Abstract
The agarophyte macroalgae Gracilaria domingensis (Kützing) Sonder ex Dickie is widely distributed along the Brazilian coast. While this species produces agarana, it is more important in the human diet. Therefore, the present study aimed to evaluate the biological effects of cadmium on its morphology and cellular organization. To accomplish this, the effects of cadmium in apical segments of G. domingensis were examined in vitro. Over a period of 16 days, the segments were cultivated and exposed to photosynthetically active radiation (PAR) at 80 μmol photons m−2 s−1, with cadmium treatments in doses of 100, 200 and 300 μM. The samples were processed for light, transmission and scanning electron microscopy. Histochemical analyses included Toluidine Blue for acidic polysaccharides, Coomassie Brilliant Blue for total protein, and Periodic Acidic Schiff for neutral polysaccharides. In all cadmium treatments, cytochemical analysis showed 1) metachromatic granulation in vacuole and lenticular thickness of the cell wall, 2) a higher concentration of cytoplasmic organelles, and 3) an increase in the number of floridean starch grains. Cadmium also caused changes in the ultrastructure of cortical and subcortical cells, including increased cell wall thickness and vacuole volume, as well as the destruction of chloroplast internal organization and increased number of plastoglobuli. In addition, treated plants showed a gradual increase in surface roughness, apparently the result of cadmium absorption. Taken together, these findings strongly suggested that cadmium negatively affects the agarophyte G. domingensis, posing a threat to the vitality of this plant species as a supplement in the human diet.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the last few years, increasing human population and industrial development have led to an increase of contaminants in aquatic systems (Rocchetta et al. 2007). Accordingly, studies reporting the effects of heavy metals on aquatic organisms are currently attracting more attention, particularly those focusing on industrial and urban pollution. The contamination of coastal waters with trace metals through sewage and other anthropogenic sources has become a severe problem (Mamboya et al. 1999). Heavy metals, such as lead, copper, cadmium, zinc, and nickel, are among the most common pollutants found in both industrial and urban effluents (Sheng et al. 2004). In low concentrations, some heavy metals (Cu, Zn, Ni, and Mn) are essential trace elements for photosynthetic organisms; however, in higher concentrations, these metals cause severe toxic effects (Hu et al. 1996). Heavy metals affect all biological organisms, especially those in the aquatic ecosystem, in many important ways. Several studies have shown such effects as decrease of macroalgae growth rates (Mamboya et al. 1999), changes in photosynthetic pigments (Rocchetta et al. 2007) and photosynthetic efficiency (Mamboya et al. 1999), as well as an increase in total proteins and lipid contents (Rocchetta et al. 2007). Finally, some reports have shown changes in the ultrastructure of the red algae Audouinella savina (F.S.Collins) Woelkerling (Talarico 2002) and Ceramium ciliatum (J. Ellis) Ducluzeau (Diannelidis and Delivopoulos 1997); the green algae Dunaliella minuta Lerche (Visvik and Rachlin 1992), Enteromorpha flexuosa (Wulfen) J.Agardh (Andrade et al. 2004) and Euglena gracilis Klebs (Rocchetta et al. 2007); and the brown alga Padina gymnospora (Kützing) Sonde (Andrade et al. 2002).
Cadmium (Cd) is one of the heavy metals most frequently implicated in environmental contaminations. This metal is utilized in the manufacture of various products, such as batteries, chipsets, pigments, television receivers, and semiconductors (Hashim and Chu 2004; Hu et al. 1996). Cd can bind to sulfated groups, as well as metalloproteins and metalloenzymes, thereby neutralizing their functions (Pinto et al. 2003). However, Cd has no nutritive value for algae (Visvik and Rachlin 1992).
The genus Gracilaria Greville is distributed worldwide from the equator to higher latitudes (Oliveira and Plastino 1994). As a source of agar extraction throughout the world, it has achieved significant economic importance (Armisen 1995). Moreover, species of this genus have been extensively studied because of the high utilization of their phycocolloids. In fact, species of Gracilaria are some of the most useful algae in the world, combining the production of the valuable polysaccharide agar with fast growth rate, ease of vegetative reproduction and other attributes favoring their cultivation (Kain and Destombe 1995).
In particular, the agarophyte macroalga Gracilaria domingensis is distributed along the Brazilian coastline from Ceara State to Santa Catarina State (Schmidt et al. 2010a). It occurs within the intertidal zone up to the lower shore. Commonly found in areas of high wave action, it has shown considerable tolerance to environmental changes, such as salinity, temperature and water circulation (Oliveira and Plastino 1994). In view of the effects of heavy metals on other species of algae, the present study aimed to evaluate the biological effects of cadmium on the cellular structure, ultrastructure and cytochemistry of the red macroalga G. domingensis.
Materials and methods
Algal material
G. domingensis samples were collected from Ponta das Canas Beach (27°23′34″S and 48°26′11″W), Florianopolis-SC, Brazil, in May 2010. The algal samples were collected from the rocks and were transported at ambient temperature in dark containers to LAMAR-UFSC (Macroalgae Laboratory, Federal University of Santa Catarina, Florianopolis, Santa Catarina, Brazil).
Unialgal culture was established as described by Oliveira et al. (1995). To avoid contamination by the presence of epiphytes, the collected algae were meticulously cleaned with a brush and filtered seawater. The apical thalli portions were maintained by immersing in seawater enriched with von Stosch medium. These segments were cultivated under the same conditions during 14 days (experimental acclimation period) before their utilization in the cadmium experiments.
Culture conditions
The apical thalli portions were selected (± 0.5 g) from the G. domingensis samples and cultivated for 16 days in Erlenmeyers flasks containing 500 mL natural sterilized seawater enriched with von Stosch medium at half strength (VSES/2) (Edwards 1970) with ±34 practical salinity units (p.s.u.). Culture room conditions were 24 °C temperature, continuous aeration, illumination from above with fluorescent lights (Philips C-5 Super 84 16 W/840, Brazil), photosynthetically active radiation (PAR) at 80 μmol photons m−2.s−1 (Li-cor light meter 250, USA) and 12 h photocycle (starting at 8 h).
The control plants (no Cd) were cultivated as described above, and for the treated plants, CdCl2 was added at graded concentrations of 100, 200 and 300 μM to the culture medium, as previously suggested by Talarico et al. (1997), Bouzon et al. (2012) with Hypnea musciformis and Xia et al. (2004) for Gracilaria lemaneiformis (Bory de Saint-Vincent) Greville, during a four-day cadmium treatment. Four replicates were made for each experimental group.
Light microscope (LM)
Samples approximately 5 mm in length were fixed in 2.5 % paraformaldehyde in 0.1 M (pH 7.2) phosphate buffer overnight (Schmidt et al. 2009). Subsequently, the samples were dehydrated in increasing series of ethanol aqueous solutions. After dehydration, the samples were infiltrated with Historesin (Leica Historesin, Heidelberg, Germany). Sections 5 μm in length were stained with different histochemical techniques and were investigated with an Epifluorescent (Olympus BX 41) microscope equipped with Image Q Capture Pro 5.1 Software (Qimaging Corporation, Austin, TX, USA).
Cytochemical staining
LM sections were stained as follows: Periodic Acid-Schiff (PAS) used to identify neutral polysaccharides (Gahan 1984); Toluidine Blue (TB-O) 0.5 %, pH 3.0 (Merck Darmstadt, Germany) used for acid polysaccharides through a metachromatic reaction (Gordon and McCandless 1973) and Coomassie Brilliant Blue (CBB) 0.02 % in Clarke’s solution (Serva, Heidelberg, Germany) used for proteins (Gahan 1984; Schmidt et al. 2009, 2010a). Controls consisted of applying solutions to sections without the staining component (e.g., omission of periodic acid application in the PAS reaction).
Transmission electron microscope (TEM)
For observation under the transmission electron microscope (TEM), samples approximately 5 mm in length were fixed with 2.5 % glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) plus 0.2 M sucrose overnight. The material was post-fixed with 1 % osmium tetroxide for four hours, dehydrated in a graded acetone series and embedded in Spurr’s resin (Schmidt et al. 2010a). Thin sections were stained with aqueous uranyl acetate followed by lead citrate, according to Reynolds (1963). Four replicates were made for each experimental group; two samples per replication were then examined under TEM JEM 1011 (JEOL Ltd., Tokyo, Japan, at 80 kV). Similarities based on the comparison of individual treatments with replicates suggested that the ultrastructural analyses were reliable.
Scanning electron microscope (SEM)
The samples were fixed for SEM observations using procedures identical to those used for TEM. The samples were dehydrated with ethanolic series, dried on a critical point table-top dryer (Leica EM CPD030, Heidelberg, Germany), then sputter-coated with gold prior to exanimation (Schmidt et al. 2012). Part o samples were cutted with razor blade to SEM. The samples were examined under SEM JSM 6390 LV (JEOL Ltd., Tokyo, Japan, at 20 kV).
Cadmium in the cell wall was analyzed using SEM (Analiser Noran System Instruments) coupled to an energy dispersive X-ray spectrometer (SEM-EDX). For SEM-EDX analysis, the samples were sputter-coated with carbon prior to examination, but they were not post-fixed with tetroxide osmium.
Results
Observations under LM and cytochemistry
When observed in transverse sections and stained with toluidine blue (TB-O), the cortical region of control of G. domingensis showed two to three layers of cells. In the outermost layer, the cells were elongated, whereas the second and third cell layers became spherical. The cortical cells were small and surrounded by a thick cell wall (2.0–3.7 μm). Metachromatic reaction in the cell wall indicated the presence of acidic polysaccharides, such as agar (Fig. 1a). An orthochromatic reaction was observed in the cytoplasm (Fig. 1a). The subcortical cells were more vacuolated when compared with the cortical cells. They exhibited different sizes and gradually increased in size toward the medullary region surrounded by a thick cell wall (6.2–9.7 μm).
Light microscopy of G. domingensis thallus transversal sections stained with TB-O. a Control cells showed metachromatic reaction in the cell wall of cortical and subcortical cells. The cytoplasm shows orthochromatic reaction. The arrows indicate the cell wall with metachromatic reaction showing lenticular thickening (arrows). b Treatment with 100 μM of cadmium. Note the greater lenticular thickness of the cell wall (arrow). Observe the increased thickness of the cortical cell wall. c Treatment with 200 μM of cadmium. Note the greater lenticular thickness of subcortical cell walls (arrow) and increased thickness cortical cell walls. d Treatment with 300 μM of cadmium. Observe the thickness of both cortical and subcortical cell walls (arrow). e Magnification of the cortical cell wall treated with 300 μM of cadmium. Observe the increased thickness of the cortical cell wall (arrow). CC: cortical cell, CW: cell wall, SC: subcortical cell
All plants were treated with graded concentrations of 100, 200 and 300 μM of cadmium. An increase in cell wall thickness was observed. Cortical cells showed an increase in size, ranging from 6.0 μm to 9.2 μm, 6.6 μm to 9.7 μm and 7.4 μm to 9.8 μm, respectively. More cell wall thickness was observed in the apical region toward the outside of the thallus. Loss of mucilage was also observed. Subcortical cells also showed an increase of cell wall thickness, ranging from 9.0 μm to 16 μm, 15 μm to 23 μm and 19 μm to 30 μm, respectively. In addition, both cortical and subcortical cells of treated plants showed a reduction in cell volume.
Those plants treated with 100, 200 and 300 μM of cadmium and also submitted to TB-O showed a metachromatic reaction in cell wall similar to that observed in control algae. However, the cytoplasm of the cortical and subcortical cells was denser compared to control (Fig. 1b–d). In cortical cells, it was possible to observe the lenticular thickness in the cell wall formed by concentric microfibrils (Fig. 1e).
Control samples of G. domingensis stained with Periodic Acid-Schiff (PAS) exhibited a positive reaction, suggesting the presence of cellulosic compounds in the cell wall. This reaction also occurred in the cytoplasm with neutral polysaccharides, especially with many floridean starch grains, the main substance reserve of red algae (Fig. 2a). By PAS reaction, it was also possible to detect a gradual increase in the density of starch grains in the cortical and subcortical cells when compared to controls (Fig. 2b–d).
Light microscopy of G. domingensis thallus transversal sections with PAS. a Control showed positive reaction, indicating cellulose (arrow) and starch grains (arrowhead). b–d Treatment with 100 μM, 200 μM and 300 μM of Cd. Reaction showed an increase in the cell wall (arrows) and number of starch grains (arrowheads)
Finally, in control samples of G. domingensis stained with Coomassie Brilliant Blue (CBB), the most external layer of the cortical region, the cytoplasm, reacted more intensely than other cells, indicating the presence of nuclei, as well as numerous organelles rich in protein. Pit connections between cortical and subcortical cells could be observed with this stain. The cytoplasm of cortical and subcortical cells of treated plants was uniformly marked with CBB, indicating a homogeneous distribution of organelles or structures rich in protein. However, the color was more intense than that observed in the cytoplasm of control cells (Fig. 3b–d). With this reaction, the nuclei became more evident (Fig. 3a–d).
Light microscopy of G. domingensis thallus transversal sections stained with CBB. a Control showed a positive reaction with the nuclei (arrowhead) and with all cellular proteins. Pit connections with positive reaction (arrow). b–d Treatment with 100 μM, 200 μM and 300 μM of Cd. The cytoplasm shows the highest density of cellular reaction. The nuclei have become more evident, and the pit connections are visible
Observations under TEM
When observed by transmission electron microscopy, the cortical and subcortical cells of control samples were somewhat vacuolated and filled with chloroplasts, floridean starch grains and nuclei close to chloroplasts (Fig. 4a). These cells were surrounded by a thick cell wall, with microfibrils having different electron densities and embedded in an amorphous matrix consisting of sulfated polysaccharides[(Figs. 4a–b).
Transmission electron microscopy (TEM) micrographic images of control G. domingensis. a Detail of cortical and subcortical cells showing numerous starch grains and chloroplast embedded in thick cell wall. The nucleus is visible near the chloroplast. b Magnification of thick cell wall with microfibrils (arrows) having different electron densities and embedded in an amorphous matrix. c Detail of cortical cell with chloroplast and vacuole. Observe the thylakoid (arrows) and plastoglobuli. d Detail of cortical cell with starch grains near the nuclei and nucleolus. Observe the chloroplast and thylakoid (arrows). C: chloroplast, N: nuclei, Nu: nucleolus, CW: cell wall, P: plastoglobuli, S: starch grain
The chloroplasts assumed the typical internal organization of the red algae with unstacked, evenly spaced thylakoids (Figs. 4c–d). Electron-dense lipid droplets described as plastoglobuli were observed between the thylakoids (Figs. 4a–c). The cortical cells had a few starch grains in a perinuclear position. Nuclei and chromatin were diffuse, and a well-developed nucleolus was always present (Figs. 4c–d).
After 16 days’ exposure to 100 μM of the cadmium, G. domingensis plants were observed to undergo some ultrastructural changes, mainly involving the cell wall and chloroplasts (Figs. 5a–d). The cell wall showed an increase in thickness with deposition of concentric microfibril layers (Fig. 5a and b). The chloroplasts showed visible changes in ultrastructural organization with irregular morphology (Fig. 5c and d). The thylakoids were disrupted, and the number of plastoglobuli was increased in the chloroplasts (Fig. 5c and d).
Transmission electron microscopy (TEM) micrographic images of G. domingensis treated with 100 μM of cadmium. a Detail the cortical cell showing numerous starch grains and chloroplast. Observe the cell wall showing an increase in thickness with deposition of concentric microfibril layers. b Cell wall with concentric microfibrils (arrows) embedded in an amorphous matrix. c Observe the increase in plastoglobuli. d Detail of the chloroplast showing disrupted thylakoids
In plants treated with 200 μM of cadmium, changes similar to plants treated with 100 μM of cadmium were observed. The cortical cells showed irregular morphology (Fig. 6a). The cell wall, which appeared with black deposits, most likely Cd, showed an increase in thickness with deposition of concentric microfibril layers (Fig. 6b). The chloroplasts were disrupted, and an increase in plastoglobuli could be observed (Fig. 6c). Compared to control cells, it was also possible to detect an increase in the density of starch grains in the cortical and subcortical cells (Fig. 6d). Finally, an increase in vacuole volume with a large quantity of electron-dense points could be seen (Fig. 6f).
Transmission electron microscopy (TEM) micrographic images of G. domingensis treated with 200 μM of cadmium. a Detail of the subcortical cell shows numerous starch grains and chloroplasts embedded in a thick cell wall. b Cell wall with concentric microfibrils embedded in an amorphous matrix with black deposits (arrows). c Disrupted chloroplast. Observe a genophore (arrowhead). d Detail of the subcortical cell shows numerous starch grains and chloroplasts embedded in a thick cell wall. e Observe the increased vacuole volume. f Magnification of the vacuole. Observe the increased vacuole volume and black deposits (arrows)
In plants treated with 300 μM of cadmium, there were more dramatic changes when compared to plants treated with 100 and 200 μM of cadmium. For example, in the cortical cells, we observed an increase in cell wall thickness (Fig.7a–b), with deposition of concentric microfibril layers and black deposits (Fig. 7b), most likely cadmium.
Transmission electron microscopy (TEM) micrographic images of G. domingensis treated with 300 μM of cadmium. a Detail of the cortical cell shows numerous disrupted chloroplasts. Observe the vacuole (arrow). b Cell wall with concentric microfibrils embedded in an amorphous matrix with black deposits (arrow). Observe chloroplast with disrupted thylakoids. c Disrupted chloroplast and higher vacuole volume with black deposits. d Cortical cell shows numerous disrupted chloroplasts and vesicles (arrows). e Observe the disrupted chloroplasts and increased plastoglobuli with different shapes
Some chloroplasts appeared without thylakoids (Fig. 7c–e) or with disrupted thylakoids, and an increase in the number of plastoglobuli with different shapes could be seen (Fig. 7e).
Observations under SEM
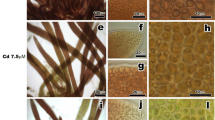
When observed by scanning electron microscopy (SEM), the surface of the tallus of the G. domingensis control showed a smooth surface in the cell wall of the cortical cells (Fig. 8a). However, plants treated with cadmium showed a gradually increasing roughness of surface (Fig. 8b–d), apparently the result of heavy metal absorption. In transverse sections (Fig. 8e–i), the cortical cells were smaller than subcortical cells in the G. domingensis control (Fig. 8e), and it was possible to observe many floridean starch grains. In treated plants, however, we observed an increase in cell wall thickness (Fig. 8f–i), as well as an increase in the number of the starch grains (Fig 8f–h).
Scanning electron microscopy (SEM) of the surface (a)–(d) and transverse sections (e)–(i) of the G. domingensis thallus. a Detail of the smooth cell wall surface of control cortical cells. b Plants treated with 100 μM of cadmium show an increased surface roughness. c Plants treated with 200 μM of cadmium show even more surface roughness. d Plants treated with 300 μM of cadmium show still more surface roughness. e Detail of control thallus transverse section showing cortical and subcortical cells with starch grains. f–h Plants treated with 100 μM, 200 μM and 300 μM of Cd of cadmium show an increase in cell wall thickness and an increase in the number of starch grains. i Detail of cell wall thickness
The results of X-ray microanalysis of G. domingensis have to be considered as qualitative. X-ray microanalysis of the control cell wall surface revealed the presence of different peaks of elements, mainly sulfur. No cadmium was detected on the surface of the control cell wall (Fig 9a). We were also unable to detect cadmium in G. domingensis treated with doses of 100 and 200 μM (Fig. 9b and c), as confirmed by X-ray microanalysis. Nonetheless, the presence of higher sulfur peaks in plants treated with 100 and 200 μM of cadmium was observed (Fig. 9b and c). Cadmium was found in the cell wall surface of G. domingensis treated 300 μM of cadmium, as well as an increase in sulfur peaks (Fig. 9d).
X-ray microanalysis of cell wall in both control and treated plants. a The control analysis is negative for cadmium. Observe the sulfur peak. (b–c) The treatment with 100 and 200 μM concentrations without cadmium peaks. Note the presence of a higher sulfur peak. d The treatment with 300 μM showed the presence the cadmium peak, as well as a higher sulfur peak
Discussion
The present study showed that increasing concentrations of cadmium induced changes in cellular organization, chloroplast morphology, as well as cell wall thickness and number of floridean starch grains, in the agarophyte G. domingensis.
The cell walls of both control and Cd-treated plants reacted positively to TB-O. This metachromatic reaction is used in histological studies as an indicator of acidic polysaccharides (Gordon-Mills et al. 1978). A violet metachromatic color with TB-O is produced by polysaccharides with carboxyl and sulfated groups (Mc Cully 1968). In our study, TB-O staining did produce a violet metachromatic reaction in the cell walls of all sections of G. domingensis similar to that seen in green, red and brown strains of Kappaphycus alvarezii (Schmidt et al. 2009, 2010b, c), Chondrus crispus (Gordon and McCandless 1973), Chondria tenuissima C. Agardh (Tsekos et al. 1985) and H. musciformis (Bouzon 2006). Since the agar present in the cell walls of G. domingensis is rich in sulfated groups (Schmidt et al. 2010a), a more intense reaction can be expected in the cell walls of both the control and treated plants. This interpretation is based on the assumption that the configuration of each sulfated tertiary polyanion exposes the polymer, forming a large amount of complex with the TB-O (Gordon and McCandless 1973). In the vacuoles, granulations appeared as reserves of complex polysaccharides and could be sites of accumulation of material which was subsequently incorporated into the cell wall. Overall, this finding indicates an increase in cell wall thickness, which was observed in some subcortical cells of treated plants after TB-O staining and by microanalysis with increased sulfur peaks.
Such increase in cell wall thickness observed in treated plants can be interpreted as a defense mechanism against exposure to cadmium (Bouzon et al. 2012). When analyzed with EDS, the cell wall of the samples cultivated with cadmium showed a high concentration of sulfur in the cell wall. The sulfated polysaccharides that compose the cell wall, such as agar and carrageenan, are responsible for most of the metal accumulation and metal-binding. In the case of carrageenan, metal-binding is associated with the degree of sulfatation (Diannelidis and Delivopoulos 1997). Thus, we concluded that the increase in sulfur concentration in the cell wall of G. domingensis must be related to cadmium retention.
Under TEM, the cell wall of control G. domingensis showed a microfibrillar texture with microfibrils structured in concentric layers with different degrees of compression. Observations of the cell wall of plants exposed to cadmium showed an increase in that structural component. It is possible that the activity of Golgi bodies is more intense in plants exposed to cadmium and that this, in turn, results in the large production of vesicles which then form at the matrix content of the cell wall (Bouzon et al. 2012). PAS reaction, as observed under LM, indicates greater deposition of neutral polysaccharides, such as cellulose. The cell walls of plants exposed to cadmium showed electron-dense granules when examined by TEM. Thus, we suggest that the granules observed in the cell walls of the samples of G. domingensis are deposits of this metal. Talarico (2002) also observed electron-dense granules in the cell wall of Audouinella saviana after cultivation in medium containing cadmium. However, the mechanism of cell wall thickness, as observed in G. domingensis, did not prevent cadmium from reaching the cytoplasm. The presence of precipitates in the vacuole of G. domingensis exposed to cadmium could be associated with a metal-immobilizing function to minimize cadmium stress. According to Talarico (2002), the electron-dense granules present in the vacuoles of Audoniella saviana are cadmium deposits.
By positive PAS reaction, numerous starch grains were found in both cortical and subcortical cells of algae exposed to cadmium. The concentration of these grains indicated that the reserves are concentrated in photosynthetic subcortical cells. This increase in the number of starch grains could be associated with morphological changes, such as a decrease of thylakoids, as observed in cells subjected to cadmium. Bouzon et al. 2012 observed a decrease in starch grains in H. musciformis after treated with different concentration of cadmium.
The ultrastructure of chloroplasts is a characteristic of great taxonomic value in algae, and the thylakoid arrangement of the different phyla is one of the most important features confirming traditional taxonomy (Dodge 1973). In red algae, the thylakoids not associated with one another are free in chloroplasts. The chloroplasts of the control G. domingensis showed a structure typical of red algae, with one peripheral thylakoid surrounded by parallel thylakoids. The number of parallel thylakoids is variable, and this number mainly depends on the spatial location of the cell in the algae. In contrast, the chloroplasts of G. domingensis exposed to cadmium showed significant structural changes, including modification in the quantity, size, and organization of thylakoids. Similar results were observed with the red macroalga Ceramium ciliatum exposed to cadmium, where the chloroplast appeared with disrupted thylakoids and an increase in plastoglobuli (Diannelidis and Delivopoulos 1997). Finally, when analyzed by TEM, G. domingensis exposed to Cd revealed an increase in the number of the plastoglobuli in the chloroplast. According to Holzinger et al. (2009), when algae are subjected to stress, nitrogen reduction and the synthesis of lipids are observed. These phenomena occur because pathways for the formation of protein-containing cell structures are suppressed.
In summary, the present study demonstrates that cadmium negatively affects the intertidal macroalgae G. domingensis. Specifically, Gracilaria domingensis treated with 100, 200 and 300 μM of cadmium after 16 days showed 1) changed morphology, 2) increased starch grains, 3) disrupted thylakoids, and 4) increased cell wall thickness and vacuole volume.
References
Andrade LR, Farina M, Amado Filho GM (2002) Role of Padina gymnospora (Dictyotales, Phaeophyceae) cell walls in cadmium accumulation. Phycologia 41:39–48
Andrade LR, Farina M, Amado Filho GM (2004) Effects of copper on Enteromorpha flexuosa (Chlorophyta) in vitro. Ecotoxicol Environ Saf 58:117–125
Armisen R (1995) Word-wide use and importance of Gracilaria. J Appl Phycol 7:231–243
Bouzon ZL (2006) Histoquímica e ultra-estrutura da ontogênese dos tetrasporângios de Hypnea musciformis (Wulfen) J. V. Lamour. (Rhodophyta, Gigartinales). Rev Bras Bot 29(2):229–238
Bouzon ZL, Ferreira EC, Santos R, Scherner F, Horta Paulo A, Maraschin M, Schmidt EC (2012) Influences of cadmium on fine structure and metabolism of Hypnea musciformis (Rhodophyta, Gigartinales) cultivated in vitro. Protoplasma. doi:10.1007/s00709-011-0301-6
Diannelidis BE, Delivopoulos SG (1997) The effects of zinc, copper and cadmium on the fine structure of Ceramium ciliatum (Rhodophyceae, Ceramiales). Mar Environ Res 44(2):127–134
Dodge JD (1973) The fine structure of algal cells. Academic, London, New York, 261 pp
Edwards P (1970) Illustrated guide to the seaweeds and sea grasses in the vicinity of Porto Aransas, Texas. Contrib Austin Mar Sci 15:1–228
Gahan PB (1984) Plant Histochemistry and cytochemistry: an introduction. Academic, London, 301 pp
Gordon EM, McCandless EL (1973) Ultrastructure and histochemistry of Chondrus crispus Stackhouse. In: Harvey MJ, McLachlan J (Eds), Chondrus Crispus: T. N. Scot. Inst. Sci., Halifax, pp 111–133
Gordon-Mills EM, Tas J, Mc Candless EL (1978) Carrageenans in the cell walls of Chondrus crispus Stack. (Rhodophyceae, Gigartinales). III. Metachromasia and the topological reaction. Phycologia 17:95–104
Hashim MA, Chu KH (2004) Biosorption of cadmium by brown, green and red seaweeds. Chem Eng J 97:249–255
Holzinger A, Roleda MY, Lütz C (2009) The vegetative arctic freshwater green alga Zygnema is insensitive to experimental UV exposure. Micron 40:831–838
Hu S, Tang CH, Wu M (1996) Cadmium accumulation by several seaweeds. Sci Tot Environ 187:65–71
Kain JM, Destombe C (1995) A rewiew of the life history, reproduction and phenology of Gracilaria. J App Phycol 7:269–281
Mamboya FA, Pratap HB, Mtolera M, Bjork M (1999) The effect of copper on the daily growth rate and photosynthetic efficiency of the brown macroalga Padina boergensenii. In: Richmond MD, Francis J (eds) Proceedings of the Conference on Advances on Marine Sciences in Tanzania pp 185–192
Mc Cully ME (1968) Histological studies on the genus Fucus. Protoplasma 62:20–40
Oliveira EC, Plastino EM (1994) Gracilariaceae. In: Akatsuka I (ed) Biology of economic seaweeds. SPB Acad. Publis., The Hague, pp 185–226
Oliveira EC, Paula EJ, Plastino EM, Petti R (1995) Metodologías para el cultivo no axenico de macroalgas marinas in vitro. In: Alveal K, Ferrario M, Oliveira E, Sar E (eds) Manual de métodos ficológicos. Universidad de Concepción, Concepción-Chile, pp 429–447
Pinto E, Sigaud-Kutner TCS, Leitão MAS, Okamoto OK, Morse D, Colepicolo P (2003) Heavy metal-induced oxidative stress in algae. J Phycol 39:1008–1018
Reynolds ES (1963) The use of lead citrate at light pH as an electron opaque stain in electron microscopy. J Cell Biol 17:208–212
Rocchetta I, Leonardi PI, Amado Filho GM, de Molina MDR, Conforti V (2007) Ultrastructure and X-ray microanalysis of Euglena gracilis (Euglenophyta) under chromium stress. Phycologia 46:300–306
Schmidt EC, Scariot LA, Rover T, Bouzon ZL (2009) Changes in ultrastructure and histochemistry of two red macroalgae strains of Kappaphycus alvarezii (Rhodophyta, Gigartinales), as a consequence of ultraviolet B radiation exposure. Micron 40:860–869
Schmidt EC, Santos R, Horta PA, Maraschin M, Bouzon ZL (2010a) Effects of UVB radiation on the agarophyte Gracilaria domingensis (Rhodophyta, Gracilariales): Changes in cell organization, growth and photosynthetic performance. Micron 41:919–930
Schmidt EC, Maraschin M, Bouzon ZL (2010b) Effects of UVB radiation on the carragenophyte Kappaphycus alvarezii (Rhodophyta, Gigartinales): changes in ultrastructure, growth, and photosynthetic pigments. Hydrobiologia 649:171–182
Schmidt EC, Nunes BG, Maraschin M, Bouzon ZL (2010c) Effect of ultraviolet-B radiation on growth, photosynthetic pigments, and cell biology of Kappaphycus alvarezii (Rhodophyta, Gigartinales) macroalgae brown strain. Photosynthetica 48:161–172
Schmidt EC, Pereira B, Mansur C, Santos R, Scherner F, Horta PA, Martins RP, Latini A, Maraschin M, Bouzon ZL (2012) Alterations in architecture and metabolism induced by ultraviolet radiation-B in the carragenophyte Chondracanthus teedei (Rhodophyta, Gigartinales). Protoplasma 249:353–367
Sheng PX, Ting Y, Chen JP, Hong L (2004) Sorption of lead, copper, cadmium, zinc and nickel by marine algal biomass: characterization of biosorptive capacity and investigation of mecanisms. J Coll Int Sci 275:131–141
Talarico L (2002) Fine structure and X-ray microanalysis of a red macrophyte cultured under cadmium stress. Environ Pollut 120:813–821
Talarico L, Bozo S, Maranzana G (1997) Preliminary observations on Audouinella saviana (Meneghini) Woelkerling (Nemaliales, Rhodophyta) cultured at increasing Cd concentrations. Phycologia 36:111
Tsekos I, Schnepf E, Makrantonakis A (1985) The ultrastructure of tetrasporogenesis in the marine red alga Chondria tenuissima (Good. Et Woodw.) (Ceramiales, Rhomelaceae). Ann Bot 55:607–619
Visvik I, Rachlin JW (1992) Ultraestructural changes in Dunaliella minuta following acute and chronic exposure to copper and cadmium. Arch Environ Contam Toxicol 23:420–425
Xia JR, Li YJ, Lu J, Chen B (2004) Effects of copper and cadmium on growth, photosyntesis, and pigment content in Gracilaria lemaneiformis. Bull Envirom Contam Toxicol 73:979–986
Acknowledgments
The authors would like to acknowledge the staff of the Central Laboratory of Electron Microscopy (LCME), Federal University of Santa Catarina, Florianopolis, Santa Catarina, Brazil, for the use of their scanning and transmission electron microscope. This study was supported in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) and Fundação de Apoio à Pesquisa Cientifica e Tecnológica do Estado de Santa Catarina (FAPESC).
Conflict of interest
The authors declare that they have no conflict of interest.
Contributors
Both authors contributed equally to this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Tsuneyoshi Kuroiwa
Rights and permissions
About this article
Cite this article
dos Santos, R.W., Schmidt, É.C. & Bouzon, Z.L. Changes in ultrastructure and cytochemistry of the agarophyte Gracilaria domingensis (Rhodophyta, Gracilariales) treated with cadmium. Protoplasma 250, 297–305 (2013). https://doi.org/10.1007/s00709-012-0412-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-012-0412-8