Abstract
Ocimum species commonly referred to as “Tulsi” are well-known for their distinct medicinal and aromatic properties. The characteristic aroma of Ocimum species and cultivars is attributed to their specific combination of volatile phytochemicals mainly belonging to terpenoid and/or phenylpropanoid classes in their essential oils. The essential oil constituents are synthesized and sequestered in specialized epidermal secretory structures called as glandular trichomes. In this comparative study, inter- and intra-species diversity in structural attributes and profiles of expression of selected genes related to terpenoid and phenylpropanoid biosynthetic pathways have been investigated. This is performed to seek relationship of variations in the yield and phytochemical composition of the essential oils. Microscopic analysis of trichomes of O. basilicum, O. gratissimum, O. kilimandscharicum, and O. tenuiflorum (green and purple cultivars) revealed substantial variations in density, size, and relative proportions of peltate and capitate trichomes among them. The essential oil yield has been observed to be controlled by the population, dominance, and size of peltate and capitate glandular trichomes. The essential oil sequestration in leaf is controlled by the dominance of peltate glandular trichome size over its number and is also affected by the capitate glandular trichome size/number with variations in leaf area albeit at lower proportions. Comprehension and comparison of results of GC-MS analysis of essential oils showed that most of the Ocimum (O. basilicum, O. tenuiflorum, and O. gratissimum) species produce phenylpropanoids (eugenol, methyl chavicol) as major volatiles except O. kilimandscharicum, which is discrete in being monoterpenoid-rich species. Among the phenylpropanoid-enriched Ocimum (O. basilicum, O. gratissimum, O. tenuiflorum purple, O. tenuiflorum green) as well, terpenoids were important constituents in imparting characteristic aroma. Further, comparative abundance of transcripts of key genes of phenylpropanoid (PAL, C4H, 4CL, CAD, COMT, and ES) and terpenoid (DXS and HMGR) biosynthetic pathways was evaluated vis-à-vis volatile oil constituents. Transcript abundance demonstrated that richness of their essential oils with specific constituent(s) of a chemical group/subgroup was manifested by the predominant upregulation of phenylpropanoid/terpenoid pathway genes. The study provides trichomes as well as biosynthetic pathway-based knowledge for genetic improvement in Ocimum species for essential oil yield and quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ocimum is an important genus belonging to family Lamiaceae and is represented by diverse species of herbs and shrubs. Many of the species of Ocimum bear a name or sub-name as “Tulsi” in traditional Indian System of Medicine (ISM), such as O. tenuiflorum green as “Rama tulsi,” O. tenuiflorum purple as “Shyama tulsi,” O. gratissimum as “Vana tulsi,” and O. kilimandscharicum as “Kapoor tulsi” (Khare 2008; Silori et al. 2009). Tulsi is considered as one of the most sacred and healthful herbs in Hindu religion and rituals. Its wide spectrum of health benefits (such as management of cough, fever, allergies, and in immuno modulatory activities) has earned it the rank of “natural medicine” and “queen of all herbs” (Singletary 2018; Sestili et al. 2018; Shirazi et al. 2014; Jayanti et al. 2018; Narendhirakannan et al. 2006). Ocimum species possess a wide variety of aroma largely depending upon the presence and proportion of phenylpropanoid and terpenoid in their essential oils (Charles and Simon 1992; Telci et al. 2006; Padalia and Verma 2011; Rao et al. 2011; Bansal et al. 2018). The pronounced perfumery, medicinal, and economic significance of the species are due to the presence of the biologically active (anticarcinogenic, antioxidant, antiviral, antifungal, antimicrobial, and wound healing) volatile compounds (Devi et al. 2000; Jayasinghe et al. 2003; Nakamura et al. 2004; Chiang et al. 2005; Okigbo and Ogbonnaya 2006; Shetty et al. 2006; Paschapur et al. 2009; Bayala et al. 2014; Jayanti et al. 2018). O. tenuiflorum and O. basilicum are among the most popular Ocimum species that are cultivated for their commercially important essential oils and/or for preparation of herbal medicines from their foliage, whereas O. gratissimum and O. kilimandscharicum are less explored species for their uses and cultivation.
Essential oils are a complex mixture of volatile aromatic compounds, belonging to different chemical classes (terpenoids, phenylpropanoids, esters, aldehydes, alcohols and ketones, etc.) (Croteau et al. 2000; Sangwan et al. 2001). The major volatile compounds, such as eugenol, methyl eugenol, methyl chavicol, camphor, 1,8-cineole, and linalool (Fig. 1), constitute essential oils from foliage of different species of Ocimum, which belong to the chemical classes of phenylpropanoids and/or terpenoids (Gang 2001; Prakash and Gupta 2005; Bansal et al. 2018).
Foliage of Ocimum species develop numerous specialized epidermal structures, which may or may not be secretory in nature and are collectively called trichomes (Werker 1993). These include the glandular trichomes for essential oil biosynthesis and accumulation and the non-glandular trichomes for defense (Oksanen 2018). The essential oil sequestering glandular or secretory trichomes possessed in essential oil bearing plants occur in two morphologically and structurally distinct types called as peltate and capitate trichomes (Navarro and EL Oualidi 2000; Ogunkunle and Oladele 2000; Adedeji et al. 2007; Bhatt et al. 2010; Naidoo et al. 2013; Yadav et al. 2017). The developmental pattern of trichomes has been shown to vary with the developmental stages of the plant or the organ (Ascensão et al. 1999; Yadav et al. 2014; Barton and Boege 2017). To sequester essential oil, peltate trichomes usually bear four-celled head structure, while capitate trichomes bear single-celled head structure. Individually, structural aspects of trichomes of O. tenuiflorum, O. basilicum, and O. gratissimum have been studied to some extent, and both peltate as well as capitate type of glandular trichomes have been reported to occur in them (Werker 1993; Kumari and Agrawal 2011; Fernandes et al. 2013). However, O. kilimandscharicum has not been studied for their trichomes so far. Variability in multiple attributes (type, size, and density) of the essential oil secretory glandular trichomes across essential oil-bearing plant species/cultivars often encountered variations in their net essential oil accumulation efficiency and/or composition. Therefore, an inter-species and intra-species comparative account of these attributes of glandular trichomes in Ocimum in the perspective of essential oil accumulation is an important domain of understanding aroma oil biogenesis. However, such an investigation of Ocimum has not been reported so far, despite substantial variations in essential oil productivity and composition among its species/cultivars. Therefore, this study is focused on comparative analysis of quantitative abundance of different trichome types as well as their size and density among selected Ocimum species and cultivars vis-à-vis yield and qualitative profiles of their essential oils. Further, correlation of the composition with expression of the key genes is important for understanding role of biosynthetic pathway. The results of comprehensive comparison of trichomes, essential oils, and gene expression profiles in Ocimum will provide newer insights into intra-specific and inter-specific variations in the essential oil biosynthesis and regulation in Ocimum.
To the best of our knowledge, this pertains to be the first comprehensive report providing a hypothesis that besides relative abundance of glandular trichome types, trichome size is an important characteristic of a species and cultivar that has an impact on their essential oil productivity. Variability in quality of essential oils with respect to nature of chemical class of their volatiles (terpenoids and phenylpropanoids) is manifested by the profiles of expression of the key genes of the relevant pathways.
Materials and methods
Plant materials
Leaf samples (third leaf from the top of the shoot for young and sixth leaf from the top for representative mature leaf, respectively) from four different species of Ocimum, viz., O. tenuiflorum (cultivars green and purple) O. gratissimum (OG), O. basilicum (OB), and O. kilimandscharicum (OK) were collected from experimental farm of CSIR-Central Institute of Medicinal and Aromatic Plants Lucknow, UP, India growing under natural conditions (soil type sandy loam, temperature 29–35 °C, and relative humidity of approximately 70–80%). Foliage sampled from 3 month-old plants was used for essential oil isolation during rainy season in the month of September. Leaf tissues of the same age from same plants were used for trichome analysis present on the abaxial and adaxial surfaces of the leaf. For molecular analysis, leaf tissues were freshly harvested, frozen immediately in liquid nitrogen, and stored in deep freezer at − 80 °C until further use in various estimations.
Measurement of leaf area
Leaf area for all the Ocimum species and cultivars were measured with at least 15 independent replicates using a Leaf Area Meter (Li-3000, Licor Inc., USA), and the average values were computed from the replicates.
Essential oil isolation and composition analysis
The essential oil was isolated from the freshly harvested foliage of all the selected Ocimum species/cultivars by hydrodistillation using a Clevenger’s apparatus as described earlier (Bose et al. 2013). The isolated essential oil was collected in glass vials in de-moistened form and stored in airtight vials for GC and GC-MS analysis.
GC and GC-MS analysis
Essential oils of different Ocimum species/cultivars were subjected to GC and GC-MS analysis for qualitative and quantitative analysis. Varian CP-3800 gas chromatograph, associated with DB-5 capillary column (dimension: 30 m × 0.25 mm × 0.25-μm film thickness), was used for analysis of the essential oils, wherein hydrogen was used as a carrier gas (flow rate of 1 ml/min), temperature was programmed at 60 to 280 °C (ramp rate of 3 °C/min), hold time was 1–5 min, and split ratio was 1:40. The temperatures of the injector and detector were programmed to be 250 and 280 °C, respectively.
For GC-MS analysis, Perkin Elmer TurboMass is attached with Auto XL GC sampler gas chromatograph, DB-5 column (dimension: 60 m × 0.25 mm × 0.25-μm film thickness), carrier gas helium (10-psi inlet pressure), flow rate 1 ml/min, and temperature programmed from 60 to 280 °C (ramp rate of 3 °C/min) for analysis of volatile constituents. The temperature of the injector port was programmed to be 250 °C. The samples were injected with a split ratio of 1:100. Mass spectra range (40 to 450 amu) was used for the identification of essential oil constituents. Identification of compounds was performed by using Wiley and NIST libraries.
Microscopic analysis
For the visualization of glandular and hairy (non-glandular) trichomes on adaxial and abaxial epidermal surfaces of plant leaf, scanning electron microscopy (SEM) as well as fluorescent microscopy (FM) were performed for all selected Ocimum species/cultivars. Fresh young and mature leaf samples were collected from the plants, and equal sections of leaves from different portions were taken in five replicates. The leaf sections were mounted on the SEM (JEOL, USA, Inc.) using carbon adhesive tape coated with gold, and images were captured. Trichome density (number of trichomes per unit area) was measured by the number of trichomes per unit area (200 μm2). The fresh young and mature leaves were stained with 1% rhodamine B (Sigma-Aldrich, USA) and visualized with fluorescence microscope (Leica, DM3000) under excitation filter wavelength (470 nm λex) and emission wavelength (535 nm λem), respectively, as described earlier (Yadav et al. 2014).
Transcriptome mining for pathway genes
The genes (Fig. 2) of phenylpropanoid pathway (PAL, C4H, 4CL, CAD, COMT and ES) and of terpenoid pathway (HMGR and DXS) were mined out from the transcriptomic SRA data (Rastogi et al. 2014). The primers for selected pathway genes and actin were designed for real-time transcript abundance analysis as described earlier (Bansal et al. 2018).
RNA extraction, cDNA synthesis, and quantitative gene expression analysis
Total RNA from the leaf tissues of all the selected Ocimum species and cultivars was isolated by CTAB method with some modifications as earlier (Bansal et al. 2018). Briefly, the tissue was ground in liquid nitrogen and suspended in CTAB buffer followed by incubation at 65 °C for 60 min with mixing at regular interval of 15 min. The CTAB buffer-sample mixture was allowed to cool at room temperature and washed (twice) with chloroform. This was followed by precipitation of RNA by addition of isopropyl alcohol and incubated at 4 °C for 30 min. Precipitates were collected by centrifugation at 12,000 rpm for 30 min in microcentrifuge tubes. The pellet was air dried and dissolved in autoclaved double distilled water. The quality of RNA was analyzed using 0.8% agarose gel by using nanodrop spectrophotometer (Thermo Scientific, Wilmington, USA).
The cDNA preparations were used for the relative quantification (RQ) analysis of transcripts among different species and cultivars of Ocimum. The 5-μg total RNA was used for the cDNA synthesis by using Thermo-Scientific Revert Aid First Strand cDNA Synthesis Kit as per the instructions of the manufacturer’s manual. The quantitative analysis of expression of genes related to biosynthesis of terpenoidal and phenypropanoidal volatiles of the essential oil was performed by real-time PCR using gene-specific primers with actin as endogenous control. The gene sequences for identifying genes were mined from transcriptomic sequences available in NCBI database. The primers were designed by Oligoanalyzer tool of IDT (https://eu.idtdna.com/calc/analyzer) and are listed in Supplementary Table 1. The real-time PCR analysis was performed using SYBR Green technology and StepOne™ Real-Time PCR System (Applied Biosystems). The PCR reaction mixture consisted of ~ 100 ng cDNA with five picomole forward and reverse primers, and 5 μl of Power SYBR Green master mix, and total reaction volume was maintained to 10 μl with nuclease free water. All the reactions were carried out in triplicates, and relative gene expression was calculated by ΔΔCT method and expressed as RQ values.
Statistical analysis
One-way ANOVA with post hoc Tukey HSD (Duncan multiple range) tests was applied for the evaluation of statistical significance at p < 0.05 level. All the experiments were performed in triplicate (n = 3). 3D scatter plots were drawn by using online software STATISTICA 13.3.
Results
Essential oil content and leaf area
Essential oil content (EOC) of Ocimum species varied among all the species/cultivars showing maximum content in O. basilicum leaves, whereas minimum values were obtained for O. tenuiflorum. Maximum leaf area was recorded to be in O. grastissimum (13.42 ± 0.79 cm2) followed by other species in the range of 2.90 ± 0.27 to 6.78 ± 1.49 cm2 (Supplementary Fig. 1).
Trichome morphology and structural attributes
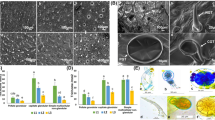
Trichomes are the surface structures predominantly present on the epidermal surface of the leaf. SEM of the leaf surfaces of the Ocimum species and cultivars revealed the presence of three types of trichomes—peltate, capitate, and hairy trichomes. Peltate trichomes were shown to possess four-celled secretory head, while capitate trichomes had one to two-celled secretory head in all the species and cultivars, studied. Hairy trichomes were found to be unicellular as well as multicellular without a secretory head (Figs. 3 and 4). Capitate trichomes were much smaller in size as compared to peltate trichomes in all the Ocimum species. Among peltate trichomes, O. basilicum possessed relatively larger sized peltate trichomes (Figs. 3 and 4). O. gratissimum had smallest unicellular hairy trichomes among all the species. In O. basilicum, hairy trichomes were noted to be present at the leaf margins as well, while in other species, these were observed to occur in a scattered pattern all over the leaf surface (Fig. 3).
Scanning electron microscopic view of trichomes on abaxial surface of young and mature leaves of Ocimum species/cultivars. (OB), O. basilicum (a, f); (OG), O. gratissimum (b, g); (OK), O. kilimandscharicum (c, h); (OSG), O. tenuiflorum green (d, i); (OSP), O. tenuiflorum purple (e, j). Scale bar = 200 μm
Analysis of diversity and density of different trichome types
The density of different types of trichomes on adaxial and abaxial surfaces of young as well as mature leaf was recorded by SEM. Trichome density was calculated as number of glandular trichomes per unit surface area (mm2) of adaxial and abaxial surfaces of leaf. The trichome density was found to be more in younger leaf compared to mature leaf for all the three type of trichomes, i.e., peltate, capitate, and hairy trichomes (Fig. 3). The maximum average density of peltate trichomes on both surfaces in two different developing stages of the leaves was recorded in OSG leaf and the minimum was noted to be in OK, whereas other three species ranged in between (Fig. 5). The maximum density of capitate glandular trichomes on both surfaces in two developing stages of the leaves was recorded in OSG and lowest values in OK, respectively. There were variations also reported in terms of surfaces with abaxial surface possessing higher density than adaxial leaf surface (Fig. 5). Size of peltate glandular trichomes was observed to be largest in OB leaves (89.22 ± 3.28 μm), followed by other species which ranged from 52.69 to 79.61 μm (Fig. 6). The size of capitate glandular trichomes was found to be maximum in OK (40.37 ± 9.28 μm) followed by other species and cultivars, wherein size varied from 28.57 to 34.28 μm (Fig. 6). Estimations of peltate glandular trichome average density and size (diameter) were also validated by fluorescence microscopy counts and measurements in all the Ocimum species/cultivars studied (Fig. 5). A decline in the trichome density with leaf maturity was observed to be a common feature for all the Ocimum species.
Glandular trichome density of adaxial (a) and abaxial (b) leaf surfaces of Ocimum species. a Peltate trichome density of young Ocimum leaves. b Capitate trichome density of young Ocimum leaves. c Peltate trichome density of mature Ocimum leaves. d Capitate trichome density of mature Ocimum leaves. (OB), O. basilicum; (OG), O. gratissimum; (OK), O. kilimandscharicum; (OSG), O. tenuiflorum green; (OSP), O. tenuiflorum purple
Variation in trichome sizes across Ocimum species. a Left side panel of the figure displays the pictorial view of size (diameter in μm) in the Ocimum species as measured by fluorescence microscope (scale bar = 1 mm). b Trichome size (peltate, capitate and hairy) in Ocimum species/cultivars. (OB), O. basilicum; (OG), O. gratissimum; (OK), O. kilimandscharicum; (OSG), O. tenuiflorum green; (OSP), O. tenuiflorum purple. Different letters (a, b, c, etc.) above the bar indicates the level of significance at p ≤ 0.05
Correlation studies
The quantitative attributes (size and density) of both the types of glandular trichomes (peltate and capitate) were subjected to estimation of their correlation with essential oil content (EOC). EOC was found to be positively correlated with glandular trichome density and size. A relationship of peltate glandular trichome density (PGTD) and peltate glandular trichome size (PGTS) with essential oil content (EOC) was also noted from the analysis, i.e., an attribute of large sized peltate trichomes correlated with more EOC despite their being in lesser number (Fig. 7a). This relationship was noted to hold true for all the species with a slight variation in OG. Contrarily, variations in capitate trichome density (CGTD) accounted for the overall EOC of species by lesser extent as capitate glandular trichome size (CGTS) was smaller as compared to peltate, thereby contributing lesser to essential oil sequestration and productivity (Fig. 7b). Thus, overall EOC varied in accordance with the number and area of the total secretory trichomes (Fig. 7a, b).
Correlation of glandular trichome attributes with essential oil content (EOC) in Ocimum. a Correlation of peltate gland trichome density (PGTD) and peltate gland trichome size (PGTS) with EOC. b Correlation of capitate gland trichome density (CGTD) and capitate gland trichome size (CGTS) with EOC; (OB), O. basilicum; (OG), O. gratissimum; (OK), O. kilimandscharicum; (OSG), O. tenuiflorum green; and (OSP), O. tenuiflorum purple
Profiles of essential oil constituents
Most of the essential oil constituents were identified and quantified in all the Ocimum species and cultivars. The identified essential oil constituents belonged to three different chemical groups/subgroups of monoterpenoids, sesquiterpenoids, and phenylpropanoids (Fig. 1). The constituents were noted to vary substantially across species as well as cultivars, viz., monoterpenoids (OB:OG:OK:OSG:OSP = 17.98%:8.88%:91.63%:1.36%:10.83%), sesquiterpenoids (OB:OG:OK:OSG:OSP = 2.76%:9.08%:5.33%:23.06%:30.37%), and phenylpropanoids (OB:OG:OK:OSG:OSP = 78.22%:75.93%:0.52%:48.22%:53.49%). Further, within the chemical group/sub-group, different species of Ocimum have distinct prime as first-tier key constituent, viz., eugenol (OG:OSG:OSP = 75.61%:48.22%:53.16%), methyl chavicol (OB = 78.20%), and camphor (OK = 53.58%). The second tier of key volatile metabolites was comprised of linalool (14.80%) and E-citral (1.45%) in O. basilicum; β-ocimene (7.26%), germacrene D (4.75%), and caryophyllene (2.20%) in O. gratissimum; D-limonene (8.65%), linalool (6.60%), camphene (4.73%), endo-borneol (4.21%), β-ocimene (3.72%), cis-sabinene hydrate (2.93%), thujol (2.17%), caryophyllene (1.77%), germacrene D (1.49%), and β-myrcene (1.20%) in O. kilimandscharicum; germacrene D (8.30%), germacrene A (5.70%), caryophyllene (3.97%), and cubidol (1.86%) in O. tenuiflorum green; and germacrene A (13.30%), β-ocimene (7.27%), germacrene D (6.99%), caryophyllene (6.40%), endo borneol (1.03%), and geranyl acetate (1.00%) in O. tenuiflorum purple (Table 1).
Real-time PCR-based expression analysis of biosynthetic pathway genes
Selected genes related to pathways of biosynthesis of essential oil constituents were analyzed for their relative transcript abundance pattern among the Ocimum species/cultivars. The genes comprised of those involved in phenylpropanoids and terpenoid biosynthetic pathways, viz., PAL (phenylalanine ammonia lyase), C4H (cinnamate-4-hydroxylase), 4CL (4-coumarate:CoA ligase), COMT (caffeoyl-CoA-O-methyl transferase), CAD (cinnamoyl alcohol dehydrogenase), and ES (eugenol synthase) related to phenylpropanoid biosynthetic pathway and HMGR (3-hydroxy-3-methyl-glutaryl coenzyme A reductase) and DXS (1-deoxy-D-xylulose 5-phosphate synthase) related to terpenoids biosynthetic pathways. The levels of expression of genes involved in phenylpropanoids biosynthesis (PAL, C4H, 4CL, COMT, CAD, and ES) were higher in OB (≈ 0.83- to 109-fold), OG (≈ 5- to 100-fold), OSG (≈ 3- to 66-fold), and OSP (≈ 4- to 88-fold) compared to OK (Fig. 8a–f). The highest expression of HMGR (about 10- to 20-fold) and DXS (about 7- to 16-fold) among the Ocimum species/cultivars was recorded in OK (Fig. 9a, b).
Real-time PCR assay based comparative profiles of expression of real-time expression analysis of genes of phenylpropanoid biosynthetic pathway in Ocimum species/cultivars (OB), O. basilicum; (OG), O. gratissimum; (OK), O. kilimandscharicum; (OSG), O. tenuiflorum green; and (OSP), O. tenuiflorum purple. PAL—phenylalanine ammonia lyase (a), C4H—cinnamate-4-hydroxylase (b), 4CL—4-coumarate CoA ligase (c), CAD—cinnamoyl alcohol dehydrogenase (d), COMT—caffeoyl-co-A-methyltransferase (e), and ES—eugenol synthase (f). Different letters (a, b, c, etc.) above the bar indicate the level of significance at p ≤ 0.05
Real-time PCR assay based comparative profiles of expression of genes of isoprenogenic pathways in Ocimum species/cultivars. (OB), O. basilicum; (OG), O. gratissimum; (OK), O. kilimandscharicum; (OSG), O. tenuiflorum green; and (OSP), O. tenuiflorum purple. HMGR-3—hydroxy-3-methyl glutaryl-Co-A reductase (a), DXS—1-deoxy-D-xylulose-5-phosphate synthase (b). Different letters (a, b, c, etc.) above the bar indicates the level of significance at p ≤ 0.05
Discussion
The present study deals with structural, physiological, and molecular aspects with respect to raison de etre of diversity in essential oil biogenesis and sequestration at inter-species/cultivar level in Ocimum species. Results of the comparative and correlative study on the commercially important set of Ocimum species and cultivars credited with medicinal and aromatic properties provided insights on (a) productivity and compositional variability of essential oils (complex mixtures of volatile secondary metabolites) biosynthesized by them, (b) inter-species and intra-species diversity of attributes (types, density and size) of glandular trichomes that are critical for essential oil biogenesis and sequestration, and (c) distinct profiles of levels of expression of genes related to the two distinct pathways of biosynthesis of volatiles (terpenoids and phenylpropanoids).
The essential oils of Ocimum species/cultivars exhibited metabolic diversity at the level of chemical groups of volatile metabolites (terpenoids and phenylpropanoids) and subgroups (monoterpenoids and sesquiterpenoids) as well as individual volatile chemical entities as prime constituent(s). The terpenoids are the largest and most diverse family of natural products biosynthesized in plants via two distinct pathways of isoprenogenesis, occurring at two distinct intra-cellular locales, i.e., cytosol and plastids (Bouvier et al. 2005). Cytosol operated acetate/mevalonate (MVA) pathway and plastid operated non-mevalonate or 1-deoxy-d-xylulose-5-phosphate (DOXP) pathway. MVA and DOXP pathways have a specificity of catering to the production of sub-class(s)/series of terpenoids with variability in the C5 building blocks (IPP & DMAPP) by iterative mechanisms via crosstalk among them (Eisenreich et al. 2004; Chaurasiya et al. 2012). Whereas, phenylpropanoids are the group of compounds biosynthesized from phenylalanine and include a number of volatiles as constituents of essential oils of plants with putative ecological functions including defense against pathogens and attractants for pollinators (Gang 2001; Gang et al. 2002).
Foliage essential oil in majority of plants is synthesized and accumulated in specialized secretory structures called essential oil glands or glandular trichomes. Accordingly, eco-physiological functions and chemotaxonomic significance of volatile secondary metabolites relate directly with glandular trichomes of the congener species (Ogunkunle and Oladele 2000). Therefore, analyzing the attributes of glandular trichomes is an important facet of understanding essential oil biogenesis. Trichomes of some families, such as Solanaceae, Lamiaceae, Rapateaceae, and Asteraceae, have been studied widely (Gairola et al. 2009; Bhatt et al. 2010; Dai et al. 2010; Huchelmann et al. 2017). Among these families, Lamiaceae has been explored in greater detail with respect to biosynthesis and sequestration of essential oil in several of its aroma oil species (Leonotis, Orthosiphon, Salvia, Mentha) owing to their commercial importance (Ascensão 1995; Shankar et al. 1999; Sharma et al. 2003; Bhatt et al. 2010; Bose et al. 2013). In the Ocimum, though several individual studies on trichomes of some species (O. tenuiflorum, O. basilicum, and O. gratissimum) have been reported earlier, O. kilimandscharicum has remained unattended. Moreover, a comparative study of all the four species with cultivars reputed for their action in traditional medicine is not reported. Further, these individual descriptions of trichomes of these specified species provide isolated botanical description of trichome structures, a species-wide comparative comprehension of diversity in attributes of glandular trichomes (structural, numerical, and dimensions) vis-à-vis phytochemicals sequestered there through, and pathway gene expression profile will provide better insights and raison de etre for the diversity in productivity and composition of essential oils. Three major/distinct types of trichomes observed on the leaf surfaces of Ocimum species, viz., peltate, capitate, and hairy trichomes (Fig. 3) are usual among aroma essential oil bearing plants. Although, other types of trichomes are reported to occur in Cucurbitaceae family (Kolb 2004), but such structures were not encountered in Ocimum species, as observed by SEM analysis of the present study. The observed structural forms of peltate, capitate, and hairy trichomes of Ocimum species as four-celled head, two-celled head, and uni- or multicellular, respectively, were in essence as reported earlier for several plants (Ascensão 1995; Ascensão et al. 1999). However, each species of Ocimum is attributed with the unique pattern of trichome arrangement, density, and proportion of peltate versus capitate trichomes and size range specificity of peltate as well as capitate trichomes. As trichome glands are strongly associated with essential oil biosynthesis and accumulation, these variations of structural types, number, density, and dimensions for glandular trichomes across Ocimum species are contributing factors in essential oil accumulation. In case of O. gratissimum, the trichome size (either glandular or non-glandular) was smallest among all the species (Fig. 5), while O. basilicum was attributed with large sized, but lesser number of peltate trichomes and, uniquely, hairy trichomes were found restricted mainly to the mid-region of the leaf. Interestingly, trichome (glandular and non-glandular) density was recorded to lessen from young to mature leaves in all Ocimum species. This signifies lack/slowing down of origin of new trichomes accompanying to the rate of leaf expansion. The substantial fall in trichome (glandular and non-glandular) density on leaf maturity has also been assigned to shedding of trichomes by the plant in view of their lowered defense-needs and ecological role(s) significance (Gairola et al. 2009). Our previous study on M. arvensis (Sharma et al. 2003) has demonstrated a characteristic phenomenon of trichome dehiscence on maturity as a perennial process all across leaf ontogeny, which becomes more impactful in causing predominant fall in trichome density in mature leaf when the ontogenic origin of trichomes almost ceases. The higher density of trichomes on the abaxial side in several plants appears to be a normal feature (Ogunkunle and Oladele 2000; Yadav et al. 2014; Yadav et al. 2017; Dhawan et al. 2016) and may have the bearing of their long existence by the lower rate of senescence/degradation from surface exposures/injuries, etc. Non-secretory hairy trichomes present on both adaxial and abaxial sides of the leaf are also considered to be involved in plant defense from the unfavorable environment, such as insects attack and frost (Wagner 1991). However, the defense and ecological mode of function of non-glandular hairs may be ubiquitous in all plant species as more of a mechanical mode of defense. This is in contrast to the glandular trichomes where species-specific specialized defense or ecological functions are performed through their complement of secondary phytochemicals. Thus, the variation in trichomes is indicative of factors controlling overall accumulation (Gianfagna et al. 1992; Sharma et al. 2003; Bhatt et al. 2010).
The patterns of variations in EOC reflected that the biogenetic efficiency was maximal in O. basilicum followed by O. kilimandscharicum then O. gratissimum, and, least but significantly variable within the two cultivars of O. tenuiflorum. These results are at slight variance from an earlier report (Padalia and Verma 2011), probably on the account of different geographical locations of the cultivation. The variability in essential oil yields may arise as a function of many factors including seasonal, geographical conditions, light, nutrition, temperature humidity, solar irradiance, and stage of harvest (Singh et al. 1989; Sangwan et al. 2001; Chang et al. 2008). Although essential oil production is the highly regulated process in plants (Sangwan et al. 2001), differences in EOC can be explained on the basis of the number of trichomes on the leaf surface and their size, which are responsible for the production of oil. Furthermore, since the capacity of peltate trichomes to produce and sequester essential oil is much higher than that of capitate, the higher proportion of peltate glands in the population of trichomes would also facilitate the higher accumulation of essential oil.
Interestingly, analysis of 3D graph (Fig. 7a, b) displaying correlation between glandular trichome density, size, and EOC reflects the glandular trichome size as a more impactful determinant of EOC than glandular trichome density. Lack of linearity or direct proportionality between EOC and trichome density is due to trichome size (in addition to trichome type), a factor also being an important determinant of net essential oil sequestration. Despite situations of low glandular trichome density, existence of larger-sized glandular trichomes could favor high EOC as observed in the case of O. basilicum and O. kilimandscharicum (Fig. 7a). Whereas, O. gratissimum despite having the higher density of trichomes possessed average content of essential oil on account of smaller sizes of trichomes. Although, capitate trichomes have much lower contribution to EOC due to their lower oil, biogenetic and accumulation capacity but their presence and population size may be the determinants of their extent of positive effect. Hence, with the increase in total trichome field area (leaf surface area), EOC also increases. Essentially, rather than merely the increase or decrease in the trichome density or size, their extent (quantitative aspects) of change govern the nature and impact on essential oil yield. This explains the situation of EOC vis-a-vis trichome attributes in O. tenuiflorum.
The observed variations in the percentage of different phytochemicals in the volatile oils of Ocimum species depend on many factors. Our results show that under North Indian conditions, four species/cultivars of Ocimum exhibited eugenol/methyl chavicol and camphor type characteristics. Although (Viña and Murillo 2003), in their study, essential oil composition of 12 varieties of O. basilicum from the urban and suburban areas of Columbia has demonstrated the methyl cinnamate, β-caryophyllene, and linalool type chemotypes. Telci et al. (2006) have reported seven chemotypes of O. basilicum with methyl eugenol, citral, methyl cinnamate, linalool, methyl cinnamate/linalool, and methyl chavicol. In the present study, camphor (a cyclic monoterpenoid) was uniquely noted to be present in O. kilimandscharicum as a chief (about 54%) constituent followed by D-limonene (a cyclic monoterpene hydrocarbon) and β-ocimene (an acyclic monoterpene hydrocarbon). However, Charles and Simon (1992) have reported a chemotype of O. kilimandscharicum rich (41 to 58%) in linalool (an acyclic monoterpene alcohol), while camphor and 1,8-cineole constituted merely 17 and 10% of the oil, respectively. O. basilicum was essentially a phenylpropanoid-rich essential oil chemotype with methyl chavicol as the major (78%) constituent and monoterpene (linalool) being a second tier phytochemical of far lower proportion. In case of O. tenuiflorum, dominance (about 48 and 53% in OSG and OSP, respectively) of eugenol as specific oil constituents was evident. Terpenoid (sesquiterpenoid subgroup) metabolites, viz., germacrene A, caryophyllene, germacrene D, and cubedol were second-tier abundance volatiles constituting about 22 and 27% of the essential oil in OSG and OSP, respectively. The major difference between purple (OSP) and green (OSG) cultivars of O. tenuiflorum was presence of significant proportion (about 10%) of monoterpenoids with predominance (7%) of monoterpenoid β-ocimene in purple cultivar and their negligible level in green cultivar of O. tenuiflorum. Earlier studies have reported essential oils of their plant of study for the species to be composed of methyl eugenol, β-caryophyllene, or even eugenol also in significant amounts (Kothari et al. 2005; Rao et al. 2011). The differences may have several possible origins like chemotype, ecotype, and geographic location (Joshi and Hoti 2014; Saharkhiz et al. 2015). It can be concluded from the foregoing discussion on comparative profiles of composition of essential oils of Ocimum species/cultivars that most of the Ocimum species produce phenylpropanoids as major (about 50 to 75%) volatiles except O. kilimandscharicum, which is discretely different in being a monoterpenoid-rich (about 90%) essential oil. Further, diversity among the phenylpropanoid dominant species/cultivars (OB, OG, OSP, OSG) resides at through the substantial presence of monoterpenoids (about 18%) in OB, sesquiterpenoids (about 20%) in OSG, and both monoterpenoids and sequiterpenoids in OG (being about 9% each) as well as OSP (being about 30 and 10%, respectively). This interesting diversity in EOC and composition can be understood, next to through diversity in glandular trichome diversity, by the studies on profiles of expression of the genes involved in terpenoid and phenylpropene biosynthesis pathways.
Functional genomics approaches are being extensively utilized for molecular understanding of biosynthetic pathways of secondary metabolites in plants. In this perspective, results of this study on levels of expression of genes responsible for the biosynthesis of phenylpropanoids (such as PAL, C4H, 4CL, CAD, COMT, and ES) and two routes of isoprenogenesis for terpenoid production (such as HMGR, DXS, the key regulatory enzymes of the mevalonate and non-mevalonate isoprenogenic pathways, respectively) provide interesting insights. The observed high levels of expression of PAL, the key regulatory gene of the phenylpropanoid pathway in O. basilicum and O. gratissimum followed by O. tenuiflorum, the phenylpropanoid-rich species, connote its significance for the characteristic oil compositional traits (Fig. 8a). Elevated expression of selected genes of intermediary and downstream steps of phenylpropanoid pathway also supplement by facilitating the production of different phenylpropanoid entities individually. The observed higher abundance of its transcripts in O. gratissimum followed by O. basilicum could be the reason for their essential oils of being phenylpropanoid-rich (with eugenol and methyl chavicol as chief phenylpropanoids, respectively). CAD, investigated in this study (Fig. 8a–f), is the important step of phenylpropanoid pathway with its expression providing flux of the intermediary metabolites to the downstream process towards the end product. The reported correlation of COMT expression with methyl chavicol and methyl eugenol in species supports the composition of essential oil (Abdollahi et al. 2017). The observed higher expression of transcripts of eugenol synthase (ES), the enzyme responsible for eugenol production in O. gratissimum and O. tenuiflorum, was in line with their phenyl propanoid-rich essential oils being dominated by eugenol.
The terpenoids are the largest and most diverse family of plant natural products encountered as a homologous series of hemi-, mono-, sesqui-, di-, tri-, and higher terpenoids with isoprene as the building block (Sangwan et al. 2001; Eisenreich et al. 2004). Plants possess two discrete routes of isoprenogenesis-mevalonate and non-mevalonate (DOXP) pathways with HMGR and DXS as the regulatory steps, respectively (Chaurasiya et al. 2012; Jadaun et al. 2017; Bansal et al. 2018). The observed lower expression of HMGR and DXS in phenylpropanoid-rich essential oil of Ocimum species reflected their limited metabolic operation resulting into relatively less representation of terpenoidal entities in their oils. Maximum expression of DXS transcripts observed in O. kilimandscharicum explains key role of DOXP pathway isoprenogenesis (Fig. 9a, b) in contributing to the essential oil majorly rich (about 90%) in monoterpenoids. This is because biosynthesis of monoterepenoid known to recruit DOXP pathway of isoprenogenesis (Jadaun et al. 2017). Variance with higher expression of DXS in O. kilimandscharicum in line with the predominant role of DOXP pathway to govern biosynthesis of its monoterpenoid-rich essential oil with the low differences in the expression of DXS and HMGR genes in the two of the phenylpropanoid type Ocimum species (O. gratissimum and O. tenuiflorum) was encountered (Bansal et al. 2018; Rastogi et al. 2014). This is understandable in the view that sesquiterpenoids also constituted a substantial complement of terpenoidal proportion of Ocimum essential oil, as tier-2 group of key metabolites which could be of either mevalonate pathway or of mixed origin (mevalonate and DOXP) Thus, possibly, possessing a considerable blend of mono- and/or sesquiterpenes in their essential oils probably invokes a significant cross-talk of the two isoprenogenic routes necessitating coordinated expression of DXS and HMGR (Jadaun et al. 2017; Chaurasiya et al. 2012). Such key studies are available in literature, where DOXP and MVA participation is elucidated in isoprenoid biosynthesis (Jadaun et al. 2017). Percentage of major constituents in the essential oils of the Ocimum species was noted to be independent of peltate or CGTS as well as their density, suggesting biosynthesis of different types of volatiles to be under overall genetic control. On the other hand, essential oil productivity rather remains as a major function controlled by specificity and abundance of structural types of biosynthetic units, i.e., glandular trichomes.
Conclusions
This study gives a comparative and correlative account of inter-species and intra-species diversity in trichome types, size, and expression profiles of key genes of biosynthetic pathways of metabolites in the perspective of diversity of essential oil productivity and composition across four Ocimum species covering two cultivars of one of the species. The study, besides giving the first account of structural features of one of the species (O. kilimandscharicum), presents comparative account of structural and quantitative aspects of glandular trichomes across Ocimum species. Study highlights trichome attributes-based trait of wide variation in essential oil yield across species. EOC has been demonstrated to be governed by the interplay of density, size, and relative proportion of peltate versus capitate oil glands as well as population size. Further, study demonstrates that richness of their essential oils with specific constituent(s) of a chemical group/subgroup was manifested by predominant upregulation of expression of genes related to upstream region of their biosynthetic pathways. The study provides trichomes as well as biosynthetic pathway-based cues for genetic improvement of essential oil in Ocimum species, which have rapidly growing attraction for food, flavor, and fragrance as well as chemical industries.
Abbreviations
- OB:
-
Ocimum basilicum
- OG:
-
Ocimum gratissimum
- OK:
-
Ocimum kilimandscharicum
- OSG:
-
Ocimum tenuiflorum (syn. Ocimum sanctum) green
- OSP:
-
Ocimum tenuiflorum (syn. Ocimum sanctum) purple
- SEM:
-
scanning electron microscopy
- PGTD:
-
peltate glandular trichome density
- PGTS:
-
peltate glandular trichome size
- CGTD:
-
capitate glandular trichome density
- CGTS:
-
capitate glandular trichome size
- PAL:
-
phenylalanine ammonia lyase
- C4H:
-
cinnamate-4-hydroxylase
- 4CL:
-
4-coumarate-CoA ligase
- CAD:
-
cinnamoyl alcohol dehydrogenase
- COMT:
-
caffeoyl-CoA-methyltransferase
- ES:
-
eugenol synthase
- HMGR:
-
3-hydroxy-3-methyl glutaryl coenzyme A reductase
- DXS:
-
1-deoxy-D-xylulose-5-phosphate synthase
References
Abdollahi MB, Eyvazpour E, Ghadimzadeh M (2017) The effect of drought stress on the expression of key genes involved in the biosynthesis of phenylpropanoids and essential oil components in basil (Ocimum basilicum L.). Phytochemistry 139:1–7. https://doi.org/10.1016/j.phytochem.2017.03.006
Adedeji O, Ajuwon OY, BO O (2007) Foliar epidermal syudies, organographic distribution of trichomes in the family Solanaceae. Int J Bot 3:276–282
Ascensão L (1995) Glandular trichomes on vegetative and reproductive organs of Leonotis leonurus (Lamiaceæ). Ann Bot 75:619–626. https://doi.org/10.1006/anbo.1995.1067
Ascensão L, Mota L, Castro MDM (1999) Glandular trichomes on the leaves and flowers of Plectranthus ornatus: morphology, distribution and histochemistry. Ann Bot 84:437–447. https://doi.org/10.1006/anbo.1999.0937
Bansal S, Narnoliya LK, Mishra B, Chandra M, Yadav RK, Sangwan NS (2018) HMG-CoA reductase from Camphor Tulsi (Ocimum kilimandscharicum) regulated MVA dependent biosynthesis of diverse terpenoids in homologous and heterologous plant systems. Sci Rep 8:3547–3561. https://doi.org/10.1038/s41598-017-17153-z
Barton KE, Boege K (2017) Future directions in the ontogeny of plant defence: understanding the evolutionary causes and consequences. Ecol Lett 20:403–411. https://doi.org/10.1111/ele.12744
Bayala B, Bassole IHN, Gnoula C, Nebie R, Yonli A, Morel L, Figueredo G, Nikiema JB, Lobaccaro JMA, Simpore J (2014) Chemical composition, antioxidant, anti-inflammatory and anti-proliferative activities of essential oils of plants from Burkina Faso. PLoS One 9:1–11. https://doi.org/10.1371/journal.pone.0092122
Bhatt A, Naidoo Y, Nicholas A (2010) An investigation of the glandular and non-glandular foliar trichomes of Orthosiphon labiatus N.E.Br. [Lamiaceae]. N Z J Bot 48:153–161. https://doi.org/10.1080/0028825X.2010.500716
Bose SK, Yadav RK, Mishra S, Sangwan RS, Singh AK, Mishra B, Srivastava AK, Sangwan NS (2013) Effect of gibberellic acid and calliterpenone on plant growth attributes, trichomes, essential oil biosynthesis and pathway gene expression in differential manner in Mentha arvensis L. Plant Physiol Biochem 66:150–158. https://doi.org/10.1016/j.plaphy.2013.02.011
Bouvier F, Rahier A, Camara B (2005) Biogenesis, molecular regulation and function of plant isoprenoids. Prog Lipid Res 44:357–429. https://doi.org/10.1016/j.plipres.2005.09.003
Chang X, Alderson PG, Wright CJ (2008) Solar irradiance level alters the growth of basil (Ocimum basilicum L.) and its content of volatile oils. Environ Exp Bot 63:216–223. https://doi.org/10.1016/j.envexpbot.2007.10.017
Charles DJ, Simon JE (1992) Essential oil constituents of Ocimum kilimandscharicum Guerke. J Essent Oil Res 4:125–128. https://doi.org/10.1080/10412905.1992.9698032
Chaurasiya ND, Sangwan NS, Sabir F, Mishra L, Sangwan RS (2012) Withanolide biosynthesis recruits both mevalonate and DOXP pathways of isoprenogenesis in ashwagandha Withania somnifera L. (Dunal). Plant Cell Rep 31:1889–1897. https://doi.org/10.1007/s00299-012-1302-4
Chiang LC, Ng LT, Cheng PW, Chiang W, Lin CC (2005) Antiviral activities of extracts and selected pure constituents of Ocimum basilicum. Clin Exp Pharmacol Physiol 32:811–816. https://doi.org/10.1111/j.1440-1681.2005.04270.x
Croteau R, Kutchan TM, Lewis NG (2000) Secondary metabolites. Biochem Mol Biol Plants 7:1250–1318. https://doi.org/10.1016/j.phytochem.2011.10.011
Dai X, Wang G, Yang DS, Tang Y, Broun P, Marks MD, Sumner LW, Dixon RA, Zhao PX (2010) Trichome: a comparative omics database for plant trichomes. Plant Physiol 152:44–54. https://doi.org/10.1104/pp.109.145813
Devi PU, Ganasoundari A, Vrinda B et al (2000) Radiation protection by the Ocimum flavonoids orientin and vicenin: mechanisms of action. Radiat Res 154:455–460. https://doi.org/10.1667/0033-7587(2000)154[0455:RPBTOF]2.0.CO;2
Dhawan SS, Shukla P, Gupta P, Lal RK (2016) A cold-tolerant evergreen interspecific hybrid of Ocimum kilimandscharicum and Ocimum basilicum: analyzing trichomes and molecular variations. Protoplasma 253:845–855. https://doi.org/10.1007/s00709-015-0847-9
Eisenreich W, Bacher A, Arigoni D, Rohdich F (2004) Biosynthesis of isoprenoids via the non-mevalonate pathway. Cell Mol Life Sci 61:1401–1426. https://doi.org/10.1007/s00018-004-3381-z
Fernandes VF, de Almeida LB, Feijó EVR d S, Silva DC, de Oliveira RA, Mielke MS, Costa LCB (2013) Light intensity on growth, leaf micromorphology and essential oil production of Ocimum gratissimum. Rev Bras Farmacogn 23:419–424. https://doi.org/10.1590/S0102-695X2013005000041
Gairola S, Naidoo Y, Bhatt A, Nicholas A (2009) An investigation of the foliar trichomes of Tetradenia riparia (Hochst.) Codd [ Lamiaceae]: an important medicinal plant of Southern Africa. Flora 204:325–330. https://doi.org/10.1016/j.flora.2008.04.002
Gang DR (2001) An investigation of the storage and biosynthesis of phenylpropenes in sweet basil. Plant Physiol 125:539–555. https://doi.org/10.1104/pp.125.2.539
Gang DR, Simon J, Lewinsohn E, Pichersky E (2002) Peltate glandular trichomes of Ocimum basilicum L. (sweet basil) contain high levels of enzymes involved in the biosynthesis of phenylpropenes. J Herbs Spices Med Plants 9:189–195. https://doi.org/10.1300/J044v09n02_27
Gianfagna TJ, Carter CD, Sacalis JN (1992) Temperature and photoperiod influence trichome density and sesquiterpene content of Lycopersicon hirsutum f. hirsutum. Plant Physiol 100:1403–1405. https://doi.org/10.1104/pp.100.3.1403
Huchelmann A, Boutry M, Hachez C (2017) Plant glandular trichomes: natural cell factories of high biotechnological interest. Plant Physiol 175:6–22. https://doi.org/10.1104/pp.17.00727
Jadaun JS, Sangwan NS, Narnoliya LK, Singh N, Bansal S, Mishra B, Sangwan RS (2017) Over-expression of DXS gene enhances terpenoidal secondary metabolite accumulation in rose-scented geranium and Withania somnifera: active involvement of plastid isoprenogenic pathway in their biosynthesis. Physiol Plant 159(4):381–400
Jayanti I, Jalaluddin M, Avijeeta A, Ramanna PK, Rai PM, Nair RA (2018) In vitro antimicrobial activity of Ocimum sanctum (Tulsi) extract on Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis. J Contemp Dent Pract 19:415–419
Jayasinghe C, Gotoh N, Aoki T, Wada S (2003) Phenolics composition and antioxidant activity of sweet basil (Ocimum basilicum L.). J Agric Food Chem 51:4442–4449. https://doi.org/10.1021/jf034269o
Joshi RK, Hoti SL (2014) Chemical composition of the essential oil of Ocimum tenuiflorum L. (Krishna Tulsi) from North West Karnataka, India. Plant Sci Today 1:99–102. https://doi.org/10.14719/pst.2014.1.3.52
Khare CP (2008) Indian medicinal plants—an illustrated dictionary. Springer-Verlag, Heidelberg
Kolb D (2004) Light, conventional and environmental scanning electron microscopy of the trichomes of Cucurbita pepo subsp. pepo var. styriaca and histochemistry of glandular secretory products. Ann Bot 94:515–526. https://doi.org/10.1093/aob/mch180
Kothari SK, Bhattacharya AK, Ramesh S, Garg SN, Khanuja SPS (2005) Volatile constituents in oil from different plant parts of methyl eugenol-rich Ocimum tenuiflorum Lf (syn. O. sanctum L.) grown in south India. J Essent Oil Res 17:656–658. https://doi.org/10.1080/10412905.2005.9699025
Kumari R, Agrawal SB (2011) Comparative analysis of essential oil composition and oil containing glands in Ocimum sanctum L. (Holy basil) under ambient and supplemental level of UV-B through gas chromatography-mass spectrometry and scanning electron microscopy. Acta Physiol Plant 33:1093–1101. https://doi.org/10.1007/s11738-010-0637-0
Naidoo Y, Kasim N, Heneidak S, Nicholas A, Naidoo G (2013) Foliar secretory trichomes of Ocimum obovatum (Lamiaceae): micromorphological structure and histochemistry. Plant Syst Evol 299:873–885
Nakamura CV, Ishida K, Faccin LC, Filho BPD, Cortez DÁG, Rozental S, de Souza W, Ueda-Nakamura T (2004) In vitro activity of essential oil from Ocimum gratissimum L. against four candida species. Res Microbiol 155:579–586. https://doi.org/10.1016/j.resmic.2004.04.004
Narendhirakannan RT, Subramanian S, Kandaswamy M (2006) Biochemical evaluation of antidiabetogenic properties of some commonly used Indian plants on streptozotocin-induced diabetes in experimental rats. Clin Exp Pharmacol Physiol 33:1150–1157. https://doi.org/10.1111/j.1440-1681.2006.04507.x
Navarro T, EL Oualidi J (2000) Trichome morphology in Teucrium L.(Labiatae). A taxonomic review. An del Jardín Botánico Madrid 57:277–297. https://doi.org/10.3989/ajbm.1999.v57.i2.203
Ogunkunle ATJ, Oladele FA (2000) Diagnostic value of trichomes in some Nigerian species of Ocimum Hyptis Jazq and Tinnea kotschy and Peys (Lamiaceae). J Appl Sci 3:1163–1180
Okigbo R, Ogbonnaya U (2006) Antifungal effects of two tropical plant leaf extracts (Ocimum gratissimum and Aframomum melegueta) on postharvest yam (Dioscorea spp.) rot. African. J Biotechnol 5:727–731
Oksanen E (2018) Trichomes form an important first line of defence against adverse environment—new evidence for ozone stress mitigation. Plant Cell Environ 41:1497–1499. https://doi.org/10.1111/pce.13187
Padalia RC, Verma RS (2011) Comparative volatile oil composition of four Ocimum species from northern India. Nat Prod Res 25:569–575. https://doi.org/10.1080/14786419.2010.482936
Paschapur MS, Patil MB, Kumar R, Patil SR (2009) Evaluation of aqueous extract of leaves of Ocimum kilimandscharicum on wound healing activity in albino wistar rats. Int J PharmTech Res 1:544–550
Prakash P, Gupta N (2005) Therapeutic uses of Ocimum sanctum Linn (Tulsi) with a note on eugenol and its pharmacological actions: a short review. Indian J Physiol Pharmacol 49:125–131. https://doi.org/10.7860/JCDR/2014/9122.4629
Rao BRR, Kotharia SK, Rajput DK, Patel RP, Darokar MP (2011) Chemical and biological diversity in fourteen selections of four Ocimum species. Nat Prod Commun 6:1705–1710
Rastogi S, Meena S, Bhattacharya A, Ghosh S, Shukla RK, Sangwan NS, Lal RK, Gupta MM, Lavania UC, Gupta V, Nagegowda DA, Shasany AK (2014) De novo sequencing and comparative analysis of holy and sweet basil transcriptomes. BMC Genomics 15:588–606. https://doi.org/10.1186/1471-2164-15-588
Saharkhiz MJ, Kamyab AA, Kazerani NK et al (2015) Chemical compositions and antimicrobial activities of Ocimum sanctum L. essential oils at different harvest stages. Jundishapur J Microbiol 8:1–16
Sangwan NS, Farooqi AHA, Shabih F, Sangwan RS (2001) Regulation of essential oil production in plants. Plant Growth Regul 34:3–21. https://doi.org/10.1023/A:1013386921596
Sestili P, Ismail T, Calcabrini C, Guescini M, Catanzaro E, Turrini E, Layla A, Akhtar S, Fimognari C (2018) The potential effects of Ocimum basilicum on health: a review of pharmacological and toxicological studies. Expert Opin Drug Metab Toxicol 14:679–692. https://doi.org/10.1080/17425255.2018.1484450
Shanker S, AjayKumar PV, Sangwan N S, Kumar Sushil, Sangwan RS (1999) Essential oil gland number and ultrastructure during Mentha arvensis leaf ontogeny. Biol Plant 42:379–387
Sharma S, Sangwan NS, Sangwan RS (2003) Developmental process of essential oil glandular trichome collapsing in menthol mint. Curr Sci 84:544–550
Shetty S, Udupa S, Udupa L, Somayaji N (2006) Wound healing activity of Ocimum sanctum Linn with supportive role of antioxidant enzymes. Indian J Physiol Pharmacol 50:163–168
Shirazi MT, Gholami H, Kavoosi G, Rowshan V, Tafsiry A (2014) Chemical composition, antioxidant, antimicrobial and cytotoxic activities of Tagetes minuta and Ocimum basilicum essential oils. Food Sci Nutr 2:146–155. https://doi.org/10.1002/fsn3.85
Silori CS, Dixit AM, Gupta L, Mistry N (2009) Observation on medicinal plant richness and associated conservation issues in district Kachchh, Gujarat. In: Trivedi PC (ed) Medicinal plants utilisation and conservation. pp 137–180
Singh N, Luthra R, Sangwan R (1989) Effect of leaf position and age on the essential oil quantity and quality in lemongrass (Cymbopogon flexuosus). Planta Med 55:254–256. https://doi.org/10.1055/s-2006-961997
Singletary KW (2018) Basil: a brief summary of potential health benefits. Nutr Today 53:92–97. https://doi.org/10.1097/NT.0000000000000267
Telci I, Bayram E, Yılmaz G, Avcı B (2006) Variability in essential oil composition of Turkish basils (Ocimum basilicum L.). Biochem Syst Ecol 34:489–497. https://doi.org/10.1016/j.bse.2006.01.009
Viña A, Murillo E (2003) Essential oil composition from twelve varieties of basil (Ocimum sp.) grown in Colombia. J Braz Chem Soc 14:744–749. https://doi.org/10.1590/S0103-50532003000500008
Wagner GJ (1991) Secreting glandular trichomes: more than just hairs. Plant Physiol 96:675–679. https://doi.org/10.1104/pp.96.3.675
Werker E (1993) Glandular hairs and essential oil in developing leaves of Ocimum basilicum L. (Lamiaceae). Ann Bot 71:43–50. https://doi.org/10.1006/anbo.1993.1005
Yadav RK, Sangwan RS, Sabir F, Srivastava AK, Sangwan NS (2014) Effect of prolonged water stress on specialized secondary metabolites, peltate glandular trichomes, and pathway gene expression in Artemisia annua L. Plant Physiol Biochem 74:70–83. https://doi.org/10.1016/j.plaphy.2013.10.023
Yadav RK, Sangwan RS, Srivastava AK, Sangwan NS (2017) Prolonged exposure to salt stress affects specialized metabolites-artemisinin and essential oil accumulation in Artemisia annua L.: metabolic acclimation in preferential favour of enhanced terpenoid accumulation accompanying vegetative to reproductive phase. Protoplasma 254:505–522. https://doi.org/10.1007/s00709-016-0971-1
Acknowledgments
SM acknowledges the Academy of Scientific and Innovative Research (AcSIR) for registration of Ph.D. program.
Funding
The authors are thankful to HCP-007, BSC-203, and BSC-107 CSIR network project for providing financial assistance. MC is thankful to CSIR, New Delhi and UGC, New Delhi for research fellowship.
Author information
Authors and Affiliations
Contributions
NSS conceived and devised the whole study plan. NSS, RSS, SM, and MC wrote the manuscript. SM, MC, RKY, and LKN conducted experiments. SM, PPS, US, DK, SB, RSS, MC, and LKN helped in resource and data generation. NSS supervised at each stage.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Handling Editor: Peter Nick
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Inter- and intra-specific diversity in phytochemicals, trichome types, and abundance
• Number and size of peltate trichomes are the key determinants of essential oil accumulation
• Major Ocimum species are phenylpropanoid-rich, whereas O. kilimandscharicum is unique in monoterpenoids
• Compositional diversity is governed by differential expression of key genes of biosynthetic pathway of phytochemical class
Electronic supplementary material
Supplementary Fig. 1
Leaf area of the Ocimum species/cultivars. (OB), O. basilicum; (OG), O. gratissimum; (OK), O. kilimandscharicum; (OSG), O. tenuiflorum green; and (OSP), O. tenuiflorum purple. (DOCX 35 kb)
Supplementary Table 1
List of primers used in real time expression analysis (DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Maurya, S., Chandra, M., Yadav, R.K. et al. Interspecies comparative features of trichomes in Ocimum reveal insights for biosynthesis of specialized essential oil metabolites. Protoplasma 256, 893–907 (2019). https://doi.org/10.1007/s00709-018-01338-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-018-01338-y