Abstract
The l-arginine/nitric oxide (L-Arg/NO) pathway regulates endothelial function and may play an important role in the pathogenesis of Duchenne muscular dystrophy (DMD). Yet, this pathway is poorly investigated in children suffering from DMD. Endothelial dysfunction can affect the perfusion of contracting muscles, thus leading to ischemia and hypoxia. In the present study, we tested the hypothesis that reduced NO production due to elevated synthesis of N G,N G-dimethyl-l-arginine (asymmetric dimethylarginine, ADMA), an endogenous inhibitor of NO synthesis, is a possible pathophysiological mechanism for progressive intramuscular muscle ischemia and disturbed endothelial function in children with DMD. Given the possible antagonistic action of homoarginine (hArg) on ADMA, we also analyzed this amino acid. We investigated 55 male patients with DMD and 54 healthy male controls (HC; aged 11.9 ± 4.8 vs. 11.1 ± 4.9 years, mean ± SD). Urinary creatinine and metabolites of the L-Arg/NO pathway were measured in plasma and urine by GC–MS or GC–MS/MS. Urine levels of ADMA and its major urinary metabolite dimethylamine (DMA), nitrite and nitrate (P < 0.001 for all) and hArg (P = 0.002) were significantly higher in DMD patients compared to HC, while the urinary DMA/ADMA molar ratio was lower (P = 0.002). In plasma, nitrate (P < 0.001), hArg (P = 0.002) and the hArg/ADMA ratio (P < 0.001) were lower in DMD than in HC. In plasma, ADMA (631 ± 119 vs. 595 ± 129 nM, P = 0.149), arginine and nitrite did not differ between DMD and HC. In DMD, positive correlations between ADMA, DMA or nitrate excretion and the stage of disease (according to Vignos and Thompson) were found. In DMD patients on steroid medication, lower concentrations of ADMA in plasma, and of DMA, ADMA, nitrate and hArg in urine were observed compared to non-treated patients. The L-Arg/NO pathway is impaired in DMD patients, with the disease progression being clinically negatively correlated with the extent of impairment. One of the underlying mechanisms in DMD may involve insufficient antagonism of ADMA by hArg. Steroids, but not creatine supplementation, seems to improve the L-Arg/NO pathway in DMD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Duchenne muscular dystrophy (DMD) is a dystrophinopathy which is inherited in an X-linked recessive manner with an incidence of about 1:3.500–6.000 in newborn boys. The disease is a result of mutations in the dystrophin gene localized in the short arm of chromosome Xp21 (Koenig et al. 1987; Annexstad et al. 2014; Mortier 1994), leading to an absence or a significant loss of functional dystrophin (Aartsma-Rus et al. 2006; Annexstad et al. 2014). Dystrophin is a structural protein and part of the dystrophin–glycoprotein complex stabilizing the sarcolemma. As a result of functional loss of dystrophin, the patients suffer from a progressive damage of muscle fibers accompanied by an increasing muscle weakness (Annexstad et al. 2014; Arahata et al. 1988). The progression of the disease shows a wide range of intra-individual differences. Thompson and Vignos established a staging (stage 0–11) for DMD regarding ambulatory ability. Stage 0 describes a patient with DMD without any clinical symptoms, while a patient at stage 11 is bedfast and not even able to sit (Thompson and Vignos 1959; Mortier 1994). DMD cannot be cured; however, a therapy with glucocorticoids can decelerate the progression of muscle weakness and even enhance muscle strength (Manzur et al. 2008; Annexstad et al. 2014). Also, administration of creatine to DMD patients has been reported to improve muscle strength in short and medium term in muscular dystrophies (Kley et al. 2013; Banerjee et al. 2010; Tarnopolsky et al. 2004).
In the 1980s, the endothelium-derived relaxing factor (EDRF) was found to be responsible for the relaxation of vascular smooth muscles by acetylcholine (ACh). EDRF was then identified as nitric oxide (NO) (Moncada and Higgs 2006; Furchgott et al. 1984; Furchgott and Zawadzki 1980; Palmer et al. 1987). NO is generated from l-arginine by oxidation of one of its terminal guanidine groups. This reaction is catalyzed by nitric oxide synthase (NOS; Palmer et al. 1988; Marletta 1993). In humans, three different isoenzymes of NOS are known, which have initially been named after the tissue in which they have been found at first: the constitutive neuronal NOS (nNOS or NOSI), endothelial NOS (eNOS or NOSIII) and the inducible NOS (iNOS or NOSII). iNOS is especially expressed in macrophages and other cells of the immune system (Förstermann et al. 1994; Moncada and Higgs 2006).
In human organisms, NO takes part in many different physiological and pathological processes. In the vascular system, NO is responsible for regulation of blood pressure by dilating vessels and for inhibition of platelet aggregation. Furthermore, NO prevents leukocyte adhesion to the blood vessel wall and vascular smooth muscle proliferation, so that NO protects against atherosclerosis (Moncada and Higgs 2006; Förstermann et al. 1994; Rees et al. 1989). In this regard, NO seems to be an important signaling molecule avoiding different cardiovascular diseases. Many of these diseases have their origin in an endothelial dysfunction due to a decrease of endothelial NO synthesis/bioavailability (Moncada and Higgs 2006). As direct measurement of authentic NO is very difficult in human biological samples including blood, nitrate and nitrite in blood and urine are determined and used for evaluating NO synthesis in vivo (Tsikas 2005).
The activity of NOS is controlled by endogenous inhibitors, among which N G,N G-dimethyl-l-arginine (asymmetric dimethylarginine, ADMA) is the most important one (Leiper and Vallance 2006). After being released in blood circulation, ADMA is partially eliminated unchanged by the kidneys. About 90 % of daily produced ADMA is first hydrolyzed to l-citrulline and dimethylamine (DMA) by the enzyme dimethylarginine dimethylaminohydrolase (DDAH). DMA is then excreted in the urine by the kidneys (Leiper and Vallance 2006; Achan et al. 2003). In adults, there are several cardiovascular, renal and other endothelial dysfunction-associated diseases, in which elevated ADMA concentrations and lower concentrations of nitrate and nitrite prevail compared to healthy subjects (Cooke 2000; Sibal et al. 2010). Elevated ADMA synthesis and consequently reduced NO synthesis are probably responsible for the development of atherosclerosis (Kielstein et al. 1999). Currently, there is feverish research on l-homoarginine (hArg) which is a homolog of l-arginine and a non-essential, non-proteinogenic cationic amino acid formed from l-lysine in the kidney (Ryan and Wells 1964; Ryan et al. 1968). hArg has been reported to be a substrate for NOS as well (Moali et al. 1998) and may therefore affect NO-dependent endothelial function (Valtonen et al. 2008). Low concentrations of hArg are positively associated with cardiovascular and all-cause mortality as well as cardiovascular diseases in humans (März et al. 2010; Pilz et al. 2011, 2014, 2015).
In recent years, the role of ADMA and other members of the L-Arg/NO pathway in children with renal and metabolic diseases has been investigated (Goonasekera et al. 1997; Lücke et al. 2006a, b, 2007, 2008; Kanzelmeyer et al. 2012, 2014). Elevated ADMA levels were found for example in children with hypertension (Goonasekera et al. 1997) and citrullinemia (Lücke et al. 2006a). Moreover, the developmental changes in the L-Arg/NO pathway from infancy to adulthood have been investigated (Lücke et al. 2007). Thus, ADMA levels in plasma were found to decrease with age, so that ADMA concentrations in young healthy controls are much higher than in healthy adults, while adolescents almost reach the levels prevailing in adulthood (Lücke et al. 2007).
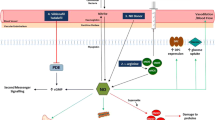
Up to now, there has been little reported data on the L-Arg/NO pathway in children with Duchenne muscular dystrophy, even though it seems to play an important role in the pathogenesis of DMD. The dystrophin complex was found to interact with the N-terminal domain of nNOS, so that the loss of dystrophin in patients with DMD leads to an absence of nNOS in skeletal muscle sarcolemma (Brenman et al. 1995). nNOS is highly expressed in mammalian skeletal muscle and NO produced by nNOS diminishes the sympathetic vasoconstriction in contracting skeletal muscles, so that blood flow is increased. This mechanism does not function in the mdx mouse which is a model of DMD with dystrophin deficiency and resulting loss of nNOS in skeletal muscles. It was supposed that the unopposed sympathetic vasoconstriction leads to progressive ischemia, hypoxia and destruction of skeletal muscles in DMD (Thomas et al. 1998; Chavoshan et al. 2002). In recent years, the NO pathway in DMD was investigated in a small number of studies. It was reported that circulating nitrite/nitrate levels in patients with DMD are lower than those in healthy controls (Gücüyener et al. 2000; Kasai et al. 2004).
In the present study, we tested the hypothesis that reduced NO synthesis due to elevated ADMA concentrations antagonizing NOS would be a possible pathophysiological mechanism for disturbed endothelial function and impaired vessel reactivity in children with DMD. Given the emerging importance of hArg in the cardiovascular system (März et al. 2010; Pilz et al. 2011, 2014, 2015) and its possible antagonistic action on ADMA (Tsikas and Kayacelebi 2014), we included circulating and urinary hArg in our investigations.
Materials and methods
Subjects
We investigated 55 male patients with DMD and 54 healthy male controls aged between 1 and 23 years. Patients with DMD were recruited during routine examinations taking place every 6 months in the Departments of Neuropediatrics of the University Children’s Hospitals in Bochum and Essen. Healthy controls were subjects undergoing little surgical operations in the Hannover Medical School and elective gastroscopies in the Children’s Hospital in Bochum. The study was designed as a cross-sectional pilot study with the intention to investigate the L-Arg/NO pathway in children with DMD. The Ethics Committees of the Faculty of Medicine at Ruhr-University Bochum and of the Hannover Medical School approved the study and written consent was given by each participant and his parents or only by the participant if he was 18 years or older.
DMD was diagnosed by molecular analysis and/or muscle biopsy. Exclusion criteria were ongoing infections, chronic kidney, liver or metabolic diseases, malignancies, epilepsy, ingestion of blood thinner and fish consumption 24 h prior to the examination. Thirty-four of our DMD patients were treated with glucocorticoids (25 with deflazacort and 9 with prednisolone), whereas 21 patients stopped or had never begun this therapy. In addition to this therapy, most of our patients received vitamin D and calcium (n = 33), and some patients (n = 7) obtained also creatine (1 or 2 g creatine monohydrate per day, without a pause or in some cases for 3 months with a pause of 1 month). Depending on heart affection, 15 of our patients took angiotensin-converting enzyme (ACE) inhibitors or ACE inhibitors plus beta-blockers. One patient suffering from a cardiac decompensation was treated with more than two antihypertensive drugs.
We defined the severity of loss of ambulation by the staging established by Thompson and Vignos (Thompson and Vignos 1959; Mortier 1994). We included three DMD patients supported by non-invasive ventilation at night and one patient fed by percutaneous endoscopic gastrostomy. Moreover, one of our patients was additionally diagnosed with a selective immunoglobulin A deficiency and another one a growth hormone deficiency.
Patients taking part in the present study were not fasting and did not follow a special diet. The only exception was avoidance of fish consumption in the last 24 h for minimizing dietary DMA intake (Tsikas et al. 2007; Tsikas 2008).
The number of investigated patients was different for the biochemical parameters we analyzed, as urine and blood samples were not available for each child. Also, we determined hArg in plasma and urine in the DMD patients and healthy subjects who had been recruited in Bochum and Essen. This information is provided in Table 1 and in the respective places of this article.
Analytical methods
Venous blood was drawn using ethylenediaminetetraacetic acid (EDTA). The blood samples were immediately put on ice, centrifuged (4500×g, 4 °C, 10 min) and the supernatant plasma samples were frozen at −80 °C until assay. In general, the time point of blood collection relative to the last meal ranged considerably in our DMD patients, but was not noted down. Urine samples from spontaneous micturition (five times obtained from the urinary bag) were frozen immediately at −20 °C until analysis. Urinary levels of ADMA, DMA, nitrate, nitrite and hArg excretion were corrected for creatinine excretion and expressed as µmol of the analyte per mmol of creatinine. Urinary creatinine was determined by GC–MS (Tsikas et al. 2010a). ADMA in plasma and urine as well as Arg in plasma was quantitated by gas chromatography–tandem mass spectrometry (GC–MS/MS) and gas chromatography (GC–MS), respectively (Tsikas et al. 2003). Nitrite and nitrate were quantified in plasma and urine simultaneously by GC–MS (Tsikas 2000). DMA in urine was determined by GC–MS (Tsikas et al. 2007). hArg in plasma and in urine was measured by GC–MS/MS (Kayacelebi et al. 2014a, 2015).
Quality control (QC) samples were analyzed alongside study samples as described previously for each analyte and biological sample. All biochemical parameters were determined in the QC samples with accuracy (bias, %) and imprecision (relative standard deviation, %) of less than ±20 and ≤20 %, respectively, indicating the validity of the analytical results in the plasma and urine samples of the study.
Statistical analysis
We used the Shapiro–Wilk and the Kolmogorov–Smirnov normality tests to evaluate data distribution. The data of our two groups (patients with DMD and healthy controls) were compared using the non-parametric Mann–Whitney test for not normally distributed data and the Student’s t test for unpaired samples for normally distributed parameters. Data are presented as mean ± standard deviation (SD). Spearman’s correlation coefficient was applied to assess correlations between the parameters and the stage of disease, according to Vignos and Thompson. Student’s t test (Mann–Whitney test for non-parametric variables) was also used for intergroup analysis of patients treated and not treated with steroids and creatine. For the comparison of two groups, a P value of <0.05 was considered statistically significant. All calculations were executed using the SPSS package (version 20, IBM Corp., Armonk, NY, USA).
Results
We measured several biochemical parameters of the L-Arg/NO pathway in plasma and urine samples of 55 male patients with DMD and 54 healthy male controls by GC–MS or GC–MS/MS methods. Yet, biological samples were not available from all children. The results of the present study are summarized in Tables 1, 2, 3 and Fig. 1.
ADMA in plasma (631 ± 119 vs. 595 ± 129 nM, P = 0.149) was higher in DMD patients than in healthy controls. Urine levels of ADMA (P < 0.001), DMA (P < 0.001), nitrite (P < 0.001), nitrate (P < 0.001) and hArg (P = 0.003) were significantly higher in DMD patients than in healthy controls. Regarding urinary ADMA, our observations confirm the increased urinary excretion of ADMA in DMD patients (Inoue et al. 1979). The DMA/ADMA molar ratio in urine (P = 0.002), nitrate in plasma (P < 0.001), hArg in plasma (P = 0.002) and the hArg/ADMA ratio in plasma (P < 0.001) were significantly lower in DMD patients than in healthy patients. There was no statistically significant difference between plasma Arg, plasma nitrite and plasma ADMA in DMD patients and healthy controls.
In DMD patients, there was a positive correlation between the stage of disease and urine DMA (r = 0.522, P < 0.001; Fig. 1d), urine ADMA (r = 0.652, P < 0.001; Fig. 1e) or urine nitrate (r = 0.507, P < 0.001; not shown). In contrast, the DMA/ADMA molar ratio in urine correlated negatively with the stage of disease (r = −0.566, P < 0.001; Fig. 1f). No significant dependence on the stage of disease was found for hArg (Fig. 1a), ADMA (Fig. 1b), hArg/ADMA ratio (Fig. 1c) in plasma, for nitrite in urine, nitrite in plasma and nitrate in plasma (data not shown).
Spearman’s correlation between stage of disease according to Vignos and Thompson and a hArg in plasma, b ADMA in plasma, c hArg/ADMA ratio in plasma, d urinary DMA (r = 0.522, P < 0.001), e urinary ADMA (r = 0.652, P < 0.001) and f DMA/ADMA ratio in urine (r = -0.566, P < 0.001) in children with DMD
In the DMD patients on steroid medication (Table 2), we measured significantly lower plasma ADMA concentrations (600 ± 119 vs. 680 ± 104 nM, P = 0.015) compared to patients not medicated with steroids. In the DMD patients on steroid medication, urinary ADMA (15.7 ± 7.95 vs. 28.1 ± 8.77 µmol/mmol creatinine, P < 0.001), DMA (65.1 ± 21.7 vs. 102 ± 25.4 µmol/mmol creatinine, P < 0.001), nitrate (188 ± 72.5 vs. 302 ± 162 µmol/mmol creatinine, P = 0.001) and hArg (0.53 ± 0.47 vs. 1.75 ± 1.92 µmol/mmol creatinine, P = 0.001) were lower than in patients not medicated by steroids. The DMA/ADMA molar ratio in urine was significantly higher in DMD patients on steroid medication compared to DMD patients without steroid medication (4.55 ± 1.27 vs. 3.79 ± 0.85, P = 0.02).
In previous studies, creatine supplementation was found to alter the plasma concentration of guanidino compounds including guanidinoacetate (Derave et al. 2004), the precursor of creatine. We compared the different biochemical parameters of the seven DMD patients being on creatine supplementation with those of DMD patients not treated with creatine (Table 3). hArg in urine (0.79 ± 0.78 vs. 1.01 ± 1.41 µmol/mmol creatinine, P = 0.887), hArg in plasma (1.20 ± 0.44 vs. 1.37 ± 0.61 µM, P = 0.403) and the plasma hArg/ADMA ratio (2.18 ± 0.78 vs. 2.18 ± 0.93, P = 0.990) did not differ between the two groups. Nearly or statistically significantly lower concentrations upon creatine treatment were obtained for ADMA (561 ± 121 vs. 641 ± 117 nM, P = 0.078) and Arg (67.9 ± 7.3 vs. 77.1 ± 16.4 µM, P = 0.062) in plasma, and for DMA (58.4 ± 30.9 vs. 81.9 ± 28.0 µmol/mmol creatinine, P = 0.036) and nitrate (160 ± 60 vs. 241 ± 130 µmol/mmol creatinine P = 0.046) in urine.
Discussion
In recent years, the L-Arg/NO pathway in childhood has been increasingly investigated. Our groups have investigated this pathway in healthy children and in children with renal and metabolic diseases (Lücke et al. 2006a, b; Kanzelmeyer et al. 2012, 2014; Chobanyan-Jürgens et al. 2012a). The L-Arg/NO pathway in children differs from that in adults. Interestingly, ADMA synthesis is higher in children compared to adults, yet without signs of cardiovascular diseases. In healthy humans, ADMA synthesis decreases steadily from infancy to adulthood (Lücke et al. 2007). We were interested to know whether the L-Arg/NO pathway is altered in children with DMD. We hypothesized that many of the symptoms seen in DMD could be due to altered L-Arg/NO pathway. We measured several biochemical parameters of the L-Arg/NO pathway in 55 DMD patients and in 54 healthy children of the same age.
There are four main findings of our study. First, urine excretion of ADMA and its main metabolite DMA are significantly higher in DMD patients than in healthy controls. The concentration of ADMA in plasma is higher in DMD compared to healthy children, but the difference did not show statistical significance. Second, nitrate in plasma is lower, but nitrate and nitrite excretion are higher in DMD patients compared to healthy controls. Third, the concentration of hArg and the hArg/ADMA molar ratio in plasma are lower in DMD compared to healthy controls. Steroids but not creatine supplementation seems to improve the L-Arg/NO pathway in DMD. These observations are discussed in the sections that follow.
ADMA and NO synthesis in DMD
The higher excretion of ADMA and its main metabolite DMA in the urine of the DMD children suggest that ADMA synthesis is elevated in DMD. The slightly higher urinary excretion of nitrate in DMD suggests that whole body NO synthesis is higher in DMD patients compared to healthy controls of our study. The higher excretion of nitrite, the lower plasma nitrite concentration and the lower nitrate-to-nitrite molar ratio in urine U NOxR in the DMD patients suggest that NO bioavailability in the renal and cardiovascular systems is impaired in DMD. Diminished NO bioavailability could be a possible mechanism for the progressive muscle ischemia and impaired vessel reactivity in DMD patients (Thomas et al. 1998; Chavoshan et al. 2002).
Diseases such as atherosclerosis, hypertension and chronic heart failure are associated with endothelial dysfunction and elevated circulating ADMA concentrations. ADMA inhibits the activity of all NOS isoforms including eNOS and nNOS (Tsikas et al. 2000; Kielstein et al. 2007). Diminished NO synthesis may therefore impair endothelial function (Böger et al. 1997; Cooke 2000; Sibal et al. 2010). In our DMD patients, elevated synthesis and presumably higher cellular ADMA concentrations may also result in endothelial dysfunction which could affect the perfusion of contracting muscles, eventually resulting in ischemia and hypoxia. This has been demonstrated in the mdx mouse, a model of DMD with dystrophin deficiency and loss of nNOS in skeletal muscles (Thomas et al. 1998; Chavoshan et al. 2002).
DMD is characterized by muscular wasting and high rates of protein degradation (Inoue et al. 1979; Tran et al. 2003; Warnes et al. 1981). Protein degradation (i.e., proteolysis) is an important source of free Arg and methylarginines including ADMA (Kakimoto and Ankazawa 1970; Tran et al. 2003). In theory, the elevated circulating and excretory ADMA concentrations measured in the DMD patients of our study could be due to increased protein degradation. However, the almost identical plasma Arg levels in DMD and healthy children argue against this possibility. Another important observation that makes proteolysis an unlikely contributor to ADMA in DMD is that the ADMA isomer symmetric dimethylarginine (SDMA, N G,N G´-dimethyl-l-arginine) was found not be elevated in urine of DMD patients (Inoue et al. 1979). As hArg is not proteinogenic, proteolysis cannot have contributed to hArg in the plasma and urine samples of our DMD patients.
Chronic inflammatory processes seem to play an important role in the pathogenesis of DMD. In dystrophic skeletal muscles a part of the progressive muscle damage is caused by activation of inflammatory cells (De Paepe and De Bleecker 2013; Evans et al. 2009; Spencer and Tidball 2001). Inflammatory cytokines may affect the homeostasis of ADMA (Zoccali et al. 2007). Also, the Arg/ADMA ratio is decreased in conditions with increased C-reactive protein and myeloperoxidase activity (van der Zwan et al. 2011). It is, therefore, possible that chronic inflammatory processes have contributed to elevated ADMA synthesis in our DMD patients.
Measurement of nitrate and nitrite in plasma and urine is commonly used to evaluate NO synthesis in vivo (Tsikas 2005). Yet, this is associated with difficulties and the use of these parameters as measures of NO synthesis and bioavailability is limited (Tsikas 2015). Our patients were not on standardized low-nitrate and low-nitrite diet and were not fasting overnight. Therefore, it is possible that dietary factors might have contributed to the elevated concentrations of nitrite and nitrate in urine. Also, 34 of the DMD patients were on glucocorticoid medication which can raise susceptibility to various infections (Cutolo et al. 2008). The comparatively higher nitrate and nitrite excretion in the urine in our DMD patients suggests that NO synthesis is elevated in DMD. The relatively lower plasma concentration of nitrite, which is considered a measure of endothelial NO production and NO bioavailability in the circulation, suggests that NO bioavailability is diminished in DMD. The higher excretion rate of nitrite in DMD compared to healthy controls suggests an impaired reabsorption of nitrite in the kidneys, finally resulting in loss of NO bioavailability mainly in the form of nitrite. In humans, renal carbonic anhydrases are involved in the reabsorption of nitrite in the proximal tubule of the nephron (Tsikas et al. 2010b; Chobanyan-Jürgens et al. 2012b). The urinary nitrate-to-nitrite molar ratio U NOxR is a useful measure of nitrite-dependent carbonic anhydrase activity (Tsikas et al. 2014). In our DMD patients, the U NOxR value was about 40 % smaller than in healthy controls suggesting considerable impairment of nitrite-dependent renal carbonic anhydrase in DMD.
In a previous study, we found that in adult patients with rheumatism the excretion rate of nitrite was elevated and correlated closely with 3-nitrotyrosine, a biomarker of oxidative stress (Pham et al. 2009). Thus, increased nitrite excretion rate in children with DMD may also be associated with enhanced oxidative/nitrosative stress in this disease, which has been reported to be exacerbated in DMD (Terrill et al. 2013).
hArg, ADMA and their relationship in DMD
Low circulating hArg concentrations are associated with cardiovascular and all-cause mortality (März et al. 2010; Pilz et al. 2011, 2014, 2015). The hArg concentration and hArg/ADMA molar ratio in plasma of DMD patients are lower than in healthy children. hArg may serve as a substrate for NOS (Moali et al. 1998) and thus improve endothelial function (Valtonen et al. 2008). Diminished hArg synthesis in DMD may decrease NO synthesis and add to the cardiovascular risk in this disease. Elevated ADMA and diminished hArg concentrations may promote synergistically the development of cardiomyopathy in DMD patients. This is supported by similar findings on hArg and ADMA in Takotsubo cardiomyopathy (Kayacelebi et al. 2014b). There are indications that hArg antagonizes the effects of ADMA in the renal and cardiovascular systems (Tsikas and Kayacelebi 2014). In the DMD patients of our study, the hArg synthesis is impaired and its antagonistic action on ADMA seems to be insufficient, which is expressed in the lower hArg/ADMA molar ratio in plasma compared to healthy controls.
The L-Arg/NO pathway in relation to the DMD stage of disease
The urinary excretion of ADMA and its metabolite DMA, as well as of nitrate, correlated positively with progressive stage of disease (according to Thompson and Vignos; Thompson and Vignos 1959; Mortier 1994) in the DMD patients. Elevated ADMA synthesis, insufficient antagonism by hArg and the diminished NO bioavailability in the DMD patients are likely to have mediated and increased progressively the endothelial dysfunction accompanied by ischemia, hypoxia and destruction of skeletal muscles. DMD patients affected more severely are usually older than less affected DMD patients. This circumstance and the age-dependent decrease of circulating ADMA in healthy children (Lücke et al. 2007) may explain in part the lack of a correlation between plasma ADMA concentration and stage of disease in the DMD patients.
Effect of steroid and creatine medication
Chronic inflammatory processes seem to play an important role in the pathogenesis of DMD (De Paepe and De Bleecker 2013; Evans et al. 2009; Spencer and Tidball 2001). Inflammatory cytokines play also a role in the L-Arg/NO pathways including ADMA synthesis (Zoccali et al. 2007). Elevated serum ADMA levels have been found in several inflammatory diseases which could be explained by a down-regulation of DDAH activity by cytokines as a result of oxidative stress (Ito et al. 1999). Glucocorticoids are anti-inflammatory drugs and used in the treatment of many chronic inflammatory diseases (Barnes 2010). The effects of steroids on the progression of DMD have been investigated by many groups. Administered glucocorticoids seem to improve muscle strength and to delay the development of cardiac and respiratory complications in DMD (Manzur et al. 2008; Annexstad et al. 2014; Leung et al. 2011). DMD patients on steroid medication were found to excrete less nitrate than DMD patients who have not been medicated with steroids. This is likely to have resulted primarily from an inhibition of iNOS expression reported for glucocorticoids (Radomski et al. 1990). It is worth mentioning that iNOS activity is several orders of magnitude higher than that of nNOS and more so of eNOS (Böhmer et al. 2014). Steroids have anti-inflammatory effects (Barnes 2010) and reduce inflammation in DMD patients. In our study, steroid administration seems to diminish inflammation and thus decrease ADMA synthesis. ADMA is a risk marker for cardiovascular disease (Sibal et al. 2010). Inhibition of ADMA synthesis by steroids could explain the attenuated progression of the disease, including reduction of cardiac and respiratory complications in patients suffering from DMD.
Routine administration of creatine to seven of our DMD patients influenced all biochemical parameters of the L-Arg/NO pathway in the same direction, i.e., it decreased their concentration in plasma and urine. Yet, creatine supplementation did not result in statistically significant changes in hArg, ADMA and their molar ratio hArg/ADMA in plasma compared to 47 of our DMD patients who were not treated with creatine. Interestingly, creatine supplementation decreased both DMA and nitrate excretion suggesting inhibition of whole body synthesis of ADMA and NO, respectively. Although not statistically significant, our results seem to confirm previous results showing an inhibitory action of creatine on the activity of the enzyme arginine:glycine amidinotransferase (AGAT) which is involved in the synthesis both of hArg and guanidinoacetate (Walker and Hannan 1976; Roberts and Walker 1985; da Silva et al. 2014), the substrate of guanidinoacetate: N-methyltransferase (GAMT) which catalyzes the N-methylation of guanidinoacetate to creatine. However, the effect of creatine on hArg and NO synthesis remains to be investigated.
Combined administration of l-arginine (3 × 2.5 g/d) and metformin (2 × 250 mg/d) for 16 weeks to five ambulatory and genetically confirmed DMD patients (age 7–10 years) was reported to increase cGMP and mitochondrial proteins of complex III and V, as well as to improve motor function and timed walking distances, without any serious side effects (Bonati et al. 2015, an Abstract). Whether the beneficial effects of the combined treatment seen in this are due to an improvement of the L-Arg/NO pathway and are mediated by l-arginine, metformin or both remains to be investigated. It is worth mentioning that metformin administration (1655 mg/d) may enhance ADMA synthesis, but decrease plasma-soluble vascular cell adhesion molecule-1 (sVCAM-1) in patients suffering from type 2 diabetes mellitus and stable coronary artery disease (Kruszelnicka et al. 2015).
Conclusions
Children with DMD have elevated synthesis of ADMA, diminished hArg synthesis and reduced NO bioavailability compared to healthy children. The extent of impairment of the L-Arg/NO pathway correlates positively with the stage of the DMD disease. Administration of steroids in DMD improves the L-Arg/NO pathway including inhibition of ADMA synthesis and exerts positive effects on the progression of the DMD disease. Administration of hArg in DMD to increase the hArg concentration might be an additional therapeutic means aiming at enhancing the antagonistic effects of hArg to ADMA in the circulation and should be investigated in future studies. Creatine supplementation in DMD seems to suppress the L-Arg/NO pathway.
Abbreviations
- ACE:
-
Angiotensin-converting enzyme
- ADMA:
-
Asymmetric dimethylarginine (N G,N G-dimethyl-l-arginine)
- AGAT:
-
Arginineglycine amidinotransferase
- DDAH:
-
Dimethylarginine dimethylaminohydrolase
- DMA:
-
Dimethylamine
- DMD:
-
Duchenne muscular dystrophy
- EDRF:
-
Endothelium-derived relaxing factor
- GAMT:
-
Guanidinoacetate N-methyltransferase
- GC–MS:
-
Gas chromatography–mass spectrometry
- GC–MS/MS:
-
Gas chromatography–tandem mass spectrometry
- hArg:
-
Homoarginine
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthase
- SDMA:
-
Symmetric dimethylarginine (N G,N G´-dimethyl-l-arginine)
- sVCAM-1:
-
Soluble vascular cell adhesion molecule-1
References
Aartsma-Rus A, Van Deutekom JC, Fokkema IF, Van Ommen GJ, Den Dunnen JT (2006) Entries in the Leiden Duchenne muscular dystrophy mutation database: an overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve 34:135–144
Achan V, Broadhead M, Malaki M, Whitley G, Leiper J, MacAllister R, Vallance P (2003) Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler Thromb Vasc Biol 23:1455–1459
Annexstad EJ, Lund-Petersen I, Rasmussen M (2014) Duchenne muscular dystrophy. Tidsskr Nor Laegeforen 134:1361–1364
Arahata K, Ishiura S, Ishiguro T, Tsukahara T, Suhara Y, Eguchi C, Ishihara T, Nonaka I, Ozawa E, Sugita H (1988) Immunostaining of skeletal and cardiac muscle surface membrane with antibody against Duchenne muscular dystrophy peptide. Nature 333(6176):861–863
Banerjee B, Sharma U, Balasubramanian K, Kalaivani M, Kalra V, Jagannathan NR (2010) Effect of creatine monohydrate in improving cellular energetics and muscle strength in ambulatory Duchenne muscular dystrophy patients: a randomized, placebo-controlled 31P MRS study. Magn Reson Imaging 28(5):698–707
Barnes PJ (2010) Mechanisms and resistance in glucocorticoid control of inflammation. J Steroid Biochem Mol Biol 120:76–85
Böger RH, Bode-Böger SM, Thiele W, Junker W, Alexander K, Frölich JC (1997) Biochemical evidence for impaired nitric oxide synthesis in patients with peripheral arterial occlusive disease. Circulation 95:2068–2074
Böhmer A, Gambaryan S, Tsikas D (2014) Human blood platelets lack nitric oxide synthase activity. Platelets. doi:10.3109/09537104.2014.974024
Bonati U, Hafner P, Erne B, Thomas E, Rutz E, Frank S, Hilker C, Deuster S, Gloor M, Bieri O, Sinnreich M, Fischmann A, Fischer D (2015) Improved muscle function in Duchenne muscular dystrophy using a combination of l-arginine and metformin. Neuropedoatrics 46:PS02-34. doi:10.1055/s-0035-1550745
Brenman JE, Chao DS, Xia H, Aldape K, Bredt DS (1995) Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell 82:743–752
Chavoshan B, Sander M, Sybert T, Hansen J, Victor R, Thomas G (2002) Nitric oxide-dependent modulation of sympathetic neural control of oxygenation in exercising human skeletal muscle. J Physiol 540(Pt 1):377–386
Chobanyan-Jürgens K, Fuchs AJ, Tsikas D, Kanzelmeyer N, Das AM, Illsinger S, Vaske B, Jordan J, Lücke T (2012a) Increased asymmetric dimethylarginine (ADMA) dimethylaminohydrolase (DDAH) activity in childhood hypercholesterolemia type II. Amino Acids 43:805–811
Chobanyan-Jürgens K, Schwarz A, Böhmer A, Beckmann B, Gutzki FM, Michaelsen JT, Stichtenoth DO, Tsikas D (2012b) Renal carbonic anhydrases are involved in the reabsorption of endogenous nitrite. Nitric Oxide 26:126–131
Cooke JP (2000) Does ADMA cause endothelial dysfunction? Arterioscler Thromb Vasc Biol 20:2032–2037
Cutolo M, Seriolo B, Pizzorni C, Secchi ME, Soldano S, Paolino S, Montagna P, Sulli A (2008) Use of glucocorticoids and risk of infections. Autoimmun Rev 8:153–155
da Silva RP, Clow K, Brosnan JT, Brosnan ME (2014) Synthesis of guanidinoacetate and creatine from amino acids by rat pancreas. Br J Nutr 111:571–577
De Paepe B, De Bleecker JL (2013) Cytokines and chemokines as regulators of skeletal muscle inflammation: presenting the case of Duchenne muscular dystrophy. Mediators Inflamm 2013:540370
Derave W, Marescau B, Vanden Eede E, Eijnde BO, De Deyn PP, Hespel P (2004) Plasma guanidino compounds are altered by oral creatine supplementation in healthy humans. J Appl Physiol 97:852–857
Evans NP, Misyak SA, Robertson JL, Bassaganya-Riera J, Grange RW (2009) Dysregulated intracellular signaling and inflammatory gene expression during initial disease onset in Duchenne muscular dystrophy. Am J Phys Med Rehabil 88:502–522
Förstermann U, Closs EI, Pollock JS, Nakane M, Schwarz P, Gath I, Kleinert H (1994) Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension 23 (6 Pt 2):1121–1131
Furchgott RF, Zawadzki JV (1980) The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288(5789):373–376
Furchgott RF, Cherry PD, Zawadzki JV, Jothianandan D (1984) Endothelial cells as mediators of vasodilation of arteries. J Cardiovasc Pharmacol 6(Suppl 2):S336–S343
Goonasekera CD, Rees DD, Woolard P, Frend A, Shah V, Dillon MJ (1997) Nitric oxide synthase inhibitors and hypertension in children and adolescents. J Hypertens 15:901–909
Gücüyener K, Ergenekon E, Erbas D, Pinarli G, Serdaroğlu A (2000) The serum nitric oxide levels in patients with Duchenne muscular dystrophy. Brain Dev 22:181–183
Inoue R, Miyake M, Kanazawa A, Sato M, Kakimoto Y (1979) Decrease of 3-methylhistidine and increase of NG, NG-dimethylarginine in the urine of patients with muscular dystrophy. Metabolism 28:801–804
Ito A, Tsao PS, Adimoolam S, Kimoto M, Ogawa T, Cooke JP (1999) Novel mechanism for endothelial dysfunction: dysregulation of dimethylarginine dimethylaminohydrolase. Circulation 99:3092–3095
Kakimoto Y, Akazawa S (1970) Isolation and identification of N-G, N-G- and N-G, N’-G-dimethyl-arginine, N-epsilon-mono-, di-, and trimethyllysine, and glucosylgalactosyl- and galactosyl-delta-hydroxylysine from human urine. J Biol Chem 245:5751–5758
Kanzelmeyer N, Tsikas D, Chobanyan-Jürgens K, Beckmann B, Vaske B, Illsinger S, Das AM, Lücke T (2012) Asymmetric dimethylarginine in children with homocystinuria or phenylketonuria. Amino Acids 42:1765–1772
Kanzelmeyer NK, Pape L, Chobanyan-Jürgens K, Tsikas D, Hartmann H, Fuchs AJ, Vaske B, Das AM, Haubitz M, Jordan J, Lücke T (2014) l-arginine/NO pathway is altered in children with haemolytic-uraemic syndrome (HUS). Oxid Med Cell Longev 2014:203512
Kasai T, Abeyama K, Hashiguchi T, Fukunaga H, Osame M, Maruyama I (2004) Decreased total nitric oxide production in patients with Duchenne muscular dystrophy. J Biomed Sci 11:534–537
Kayacelebi AA, Nguyen TH, Neil C, Horowitz JD, Jordan J, Tsikas D (2014a) Homoarginine and 3-nitrotyrosine in patients with takotsubo cardiomyopathy. Int J Cardiol 173:546–547
Kayacelebi AA, Beckmann B, Gutzki FM, Jordan J, Tsikas D (2014b) GC-MS and GC-MS/MS measurement of the cardiovascular risk factor homoarginine in biological samples. Amino Acids 46:2205–2217
Kayacelebi AA, Knöfel A-K, Beckmann B, Hanff E, Warnecke G, Tsikas D (2015) Measurement of unlabeled and stable isotope-labeled homoarginine, arginine and their metabolites in biological samples by GC–MS and GC–MS/MS. Amino Acids. doi:10.1007/s00726-015-1984-3
Kielstein JT, Böger RH, Bode-Böger SM, Schäffer J, Barbey M, Koch KM, Frölich JC (1999) Asymmetric dimethylarginine plasma concentrations differ in patients with end-stage renal disease: relationship to treatment method and atherosclerotic disease. J Am Soc Nephrol 10:594–600
Kielstein A, Tsikas D, Galloway GP, Mendelson JE (2007) Asymmetric dimethylarginine (ADMA)–a modulator of nociception in opiate tolerance and addiction? Nitric Oxide 17:55–59
Kley RA, Tarnopolsky MA, Vorgerd M (2013) Creatine for treating muscle disorders. Cochrane Database Syst Rev 6:CD004760. doi:10.1002/14651858.CD004760.pub4
Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C, Kunkel LM (1987) Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell 50:509–517
Kruszelnicka O, Chyrchel B, Golay A, Surdacki A (2015) Differential associations of circulating asymmetric dimethylarginine and cell adhesion molecules with metformin use in patients with type 2 diabetes mellitus and stable coronary artery disease. Amino Acids. doi:10.1007/s00726-015-1976-3
Leiper J, Vallance P (2006) The synthesis and metabolism of asymmetric dimethylarginine (ADMA). Eur J Clin Pharmacol 62:33–38
Leung DG, Germain-Lee EL, Denger BE, Wagner KR (2011) Report on the second endocrine aspects of Duchenne muscular dystrophy conference December 1–2, 2010, Baltimore, Maryland, USA. Neuromuscul Disord 21:594–601
Lücke T, Tsikas D, Kanzelmeyer N, Vaske B, Das AM (2006a) Elevated plasma concentrations of the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine in citrullinemia. Metabolism 55:1599–1603
Lücke T, Tsikas D, Kanzelmeyer NK, Boerkoel CF, Clewing JM, Vaske B, Ehrich JH, Das AM (2006b) Vaso-occlusion in Schimke-immuno-osseous dysplasia: is the NO pathway involved? Horm Metab Res 38:678–682
Lücke T, Kanzelmeyer N, Kemper MJ, Tsikas D, Das AM (2007) Developmental changes in the l-arginine/nitric oxide pathway from infancy to adulthood: plasma asymmetric dimethylarginine levels decrease with age. Clin Chem Lab Med 45:1525–1530
Lücke T, Kanzelmeyer N, Chobanyan K, Tsikas D, Franke D, Kemper MJ, Ehrich JH, Das AM (2008) Elevated asymmetric dimethylarginine (ADMA) and inverse correlation between circulating ADMA and glomerular filtration rate in children with sporadic focal segmental glomerulosclerosis (FSGS). Nephrol Dial Transplant 23:734–740
Manzur AY, Kuntzer T, Pike M, Swan A (2008) Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev (1):CD003725. doi:10.1002/14651858.CD003725.pub3
Marletta MA (1993) Nitric oxide synthase structure and mechanism. J Biol Chem 268:12231–12234
März W, Meinitzer A, Drechsler C, Pilz S, Krane V, Kleber ME, Fischer J, Winkelmann BR, Böhm BO, Ritz E, Wanner C (2010) Homoarginine, cardiovascular risk, and mortality. Circulation 122:967–975
Moali C, Boucher JL, Sari MA, Stuehr DJ, Mansuy D (1998) Substrate specificity of NO synthases: detailed comparison of l-arginine, homo-l-arginine, their N omega-hydroxy derivatives, and N omega-hydroxynor-l-arginine. Biochemistry 37(29):10453–10460
Moncada S, Higgs EA (2006) The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol 147(Suppl 1):S193–S201
Mortier W (1994) Muskel- und Nervenerkrankungen im Kindesalter. Thieme, Stuttgart
Palmer RM, Ferrige AG, Moncada S (1987) Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327(6122):524–526
Palmer RM, Ashton DS, Moncada S (1988) Vascular endothelial cells synthesize nitric oxide from l-arginine. Nature 333(6174):664–666
Pham VV, Stichtenoth DO, Tsikas D (2009) Nitrite correlates with 3-nitrotyrosine but not with the F(2)-isoprostane 15(S)-8-iso-PGF(2alpha) in urine of rheumatic patients. Nitric Oxide 21:210–215
Pilz S, Meinitzer A, Tomaschitz A, Drechsler C, Ritz E, Krane V, Wanner C, Boehm BO, März W (2011) Low homoarginine concentration is a novel risk factor for heart disease. Heart 97(15):1222–1227
Pilz S, Teerlink T, Scheffer PG, Meinitzer A, Rutters F, Tomaschitz A, Drechsler C, Kienreich K, Nijpels G, Stehouwer CD, März W, Dekker JM (2014) Homoarginine and mortality in an older population: the Hoorn study. Eur J Clin Invest 44:200–208
Pilz S, Meinitzer A, Gaksch M, Grübler M, Verheyen N, Drechsler C, Hartaigh BÓ, Lang F, Alesutan I, Voelkl J, März W, Tomaschitz A (2015) Homoarginine in the renal and cardiovascular systems. Amino Acids. doi:10.1007/s00726-015-1993-2
Radomski MW, Palmer RM, Moncada S (1990) Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci 87:10043–10047
Rees DD, Palmer RM, Moncada S (1989) Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci 86:3375–3378
Roberts JJ, Walker JB (1985) Higher homolog and N-ethyl analog of creatine as synthetic phosphagen precursors in brain, heart, and muscle, repressors of liver amidinotransferase, and substrates for creatine catabolic enzymes. J Biol Chem 260:13502–13508
Ryan WL, Wells IC (1964) Homocitrulline and homoarginine synthesis from lysine. Science 144(3622):1122–1127
Ryan WL, Barak AJ, Johnson RJ (1968) Lysine, homocitrulline, and homoarginine metabolism by the isolated perfused rat liver. Arch Biochem Biophys 123:294–297
Sibal L, Agarwal SC, Home PD, Boger RH (2010) The role of asymmetric dimethylarginine (ADMA) in endothelial dysfunction and cardiovascular disease. Curr Cardiol Rev 6:82–90
Spencer MJ, Tidball JG (2001) Do immune cells promote the pathology of dystrophin-deficient myopathies? Neuromuscul Disord 11:556–564
Tarnopolsky MA, Mahoney DJ, Vajsar J, Rodriguez C, Doherty TJ, Roy BD, Biggar D (2004) Creatine monohydrate enhances strength and body composition in Duchenne muscular dystrophy. Neurology 62:1771–1777
Terrill JR, Radley-Crabb HG, Iwasaki T, Lemckert FA, Arthur PG, Grounds MD (2013) Oxidative stress and pathology in muscular dystrophies: focus on protein thiol oxidation and dysferlinopathies. FEBS J 280:4149–4164
Thomas GD, Sander M, Lau KS, Huang PL, Stull JT, Victor RG (1998) Impaired metabolic modulation of alpha-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc Natl Acad Sci USA 95:15090–15095
Thompson RA, Vignos PJ Jr (1959) Serum aldolase in muscle disease. AMA archives of internal medicine 103(4):551–564
Tran CT, Leiper JM, Vallance P (2003) The DDAH/ADMA/NOS pathway. Atheroscler Suppl 4:33–40
Tsikas D (2000) Simultaneous derivatization and quantification of the nitric oxide metabolites nitrite and nitrate in biological fluids by gas chromatography/mass spectrometry. Anal Chem 72:4064–4072
Tsikas D (2005) Methods of quantitative analysis of the nitric oxide metabolites nitrite and nitrate in human biological fluids. Free Radic Res 39:797–815
Tsikas D (2008) A critical review and discussion of analytical methods in the l-arginine/nitric oxide area of basic and clinical research. Anal Biochem 379:139–163
Tsikas D (2015) Circulating and excretory nitrite and nitrate: their value as measures of nitric oxide synthesis, bioavailability and activity is inherently limited. Nitric Oxide 45:1–3
Tsikas D, Kayacelebi AA (2014) Do homoarginine and asymmetric dimethylarginine act antagonistically in the cardiovascular system? Circ J 78:2094–2095
Tsikas D, Sandmann J, Savva A, Luessen P, Böger RH, Gutzki FM, Mayer B, Frölich JC (2000) Assessment of nitric oxide synthase activity in vitro and in vivo by gas chromatography-mass spectrometry. J Chromatogr B 742:143–153
Tsikas D, Schubert B, Gutzki FM, Sandmann J, Frölich JC (2003) Quantitative determination of circulating and urinary asymmetric dimethylarginine (ADMA) in humans by gas chromatography-tandem mass spectrometry as methyl ester tri(N-pentafluoropropionyl) derivative. J Chromatogr B 798:87–99
Tsikas D, Thum T, Becker T, Pham VV, Chobanyan K, Mitschke A, Beckmann B, Gutzki FM, Bauersachs J, Stichtenoth DO (2007) Accurate quantification of dimethylamine (DMA) in human urine by gas chromatography-mass spectrometry as pentafluorobenzamide derivative: evaluation of the relationship between DMA and its precursor asymmetric dimethylarginine (ADMA) in health and disease. J Chromatogr B 851:229–239
Tsikas D, Wolf A, Mitschke A, Gutzki FM, Will W, Bader M (2010a) GC-MS determination of creatinine in human biological fluids as pentafluorobenzyl derivative in clinical studies and biomonitoring: inter-laboratory comparison in urine with Jaffé, HPLC and enzymatic assays. J Chromatogr B 878:2582–2592
Tsikas D, Schwarz A, Stichtenoth DO (2010b) Simultaneous measurement of [15N]nitrate and [15N]nitrite enrichment and concentration in urine by gas chromatography mass spectrometry as pentafluorobenzyl derivatives. Anal Chem 82:2585–2587
Tsikas D, Niemann J, Flentje M, Schwarz A, Tossios P (2014) N-Acetylcysteine (NAC) inhibits renal nitrite and nitrate reabsorption in healthy subjects and in patients undergoing cardiac surgery: risk of nitric oxide (NO) bioavailability by NAC? Int J Cardiol 177:30–33
Valtonen P, Laitinen T, Lyyra-Laitinen T, Raitakari OT, Juonala M, Viikari JS, Heiskanen N, Vanninen E, Punnonen K, Heinonen S (2008) Serum L-homoarginine concentration is elevated during normal pregnancy and is related to flow-mediated vasodilatation. Circ J 72:1879–1884
van der Zwan LP, Scheffer PG, Dekker JM, Stehouwer CD, Heine RJ, Teerlink T (2011) Systemic inflammation is linked to low arginine and high ADMA plasma levels resulting in an unfavourable NOS substrate-to-inhibitor ratio: the Hoorn Study. Clin Sci (Lond) 121:71–78
Walker JB, Hannan JK (1976) Creatine biosynthesis during embryonic development. False feedback suppression of liver amidinotransferase by N-acetimidoylsarcosine and 1-carboxymethyl-2-iminoimidazolidine (cyclocreatine). Biochemistry 15:2519–2522
Warnes DM, Tomas FM, Ballard FJ (1981) Increased rates of myofibrillar protein breakdown in muscle-wasting diseases. Muscle Nerve 4:62–66
Zoccali C, Maas R, Cutrupi S, Pizzini P, Finocchiaro P, Cambareri F, Panuccio V, Martorano C, Schulze F, Enia G, Tripepi G, Boger R (2007) Asymmetric dimethyl-arginine (ADMA) response to inflammation in acute infections. Nephrol Dial Transplant 22:801–806
Acknowledgments
The authors thank A. Mitschke and M. T. Suchy for excellent laboratory assistance and F. M. Gutzki for performing GC–MS and GC–MS/MS analyses. We are also grateful for the support in recruiting DMD patients from the Department of Neuropediatrics in the University Hospital Essen.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
The Ethics Committees of the Faculty of Medicine at Ruhr-University Bochum and of the Hannover Medical School approved the study. Written consent was given by each participant and his parents or only by the participant if he was 18 years or older.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hörster, I., Weigt-Usinger, K., Carmann, C. et al. The l-arginine/NO pathway and homoarginine are altered in Duchenne muscular dystrophy and improved by glucocorticoids. Amino Acids 47, 1853–1863 (2015). https://doi.org/10.1007/s00726-015-2018-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-015-2018-x