10.1093/hmg/ddq306
Klivenyi P, Ferrante RJ, Matthews RT, Bogdanov MB, Klein AM, Andreassen OA, Mueller G, Wermer M, Kaddurah-Daouk R, Beal MF (1999) Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nat Med 5(3):347–350
Klivenyi P, Gardian G, Calingasan NY, Yang L, Beal MF (2003) Additive neuroprotective effects of creatine and a cyclooxygenase 2 inhibitor against dopamine depletion in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease. J Mol Neurosci 21(3):191–198
Klivenyi P, Kiaei M, Gardian G, Calingasan NY, Beal MF (2004) Additive neuroprotective effects of creatine and cyclooxygenase 2 inhibitors in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurochem 88(3):576–582
Klopstock T, Elstner M, Bender A (2011) Creatine in mouse models of neurodegeneration and aging. Amino Acids 40(5):1297–1303. doi:10.1007/s00726-011-0850-1
Lawler JM, Barnes WS, Wu G, Song W, Demaree S (2002) Direct antioxidant properties of creatine. Biochem Biophys Res Commun 290(1):47–52. doi:10.1006/bbrc.2001.6164
Li Z, Wang P, Yu Z, Cong Y, Sun H, Zhang J, Zhang J, Sun C, Zhang Y, Ju X (2015) The effect of creatine and coenzyme q10 combination therapy on mild cognitive impairment in Parkinson’s disease. Eur Neurol 73(3–4):205–211. doi:10.1159/000377676
Lodi R, Schapira AH, Manners D, Styles P, Wood NW, Taylor DJ, Warner TT (2000) Abnormal in vivo skeletal muscle energy metabolism in Huntington’s disease and dentatorubropallidoluysian atrophy. Ann Neurol 48(1):72–76
Lowe MT, Faull RL, Christie DL, Waldvogel HJ (2015) Distribution of the creatine transporter throughout the human brain reveals a spectrum of creatine transporter immunoreactivity. J Comp Neurol 523(5):699–725. doi:10.1002/cne.23667
Lyoo IK, Kong SW, Sung SM, Hirashima F, Parow A, Hennen J, Cohen BM, Renshaw PF (2003) Multinuclear magnetic resonance spectroscopy of high-energy phosphate metabolites in human brain following oral supplementation of creatine-monohydrate. Psychiatry Res 123(2):87–100
Mak CS, Waldvogel HJ, Dodd JR, Gilbert RT, Lowe MT, Birch NP, Faull RL, Christie DL (2009) Immunohistochemical localisation of the creatine transporter in the rat brain. Neuroscience 163(2):571–585. doi:10.1016/j.neuroscience.2009.06.065
Matthews RT, Yang L, Jenkins BG, Ferrante RJ, Rosen BR, Kaddurah-Daouk R, Beal MF (1998) Neuroprotective effects of creatine and cyclocreatine in animal models of Huntington’s disease. J Neurosci 18(1):156–163
Matthews RT, Ferrante RJ, Klivenyi P, Yang L, Klein AM, Mueller G, Kaddurah-Daouk R, Beal MF (1999) Creatine and cyclocreatine attenuate MPTP neurotoxicity. Exp Neurol 157(1):142–149. doi:10.1006/exnr.1999.7049
National Academies of Sciences, Engineering, and Medicine (2015) Reproducibility issues in research with animals and animal models: workshop in Brief. The National Academies Press, Washington, DC, 1–8. ISBN 978-0-309-38017-1
NINDS NET-PD Investigators (2006) A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology 66(5):664–671
O’Gorman E, Beutner G, Dolder M, Koretsky AP, Brdiczka D, Wallimann T (1997) The role of creatine kinase in inhibition of mitochondrial permeability transition. FEBS Lett 414(2):253–257
Olanow CW (2007) The pathogenesis of cell death in Parkinson’s disease—2007. Mov Disord 22(Suppl 17):S335–S342
Pastula DM, Moore DH, Bedlack RS (2012) Creatine for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev 12:CD005225. doi:10.1002/14651858.CD005225.pub3
Perasso L, Cupello A, Lunardi GL, Principato C, Gandolfo C, Balestrino M (2003) Kinetics of creatine in blood and brain after intraperitoneal injection in the rat. Brain Res 974(1–2):37–42
Perasso L, Lunardi G, Risso F, Pohvozcheva A, Leko M, Gandolfo C, Florio T, Cupello A, Burov S, Balestrino M (2008) Protective effects of some creatine derivatives in brain tissue anoxia. Neurochem Res 33(5):765–775
Perasso L, Adriano E, Ruggeri P, Burov SV, Gandolfo C, Balestrino M (2009) In vivo neuroprotection by a creatine-derived compound: phosphocreatine-Mg-complex acetate. Brain Res 1285:158–163
Perasso L, Spallarossa P, Gandolfo C, Ruggeri P, Balestrino M (2013) Therapeutic use of creatine in brain or heart ischemia: available data and future perspectives. Med Res Rev 33(2):336–363. doi:10.1002/med.20255
Peters OM, Ghasemi M, Brown RH Jr (2015) Emerging mechanisms of molecular pathology in ALS. J Clin Invest 125(5):1767–1779. doi:10.1172/JCI71601
Poortmans JR, Francaux M (2000) Adverse effects of creatine supplementation: fact or fiction? Sports Med 30(3):155–170
Rosas HD, Doros G, Gevorkian S, Malarick K, Reuter M, Coutu JP, Triggs TD, Wilkens PJ, Matson W, Salat DH, Hersch SM (2014) Precrest: a phase II prevention and biomarker trial of creatine in at-risk Huntington disease. Neurology 82(10):850–857. doi:10.1212/wnl.0000000000000187
Rosenfeld J, King RM, Jackson CE, Bedlack RS, Barohn RJ, Dick A, Phillips LH, Chapin J, Gelinas DF, Lou JS (2008) Creatine monohydrate in ALS: effects on strength, fatigue, respiratory status and ALSFRS. Amyotroph Lateral Scler 9(5):266–272. doi:10.1080/17482960802028890
Schapira AH (2008) Mitochondria in the aetiology and pathogenesis of Parkinson's disease. Lancet Neurol 7(1):97–109
Scott S, Kranz JE, Cole J, Lincecum JM, Thompson K, Kelly N, Bostrom A, Theodoss J, Al-Nakhala BM, Vieira FG, Ramasubbu J, Heywood JA (2008) Design, power, and interpretation of studies in the standard murine model of ALS. Amyotroph Lateral Scler 9(1):4–15. doi:10.1080/17482960701856300
Shefner JM, Cudkowicz ME, Schoenfeld D, Conrad T, Taft J, Chilton M, Urbinelli L, Qureshi M, Zhang H, Pestronk A, Caress J, Donofrio P, Sorenson E, Bradley W, Lomen-Hoerth C, Pioro E, Rezania K, Ross M, Pascuzzi R, Heiman-Patterson T, Tandan R, Mitsumoto H, Rothstein J, Smith-Palmer T, MacDonald D, Burke D, NEALS Consortium (2004) A clinical trial of creatine in ALS. Neurology 63(9):1656–1661
Smith RN, Agharkar AS, Gonzales EB (2014) A review of creatine supplementation in age-related diseases: more than a supplement for athletes. F1000Res 3:222. doi:10.12688/f1000research.5218.1
Snow RJ, Turnbull J, da Silva S, Jiang F, Tarnopolsky MA (2003) Creatine supplementation and riluzole treatment provide similar beneficial effects in copper, zinc superoxide dismutase (G93A) transgenic mice. Neuroscience 119(3):661–667
Stevens PR, Gawryluk JW, Hui L, Chen X, Geiger JD (2014) Creatine protects against mitochondrial dysfunction associated with HIV-1 Tat-induced neuronal injury. Curr HIV Res 12(6):378–387
Tabrizi SJ, Cleeter MW, Xuereb J, Taanman JW, Cooper JM, Schapira AH (1999) Biochemical abnormalities and excitotoxicity in Huntington’s disease brain. Ann Neurol 45(1):25–32
Tarnopolsky MA (2010) Caffeine and creatine use in sport. Ann Nutr Metab 57(Suppl 2):1–8. doi:10.1159/000322696
Thorsteinsdottir B, Grande JP, Garovic VD (2006) Acute renal failure in a young weight lifter taking multiple food supplements, including creatine monohydrate. J Ren Nutr 16(4):341–345. doi:10.1053/j.jrn.2006.04.025
Turner CE, Byblow WD, Gant N (2015) Creatine supplementation enhances corticomotor excitability and cognitive performance during oxygen deprivation. J Neurosci 35(4):1773–1780. doi:10.1523/jneurosci.3113-14.2015
Verbessem P, Lemiere J, Eijnde BO, Swinnen S, Vanhees L, Van Leemputte M, Hespel P, Dom R (2003) Creatine supplementation in Huntington’s disease: a placebo-controlled pilot trial. Neurology 61(7):925–930
Wallimann T, Tokarska-Schlattner M, Schlattner U (2011) The creatine kinase system and pleiotropic effects of creatine. Amino Acids 40(5):1271–1296. doi:10.1007/s00726-011-0877-3
Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, Sisodia SS, Cleveland DW, Price DL (1995) An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron 14(6):1105–1116
Xiao Y, Luo M, Luo H, Wan J (2014) Creatine for Parkinson’s disease. Cochrane Database Syst Rev 6:CD009646. doi:10.1111/j.1471-4159.2009.06074.x
Yang L, Calingasan NY, Wille EJ, Cormier K, Smith K, Ferrante RJ, Beal MF (2009) Combination therapy with coenzyme Q10 and creatine produces additive neuroprotective effects in models of Parkinson’s and Huntington’s diseases. J Neurochem 109(5):1427–1439. doi:10.1111/j.1471-4159.2009.06074.x
Yazigi Solis M, de Salles Painelli V, Giannini Artioli G, Roschel H, Concepción Otaduy M, Gualano B (2014) Brain creatine depletion in vegetarians? A cross-sectional ¹H-magnetic resonance spectroscopy (¹H-MRS) study. Br J Nutr 111(7):1272–1274. doi:10.1017/S0007114513003802
Zhang W, Narayanan M, Friedlander RM (2003) Additive neuroprotective effects of minocycline with creatine in a mouse model of ALS. Ann Neurol 53(2):267–270. doi:10.1002/ana.10476
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AB and TK were invited speakers with travel grants at creatine conferences, sponsored by one of the creatine manufacturers, Alzchem, Trostberg, Germany.
Ethical standard statement
This review article does not contain any studies with human participants performed by any of the authors.
Informed consent statement
The authors of this review article did not perform research on participants. Obtaining informed consent was therefore not applicable.
Additional information
Handling Editor: T. Wallimann and R. Harris.
Rights and permissions
About this article
Cite this article
Bender, A., Klopstock, T. Creatine for neuroprotection in neurodegenerative disease: end of story?. Amino Acids 48, 1929–1940 (2016). https://doi.org/10.1007/s00726-015-2165-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-015-2165-0