Abstract
Copper and lead, as remnants of industrial activities and urban effluents, have heavily contaminated many aquatic environments. Therefore, this study aimed to determine their effects on the physiological, biochemical, and cell organization responses of Hypnea musciformis under laboratory conditions during a 7-day experimental period. To accomplish this, segments of H. musciformis were exposed to photosynthetic active radiation at 80 μmol photons m−2 s−1, Cu (0.05 and 0.1 mg kg−1), and Pb (3.5 and 7 mg kg−1). Various intracellular abnormalities resulted from exposure to Cu and Pb, including a decrease in phycobiliproteins. Moreover, carotenoid and flavonoid contents, as well as phenolic compounds, were decreased, an apparent reflection of chemical antioxidant defense against reactive oxygen species. Treatment with Cu and Pb also caused an increase in the number of floridean starch grains, probably as a defense against nutrient deprivation. Compared to plants treated with lead, those treated with copper showed higher metabolic and ultrastructural alterations. These results suggest that H. musciformis more readily internalizes copper through transcellular absorption. Finally, as a result of ultrastructural damage and metabolic changes observed in plants exposed to different concentrations of Cu and Pb, a significant reduction in growth rates was observed. Nevertheless, the results indicated different susceptibility of H. musciformis to different concentrations of Cu and Pb.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As an essential micronutrient for plants, Cu is a structural element in regulatory proteins (Yruela 2005). However, when present in excess in the environment, Cu affects growth and impairs important cellular processes like photosynthetic electron transport, photosynthesis, and respiration. Membrane transport systems seem to be a target of this metal, playing a central role in toxicity processes (Yruela 2005). Industrial and domestic waste, agricultural practices, Cu marine drainage, Cu-based pesticides, and antifouling paints have all led to a clear increase in Cu concentrations in aquatic environments (Callow and Callow 2002).
Lead (Pb) is not an essential micronutrient for plants (Yruela 2005). Pb is among the most common pollutants found in both industrial and urban effluents (Sheng et al. 2004). Anthropic activities, such as combustion of leaded gasoline and the use of Pb-aerosol, have promoted the discharge of Pb in the environment throughout the twenteith century (Pourrut et al. 2011; Horta-Puga and Carriquiry 2014). As a stressor in plants, it promotes changes in chlorophyll–protein complexes (Janik et al. 2013), decreases nitrogen metabolism (Pourrut et al. 2011), and reduces the rate of electron transport of photosystem II in red algae (Gouveia et al. 2013).
Several studies with heavy metals have shown such effects as decreasing macroalgal growth rates (Bouzon et al. 2012a; Gouveia et al. 2013; Mamboya et al. 1999; Santos et al. 2012) and changes in photosynthetic pigments (Bouzon et al. 2012a; Gouveia et al. 2013; Rocchetta et al. 2007; Santos et al. 2012). Other studies showed that heavy metals promote negative effects in photosynthetic efficiency (Bouzon et al. 2012a; Mamboya et al. 1999). Rocchetta et al. (2007) verified the increase in total proteins and lipid contents in Euglena gracilis Klebs. Some reports on heavy metals have shown changes in the ultrastructure of the red algae Audouinella savina (F.S. Collins) Woelkerling (Talarico 2002), Ceramium ciliatum (J. Ellis) Ducluzeau (Diannelidis and Delivopoulos 1997), Hypnea musciformis (Wulfen) Lamouroux (Bouzon et al. 2012a), Gracilaria domingensis (Kützing) Sonder ex Dickie (Santos et al. 2013), and Gracilaria domingensis (Gouveia et al. 2013).
Pollutants, like heavy metals, promote direct and indirect effects on an ecosystem by changing rates of decomposition, nutrient cycling, and oxygen dynamics (Fleeger et al. 2003). In the environment, red algae accumulate heavy metals and are effective biosorbents for Cu and Pb (Garcia-Rios et al. 2007; Vilar et al. 2008). As a result, heavy metals that accumulate in cell walls can act as good biomarkers (Sheng et al. 2004). On the other hand, the metals which enter the cell can promote alterations in subcellular structures (Bouzon et al. 2012b; Santos et al. 2012; Gouveia et al. 2013) and promote oxidative stress (Liu and Pang 2010).
Hypnea is a source of kappa carrageenan and phycocolloids throughout the world, presenting significant economic importance (Bouzon 2006). Among the many macroalgae found in coastal systems, Hypnea J.V. Lamouroux is the biomarker of most probable consequence, owing to its worldwide distribution in the Atlantic, Indian, and Pacific Oceans (Bouzon 2006; Schmidt et al. 2012b). Hypnea musciformis (Wulfen) J.V. Lamouroux is the best-known species in the genus Hypnea and has been reported to occur in many tropical and subtropical shores (Silva et al. 1996). In Brazil, it is widespread along the Brazilian coast (Berchez and Oliveira 1989). Thus, in this study, we investigated the in vitro effect of Cu and Pb on H. musciformis by evaluating (1) algal growth rates relative to Cu and Pb uptake, (2) photosynthetic pigments, phenolic compounds, total proteins, and metabolic profile, (3) photosynthetic performance, and (4) morphology and ultrastructure.
Materials and methods
Algal material
H. musciformis samples were collected from Pontas das Canas Beach (27°23′34″ S, 48°26′11″ W), Florianopolis-SC, Brazil, in January 2012, during the summer season. The algal samples were collected from the intertidal zone during low tide and transported at ambient temperature in dark containers to the Plant Cell Biology Laboratory, Federal University of Santa Catarina, Florianopolis, Santa Catarina, Brazil (LaBCeV-UFSC). Macroepiphytes from macroalgal samples were meticulously eliminated by cleaning with a brush and filtered seawater. Apical portions were maintained in culture medium with filtered seawater plus von Stosch enrichment solution at half strength (VSES/2; Edwards 1970) and cultivated under laboratory-controlled conditions during 7 days before experimental treatment with Cu and Pb.
Culture conditions, lethality tests, and experimental treatments
Apical thallus portions were selected (±1.0 g) from the acclimated H. musciformis plants, cultivated for 7 days under experimental conditions with heavy metals in Erlenmeyer flasks containing 500 mL of natural sterilized seawater and salinity of 34 practical salinity units (p.s.u.), and enriched with VSES/2 (without ethylene diamine tetraacetic acid (EDTA)). Laboratory-controlled conditions were 24 ± 2 °C, continuous aeration, 80 ± 5 μmol photons m−2 s−1 (Philips C-5 Super 84 16 W/840 fluorescent lamps; LI-COR light meter 250, USA), and 12-h photocycle (starting at 8 h). Lethality tests (Supplementary Table 1) were performed using Cu and Pb from 0.05 to 25 mg kg−1 for each metal. Experimental treatments were carried out with a control (i.e., no metal addition) and Cu and Pb supplied individually as CuCl2 (0.05 and 0.1 mg kg−1) and PbCl2 (3.5 and 7 mg kg−1). Twelve replicates were made for each experimental group (total of five treatments).
Concentration of Cu and Pb in seawater and algal samples
The concentrations of Cu and Pb in seawater and algal samples (initial and end of experiment) were analyzed by inductivity-coupled plasma atomic emission spectrometry (Perkin Elmer, model AAnalyst 100, Shelton, USA), using the following analyte line: Cu 324.7 nm and Pb 217.0 nm plasma view-axial, with a detection limit of 0.001 mg kg−1 for Cu and Pb. The algal samples exposed to Cu and Pb (750 mg) were washed in distilled water, dried at 50 ± 5 °C, and digested in nitric acid. Seawater samples (50 mL) were digested using concentrated nitric acid with ultrasonic extraction. Total metal absorption rate was expressed in % and calculated using the following equation: W m/Wf × 100, where W m = mg of Cu or Pb in 500 mL of water and Wf = respective mg of Cu or Pb in mg of algae wet weight. Bioconcentration factor (BCF) was calculated using the following equation: C r × W w/C i, where C r = remaining metal concentration, W w = wet weight of plant biomass (expressed in mg kg−1), and C i = initial concentration of metal added in the culture medium. All analyses were performed in quadruplicate.
Growth rates
Growth rates (GRs) were calculated using the following equation: GR (% day−1) = [(Wt/Wi)1/t − 1] × 100, where Wi = initial wet weight, Wt = wet weight after 7 days, and t = experimental time in days (Penniman et al. 1986).
Pigment analyses
The contents of photosynthetic pigments (chlorophyll a, phycobiliproteins, and carotenoids) and flavonoids of H. musciformis were analyzed from frozen fresh samples (n = 4) kept at −40 °C until use.
Chlorophyll a was extracted from approximately 1 g of algal material in 3 mL of dimethylsulfoxide (Merck, Darmstadt, FRG) at 40 °C, during 30 min, using a glass tissue homogenizer (Hiscox and Israelstam 1979; Schmidt et al. 2010). The homogenates were centrifuged at 2000g for 20 min, and the supernatant containing the pigment was quantified spectrophotometrically, according to Wellburn (1994).
Phycobiliproteins were extracted from about 1 g of algal material ground to a powder with liquid nitrogen and extracted at 4 °C in darkness in 0.1 M phosphate buffer, pH 6.4. The homogenates were centrifuged at 2000g for 20 min. Phycobiliprotein levels [allophycocyanin (APC), phycocyanin (PC), and phycoerythrin (PE)] were determined by UV–vis spectrophotometry (Multireader Infinite M200, TECAN), and calculations were performed using the equations of Kursar et al. (1983).
Carotenoids were extracted from 1 g of algal material by using hexane/acetone (1:1, v/v) containing 100 mg L−1 tert-butyl hydroxytoluene. The homogenates were filtered through a cellulose membrane to remove particles, and the organosolvent was evaporated under N2 flux. The residues were dissolved in hexane (3 mL), and carotenoid contents were determined through UV–visible spectrophotometry (SF200ADV, Bel) by measuring the absorbance at 450 nm (three lectures per sample) (Multireader Infinite M200, TECAN) (Aman et al. 2005). Carotenoid quantification was based on β-carotenestandard curve (1–50 μg mL−1; y = 0.055x; r 2 = 0.999).
Total flavonoid assay
Flavonoids were extracted from 1 g fresh frozen samples (n = 4) using 10 mL 80 % aqueous methanol. The homogenates were filtered through a cellulose membrane to remove particles, and one aliquot (2 mL) of the extract was centrifuged for 5 min at 4000 rpm. The total flavonoid content was determined by aluminum chloride colorimetric method (Zacarias et al. 2007). Briefly, an aliquot of 0.5 mL of extracts was added to 2.5 mL of ethanol and 0.5 mL of 2 % aluminum chloride hexahydrate (AlCl3 · 6H2O). After incubation at room temperature for 1 h, absorbance of the reaction mixture was measured at 420 nm (Hitachi, Model 100–20). Flavonoid quantification was based on the quercetin standard curve (10–200 μg mL−1; y = 0.010x; r 2 = 0.999).
Phenolic compounds
The total phenolic compounds were extracted from 1 g fresh frozen samples of H. musciformis for each treatment (n = 4) after the experimental period. Samples were triturated with a mixer (0.5 g) in 5 mL of 0.1 M phosphate buffer, pH 6.4, with 0.2 % TRITON X-100. After that, the homogenates were centrifuged at 3000g for 20 min, and the supernatant containing the total phenolic compounds was quantified spectrophotometrically, according to the Folin–Ciocalteu method (Singleton et al. 1999). Quantification of total phenolic compounds was based on the standard curve (y = 0.0671x + 0.068; r 2 = 0.995) obtained using phenol (Sigma, USA).
Photosynthetic performance
Experiments were followed by measurements of chlorophyll a fluorescence using a pulse amplitude-modulated fluorometer (Diving-PAM underwater fluorometer; Walz, Effeltrich, Germany). PAM optimal configurations were previously evaluated for H. musciformis under laboratory conditions as follows: sat pulse int 9 (4000 μmol photons m−2 s−1), duration 0.8 s, gain 3, damping 3, and ETR-F 1.00. After measurements of yield (sat pulse), algae samples were acclimated to dark for 10 min, followed by application of rapid light curves (RLC). These curves were performed with nine different actinic light irradiances: 0, 70, 115, 160, 250, 340, 550, 841, and 1180 μmol photons m−2 s−1. After acclimation to dark, the first saturation pulse allowed calculation of basal chlorophyll fluorescence (F 0) and maximal chlorophyll fluorescence (F m). These were followed by increasing actinic light irradiance, lasting 20 s each. After exposure to each irradiation value, saturating pulses were applied, allowing calculation of F and F m′. Maximum quantum yield was calculated as F v/F m = (F m − F o)/F m. Effective quantum yield was obtained as Y(II) = (F m′ − F)/F m′. Absorbance factors (A) were then calculated, i.e., the relationship between the radiation absorbed by algae and the total incident radiation, following the formula A = 1 − (E t/E o), where E t is the amount of irradiance transmitted by thalli sections, and E o is the initial irradiance. ETRreal values were calculated using the following equation: ETRreal = Y(II) * PAR * absorbance * 0.15. RLC allowed obtaining curve fitting parameters, such as photosynthetic efficiency (α ETR) and the maximum rate of electron transport (ETRmax). These values were obtained by fitting RLCs according to the equation of Platt et al. (1980). Other parameters were calculated, such as non-photochemical quenching (NPQ) (Maxwel and Jhonson 2000) and regulated energy dissipated (Y(NPQ)). The maximum quantum yield was calculated as [(F m − F o)/F m)]. Definite integral (DI) was calculated with Y(II), Y(NO), and Y(NPQ) values.
Total proteins
H. musciformis samples were triturated with mixer (0.5 g) in 5 mL of 0.1 M phosphate buffer, pH 6.4, with 0.02%Triton™ X-100 (Sigma, USA). The homogenates were centrifuged at 3000×g for 20 min. The supernatant containing the total proteins was quantified spectrophotometrically, according to Bradford (1976), with standard curve (y = 0.00022x; r 2 = 0.997), using Albumin (Sigma, USA) as the standard protein.
Light microscopy
Samples approximately 5 mm in length were fixed in 2.5 % paraformaldehyde in 0.1 M (pH 7.2) phosphate buffer overnight. Subsequently, the samples were dehydrated in increasing series of ethanol aqueous solutions. After dehydration, the samples were infiltrated with Historesin (Leica Historesin, Heidelberg, Germany). Sections 4 μm in length were stained with periodic acid–Schiff (PAS) to identify neutral polysaccharides (Schmidt et al. 2009) and investigated with an Epifluorescent (Olympus BX 41) microscope equipped with Image Q Capture Pro 5.1 Software (Qimaging Corporation, Austin, TX, USA).
Transmission electron microscopy
For observation under transmission electron microscopy (TEM), samples approximately 5 mm in length were fixed with 2.5 % glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) plus 0.2 M sucrose overnight as described in Schmidt et al. (2009). The material was post-fixed with 1 % osmium tetroxide for 4 h, dehydrated in a graded acetone series, and embedded in Spurr’s resin, following Schmidt et al. (2010). Ultrathin sections (70 nm) were stained with aqueous uranyl acetate, followed by Pb citrate, according to Reynolds (1963). Four replicates were made for each experimental group; two samples per replication were then examined and photographed under TEM JEM 1011 (JEOL Ltd., Tokyo, Japan, at 80 kV). Similarities based on the comparison of individual treatments with replicates suggested that the ultrastructural analyses were reliable.
Scanning electron microscopy
Samples of H. musciformis control and those treated with Cu and Pb for scanning electron microscopy (SEM) observations were fixed using procedures identical to those used for TEM. The samples were dehydrated with ethanolic series, dried on Leica EM CPD300 CriticalPoint Dryer (Leica, Heidelberg, Germany), and then sputter-coated with gold prior to examination, according to Schmidt et al. (2012a). The samples were examined under SEM JSM 6390 LV (JEOL Ltd., Tokyo, Japan, at 10 kV). Cu and Pb were analyzed in the cell wall using SEM (NORAN System 7, Thermo Scientific Instruments) coupled to an energy dispersive X-ray spectrometer (SEM-EDS), without post-fixing the samples in osmium tetroxide or coating with gold.
Statistical analysis
Data were analyzed by unifactorial analysis of variance (ANOVA) and Newman–Keuls aposteriori test using Statistica software (Release 10.0), considering p ≤ 0.05. Statistical comparisons were performed to evaluate variations in the lethality tests, bioabsorption under Cu and Pb treatments, growth rates, concentration of photosynthetic pigments, flavonoid contents, total proteins, photosynthetic performance, and EDS analyses.
Results
Copper and lead uptake
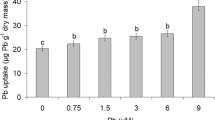
After the experimental period, an analysis of absorption percentage and BCF of Cu and Pb for respective uptake in H. musciformis did not result in the detection of either metal in the control plants (Fig. 1a). However, plants cultivated with Cu and Pb showed significant differences in absorption rate (Fig. 1a) and BCF values (Fig. 1b). The samples treated with Cu showed bioabsorption rates of 32.49 and 20.90 % for 0.05 and 0.1 mg kg−1 Cu, respectively. For Pb-treated samples, lower bioabsorption values of 6.99 and 16.93 % for 3.5 and 7 mg kg−1, respectively, were observed. Therefore, the plants cultivated with Cu showed higher bioconcentration factor values, such as 398.33 and 514.9, compared with control and Pb-treated plants.
Growth rates
After the experimental period, H. musciformis showed statistical differences (p ≤ 0.05) in GR values between control plants and thalli cultivated with different concentrations of Cu and Pb (Fig. 2). The control samples showed 8.14 % day−1, whereas Cu-treated plants at both 0.05 and 0.1 mg kg−1 exhibited a significant reduction in GRs when compared to control, specifically a decrease of 44.85 and 48.04 % of growth rates, respectively, compared to control conditions. H. musciformis treated with Pb showed a reduction in growth rate compared with control samples in that algae treated with 3.5 and 7 mg kg−1 (See Fig. 1.) of Pb showed 21.98 and 19.05 % lower growth rates, respectively, when compared with control.
After the experimental period, H. musciformis control samples presented typical reddish branches (Supplementary Fig. 1a). Evident bleaching and depigmentation of the apical segments were observed after exposure to 0.05 mg kg−1 Cu (Supplementary Fig. 1b), but more extensive bleaching was observed after treatment with 0.1 mg kg−1 Cu (Supplementary Fig. 1c). Treatment with 3.5 and 7 mg kg−1 of Pb (Supplementary Fig. 1d, e) showed a reduction in the amount of branches, but the color was similar to that of control.
Photosynthetic pigments, phenolics, flavonoids, and total proteins
Control and Cu- and Pb-treated samples of H. musciformis showed modifications in the content of photosynthetic pigments (Table 1). Chlorophyll a concentration decreased after exposure to Cu and Pb, while the samples treated with 7 mg kg−1 of Pb showed an increase of chlorophyll a of 31.75 %. The concentrations of phycobiliproteins (APC, PC, and PE) and phenolic compounds from H. musciformis showed a larger reduction for both concentrations of Cu than that observed for either concentration of Pb. On the other hand, the concentration of flavonoids and total carotenoids (Table 1) in plants exposed to Cu and Pb showed a significant decrease compared with control.
Total protein concentration was decreased by 26.21 % in samples of H. musciformis treated with 0.1 mg kg−1 Cu, while samples treated with 7 mg kg−1 Pb resulted in a 17.84 % decrease (Table 1).
Photosynthetic parameters
Samples of H. musciformis showed significant differences in photosynthetic parameters, such as ETRreal (Fig. 3a), which decreased in treatments with Cu. The ETRmax (Fig. 3b) showed a decrease only for treatment with 0.1 ppm Cu. F v/F m increased in treatments with Cu, but not in treatments with Pb (Fig. 3c). Among all treatments, E k (Fig. 3d) and α (Fig. 3e) showed no significant differences. YII (Fig. 4a), YNO (Fig. 4b), and YNPQ (Fig. 4c) were utilized to calculate the DI of photosystem II Yield (DIYII), and no significant differences were shown among the treatments. However, Cu promoted an increase in DIYNO and a decrease in DIYNPQ (Table 2). While treatments with Pb showed no significant changes in DIYNO, a decrease in DIYNPQ was noted (Table 2).
The ETR curve (Fig. 5) showed that the samples of H. musciformis treated with 0.1 ppm of Cu presented a decrease after 7 days of experimentation. However, the treatments of Pb showed no changes in ETR curve.
Relative rate of photosynthetic electron transport versus light emitted and measured by PAM on samples of H. musciformis treated for 7 days with 0.05 and 0.1 mg kg−1 of Cu and 3.5 and 7 mg kg−1 of Pb. The data represent the average of four replicates done with each treatment. The values represent ± SD for means (n = 4)
Observation under light microscopy and cytochemistry
Control samples of H. musciformis stained with PAS exhibited a positive reaction (Fig. 6a). This reaction indicated the presence of neutral polysaccharides, with floridean starch grains, suggesting the presence of cellulosic compounds in the cell wall. Using this same cytochemical technique, no discernible change was observed when compared with the amount of starch detected in samples treated with lower concentrations of Cu (Fig. 6c) and Pb (Fig. 6e). In contrast, plant cells treated with 0.1 mg kg−1 of Cu and 7 mg kg−1 of Pb showed an increasing number of starch grains, especially in the cortical cells (Fig. 6b, d). Treated samples showed no differences in the deposition of cellulosic compounds in the cell wall (Fig. 6b–e).
Light microscopy of H. musciformis thallus transversal sections with PAS after 7 days of treatments. PAS showed neutral polysaccharides in cell walls (cellulose) and cytoplasm (starch grains). a Control; observe the cell wall (arrow) and floridean starch grains (S). b Sample treated with 0.05 mg kg−1 of Cu: cell walls (arrow), S (starch grains). c Sample treated with 0.1 mg kg−1 of Cu: cell walls (arrow), S (starch grains). d Sample treated with 3.5 mg kg−1 of Pb: cell walls (arrow), S (starch grains). e Sample treated with 7 mg kg−1 of Pb: cell walls (arrow), S (starch grains)
Observations under transmission electron microscopy
Observation by TEM of control samples of H. musciformis showed cortical cells with chloroplasts and a large quantity of floridean starch grains close to the chloroplasts (Fig. 7a) surrounded by a thick cell wall with concentric microfibril layers. In the chloroplasts, a few plastoglobuli were observed between the thylakoids (Fig. 7b). Small mitochondria were present in association with the chloroplasts (Fig. 7c).
TEM micrographic images of control and Cu treatments of H. musciformis after 7 days of treatments. a–c Control samples. a Cortical cell with cell wall (CW), chloroplasts (C), and starch grains (S). b Detail of chloroplast (C), showing the plastoglobuli (P). Microfibrils of cellulose (arrows). c Observe the mitochondria (M). d–h Detail of cortical cells treated with 0.05 mg kg−1 of Cu. d Cortical cell showing cell walls with electron-dense points (arrowheads) and vacuolization (V). e Observe the vesicles of cell wall matrix deposition (head). f Chloroplast (C) presented spacing between the thylakoid (heads). g The vacuole presented deposition of electron-dense material (arrows). h–j Detail of cortical cells treated with 0.1 mg kg−1 of Cu. h Cortical cell; observe the vacuole (V). i Observe deposit of electron-dense points in the cell wall (arrow); observe the irregularity of thylakoids (arrows). j Vacuole (V)
In the samples treated with 0.05 mg kg−1 of Cu, cortical cells appeared to be vacuolated (Fig. 7d), presenting electron-dense points in the cell wall. Chloroplasts showed a few changes in ultrastructural organization (Fig. 7e). In particular, the vacuole presented deposition of electron-dense material (Fig. 7f). Cortical cells in H. musciformis samples treated for 7 days with 0.1 mg kg−1 of Cu also presented vacuolization (Fig. 7 g). The cortical cell wall exhibited deposition of electron-dense points (Fig. 7 h), and chloroplasts showed a few changes in ultrastructural organization (Fig. 7 h). The vacuole presented deposition of electron-dense material (Fig. 7i). Moreover, samples treated with Pb at 3.5 mg kg−1 showed some noticeable changes in the shape of cortical cells (Fig. 8a). Some electron-dense precipitates were observed in the cell wall (Fig. 8b). Chloroplasts showed a few changes in ultrastructural organization (Fig. 8c), and some starch grains were observed near the chloroplasts (Fig. 8c). The vacuole presented deposition of electron-dense material (Fig. 8d). Cortical cells of H. musciformis treated with 7 mg kg−1 of Pb presented numerous electron-dense precipitates in the cell wall (Fig. 8e, f). Chloroplasts showed changes in ultrastructural organization (Fig. 8 g), and the vacuole presented deposition of electron-dense material (Fig. 8 h).
TEM micrographic images of Pb treatments of H. musciformis after 7 days of treatments. a–d Cortical cells treated with 3.5 mg kg−1 of Pb. a Cortical cell. b Detail of cell wall; deposit of electron-dense points (arrow). c Detail of the chloroplast with alterations in thylakoids (arrows). d Magnification of vacuole with deposit of electron-dense points. e–i Cortical cells treated with 7 mg kg−1 of Pb. e Cortical cell. f Detail of cell wall; deposit of electron-dense points (arrow). g Detail of the chloroplast with alterations in thylakoids (arrows). h Magnification of vacuole with deposit of electron-dense points (arrows)
Scanning electron microscopy
When observed by SEM after 7 days of culture, the surface H. musciformis thallus in control and Cu- and Pb-treated plants showed different results (Fig. 9a–e). The surface of control samples showed a wrinkled appearance (Fig. 9a). However, with the increase of Cu concentration in the cultures, the surface became corrugated (Fig. 9b, c). On the other hand, the samples treated with Pb at 3.5 mg kg−1 showed a flaky formation on the surface (Fig. 9d), while formation of larger scales was observed as a result of Pb treatment at 7 mg kg−1 (Fig. 9e).
SEM of the surface of H. musciformis after 7 days of treatment. a Detail of the roughness of cell wall surface of control cortical cells. b Plants treated with 0.05 mg kg−1 of Cu show a decreased surface roughness. c Plants treated with 0.1 mg kg−1 of Cu show a decreased surface roughness. d Plants treated with 3.5 mg kg−1 of Pb show a scaly surface. e Plants treated with 7 mg kg−1 of Pb show an increase in scaly surface. Bars = 10 μm
The results of X-ray microanalysis of H. musciformis was qualitative (Table 3). X-ray microanalysis of the control cell wall surface revealed the presence of different percentages of elements, such as carbon, nitrogen, oxygen, sodium, magnesium, and calcium, in all samples. Cu and Pb were not detected on the surface of the control cell wall, but the presence of these metals in their respective treatments was detected (Table 3).
Discussion
The present study showed that Cu and Pb induce different levels of physiological, biochemical, and cell organization responses in the red macroalga H. musciformis, indicating the activation of different mechanisms that promote the uptake and toxicity of Cu and Pb.
Bioabsorption assays of H. musciformis showed an accumulation of 32.5 % for Cu at 0.05 mg kg−1, but only 16.93 % for Pb at 7 mg kg−1. The percentage of absorption represents the capacity of H. musciformis to capture ions of Cu and Pb in the environment. Generally, both treatments with Cu showed major absorption values, but at 0.1 mg kg−1, Cu showed a lower absorption than Cu at 0.05 mg kg−1. The absence of proportional increase of bioabsorption with increasing Cu concentration suggests that the mechanism of metal uptake became saturated. On the other hand, treatments with Pb showed a minor percentage of absorption, but this percentage increased with increasing Pb concentration. BCF represents the affinity of a pollutant for an organism. Our results showed that the increase in Cu concentration promoted an increase in BCF. However, in treatments with Pb, an increase in concentration of Pb promoted a decrease in BCF, possibly indicating that the capacity of H. musciformis to assimilate Cu is greater than its capacity to assimilate Pb.
X-ray analysis with SEM-EDS detected the presence of Cu and Pb in all treatments, with a major presence of Pb on the surface of the samples. These data suggest that the interaction between Pb and carrageenan polysaccharide composition of cell wall matrix is greater than its interaction with either Cu or Pb, and that must have been the likely cause of higher Cu uptake, when compared to Pb, as reflected by BCF. Under TEM, we also observed the presence of some electron-dense particles in the cell wall for all treatments, most likely deposits of Cu and Pb. Talarico (2002) observed similar deposition of electron-dense particles in the cell wall in Cd-treated Audouiella saviana, which was attributed to Cd. The same phenomenon was observed in H. musciformis treated for 7 days with Cd (Bouzon et al. 2012a) and in G. domingensis (Santos et al. 2013) and P. capillacea (Felix et al. 2014) treated for 7 days with Cd. The images of the external cell wall surface of H. musciformis analyzed by SEM show the surface roughness of the control sample. Cu and Pb treatment resulted in the changes in surface morphology.
After treatments with both Cu and Pb, TEM analysis showed increased vacuolization and deposition of metals in both cell wall and vacuole. The same finding was reported by Santos et al. (2013) in G. domingensis treated with Cd, Santos et al. (2014) in G. floridanum treated with Cd, Cu, and Pb, and Bouzon et al. (2012b) in H. musciformis treated with Cd. In the present study, such damage indicates that the chelation of metals by the cell walls of H. musciformis was not sufficient. Thus, with the presence of transporters in the plasma membrane, metals like Cu and Pb can easily enter the cell, promoting alterations in organelles, as well as proteins, such as pigments. Our analyses did not show any increase in the thickness of the cell wall. Increasing cell wall thickness is a host defense strategy, in which sulfated compounds present in cell walls of algae can chelate metals (Diannelidis and Delivopoulos 1997), preventing metal–amino acid chelates from entering the cell (Andrade et al. 2010; Santos et al. 2013). Other researches with red algae treated with Cd also found an increase in cell wall thickness (Bouzon et al. 2012a; Santos et al. 2013).
After 7 days of treatment, the control samples of H. musciformis presented a significant increase in biomass, whereas the samples treated with Cu and Pb showed less increase in biomass. The growth rate was even lower in Cu treatments. To account for these results, it can be hypothesized that the metabolic routes were most likely diverted to protect the photosystem apparatus, suggesting a physicochemical strategy of circumventing the harmful effects of metals in the cell. At the same time, however, this defense strategy may have altered the growth rates of H. musciformis samples treated with Cu and Pb. Gouveia et al. (2013) demonstrated that G. domingensis cultivated during 7 days with 5 and 10 μM Cu showed different results, with negative GRs and reduction in algal biomass. The fact that Cu is an essential nutrient in the formation of metalloenzymes indicates the presence of Cu transport proteins in all cell membranes (Yruela 2005).
Quantification of chlorophyll a showed a decrease for treatments with Cu. While treatments of Pb at 3.5 mg kg−1 had no significant effects, Pb at 7 mg kg−1 showed an increase in the amount of chlorophyll a. Probably, treatments with Cu most likely promoted a reduction in enzymatic activity (Stobart et al. 2006; Xia et al. 2004) or promoted the deficiency of Mg and Fe in the biosynthesis of chlorophyll a (Greger and Ogren 1991; Xia et al. 2004).
Samples of H. musciformis after 7 days of treatment presented alteration in some photosynthetic efficiency parameters. Cu-treated samples presented an increase in quantum yield quenching (F v/F m), while Pb treatments showed no significant changes. Cu and Pb promoted no significant changes in photosystem II based on the quantum effective yield (YII). Cu promoted a decrease in unregulated nonphotochemical quenching (YNO), while Pb promoted a decrease in regulated nonphotochemical quenching (YNPQ). ETR was decreased only with treatment of 0.1 mg kg−1 Cu. Results suggest that these plants adopt different strategies in order to maintain photosynthetic efficiency during stress. Thus, for example, while chlorophyll a decreased, along with a major decrease in phycobiliproteins, in samples treated with Cu, an increase in F v/F m and Y(NPQ) was also seen. However, Pb promoted an increase in Y(NO), as well as an increase in chlorophyll a that may have compensated for the damage promoted by Pb. Phycobiliproteins were degraded by decreasing their quantity, probably as a mechanism deployed to prevent the excess of excitation energy that causes serious oxidative damage to several biological compounds, such as lipids, nucleic acids, and proteins. This corroborates our results showing that 0.1 mg kg−1 of Cu and 3.5 mg kg−1 of Pb promote an increase in the amount of proteins.
TEM analyses showed that the chloroplast of samples treated with Cu and Pb did not undergo many changes, including the plastoglobuli. Treatments with both Cu and Pb promoted some alterations in thylakoids. On the other hand, the small deformations in the membranes of thylakoids likely contributed to changes in photosynthetic efficiency as a result of differences in the diffraction and reflection of light rays in the thylakoid membrane. In other experiments with Cd in red algae, structural deformation of the chloroplast was also observed (Talarico 2002; Bouzon et al. 2012a; Santos et al. 2013). Santos et al. (2014) observed alterations in thylakoids of G. floridanum treated with Cd, Cu, and Pb. The plastoglobuli are considered lipidic material (Bouzon et al. 2012b), and an increase in the amount of plastoglobuli was also observed in C. ciliatum exposed to Cd (Diannelidis and Delivopoulos 1997).
Compared to Pb treatments, the amount of carotenoids in H. musciformis showed reduction. Carotenoids can act as an antioxidant mechanism, and they are synthesized and stored in chloroplasts as lipophilic antioxidants (Römer et al. 2002). According to Gouveia et al. (2013), the production of carotenoids is a strategy to prevent the effects of ROS. In the present study, the amount of flavonoids and phenolics decreased as a result of treatment with Cu and Pb. According to Collén et al. (2003), flavonoids were synthesized for the purpose of preventing oxidative stress. In Porphyra acanthophora var. brasiliensis E.C. Oliveira & Coll, Bouzon et al. (2012b) observed that the amount of flavonoids in ambient samples was greater than that of either control or UVB-treated samples. As determined in the present study, flavonoids were not synthesized in substantial amounts; therefore, it is possible that the action of flavonoids as an antioxidant in H. musciformis under metal stress is not as effective as that demonstrated in other plants.
Conclusion
In summary, the present study demonstrates that Cu and Pb treatments in H. musciformis induce many different changes in growth rates, photosynthetic pigments, photosynthetic performance, as well as ultrastructural features, and that these changes became obvious after an experimental period of 7 days. Damage to the photosynthetic apparatus and thylakoid membranes was evidenced by discoloration, pigment reduction, and biosynthesis of starch grains. However, H. musciformis did not show cell wall thickness to prevent the entry of Cu and Pb in the cytoplasm. Consequently, cortical cells became vacuolized, and under transmission microscopy, metals were observed in the vacuole. Treatment with both Cu and Pb generally resulted in a decreased amount of chemical antioxidant defense against ROS, including carotenoids, flavonoids, and phenolic compounds, indicating that Cu is more toxic to H. musciformis than Pb.
References
Aman R, Carle R, Conrad J, Beifuss U, Schieber A (2005) Isolation of carotenoids from plant materials and dietary supplements by high-speed counter-current chromatography. J Chromatogr A 1074:99–105
Andrade LR, Leal R, Noseda M, Duarte MER, Pereira MS, Mourão PAS, Farina M, Filho GMA (2010) Brown algae overproduce cell wall polysaccharides as a protection mechanism against the heavy metal toxicity. Mar Poll Bull 60:1482–1488
Berchez AS, Oliveira EC (1989) Maricultural assays with the carrageenophyte Hypnea musciformis in São Paulo, Brazil. Hydrobiologia 260–261:255–261
Bouzon ZL (2006) Histochemistry and ultrastructure of the ontogenesis of the tetrasporangia of Hypnea musciformis (Wulfen) J. V. Lamour. (Gigartinales, Rhodophyta). Revista Brasil Bot 29(2):229–238 [in Portuguese]
Bouzon ZL, Ferreira EC, Santos RW, Scherner F, Horta PA, Maraschin M, Schmidt EC (2012a) Influences of cadmium on fine structure and metabolism of Hypnea musciformis Rhodophyta, Gigartinales cultivated in vitro. Protoplasma 249:637–650
Bouzon ZL, Chow F, Simioni C, Santos RW, Ouriques LC, Felix MRL, Osorio LKP, Gouveia C, Martins RP, Latini A, Ramlov F, Maraschin M, Schmidt EC (2012b) Effects of natural radiation, photosynthetically active radiation and artificial ultraviolet radiation-B on the chloroplast organization and metabolism of Porphyra acanthophora var. brasiliensis (Rhodophyta, Bangiales). Microsc Microanal 18:1467–1479
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Callow ME, Callow J (2002) Marine biofouling: a sticking problem. Biologist 49:1–5
Collén J, Pinto E, Pedersén M, Colepicolo P (2003) Induction of oxidative stress in the red macroalgae Gracilaria tenuisitipitata by pollutant metals. Arch Environ Contam Toxicol 45:337–342
Diannelidis BE, Delivopoulos SG (1997) The effects of zinc, copper and cadmium on the fine structure of Ceramium ciliatum (Rhodophyceae, Ceramiales). Mar Environ Res 44(2):127–134
Edwards P (1970) Illustrated guide to the seaweeds and sea grasses in the vicinity of Porto Arkansas, Texas. Contrib Mar Sci 15:1–228
Felix MRL, Osorio LKP, Ouriques LC, Farias-Soares FL, Steiner N, Kreusch M, Pereira DT, Simioni C, Costa GB, Horta PA, Chow F, Ramlov F, Maraschin M, Bouzon ZL (2014) The Effect Of Cadmiumunder Different Salinity Conditions On The Cellular Architecture And Metabolism In The Red Alga Pterocladiella Capillacea (rhodophyta, Gelidiales) Microsc Microanal 20(5):1411–1424
Fleeger JW, Carman KR, Nisbet RM (2003) Indirect effects of contaminants in aquatic ecosystems. Sci Tot Environ 317:207–233
Garcia-Rios V, Freile-Pelegrın Y, Robledo D, Mendoza-Cozat D, Moreno-Sanchez R, Gold-Bouchota G (2007) Cell wall composition affects Cd2+ accumulation and intracellular thiol peptides in marine red algae. Aquat Toxicol 81:65–72
Gouveia C, Kreusch M, Schmidt EC, Felix MRL, Osorio LKP, Pereira DT, Santos R, Ouriques LC, Martins RP, Latini A, Ramlov F, Carvalho TJG, Chow F, Maraschin M, Bouzon ZL (2013) The effects of lead and copper on the cellular architecture and metabolism of the red alga Gracilaria domingensis. Microsc Microanal 19:513–524
Greger M, Ogren E (1991) Direct and indirect effects of Cd2+ on photosynthesis in sugar beet (Beta vulgaris). Plant Physiol 83(1):129–135
Hiscox JD, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1332–1334
Horta-Puga G, Carriquiry JD (2014) The last two centuries of lead pollution in the Southern Gulf of Mexico recorded in the annual bands of the scleractinian coral Orbicella faveolata. Bull Environ Contam Toxicol 92:567–573
Janik E, Szczepaniuk J, Maksymiec W (2013) Organization and functionality of chlorophyll–protein complexes in thylakoid membranes isolated from Pb-treated Secale cereal. J Photochem Photobiol B Biol 125:98–124
Kursar TA, van Der Meer J, Alberte RS (1983) Light-harvesting system of the red alga Gracilariatikvahiae. I. Biochemical analyses of pigment mutations. Plant Physiol 73:353–360
Liu F, Pang SJ (2010) Stress tolerance and antioxidant enzymatic activities in the metabolisms in reactive oxygen species in two intertidal red algae Grateloupia turuturu and Palmariapalmata. J Exper Mar BiolEcol 382:82–87
Mamboya FA, Pratap HB, Mtolera M, Bjork M (1999) The effect of copper on the daily growth rate and photosynthetic efficiency of the brown macroalga Padinaboergensenii. In: Richmond M.D., Francis J. (eds.) Proceedings of the conference on advances on marine sciences in Tanzania. pp. 185–192.
Maxwel K, Jhonson GN (2000) Review article: chlorophyll fluorescence—a practical guide. J Exper Bot 51(345):659–668
Penniman CA, Mathieson AC, Penniman CE (1986) Reproductive phenology and growth of Gracilaria tikvahiae McLachlan (Gigartinales, Rhodophyta) in the Great Bay Estuary. New Hampshire. Bot Mar 29:47–154
Platt T, Gallegos CL, Harrison WG (1980) Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J Mar Res 38(4):687–701
Pourrut B, Shahid M, Dumat C, Winterton P, Pinelli E (2011) Lead uptake, toxicity, and detoxification in plants. Rev Environ Contam Toxicol 213:113–136
Reynolds ES (1963) The use of lead citrate at light pH as an electron opaque stain in electron microscopy. J Cell Biol 17:208–212
Rocchetta I, Leonardi PI, Amado Filho GM, Molina MDR, Conforti V (2007) Ultrastructure and X-ray microanalysis of Euglena gracilis (Euglenophyta) under chromium stress. Phycologia 46:300–306
Römer S, Lubeck J, Kauder F, Steiger S, Adomat C, Sandmannz G (2002) Metabolic Genetic Engineering of a Zeaxanthin-rich Potato by (2002) Metabolic Genetic Engineering of a Zeaxanthin-rich Potato by Antisense Inactivation and Co-suppression of Carotenoid Epoxidation. Eng 4:263–272
Santos RW, Schmidt EC, Paula MR, Latini A, Horta PA, Maraschin M, Bouzon ZL (2012) Effects of cadmium on growth, photosynthetic pigments, photosynthetic performance, biochemical parameters and structure of chloroplasts in the agarophyte Gracilaria domingensis Rhodophyta, Gracilariales. Am J Plant Sci 3:1077–1084
Santos RW, Schmidt EC, Bouzon ZL (2013) Changes in ultrastructure and cytochemistry of the agarophyte Gracilaria domingensis (Rhodophyta, Gracilariales) treated with cadmium. Protoplasma 250:297–305. doi:10.1007/s00709-012-0412-8
Santos RW, Schmidt EC, Felix MRL, Osorio LKP, Kreusch M, Pereira DT, Simioni C, Chow Ho FF, Ramlov F, MaraschinM BZL (2014) Bioabsorption of cadmium, copper and lead by the red macroalga Gelidium floridanum: physiological responses and ultrastructure features. Ecotox Environ Saf 105:80–89
Schmidt EC, Scariot LA, Rover T, Bouzon ZL (2009) Changes in ultrastructure and histochemistry of two red macroalgae strains of Kappaphycus alvarezii (Rhodophyta, Gigartinales), as a consequence of ultraviolet B radiation exposure. Micron 40(8):860–869
Schmidt EC, Nunes BG, Maraschin M, Bouzon ZL (2010) Effect of ultraviolet-B radiation on growth, photosynthetic pigments, and cell biology of Kappaphycus alvarezii, Rhodophyta, Gigartinales, macroalgae brown strain. Photosynthetica 48:161–172
Schmidt EC, Pereira B, Santos R, Gouveia C, Costa GB, Faria GSM, Scherner F, Horta PA, Paula MR, Latini A, Ramlov F, Maraschin M, Bouzon ZL (2012a) Responses of the macroalgae Hypnea musciformis after in vitro exposure to UV-B. Aquatic Bot 100:8–17
Schmidt EC, Santos R, Faveri C, Horta PA, Paula MR, Latini A, Ramlov F, Maraschin M, Bouzon ZL (2012b) Response of the agarophyte Gelidium floridanum after in vitro exposure to ultraviolet radiation B: changes in ultrastructure pigments, and antioxidant systems. J Appl Phycol 24:1341–1352
Sheng PX, Ting Y, Chen JP, Hong L (2004) Sorption of lead, copper, cadmium, zinc and nickel by marine algal biomass: characterization of biosorptive capacity and investigation of mechanisms. J Coll Interf Sci 275:131–141
Silva PC, Basson PW, Moe RL (1996) Catalogue of the marine algae of the Indian Ocean. UnivCalif Public Bot 79:1–1259
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzimol 299:152–178
Stobart AK, Griffiths WT, Ameen-Bukhari I, Sherwood RP (2006) The effect of Cd2+ on the biosynthesis of chlorophyll in leaves of barley. Physiol Plant 63(3):293–298
Talarico L (2002) Fine structure and X-ray microanalysis of a red macrophyte cultured under cadmium stress. Environ Pollut 120:813–821
Vilar VJP, Botelho CMS, Boaventura RAR (2008) Lead and copper biosorption by marine red algae Gelidium and algal composite material in a CSTR (“Carberry” type). Chem Eng J 138:249–257
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Xia JR, Li YJ, Lu J, Chen B (2004) Effects of copper and cadmium on growth, photosynthesis, and pigment content in Gracilaria lemaneiformis. Bull Environ Contam Toxicol 73:979–986
Yruela I (2005) Copper in plants. Braz J Plant Physiol 17:145–146
Zacarias AA, Moresco HH, Horst H, Brighente IMC, Marques MCA, Pizzollati MG (2007) Determinação do teor de fenólicos e flavonóides no extrato e frações de Tabebuia heptaphylla. 30a Reunião Anual da Sociedade Brasileira de Química, Santa Maria, Rio Grande do Sul.
Acknowledgments
The authors would like to acknowledge the staff of the Central Laboratory of Electron Microscopy (LCME), Federal University of Santa Catarina, Florianopolis, Santa Catarina, Brazil, for the use of their scanning and transmission electron microscopes. This study was supported, in part, by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) and Fundação de Apoio à Pesquisa Cientifica e Tecnológica do Estado deSanta Catarina (FAPESC, Brazil).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Néstor Carrillo
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Morphology of H. musciformis samples after 7 days of treatments. a Control. b, c Samples present bleaching of thallus (arrow). d No bleaching is evident. e Sample showed no bleaching (JPEG 336 kb)
Supplementary Table 1
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Santos, R.W., Schmidt, É.C., Vieira, I.C. et al. The effect of different concentrations of copper and lead on the morphology and physiology of Hypnea musciformis cultivated in vitro: a comparative analysis. Protoplasma 252, 1203–1215 (2015). https://doi.org/10.1007/s00709-014-0751-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-014-0751-8