Abstract
The present study has been carried out to investigate the role of taurine (2-aminoethanesulfonic acid), a conditionally essential amino acid, in ameliorating cadmium-induced renal dysfunctions in mice. Cadmium chloride (CdCl2) has been selected as the source of cadmium. Intraperitoneal administration of CdCl2(at a dose of 4 mg/kg body weight for 3 days) caused significant accumulation of cadmium in renal tissues and lessened kidney weight to body weight ratio. Cadmium administration reduced intracellular ferric reducing/antioxidant power (FRAP) of renal tissues. Levels of serum marker enzymes related to renal damage, creatinine and urea nitrogen (UN) have been elevated due to cadmium toxicity. Cadmium exposure diminished the activities of enzymatic antioxidants, superoxide dismutase (SOD), catalase (CAT), glutathione-S-transferase (GST), glutathione reductase (GR), glutathione peroxidase (GPx) and glucose-6-phosphate dehydrogenase (G6PD) as well as non-enzymatic antioxidant, reduced glutathione (GSH) and total thiols. On the other hand, the levels of oxidized glutathione (GSSG), lipid peroxidation, protein carbonylation, DNA fragmentation, concentration of superoxide radicals and activities of cytochrome P450 enzymes (CYP P450s) have been found to increase due to cadmium intoxication. Treatment with taurine (at a dose of 100 mg/kg body weight for 5 days) before cadmium intoxication prevented the toxin-induced oxidative impairments in renal tissues. The beneficial role of taurine against cadmium-induced renal damage was supported from histological examination of renal segments. Vitamin C, a well-established antioxidant was used as the positive control in the study. Experimental evidence suggests that both taurine and vitamin C provide antioxidant defense against cadmium-induced renal oxidative injury. Combining all, results suggest that taurine protects murine kidneys against cadmium-induced oxidative impairments, probably via its antioxidative property.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metals are probably the oldest toxins known to human population. Cadmium (Cd) is one of the most environmentally abundant toxic metals affecting numerous organs of the body (WHO 1992; US Department of Health and Human Services 1997). In addition to occupational exposure, its exposure also occurs from inhalation and primarily, by ingestion of contaminated food and drinking water. Symptoms associated with acute Cd exposure are dysuria, polyuria, dyspnea, chest pain, irritability, fatigue, headache and dizziness (Wittman and Hu 2002). Kidneys are critically affected by Cd exposure (Jin et al. 2002). The mechanisms of metal-induced nephrotoxicity at molecular level have been studied for decades, but still are poorly understood. After intake, Cd enters the blood where it binds with erythrocyte membrane and plasma albumin and circulates throughout the body (Bauman et al. 1993). In liver, Cd forms complex with metallothionein, a cystein-rich protein (Klaassen et al. 1999). This Cd–metallothionein complex is slowly released from liver and circulates to the kidney. In renal cells, the complex is dissolved; free Cd is released and absorbed in proximal tubules. If kidney’s metallothionein defense and detoxification system are overwhelmed, free Cd can damage renal tubules (Patrick 2003). According to urinary data, proximal tubule is the major target site for metal-induced nephrotoxicity (Sabolic 2006). The common event in nephrotoxic action of metals (including Cd) in proximal tubule is the generation of oxidative stress (Szuster-Ciesielska et al. 2000). Earlier investigations provide a number of epidemiological evidences on Cd-induced renal toxicity (Ahn et al. 1999; WHO 1992). Hu (2000) reported that occupational exposure to Cd causes kidney stones and glomeruler damage. Cd-intoxication evokes various cellular responses to protect the cell from the metal-induced toxicity (Beyersmann and Hechtenberg 1997).
Considering the relationship between Cd exposure and oxidative stress, it is reasonable that administration of some antioxidant should be an important therapeutic approach. Taurine (2-aminoethanesulfonic acid), a conditionally essential amino acid, has been considered as an antioxidant (Huxtable 1992; Son et al. 2007). It is a derivative of the sulphur-containing amino acid, cysteine and is present in many tissues of mammals with high concentrations. It differs from most biological amino acids, as it is a sulfonic acid rather than a carboxylic acid; it is a beta-amino acid rather than an alpha-amino acid. It is not incorporated into proteins, but it plays many roles in the body, including bile acid conjugation, detoxification, membrane stabilization, osmoregulation, and modulation of excitatory neurotransmission and intracellular calcium levels (Huxtable 1992; Wessberg et al. 1983). A number of investigators reported that taurine protects many of the body’s organs against toxicity and oxidative stress due to heavy metals and other toxin as well as drugs (Dogru-Abbasoglu et al. 2001; Hwang et al. 1998; Gurer et al. 2001; Tabassum et al. 2006). Although biochemical and physiologic function of taurine is still undefined, considerable evidence shows that it can act as a direct antioxidant by scavenging ROS or as an indirect antioxidant by preventing changes in membrane permeability due to oxidative impairment (Timbrell et al. 1995; Wright et al. 1986).
Cd-induced renal disorder is a well-known problem. Antioxidant property of taurine is also well established. Hence, it may be hypothesized that taurine could play a preventive role against Cd-induced nephrotoxicity. Thus, the present study has been undertaken to evaluate the beneficial role of taurine against cadmium-induced renal damages in mice. Distribution of cadmium in kidney tissues has been measured by atomic absorption spectroscopy. The in vivo antioxidant power of taurine in renal tissue was determined by ferric reducing/antioxidant power (FRAP) assay. The extent of renal damages caused by cadmium and the protective role of taurine was evaluated by measuring the (a) kidney weight, body weight and kidney weight to body weight ratios in experimental animals; (b) the activities of serum marker enzymes related to renal dysfunction; (c) activities of intracellular antioxidant enzymes; (d) the levels of cellular metabolites; (e) the extent of lipid peroxidation and protein carbonylation; (f) the extent of DNA fragmentation; (g) activities of cytochrome P450 (CYP P450s) enzymes and (h) the concentration of intracellular superoxide radical anion. In addition, histological studies were carried out to assess the ultrastructural changes in murine kidneys.
Materials and methods
Animals
Male Swiss-albino mice weighing between 20–25 g were acclimatized under laboratory condition for 2 weeks before starting the experiments. They were maintained under standard conditions of temperature (25 ± 1°C) and humidity (30%) with an alternating 12 h light/dark cycles. The animals had free access to standard diet and water ad libitum. All the studies were performed in conformity with the guidance for care and standard experimental animals study ethical protocols.
Chemicals
Bovine serum albumin (BSA) and Bradford reagent, taurine (2-aminoethane sulfonic acid) were purchased from Sigma-Aldrich Chemical Company (St Louis, MO, USA). Kits for creatinine and urea nitrogen (UN) measurements were purchased from Span diagnostic Ltd., India. Cadmium chloride (CdCl2) and all other necessary reagents of analytical grade were bought from Sisco research laboratory, India.
Determination of dose-dependent activity of cadmium by SOD assay
SOD assay was performed to determine optimum dose of cadmium needed for maximum renal damage. Animals were randomly distributed into seven groups consisting of six animals in each. The first group served as normal control and received water as vehicle. The remaining six groups of animals were treated with six different doses of CdCl2 (0.5, 1, 2, 4, 6 and 8 mg/kg body weight) intraperitoneally for 3 days. Twenty-four hours after the final dose of CdCl2 intoxication, all the animals were killed, kidneys were collected and SOD assay were performed with kidney tissue homogenate.
Determination of dose-dependent activity of taurine by ferric reducing/antioxidant power (FRAP) assay
FRAP assay was performed to determine the optimum dose of taurine necessary for the protection of murine kidneys against CdCl2-induced oxidative impairment. For this purpose, mice were divided into eight groups each consisting of six animals. The first two groups served as normal control (received only water as vehicle) and toxin control (received CdCl2 at a dose of 4 mg/kg body weight for 3 days, i.p.), respectively. The remaining six groups of animals were treated with six different doses of taurine (10, 25, 50, 75, 100 and 150 mg/kg body weight for 5 days, i.p.) followed by CdCl2 intoxication (4 mg/kg body weight for 3 days, i.p., once daily). Twenty-four hours after the final dose of CdCl2 intoxication, all the animals were killed, kidneys were collected and FRAP assay was performed with the kidney tissue homogenates.
Experimental design
The animals were divided into four groups, consisting of six mice in each group and they were treated as follows:
- Group 1:
-
Normal control (animals received only water as vehicle).
- Group 2:
-
Toxin control (animals received CdCl2 intraperitoneally at a dose of 4 mg/kg body weight for 3 days, once daily).
- Group 3:
-
Animals were treated with a single dose of taurine (i.p., at a dose of 100 mg/kg body weight, once daily) for 5 days followed by CdCl2 (i.p., 4 mg/kg body weight, once daily) intoxication for next 3 days.
- Group 4:
-
Vitamin C was administered at a dose of 100 mg/kg body weight orally for 5 days prior to CdCl2 (i.p., 4 mg/kg body weight for 3 days, once daily) intoxication and served as positive control.
The animals were killed under light ether anesthesia and kidneys were collected.
Estimation of renal cadmium content
The cadmium contents in renal tissues of all experimental animals were analyzed following the method of Pari et al. (2007) with some modifications. Briefly, a part of the tissue was digested three times with a mixture of deionized water; HNO3 and H2O2 until almost dry The residual mass was finally dissolved in 1% HNO3 and the solution was used for the estimation of cadmium content by atomic absorption spectrophotometer (Perkin Elmer Model No. 3100) furnished with a cadmium hollow cathode lamp.
Determination of kidney weight, body weight and kidney weight to body weight ratio
Body weight of each animal was taken. After sacrifice, the kidneys from experimental animals were quickly excised and weighed. Then the ratio of kidney weight to body weight was measured for each animal.
Assessment of serum specific markers related to renal dysfunction
For assessment of serum-specific markers (creatinine and UN levels) related to renal damage, blood samples were collected by puncturing hearts of all experimental animals, kept overnight for clotting and then centrifuged at 3,000g for 10 min. Creatinine and UN levels in the sera were measured by using standard kits.
Preparation of kidney homogenate
Kidneys were homogenized using glass homogenizer in 100 mM potassium phosphate buffer containing 1 mM EDTA, pH 7.4 and centrifuged at 12,000g for 30 min at 4°C. The supernatant was collected and used for the experiments.
Determination of protein content
The protein content of the experimental samples was measured by the method of Bradford (1976) using crystalline BSA as standard.
Determination of in vivo antioxidant power by ferric reducing/antioxidant power (FRAP) assay
The FRAP assay measures the change in absorbance at 593 nm due to the formation of a blue-colored FeII-tripyridyltriazine compound from the colorless oxidized FeIII form by the action of electron donating antioxidants (Benzie and Strain 1999). Briefly, 50 μl of sample was added to 1.5 ml freshly prepared and prewarmed (37°C) FRAP reagent (300 mM acetate buffer, pH 3.6, 10 mM TPTZ in 40 mM HCl and 20 mM FeCl3·6H2O in the ratio of 10:1:1) and incubated at 37°C for 10 min. The absorbance of the sample was recorded against reagent blank (1.5 ml FRAP reagent + 50 μl distilled water) at 593 nm.
Estimation of MDA and lipid hydroperoxide
The extent of lipid peroxidation in terms of malondialdehyde (MDA) formation was measured according to the method of Esterbauer and Cheeseman (1990). Sample containing 1 mg protein was mixed with 1 ml TCA (20%), 2 ml TBA (0.67%) and heated for 1 h at 100°C. After cooling, the precipitate was removed by centrifugation. The absorbance of the sample was measured at 535 nm using a blank containing all the reagents except the sample. MDA content of the sample was calculated using the extinction co-efficient of MDA, which is 1.56 × 105 M−1 per cm.
The concentration of lipid hydroperoxide in the experimental sample was estimated by the FOX assay described by Jiang et al. (1992). For this purpose the tissue homogenate was mixed with FOX reagent [88 mg of butylated hydroxy toluene, 7.6 mg xylenol orange and 9.8 mg of ammoniam iron (II) sulphate in 90 ml methanol and 10 ml of H2SO4]. After 30 min the absorbance of the solution was read at 560 nm. The amount of hydroperoxide produced was calculated using the molar extinction coefficient of 4.6 × 104 M−1 per cm.
Estimation of protein carbonyl content
Protein carbonyl contents were determined according to the methods of Uchida and Stadtman (1993). The sample was treated with an equal volume of 0.1% (w/v) 2,4-DNPH in 2 N HCl and incubated for 1 h at room temperature and then treated with 20% TCA. After centrifugation, the precipitate was washed three times with EtOH/EtOAc and dissolved in 8 M guanidine hydrochloride in 133 mM Tris solution containing 13 mM EDTA. The absorbance was recorded at 365 nm. The results were expressed as nmol of DNPH incorporated/mg protein based on the molar extinction coefficient of 22,000 M−1 per cm for aliphatic hydrazones.
Assay of antioxidant enzymes
The activities of antioxidant enzymes, SOD, CAT, GST, GR, GPx and G6PD have been measured in kidney homogenates of all experimental animals.
SOD activity has been measured by following the method originally developed by Nishikimi (1972) and then modified by Kakkar (1984). One unit of SOD activity is defined as the enzyme concentration required inhibiting chromogen production by 50% in 1 min under the assay conditions.
CAT activity was determined by following the decomposition of H2O2 at 240 nm for 10 min and it was monitored spectrophotometrically according to the method of Bonaventura et al. (1972). One unit of CAT activity is defined as the amount of enzyme, which reduces 1 μmol of H2O2 per minute.
GST activity was assayed based on the conjugation reaction with glutathione in the first step of mercapturic acid synthesis (Habig et al. 1974). The GST activity was expressed as μmoles of CDNB conjugate formed per min/mg protein.
GR activity was determined according to the method of Smith et al. (1988). The increase in absorbance at 412 nm was monitored spectrophotometrically for 3 min at 24°C. The enzyme activity was calculated using molar extinction coefficient of 13,600 M−1 per cm. One unit of enzyme activity is defined as the amount of enzyme, which catalyzes the oxidation of 1 μmol NADPH per minute.
GPx activity was measured by following the method of Flohe and Gunzler (1984) using H2O2 and NADPH as substrates. The conversion of NADPH to NADP+ was observed by recording the changes in absorption intensity at 340 nm and 1 U of enzyme activity is defined as the amount of enzyme that catalyzes the oxidation of 1 μmol NADPH per minute.
G6PD activity was determined as described by Lee (1982) in 0.1 M Tris–HCl buffer, pH 8.0, containing 1 mM glucose-6-phosphate, 1 mM NADP+ and suitable amount of protein sample. One unit of G6PD activity was calculated as 1 nmol of NADP+ converted in NADPH per minute.
Assay of cellular metabolites
GSH level was measured according to the method of Ellman (1959) by using DTNB (Ellman’s reagent) as the key reagent. DTNB forms a yellow-colored complex with GSH and the absorbance was measured at 412 nm. A standard curve was drawn using different known concentrations of GSH solution. With the help of this standard curve, GSH contents were calculated.
GSSG contents were determined by following the method of Hissin and Hilf (1976) using 0.04 M NEM, of 0.3 M Na2HPO4 and DTNB. The results were expresses as nmol per mg protein.
Total thiols (total sulfhydryl groups) content was measured according to the method of Sedlak and Lindsay (1958) with some modifications. The content of total thiols was calculated using molar extinction coefficient of 13,600 M−1 per cm.
Estimation of intracellular superoxide radical anion concentration
The concentration of intracellular super oxide radical anion was measured by the method of Madesh and Balasubramanian (1997). About 100 μl of tissue homogenate was mixed with 6 ml of MTT solution (1.25 mM in PBS, pH 7.4) and the mixture was incubated at 37°C for 30 min. After incubation, the formazan formed due to the reduction of MTT was dissolved in 150 μl DMSO and the absorbance of the solution was measured at 570 nm. The amount of superoxide radical anion generated was calculated using the molar extinction coefficient of MTT formazan 17,000 M−1 per cm at pH 7.4–8.0.
Estimation of CYP activity from kidney microsomes
The reaction mixture contained 100 μg microsomal proteins in a100 μl reaction system containing 0.4 mM p-nitrophenol and 1 mM NADPH. The reaction was incubated at 37°C and stopped after 60 min by addition of 30 ml 20% TCA and placed on ice. Briefly after centrifugation, the sup was taken and mixed with 2 M NaOH and the absorbance measured at 546 nm. 4-Nitrocatechol formation was quantitated by using an extinction coefficient of 10.28 mM−1 per cm (Patten et al. 1992).
DNA fragmentation assay
The extent of DNA fragmentation in the kidney tissue was determined by the method as described by Lin et al. (1997). Briefly, kidney tissue homogenates were treated with 100 mM Tris buffer, pH 8.0, 1 mM EDTA and 0.5% triton X-100 and centrifuged. The supernatant was transferred carefully in a tube and 1 ml of 25% TCA was added to it; the mixture was vortexed vigorously and incubated overnight at 4°C. Quantitative analysis of DNA was carried out by diphenylamine reaction. The percentage of fragmentation was calculated from the ratio of DNA in supernatant to the total DNA.
The extent of DNA fragmentation has also been assayed by electrophoresing genomic DNA samples, isolated from normal as well as experimental mouse kidney, on agarose/EtBr gel by the procedure described by Sellins and Cohen (1987).
Histological studies
Kidneys from the normal and experimental mice were fixed in 10% buffered formalin and were processed for paraffin sectioning. Sections of about 5-μm thickness were stained with hematoxylin and eosin to evaluate under light microscope.
Statistical analysis
All the values are represented as mean ± SD (n = 6). Data on biochemical investigation were analyzed using analysis of variance (ANOVA) and the group means were compared by Duncan’s multiple range test (DMRT). P values of 0.05 or less were considered significant.
Results
Dose dependant activity of CdCl2
Figure 1 illustrates dose-dependent activity of CdCl2 by SOD assay. Cadmium intoxication reduced SOD activity linearly up to a dose of 4 mg/kg body weight. This dose was chosen as the optimum dose of CdCl2 throughout the study.
Dose-dependent in vivo antioxidant power of taurine
Figure 2 represents the dose-dependent in vivo ferric reducing antioxidant power of taurine against Cd-induced renal toxicity. Cadmium intoxication significantly (P < 0.01) attenuated the intracellular ferric-reducing antioxidant power. Administration with taurine prior to toxin exposure showed significant increase in antioxidant power linearly up to a dose of 100 mg/kg body weight.
Dose-dependent effect of taurine on intracellular antioxidant power against cadmium-induced toxicity in the kidney tissue of the experimental mice. Cont antioxidant power in normal mice; Cd antioxidant power in cadmium treated mice; TAU-10 + Cd, TAU-25 + Cd, TAU-50 + Cd, TAU-75 + Cd, TAU-100 + Cd and TAU-150 + Cd antioxidant power in taurine (TAU) treated mice for 5 days at a dose of 10, 25, 50, 75, 100 and 150 mg/kg body weight prior to cadmium administration. Each column represents mean ± SD, n = 6. a Significant difference between the vehicle control and toxin treated groups and b significant difference between the toxin treated and taurine-treated groups (P a < 0.01, P b < 0.01)
Effects of taurine on intracellular Cd concentration
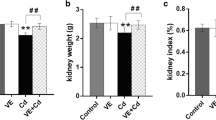
Table 1 shows that the toxin administration significantly (P < 0.01) increased the intracellular cadmium accumulation in the renal tissue of the Cd-intoxicated animals. The metal (Cd) toxicity also reduced the kidney weight to body weight ratio. Taurine treatment could prevent the increased accumulation of cadmium as well as the reduced level of kidney weight to body weight ratio.
Effect on body weight, kidney weight and their ratios
Table 1 represents the body weight, kidney weight and their ratios in all experimental mice. It has been observed that Cd intoxication reduced the body weight; kidney weight as well as their ratios and that could be prevented by the taurine pretreatment.
Assessment of serum specific renal functional markers
A significant increased level of serum urea and creatinine has been observed in the serum sample of Cd-intoxicated experimental mice (Table 2). Administration with taurine at a dose of 100 mg/kg body weight for 5 days prior to toxin exposure prevented the increased levels of both serum urea and creatinine.
Estimation of lipid peroxidation and protein carbonylation
Table 3 shows that exposure of cadmium to mice caused significant (P < 0.01) increase in the levels of MDA, lipid hydroperoxide and protein carbonyl content in the kidney tissue of the experimental mice. Treatment with taurine, however, could prevent toxin induced increased level of both lipid peroxidation and protein carbonylation.
Activities of antioxidant enzymes
The effects of taurine on activities of antioxidant enzymes, namely, SOD, CAT, GST, GR, GPx and G6PD against cadmium toxicity have been represented in Table 4. A significant reduction in the activities of all antioxidant enzymes has been observed in the kidney tissue of the cadmium-intoxicated experimental animals. Activities of the antioxidant enzymes in taurine-pretreated group are almost close to that of the normal control.
Levels of cellular metabolites
Levels of non-enzymatic antioxidants and their metabolites have been represented in Table 5. Cadmium intoxication decreased the levels of total thiols and GSH along with increased the level of its metabolite, GSSG. Pretreatment with taurine could prevent the toxin-induced alterations and kept the intracellular thiol status almost close to normal range.
Effect on the other oxidative stress related parameters
Table 6 depicts the concentration of intracellular super oxide radical anion and the extent of cytochrome P450s activity in the kidney tissue of the experimental animals. Exposure to Cd increased the concentration of intracellular super oxide radical anion as well as the extent of cytochrome P450s activity. Administration of taurine might keep the status of these two parameters nearly close to normal against cadmium toxicity.
Assessment of DNA fragmentation
Figure 3 represents the extent of DNA fragmentation. In Fig. 3a, a smear on agarose gel has been observed in cadmium-treated group, indicating random DNA degradation, a hallmark of necrosis. Taurine pretreatment was found to be effective to prevent the toxin-induced smear formation.
a DNA fragmentation pattern of the Cd-induced renal damage on agarose/EtBr gel. DNA isolated from experimental kidney tissues was loaded onto 1% (w/v) agarose gels. Lane 1 marker (1 kb DNA ladder), lanes 2, 3 DNA isolated from normal kidney, lanes 4, 5 DNA isolated from CdCl2 intoxicated kidney, lanes 6, 7 DNA isolated from taurine pretreated kidney samples. b Effect of taurine (TAU) on the extent of DNA fragmentation in the kidney tissue of the experimental mice. Cont normal mice, Cd CdCl2-treated mice, TAU + Cd mice treated with taurine prior to cadmium administration. Each column represents mean ± SD, n = 6. a Significant difference between the vehicle control and toxin-treated groups and b significant difference between the toxin-treated and taurine-treated groups (P a < 0.01, P b < 0.01)
In addition, quantitative measurement of DNA fragmentation (by the colorimetric diphenylamine reaction) has also been represented by Fig. 3b. In agreement with the above findings, cadmium intoxication increased the extent of DNA fragmentation that could be prevented by the pretreatment with taurine.
A well-known antioxidant, vitamin C, has been included in the present study as a positive control. Being an antioxidant, vitamin C could prevent cadmium-induced renal oxidative dysfunction.
Histological assessment
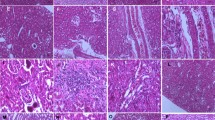
Histological studies showed that Cd intoxication caused multiple foci of hemorrhage necrosis and cloudy swelling of tubules in the kidney tissue (Fig. 4b). Treatment with taurine prior to cadmium toxicity remarkably reduced the Cd-induced pathological lesions (Fig. 4c), which is in agreement with the results of the other parameters.
Hematoxylin and eosin-stained kidney section of a normal mice (×10), showing appearance of glomeruli (marked with arrows); b toxin treated mice, showing multiple foci of hemorrhage, necrosis and cloudy swelling of tubules (×10) and c kidney section pretreated with taurine at a dose of 100 mg/kg body weight for 5 days followed by CdCl2 intoxication (×10) showing almost normal appearance of glomeruli (marked with arrows) and tubules in kidney
Discussion
In the present study, we observed that Cd-toxicity caused renal dysfunction by disturbing its antioxidant defense system. Results suggest that taurine treatment, prior to Cd-intoxication could prevent that toxin-induced alterations of the prooxidant–antioxidant related parameters in the experimental animals.
Waisberg et al. (2003) and Watjen and Beyermann (2004) reported that interaction between the components of the cellular antioxidant defense system and Cd produces reactive oxygen species (ROS), which may act as a signaling molecule in the induction of cell death. Stohs et al. (2000) indicated that Cd-intoxication itself does not generate free radicals directly but the metal indirectly generates different radicals like hydroxyl, superoxide and nitrosyl. In our present study, we found that Cd-administration decreased the kidney weight to body weight ratio and increased the intracellular Cd concentration in the experimental animals. Treatment with tautrine prior to the metal could, however, prevent the organ from dysfunction probably by its interaction with Cd. Binding of Cd to the anionic sites of the membrane phospholipids causes superficial irregularities on the plasma membrane (Sorensen et al. 1984) and thereby alters membrane fluidity and cellular homeostasis which ultimately leads to organ dysfunction (Rong et al. 1996). The increased lipid peroxidation and protein carbonyl contents have been observed in the kidney tissues of the experimental mice after Cd exposure in the present experiments. The toxin also increased the level of urea and creatinine in the serum. Treatment with taurine prior to Cd administration, however, prevented the enhancement in the lipid peroxidation as well as in the protein carbonyl contents and kept the organ close to its normal physiological state.
Quig (1998) reported that Cd exposure inactivates most of the antioxidant enzymes by either the direct binding of the metal to the active sites of the enzymes containing –SH groups or by the displacement of the metal cofactors from the active sites (Casalino et al. 2000). In another report, Moskovitz et al. (2002) suggested that increased level of protein carbonylation and decreased level of protein thiols in Cd-toxicity cause oxidative modification of many enzymes. We observed the decreased activities of the antioxidant enzymes (SOD, CAT, GST, GR, GPx and G6PD) in the renal tissues of the Cd-intoxicated animals. Pretreatment with taurine, however, prevented the Cd-induced alterations and kept the activities of the antioxidant enzymes close to those of the normal animals.
Valko et al. (2005) reported that depletion of intracellular thiol groups due to Cd-toxicity is the prerequisite for ROS generation. In addition, the levels of the non-enzymatic antioxidants, vitamin C, vitamin E and GSH have also been decreased during Cd-toxicity (Sunitha et al. 2001; Pari and Murugavel 2005). In the present study, a significant decrease in the levels of GSH as well as total thiols and increase in GSSG levels have been observed in the kidney tissues of Cd-exposed animals. These phenomena increase the susceptibility of the organ towards free radical damage. Administration of taurine could inhibit the Cd-induced oxidative threat and thereby maintain the levels of the non-enzymatic antioxidants in the kidney tissue nearly close to that in normal animals.
Free radical-induced oxidative stress has been implicated in the etiology of kidney diseases (Scibior and Zaporowska 2007; Barrera et al. 2003). Halliwell and Gutleridge (1990) reported that excessive ROS generation destroys proteins, lipids, and DNA by oxidation. In the present study, the results showed that Cd increased the generation of superoxides and elevated DNA fragmentation. Taurine pretreatment, however, prevented the Cd-induced accumulation of super oxides and elevated degree of DNA fragmentation. It has been generally accepted that cytochrome P-450s (CYPs) are involved in the activation as well as in the detoxification processes of xenobiotics. CYP-dependent monooxygenases could convert the xenobiotics to reactive intermediates, which can either initiate lipid peroxidation or bind covalently to macromolecules like DNA, proteins, etc. (Gut et al. 1996). We found that Cd increased the activities of the CYPs in the renal tissues of the experimental mice and that increment could be almost blocked by the pretreatment with taurine.
Histological examination revealed that cadmium caused a significant damage in renal ultra structure showing marked tubular damages. Complete loss of brush borders, extensive tubular casts and debris as well as tubular dilatations was observed. Treatment with taurine prevented any such toxin-induced alterations and kept the kidney histologically almost normal.
Based on the results of present as well as previous studies the following mechanisms have been proposed for the antioxidant effects of taurine. (a) As a direct antioxidant, taurine could quench and detoxify several reactive intermediates, like hypochlorous acid (HOCl) generated by myeloperoxidase (Timbrell et al. 1995; Huxtable 1992), nitric oxide (Redmond et al. 1996), H2O2 (Cozzi et al. 1995) and hydroxyl radical (·OH) (Aruoma et al. 1988). (b) Being an indirect antioxidant, taurine could prevent the changes in membrane permeability due to oxidative injury via intercalating into the membrane and stabilizing it (Timbrell et al. 1995; Gordon et al. 1992). Timbrell et al. (1995) and Wright et al. (1986) reported that the membrane stabilizing effect of taurine is linked to an action on permeability of ions and water. (c) The sulphonate group of taurine is a strong acid that changes the molecule completely to zwitterionic form in the physiological pH range (Huxtable 1992). The direct interaction between taurine and metal ion is, thus, mainly attributed to the electrostatic association (Fig. 5a) between the sulphonate ion and the metal cation (Wright et al. 1986).
Vitamin C (ascorbic acid) is an important water-soluble antioxidant which scavenges free radicals and protects oxidative damage (Fraga et al. 1991). Vitamin C, after being oxidized to dehydroascorbic acid by free radicals (Fig. 5b), is regenerated via the glutathione enzyme complex (Halliwell et al. 1987). Gupta and Kar (1998) reported that vitamin C could prevent Cd-induced increased lipid peroxidation. Although there is no structural similarity among the functional groups of these two molecules, taurine provides antioxidant defense against Cd-induced oxidative stress as comparable to that of vitamin C. Hence vitamin C was chosen as the positive control throughout the study.
In agreement with the hypothesis, our study suggests that taurine plays a protective role against Cd-induced renal oxidative damages. Further studies are, however, necessary to find out the exact mechanism of nephro-protective activity of taurine.
Change history
20 March 2024
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s00726-024-03390-w
Abbreviations
- BSA:
-
Bovine serum albumin
- CdCl2 :
-
Cadmium chloride
- CDNB:
-
1-Chloro-2,4-dinitrobenzene
- DNPH:
-
2,4-Dinitro phenyl hydrazine
- DTNB:
-
5,5′-Dithiobis(2-nitrobenzoic acid) [Ellman’s reagent]
- EDTA:
-
Ethylene diamine tetraacetic acid
- FeCl3 :
-
Ferric chloride
- FRAP:
-
Ferric reducing/antioxidant power
- GSH:
-
Glutathione
- GSSG:
-
Glutathione disulfide
- H2O2 :
-
Hydrogen peroxide
- MDA:
-
Malonaldehyde
- NEM:
-
N-ethylmaleimide
- NADH:
-
Nicotinamide adenine dinucleotide reduced disodium salt
- NBT:
-
Nitro blue tetrazolium chloride
- PMT:
-
Phenazine methosulphate
- ROS:
-
Reactive oxygen species
- NaN3 :
-
Sodium azide
- TBA:
-
Thiobarbituric acid
- TCA:
-
Trichloroacetic acid
- TPTZ:
-
2,4,6-Tripyridyl-1,3,5-triazine
- UN:
-
Urea nitrogen
References
Ahn DW, Kim MY, Kim KR, Park YS (1999) Cadmium binding and sodium dependent solute transport in renal brush border membrane vesicles. Toxicol Appl Pharmacol 154:212–218
Aruoma OI, Halliwell B, Hoey BM, Butler J (1988) The antioxidant action of taurine, hypotaurine and their metabolic precursors. Biochem J 256:251–255
Barrera D et al (2003) HO-I induction attenuates renal damage and oxidative stress induced by K2Cr2O7. Free Radic Biol Med 34:1390–1398
Bauman JW, Liu J, Klaassen CD (1993) Production of metalothionein and heat-shock proteins in response to metals. Fundam Appl Toxicol 21:15–22
Benzie IFF, Strain JJ (1999) Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol 299:15–27
Beyersmann D, Hechtenberg S (1997) Cadmium, gene regulation, and cellular signaling in mammalian cells. Toxicol Appl Pharmacol 144:247–261
Bonaventura J, Schroeder WA, Fang S (1972) Human erythrocyte catalase:an improved method of isolation and a revaluation of reported properties. Arch Biochem Biophys 150:606–617
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Casalino E, Calzaretti G, Sblano L, Landriscina C (2000) Cadmium dependent enzyme activity alteration is not imputable to lipid peroxidation. Arch Biochem Biophys 383:288–295
Cozzi R, Ricordy R, Bartolini F et al (1995) Taurine and ellagic acid: two differently-acting natural antioxidants. Environ Mol Mutagen 26:248–254
Dogru-Abbasoglu S, Kanbagli O, Balkan J, Cevikbas U, Aykac-Toker G, Uysal M (2001) The protective effect of taurine against thioacetamide hepatotoxicity of rats. Hum Exp Toxicol 20:23–27
Ellman GL (1959) Tissue sulfhydryl group. Arch Biochem Biophys 82:70–77
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421
Flohe L, Gunzler WA (1984) Assay of glutathione peroxidase. Methods Enzymol 105:114–121
Fraga CG, Motchnik PA, Shigenaga MK, Helbock HJ, Jacob RA, Ames BN (1991) Ascorbic acid protects against endogenous oxidative damage in human sperm. Proc Natl Acad Sci USA 88:11003–11006
Gordon RE, Heller RF, Heller RF (1992) Taurine protection of lungs in hamster models of oxidant injury: a morphologic time study of paraquat and bleomycin treatment. In: Lombardini JB, Schaffer SW, Azuma J (eds) Taurine: nutritional value and mechanisms of action. Plenum Press, New York, pp 319–323
Gupta P, Kar A (1998) Role of ascorbic acid in cadmium induced thyroid dysfunction and lipid peroxidation. J Appl Toxicol 18:317–320
Gurer H, Ozgunes H, Saygin E, Ercal N (2001) Antioxidant effect of taurine against lead-induced oxidative stress. Arch Environ Contam Toxicol 41:397–402
Gut I, Nedelcheval V, Soucek P, Stopka P, Tichavska B (1996) Cytochrome P450 in benzene metabolism and involvement of their metabolites and reactive oxygen speciesin toxicity. Environ Health Perspect 104:1211–1218
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Halliwell B, Gutteridge JMC (1990) Free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol 186:1–85
Halliwell B, Wasil M, Grootveld M (1987) Biologically significant scavenging of the myeloperoxidase derived oxidant hypochlorous acid by ascorbic acid. FEBS Lett 213:15–17
Hissin PJ, Hilf RA (1976) A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem 74:214–226
Hu H (2000) Exposure to metals. Primary Care 27:983–996
Huxtable RJ (1992) Physiological action of taurine. Physiol Rev 72:101–163
Hwang DF, Wang L, Cheng HM (1998) Effect of taurine on toxicity of copper in rats. Food Chem Toxicol 36:239–244
Jiang ZY, Hunt JV, Wolff SP (1992) Detection of lipid hydroperoxide using the FOX method. Anal Biochem 202:384–389
Jin T, Nordberg M, Frech W, Dumont X, Bernard A, Ye TT, Kong Q, Wang Z, Li P, Lundstrom NG, Li Y, Nordberg GF (2002) Cadmium biomonitoring and renal dysfunction among a population environmentally exposed to cadmium from smelting in China (ChinaCad). Biometals 15:397–410
Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 21:130–132
Klaassen CD, Liu J, Choudhuri S (1999) Metallothionein: an intracellular protein to pretect against cadmium toxicity. Annu Rev Pharmacol Toxicol 39:267–294
Lee CY (1982) Glucose-6-phosphate dehydrogenase from mouse. Methods Enzymol 89:252–257
Lin KT, Xue JY, Sun FF, Wong PYK (1997) Reactive oxygen species participate in peroxinitrile induced apoptosis in HL 60 cells. Biochem Biophys Res Commun 230:115–119
Madesh M, Balasubramanian KA (1997) A microlitre plate assay for superoxide using MTT reduction method. Indian J Biochem Biophys 34:535–539
Moskovitz J, Yim MB, Chock BP (2002) Free radicals and disease. Arch Biochem Biophys 397:354–359
Nishikimi M, Rao NA, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46:849–854
Pari L, Murugavel P (2005) Role of diallyl tetrasulfide in ameliorating the cadmium induced biochemical changes in rats. Environ Toxicol Pharmacol 20:493–500
Pari L, Murugavel P, Sitasawad SL, Kumar KS (2007) Cytoprotective and antioxidant role of diallyl tetrasulfide on cadmium induced renal injury: an in vivo and in vitro study. Life Sci 80:650–658
Patrick L (2003) Toxic metals and antioxidants: Part II. The role of antioxidants in arsenic and cadmium toxicity. Altern Med Rev 8:106–128
Patten CJ, Ishizaki H, Aoyama T, Lee M, Ning SM, Huang W (1992) Catalytic properties of human cytochrome P450 2E1 produced by cDNA expression in mammalian cells. Arch Biochem Biophys 299:163–171
Quig D (1998) Cysteine metabolism and metal toxicity. Altern Med Rev 3:262–270
Redmond HP, Wang JH, Bouchier-Hayes D (1996) Taurine attenuates nitric oxide- and reactive oxygen intermediate-dependent hepatocyte injury. Arch Surg 131:1287–1288
Rong Y, Geng Z, Lau BSH (1996) Ginko biloba attenuates oxidative stress in macrophages and endothelial cells. Free Radic Biol Med 20:121–127
Sabolic I (2006) Loss of basolateral invaginations in proximal tubules of cadmium-intoxicated rats is independent of microtubules and clathrin. Toxicology 218:149–163
Scibior A, Zaporowska H (2007) Effects of vanadium(V) and/or chromium(III) on l-ascorbic acid and glutathione as well as iron, zinc, and copper levels in rat liver and kidney. J Toxicol Environ Health A 70:696–704
Sedlak J, Lindsay RH (1958) Estimation of total, protein-bound, and non protein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 24/25:192–205
Sellins KS, Cohen JJ (1987) Gene induction by gamma-irradiation leads to DNA fragmentation in lymphocytes. J Immunol 139:3199–3206
Smith IK, Vierheller TL, Thorne CA (1988) Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis (2-nitrobenzoic acid). Anal Biochem 175:408–413
Son HY, Kim H, Kwon YH (2007) Taurine prevents oxidative damage of high glucose-induced cataractogenesis in isolated rat lenses. J Nutr Sci Vitaminol 53:324–330
Sorensen EMB, Smith NKR, Boecker CS, Acosta D (1984) Calcium amelioration of cadmium induced cytotoxicity in cultured rat hepatocytes. In Vitro 20:771–779
Stohs SJ, Bagchi D, Hassoun E, Bagchi M (2000) Oxidative mechanisms in the toxicity of chromium and cadmium ions. J Environ Pathol Toxicol Oncol 19:201–213
Sunitha S, Nagaraj M, Varalakshmi P (2001) Hepatoprotective effect of lupeol and lupeol linoleate on tissue antioxidant defence system in cadmium induced hepatotoxicity in rats. Fitoterapia 72:516–523
Szuster-Ciesielska A, Stachura A, Slotwinska M, Kaminska T, Sneizko R, Paduch, Abramczyk D, Filar J, Kandefer-Szerszen M (2000) The inhibitory effect of zinc on cadmium-induced cell apoptosis and reactive oxygen species (ROS) production in cell cultures. Toxicology 145:159–171
Tabassum H, Rehman H, Banerjee BD, Raisuddin S, Parvez S (2006) Attenuation of tamoxifen-induced hepatotoxicity by taurine in mice. Clin Chim Acta 370:129–136
Timbrell JA, Seabra V, Watereld CJ (1995) The in vivo and in vitro protective properties of taurine. Gen Pharmacol 26:453–462
Uchida K, Stadtman ER (1993) Covalent attachment of 4-hydroxynonenal to glyceraldehydes-3-phosphate dehydrogenase. J Biol Chem 268:6388–6393
US Department of Health, Human Services (1997) Toxicological profile for cadmium. Draft for Public Comment, Agency for Toxic Substances and Disease Registry, Atlanta
Valko M, Morris H, Cronin MTD (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12:1161–1208
Waisberg M, Joseph P, Hale B, Beyersmann D (2003) Molecular mechanisms of cadmium carcinogenesis. Toxicology 192:95–117
Watjen W, Beyermann D (2004) Cadmium induced apoptosis in C6 glioma cells: influence of oxidative stress. Biometals 17:65–78
Wessberg P, Hedner T, Hedner J, Jonason J (1983) Effects of taurine and taurine antagonists on some respiratory and cardiovascular parameters. Life Sci 33:1649–1656
WHO (1992) Environmental health criteria 134, cadmium, 1st edn. World Health Organization, Geneva
Wittman R, Hu H (2002) Cadmium exposure and nephropathy in a 28-year-old female metals worker. Environ Health Perspect 110:1261–1266
Wright CE, Tallan HH, Linn YY (1986) Taurine: biological update. Annu Rev Biochem 55:427–453
Acknowledgment
The authors are grateful to Mr. Prasanta Pal for excellent technical assistance for the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Prasenjit Manna and Mahua Sinha contributed equally in the study.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s00726-024-03390-w
About this article
Cite this article
Manna, P., Sinha, M. & Sil, P.C. RETRACTED ARTICLE: Taurine plays a beneficial role against cadmium-induced oxidative renal dysfunction. Amino Acids 36, 417–428 (2009). https://doi.org/10.1007/s00726-008-0094-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-008-0094-x