Abstract
The primary patterns of alterations in the primnesiophyte microalga Isochrysis galbana’s morphological, structural-functional, fluorescence, and cytometric characteristics under the impact of various concentrations of copper in the ionic and nanoform have been investigated. It has been demonstrated that copper in the form of nanoxide is approximately 10 times less hazardous to microalgae than copper in its ionic form. The two types of copper under investigation had similar effects on cells, including growth inhibition, a reduction in the effectiveness of the photosynthetic apparatus, an increase in the volume and intracellular concentration of chlorophyll a, an increase in the production of reactive oxygen species, and a decrease in the activity of the enzyme esterase. The surface of the cell wall is significantly affected mechanically by CuO NPs, which is a distinguishing characteristic. The final density of cultures in the stationary growth phase depended on the introduced concentration of the contaminant and, naturally, decreased with an increase in the initial copper content in the medium. At sublethal concentrations of pollutants, I. galbana culture was divided into two subpopulations of cells with different functional activities: sensitive and resistant to copper. The latter are able to ensure the survival and subsequent recovery of the I. galbana population under favorable growth conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The widespread use of nanotechnology products in various fields of human activity makes the issue of assessing its impact on the environment particularly relevant. At the same time, the front of experimental studies on the assessment of the toxicity of NPs is noticeably lagging behind the rapid progress of nanotechnology. The growth in the production of nanomaterials and their successful implementation cause natural concerns about the manifestation of specific nanoscale effects for living organisms. The inhabitants of aquatic ecosystems are in a dangerous risk zone. World annual production of copper oxide NPs (CuO NPs) in 2014 was approximately 570 t/year and, according to forecasts, it will be 1600 t/year by 2025 (Keller et al., 2013). CuO NPs are mainly used as biocides (Ivask et al., 2014), in Xiong et al. (2017), and are included in most antifouling paints (Adeleye et al., 2016), as a result of which they fall into reservoirs, thereby creating a potential danger to aquatic organisms.
Phytoplankton is an important component of aquatic ecosystems, is the primary producer of organic matter, and is the basis of all trophic interactions. As a result, microalgae are potentially the most susceptible to NPs at different levels: reproductive (population growth), structural, and metabolic (Navarro et al., 2008a, b). The main mechanisms of the NPs’ effect on algae are adsorption on cell walls, which can subsequently lead to the destruction of membranes or self-shadowing of cells; the release of metal ions; and the internalization of nanoparticles into algae cells (Zhang et al., 2020). In addition, cultures of oxygenic phototrophic organisms, mostly microalgae, are widely used in the field of environmental protection, which is due to the ability to trace the impact of an adverse factor on both the cell and the population as a whole for many generations and to identify the long-term consequences of such an impact (Vasilieva et al., 2022). In modern bioanalysis of water toxicity, in which microalgae are used as test objects, the growth rate, the number of cells, and their viability are the most widely controlled parameters. Of course, these parameters are informative from an ecological point of view since they allow us to identify and assess the degree of negative influence of a particular pollutant on a living organism (Prado et al., 2012).
However, this approach shows only the general reaction of the algae culture to the presence of pollutants in the environment and does not allow obtaining information about the effect of the toxicant on individual cells, which can vary greatly in the degree of resistance to the action of the toxicant (Traller & Hildebrand, 2013). In turn, the study of the morphological and physiological properties of individual cells under optimal and stressful growth conditions allows us to identify the mechanisms of acclimation to stress factors and, as a consequence, the survival of the population in changing environmental conditions (Armbrust & Chisholm, 1992; Bucci et al., 2012). However, experimental studies showing that algae cells can have different physiological statuses under the same stress are fragmentary.
Another important aspect touched upon in this paper is the choice of the object of research. The primnesiophyte microalgae Isochrysis galbana (Haptophyta) is a widely used food object and is included in the diet of most mollusk larvae, zooplankton, as well as fish and crustaceans in the early stages of growth due to its optimal cell size for absorption and high nutritional value (Aganesova, 2021; Mzozo et al., 2019). There are very few works in the literature devoted to assessing the effect of metal nanoparticles on this type of microalgae, which can be considered one of the carriers of nanoparticles along the trophic chain as a result of their adsorption on the cell surface.
The purpose of this study was to evaluate the physiological response of the primnesiophyte microalgae Isochrysis galbana to the effects of copper oxide nanoparticles and copper ions of different concentrations. To achieve this goal, the following tasks were solved: (1) to evaluate the dynamics of growth, structural, fluorescent, and cytometric indicators of algae at different concentrations of copper in the ionic and nanoform; (2) to evaluate the effect of the introduced concentration of the pollutant on the final density of the culture in the stationary phase of growth; and (3) to determine the threshold concentrations of Cu2+ and CuO NPs that inhibit the growth and potential photosynthetic activity of Isochrysis galbana and the possible adaptive potential of algae at these values.
2 Materials and Methods
2.1 Object of Investigation
An algologically pure culture of the primnesiophyte microalgae Isochrysis galbana Parke, 1949, from the collection of marine planktonic microalgae of the Scientific and Educational Center for Collective Use “Hydrobionts of the World Ocean” (WDCM No. 1201) of A.O. Kovalevsky Institute of Biology of the Southern Seas of RAS (IBSS) was used as an object of study.
2.2 Experimental Conditions
During the experiment, algae were grown on a fivefold diluted Goldberg nutrient medium (Kabanova, 1961). The concentrations of components in final medium were KNO3 40.4 mg·L−1, NaH2PO4 1.42 mg·L−1, FeCl3·6H2O 0.054 mg·L−1, MnCl2·4H2O 0.04 mg·L−1, and CoCl2·6H2O 0.048 mg·L−1. The use of a diluted nutrient medium in the experiment allowed to avoid a large cell abundance of I. galbana during cultivation. The study of copper toxicity in dense cultures is impractical, since extracellular organic substances released by algae are able to bind copper ions into chelate complexes, reducing its toxicity, and therefore the results of the experiment may be distorted. The initial cell concentration of I. galbana in all variants of the experiment was 6·104 cells·mL−1.
Algae were cultivated in conical flasks with a culture suspension volume of 150 mL at an illumination intensity of 85 µE·m−2·s−1, a light-dark period of 14/10 h, and a temperature of 20 °C. The flasks were illuminated from below with cold light LEDs. The illumination level was determined by a 4π sensor of a QSL 2101 quantometer (Biospherical Instruments Inc., USA). The cultures were bubbling with aquarium compressors; the pH value of the culture medium was in the range of 8.2–8.4. Prior to the start of the experiment, the algae were adapted to the specified abiotic growth conditions for 3 days. At the same time, the density of the cultures was maintained at approximately the same level by daily dilution with fresh nutrient medium. On the fourth day of the experiment, pollutant solutions were added to the flasks. Further cultivation of algae was carried out in a cumulative mode for 9 days until the algae reached a stationary phase of growth. Measurement of all the studied parameters was carried out daily. This approach made it possible to evaluate the dynamics of the main structural and functional indicators of algae at different concentrations of pollutants in the medium at different stages of cumulative growth (from the log-phase to the stationary phase).
Solutions of toxicants were prepared using copper sulfate CuSO4 × 5H2O (ACS reagent, ≥ 98.0%, Sigma-Aldrich, USA) and copper oxide nanopowder with an average particle size of 50-80 nm (99.8% purity, Sigma-Aldrich, USA). Toxicant solutions were prepared from the CuSO4 × 5H2O mother solution with concentrations of 75, 150, 225, and 375 μg·L−1 (in terms of Cu2+ ions). The solution containing copper oxide nanoparticles was preliminarily sonicated using a Sapphire ultrasonic bath for 1 h to disperse the particles. The concentration of copper oxide nanoparticles in different variant of the experiment was 1000, 2500, and 3750 μg·L−1 (in terms of Cu2+ ions). The choice of the toxicant concentrations is due to the previously obtained data on the degree of copper toxicity to I. galbana. The selected ranges of pollutant concentrations made it possible to determine the threshold values of Cu2+ and CuO NPS that inhibit the growth and potential photosynthetic activity of I. galbana, as well as to calculate the EC50 and LC50 values for this species. Algae cultivated on a medium without of copper were used as a control. Three experimental flasks were used in each variant of the experiment.
2.3 Measurement Methods
Cytometric analysis of samples was carried out at the Center for Collective Use Spectrometry and Chromatography of A.O. Kovalevsky Institute of Biology of the Southern Seas of RAS, using a MACS Quant Analyzer flow cytometer (Miltenyi Biotec, Germany) equipped with three lasers (405 nm, 488 nm, 635 nm). The FSC Express 7 Research Edition software was used for data processing. The total number of microalgae in cultures was determined according to the method described by us earlier (Solomonova & Mykhanov, 2011).
The specific growth rate of microalgae was calculated using the following Eq. (1):
where µ is the specific growth rate of algae, days−1; N0 and Nt are initial concentration of cells and their number after time t, cells·mL−1; and t is the time between measurements, days.
Production of reactive oxygen species (ROS) in I. galbana was evaluated using the fluorescence of 2–7-dichlorofluoresceindiacetate dye (H2DCF-DA; Merck, Germany). H2DCF-DA is cell-permeable indicator of ROS, most often used for their quantitative assessment (Wang & Joseph, 1999). The cell suspension was stained according to (Wang & Joseph, 1999). Fluorescence intensity H2DCF-DA was evaluated by the fluorescence of cells in the green region of the spectrum, 525 nm; the autofluorescence of I. galbana cells in this region of the spectrum was preliminarily subtracted.
Cell metabolic activity (relative value) was assessed using two parametric cytograms by FDA fluorescence (fluorescein diacetate) (FL1 channel in the green region of the spectrum, 525 nm) and cell autofluorescence in the red region (FL4) of the spectrum on dimensionless logarithmic scales. FDA, which contains a substrate, is specific to enzymes of the esterase group; it is a marker of metabolic activity in living cells, and the intensity of its fluorescence is proportional to the physiological activity of each of the studied cells (Solomonova & Mykhanov, 2011). Algae were stained with the vital dye fluorescein diacetate (FDA) according to the protocol (Solomonova & Mykhanov, 2011). The average values of the accumulated FDA fluorescence per cell were calculated, the I. galbana cell autofluorescence values in this region of the spectrum were preliminarily subtracted. The ratio of painted to unpainted cells was used to determine the percentage of live cells in the solution.
The fluorescence of the algae pigment complex was measured using a Mega-25 m fluorometer with pulsed excitation light modulation at a wavelength of 455 nm, developed at the Department of Biophysics, Faculty of Biology, Moscow State University (OOO Gene and Cell Therapy, Russia) (Pogosyan & Matorin, 2005). Before measurement, the samples were kept in the dark for 15 min.
The relative variable fluorescence, which characterizes the maximum quantum efficiency of the use of light energy, was calculated using Formula (2):
where F0 is chlorophyll a fluorescence with open reaction centers, measured after 2.5 µs from the beginning of irradiation with an intensity of 5000 µE·m−2·s−1; Fm is maximum chlorophyll a fluorescence with closed reaction centers, measured after 1 s from the beginning of irradiation with an intensity of 5000 µE·m−2·s−1.
Maximum relative electron transport rate of PS II at light intensity 500 µE·m−2·s−1 and exposure time of 40 s were calculated using the following Formula (3):
where F'm is maximum chlorophyll a fluorescence against the background of constant lighting, F'0 is zero chlorophyll a fluorescence with constant lighting, and I is the intensity of constant illumination.
Determination of chlorophyll a concentration in the sample was carried out using the fluorimetric method (Jeffrey et al., 1997). Fluorescence measurements were carried out using a Mega-25 m laboratory fluorometer with pulse modulation of exciting light at a wavelength of 455 nm (Pogosyan & Matorin, 2005). The preliminary calibration of the fluorimeter was carried out using chromatographically pure chlorophyll a (Sigma, USA), the initial concentration of which was determined using an SF-2000 spectrophotometer (LOMO, Russia). Calculation of the specific chlorophyll a content (pg/cell) in algal cells was made by dividing the total concentration of a given pigment by the number of culture cells for each individual case.
Microscopy
Light microscopy (LM) was performed using a Mikmed-6 microscope (LOMO, Russia) at × 1000 magnification with an MS-6.3 digital camera (LOMO, Russia) equipped with MCVeiw software (LOMO-Microsystems, Russia)on the basis of the Center for Collective Use Molecular Structure of Matter of the Sevastopol State University. Cell sizes were determined based on micrographs using the Image J 1.50i program (National Institutes of Health, United States, Java 1.6.0_20 (32-bit). The volume was calculated using the formula (Bryantseva et al., 2005).
The protocol (Murtey & Ramasamy, 2016) was used to prepare samples for a scanning electron microscope (SEM). The samples were dried at the critical point (1.5–2.5 h) using a Leica EM CPD300 device (Germany). For sputtering (Au/Pd; 0.5–1.0 min), a Leica EM ACE200 device (Germany) was used. The samples were examined using a Hitachi SU3500 scanning electron microscope (Japan) at a magnification from × 500 to × 5000. Energy-dispersive X-ray spectroscopy was performed using an Oxford instruments ULTIM MAX 65 X-ray detector (UK).
Statistical Analysis
Statistical data processing was carried out using standard software packages Microsoft Excel 7.0 (Microsoft Office) and Grapher-16 (Golden Software). The values shown in the graphs and in the table are the average of three measurements. The significance of differences between the samples was assessed by Student’s t-test with a significance level of P < 0.05.
3 Results and Discussion
3.1 The Effects of Copper Ions (Cu2+) and Nanoparticles of Copper Oxide (CuO) on the Dynamics of the Number of Isochrysis galbana Cells Under Enrichment Cultivation Conditions
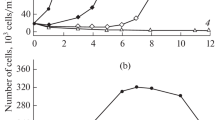
Figure 1 shows the dynamics of changes in the number of I. galbana cultures when Cu2+ copper ions and copper oxide nanoparticles of different concentrations are added to the medium. The introduction of Cu2+ into the cultivation medium at concentrations of 75 and 150 μg·L−1 led to a reliably slowdown in the growth rate of algae (unpaired t-test, p < 0.05); nevertheless, the increase in the number of crops was carried out throughout the experiment (Fig. 1a). At a concentration of copper ions of 225 μg·L−1, a slight growth of algae was observed on the first day of the experiment; a growth arrest was noted on day 2, while the number of cells remained almost at the same level (11–12·104 cells·mL−1) until the end of the exposure. Three days after the introduction of the pollutant, the specific growth rate of I. galbana was 0.67, 0.51, 0.31, and 0.12 day−1 at a copper concentration of 0, 75, 150, and 225 μg·L−1, respectively (Table 1). The copper concentration of 375 μg·L−1 was lethal for I. galbana, causing rapid and irreversible degradation of the culture under study. The EC50 and LC50 values were 105 and 345 μg·L−1, respectively. These values were calculated based on cell abundance 72 h after Cu2+ addition. When CuO nanoparticles were added to the medium at concentrations of 1000, 2500, and 3750 μg·L−1 on the first day of the experiment, the growth rate of algae did not significantly differ from the control (unpared t-test, p < 0.05) (Fig. 1b). On the second day, a concentration-dependent decrease in the growth rate of algae was observed with an increase in the content of CuO NPs in the medium. Starting on the third day of the experiment, at a concentration of CuO nanoparticles of 1000 μg·L−1, a slowdown in the rate of cell division and a gradual transition of the culture to a stationary growth phase were observed. At concentrations of 2500 and 3750 μg·L−1, a progressive decrease in the number of cells in the culture suspension was noted. The EC50 value calculated based on cell abundance 72 h after CuO NPs addition were 1300 μg·L−1.
The final density of cultures in the stationary growth phase depended on the introduced concentration of the pollutant; it naturally decreased with an increase in the initial copper content in both ionic and nanoform in the medium. In the control version of the experiment without the addition of copper, the number of I. gabbana cells in the stationary growth phase was 13.5·105 cells·mL−1, while Cu2+ was added to the medium at concentrations of 75, 150, and 225 μg·L−1, the final density of the cultures decreased to 50, 30, and 10·104 cells·mL−1, respectively. The maximum number of I. galbana with the introduction of CuO NPs at concentrations of 1000 and 2500 μg·L−1 was 35 and 16·104 cells·mL−1, respectively. In a cumulative algae culture, the final cell density is determined mainly by the exhaustion of one or more nutrients in the medium (Yaakob et al., 2021). The difference we observed in the final density of the I. galbana culture is probably explained by the elimination of a significant proportion of cells in the first hours after the introduction of pollutants into the culture medium.
3.2 The Impact of Copper Ions and Copper Nanoparticles on the Pigment Content and Efficiency of the Photosynthetic Apparatus in Isochrysis galbana
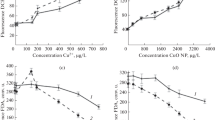
An increase in the concentration of Cu2+ in the medium from 0 to 225 μg·L−1 and CuO NPs from 0 to 2500 μg·L−1 led to an reliably increase in the intracellular concentration of chlorophyll a from 0.5 to 1.1 pg·cell−1 (unpared t-test, p < 0.05) (Table 1). At a concentration of Cu2+ of 375 μg·L−1 and CuO NPs of 3750 μg·L−1, on the contrary, the specific content of chlorophyll in cells decreased to 0.3 pg·cell−1, indicating the degradation of chlorophyll molecules and the suppression of pigment biosynthesis under the action of a pollutant.
An increase in the concentration of copper in the medium led to a decrease in the efficiency of the photosynthetic apparatus of I. galbana. In the native sample (without the addition of copper), the variable fluorescence index of chlorophyll Fv/Fm, characterizing the quantum efficiency of photosystem 2, had a value of 0.75, and the relative rate of electronic transport rETR was 0.3. CuO NPs at a concentration of 1000 μg·L−1 and Cu2+ up to 150 μg·L−1 did not significantly affect Fv/Fm and rETR* cells. While the introduction of pollutants into the medium in a higher concentration led to a prolonged reliable decrease in Fv/Fm to 0.4–0.5 (unpared t-test, p < 0.05) and a drop in the relative speed of electronic transport to values less than 0.1 (Fig. 2).
Thus, inhibition of the growth of I. galbana manifests itself at a lower concentration of copper than a change in the photosynthetic activity of cells. The separation of the rates of photosynthesis and growth leads to the fact that cells continue to fix carbon, but at the same time they cannot divide or divide at a low rate. This is confirmed by a significant increase in cell volumes and the specific content of chlorophyll a in them when exposed to copper. Inhibition of algae growth against the background of high photosynthetic activity in cells may be associated with the interaction of copper ions and CuO nanoparticles with a protein responsible for regulating cell cycle events such as DNA replication and cell division (Magdolenova et al., 2014). According to the study (Schiavo et al., 2016), a twofold decrease in the rate of algae division was noted as the primary response to the effect of ZnO NPs. The authors explain this as a violation of the cell division cycle. While the effect of ZnO NPs on other physiological parameters of cells, including the structure of the DNA molecule, was noted at higher concentrations of the pollutant. Another possible reason for growth inhibition may be the blocking by a pollutant of the production of methionine involved in the process of cell division; as a result, cells do not divide but are characterized by high photosynthetic activity (Stauber & Florence, 1987).
A decrease in the efficiency of the I. galbana photosynthetic apparatus was noted at copper concentrations, causing pronounced inhibition of algae growth. In earlier studies, we have shown that the fluorescent parameters Fv/Fm and rETR* cannot be used as universal indicators of the physiological state of cells in conditions of copper contamination (Akimov et al., 2023; Shoman et al., 2022; Solomonova et al., 2022). This is due to the fact that copper (both in ionic form and in the form of nanoxide) has different effects on the efficiency of the photosynthetic apparatus in different types of algae. For example, in the green algae Dunaliella salina and the dinoflagellate Prorocentrum cordatum, the Fv/Fm coefficient was weakly dependent on the concentration of the pollutant in the medium and decreased only under conditions of crop elimination. At the same time, the Thalassiosira weissflogii diatom showed a regular decrease in Fv/Fm and rETR* parameters with an increase in the concentration of Cu2+ and CuO NPs in the medium. Analysis of the literature data also revealed a large disparity of results in the issue under study (Barreto & Lombardi, 2016; Xu et al., 2020).
3.3 The Impact of Copper Ions and Copper Oxide Nanoparticles on Isochrysis galbana FDA Fluorescence and ROS Generation
The introduction of CuO NPs and Cu2+ into the culture medium at concentrations of 1000 and 75–150 μg·L−1, respectively, did not affect the enzymatic activity of cells or the production of reactive oxygen species. At the indicated concentrations of pollutants, the values of FDA and D2CFH-DA fluorescence in I. galbana did not significantly differ from the control (unpared t-test, p < 0.05) and amounted to 70–80 and 15–25 r.u., respectively (Table 1). At higher concentrations of pollutants, a prolonged drop in the enzymatic activity of cells and an increase in the production of reactive oxygen species (ROS) were observed. At maximum concentrations of copper on the 3rd day of the experiment, a twofold decrease in FDA fluorescence and an increase in ROS production by about three times compared to the control were noted, indicating the development of oxidative stress in cells. An increase in ROS production is one of the first physiological responses of a cell to an adverse external influence. With an excessive amount of ROS, they damage proteins, nucleic acids, and membrane lipids and, ultimately, can cause apoptosis in cells (Sendra et al., 2018; Zhang et al., 2018). In turn, the decrease in fluorescence of the FDA indicates a decrease in the activity of enzymes in the group of esterases, cytoplasmic enzymes that hydrolyze esters into alcohols and acids and are an important element of the metabolism of phospholipids in cell membranes (Dorsey et al., 1989). The results obtained show the expediency of using changes in ROS production and esterase activity of cells as indicators of sublethal toxicity of copper, both in ionic form and in the form of CuO NPs.
3.4 The Effect of Copper Oxide Nanoparticles and Copper Ions on the Functional Heterogeneity of Isochrysis galbana cells
The physiological heterogeneity of a population manifests itself in the process of phylogeny, when specific and non-specific mechanisms of its adaptation to changing environmental conditions are formed. From the data presented in Fig. 3, it can be seen that in the native sample without the addition of copper, the population is 93% represented by cells with a high content of chlorophyll. At sublethal concentrations of pollutants (μg·L−1 Cu2+ and 2500 μg·L−1 CuO NPs) on the 3rd day of the experiment, the I. galbana culture was divided into two groups of cells: cells with high (region A) and low (region B) pigment content (Fig. 3b, c). At the same time, the number of cells in region A was 42 and 31% of the total number of living cells in culture with the addition of Cu2+ and CuO NPs, respectively. Cells from regions A and B reliably differed not only in chlorophyll content but also in size and fluorescence intensity of the FDA and ROS products (unpared t-test, p < 0.05) (Table 1, values indicated by an asterisk). So, in cells from region B, the chlorophyll content was 3 times lower, ROS production was 3 times higher than in cells from region A, and the fluorescence of the FDA was less than 20 r.u. In addition, cells from region B were characterized by a smaller size and greater granularity.
It should be noted that the division of the culture into two subpopulations of cells with different functional activities was noted only at sublethal concentrations of copper. The observed effect probably indicates the development of adaptation mechanisms in I. galbana, which contribute to maintaining the viability of the population in extremely unfavorable growth conditions due to the formation of copper-resistant cells. Thus, in the process of primary intoxication, the mass death of copper-sensitive cells is observed. However, exposure to high concentrations of Cu2+ and CuO NPs does not lead to complete elimination of the population, which is due to the presence in the culture of cells resistant to copper and capable of ensuring the survival and subsequent recovery of the I. galbana population under favorable growth conditions. Probably, these resistant cells appear in the population by chance through rare spontaneous mutations that occur upon contact with a pollutant and have more advanced mechanisms for its detoxification (Ipatova et al., 2018). On the other hand, it is known that in any population, there is a pool of initially resistant cells that also ensure the safety of the population (Ipatova et al., 2015).
Cells from region B are characterized by pronounced heterogeneity (CV = 88–112%) along the FS axis. This shows that the described group of algae is a “population of cells of various functional states, from live active to inactive, destroyed, and individual large fluorescent fragments that have preserved photosynthetic pigments. High CV values are characteristic of cultures at the elimination stage and, in the context of this experiment, indicate the mass death of copper-sensitive I. galbana cells.
3.5 Copper Ions and Copper Oxide Nanoparticles’ Effects on Cell Morphology Isochrysis galbana
Morphometric studies of the native sample (control) of I. gabbana showed that the cells had a spherical shape with two closely spaced flagella for movement, up to 7 microns long. The chloroplast is single and yellow-brown in color (Fig. 4a, e) (Tomas, 1997). In cells exposed to sublethal concentrations of the studied pollutants (Cu2+ = 225 μg·L−1 and CuO NPs = 2500 μg·L−1), the cytoplasmic membrane protruded beyond the cell wall (Fig. 3f, g). In the literature, this phenomenon is known as “blebbing” and is one of the distinguishing features of cell apoptosis (Wickman et al., 2013). During apoptosis, the cytoskeleton of the cell is destroyed, which causes the membrane to bulge out, forming apoptotic vesicles.
Morphological changes in Isochrysis galbana cells under different cultivation conditions according to data of scanning electron microscopy (a–d, h) and light microscopy (e-g): the native sample (the control) (a, e); adding of 225 µg·L−1 Cu2+ (b, f); adding of 2500 µg·L−1 CuO NPs (c, d); energy-dispersive X-ray spectroscopy (h). The scale bars correspond to 5 μm
CuO NPs had a mechanical effect on the surface of the cell wall of I. galbana. However, the deformation of cells under the action of NPs was expressed to a much lesser extent than in the previously described species, Prorocentrum cordatum, Thalassiosira weissflogii, and Dunaliella salina (Akimov et al., 2023; Shoman et al., 2022; Solomonova et al., 2022). Probably, this effect can be explained by the fact that the destruction of cells I. galbana as a result of the mechanical action of nanoparticles is accompanied by intensive lysis with rapid disintegration and dissolution of the destroyed components of algae cells, which is associated with the morphological features of this species. I. galbana are small-celled algae with an ultrathin cell membrane surrounding the cytoplasmic membrane; therefore, under lysis conditions, the plasmalemma makes up an insignificant part of the total substance in the cell. Nevertheless, according to SEM data, some I. galbana cells had perforations of the plasma membrane. White arrows indicate sites of damage evident in micrographs (Fig. 3b, c). In most I. galbana cells, adsorption of aggregates, presumably CuO NPs, on the surface of the cell walls was observed (Fig. 3d). Energy-dispersive X-ray spectroscopy of the elemental composition of these aggregates confirmed the presence of copper in their composition (Fig. 3h). NPs adsorption occurs due to electrostatic interactions and chemical bonds on the surface of microalgae cells (Adeleye & Keller, 2016). This kind of reaction to the presence of metallic nanoparticles in the medium is typical and is observed in many other species of microalgae (Aruoja et al., 2009; Sendra et al., 2018; Zhang et al., 2018). The danger of such interaction lies in the shading of cells as well as the deterioration of the integrity, permeability, and respiratory function of cell membranes (Adeleye et al., 2016).
The average cell volume in the control was 55 ± 3 µm3 (Table 1). When exposed to the sublethal concentration of copper ions (225 µg·L−1), it increased slightly to 63 ± 9 µm3. When adding CuO NPs to the medium at a concentration of 2500 µg·L−1, the average volume of I. galbana cells significantly increased by 1.4 times compared to the control, 77 ± 13 µm3 (unpaired t-test, p < 0.05). The addition of copper ions at a concentration of 0-375 µg·L−1 did not affect the lateral light scattering of SS cells, which characterizes their granularity; it did not differ from the control and amounted to 3–5 r.u. (Table 1). Cultivation of algae with CuO NPs at a concentration of 1000 µg·L−1 led to a prolonged increase in the values of lateral light scattering (SS) cells three times on the third day of the experiment and five times on the ninth. At a sublethal concentration of CuO NPs 2500 µg·L−1, as already noted above, the culture was divided into two subpolulations, differing in chlorophyll fluorescence, FDA fluorescence values, and SS values. In the group of cells that corresponded to the control in terms of fluorescent characteristics, the SS parameter did not change, whereas for the second subpopulation, a five- and six-fold increase in the lateral light scattering signal was noted on the third and ninth days of the experiment, respectively. At the lethal concentration of nanoparticles (3750 µg·L−1), respectively, a significant increase in SS values was observed from the first day of testing (Table 1). An increase in cell granularity upon exposure to nanoparticles has been reported multiple times in the literature for other algal species (Franklin et al., 2001; Pulido-Reyes et al., 2015). We have also shown this effect for other representatives of phytoplankton (Shoman et al., 2022; Solomonova et al., 2022). This is due to a number of effects that nanoparticles have on algae cells. The first reason is the deformation of the cell surface as a result of the mechanical action of metal nanoparticles on it. Another possible reason for the increase in the SS signal may be ultrastructural changes (mainly in lysosomes and vacuoles) due to the toxic effect of copper and/or internalization of individual nanoparticles inside the cell (Jamers et al., 2013).
4 Conclusion
The main regularities of changes in morphological, structural-functional, fluorescent, and cytometric parameters in the primnesiophyte microalgae Isochrysis galbana under the influence of different concentrations of copper in the ionic and nanoforms have been established. It has been shown that copper in ionic form is about 10 times more toxic to microalgae than in the form of nanoxide. Copper in the two studied forms had a similar effect on cells: inhibition of growth, decrease in the efficiency of the photosynthetic apparatus, increase in the volume and intracellular concentration of chlorophyll a, increase in ROS production, and drop in enzymatic (esterase) activity. A distinctive feature of CuO NPs is a pronounced mechanical effect on the surface of the cell wall. The final density of cultures in the stationary growth phase depended on the introduced concentration of the pollutant and naturally decreased with an increase in the initial copper content in the medium. At sublethal concentrations of pollutants, the I. galbana culture was divided into 2 subpopulations of cells with different functional activities: sensitive and resistant to the effects of copper. The latter are able to ensure the survival and subsequent recovery of the I. galbana population under favorable growth conditions.
Despite the fact that copper in the nanoform is less toxic to microalgae, it has both mechanical and toxic effects on cells. Therefore a progressive increase in nanodispersed pollutants in the marine environment is a potential hazard both for primary production and for subsequent links in the trophic chain. This emphasizes the need for further in-depth studies on the assessment of the physiological response and possible adaptation changes in the functional state of oxygenic phototrophs under the effect of nanodispersed pollutants. In addition, it is necessary to compile a comprehensive toxicological and ecological characteristic of nanomaterials using biological objects of different trophic affiliation.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Adeleye, A. S., & Keller, A. A. (2016). Interactions between algal extracellular polymeric substances and commercial TiO2 nanoparticles in aqueous media. Environmental Science & Technology, 50(22), 12258–12265.
Adeleye, A. S., Conway, J. R., Garner, K., Huang, Y., Su, Y., & Keller, A. A. (2016). Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chemical Engineering Journal, 286, 640–662.
Aganesova, L. O. (2021). The reproduction and development of brackish-water copepods that were fed microalgae of different species. Russian Journal of Marine Biology, 47(2), 114–120.
Akimov, A., Solomonova, E., Shoman, N., Rylkova, O. (2023). Comparative evaluation of the effect of copper oxide nanoparticles and copper sulfate on the structural and functional characteristics of Thalassiosira weissflogii under conditions of cumulative cultivation. Russian Journal of Plant Physiology, 70(5), 494–505.
Armbrust, E. V., & Chisholm, S. W. (1992). Patterns of cell size change in a marine centric diatom: Variability evolving from clonal isolates 1. Journal of Phycology, 28(2), 146–156.
Aruoja, V., Dubourguier, H. C., Kasemets, K., & Kahru, A. (2009). Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Science of the Total Environment, 407(4), 1461–1468.
Barreto, D. M., & Lombardi, A. T. (2016). Environmentally relevant concentrations of TiO2 nanoparticles affected cell viability and photosynthetic yield in the Chlorophyceae Scenedesmus bijugus. Water, Air, & Soil Pollution, 227, 1–11.
Bryantseva, Yu., Lyakh, A., & Sergeeva, A. (2005). Calculation of volumes and surface areas of unicellular algae of the Black Sea. NAS of Ukraine, Institute of Biology of the Southern Seas, (In Russian).
Bucci, V., Nunez-Milland, D., Twining, B. S., & Hellweger, F. L. (2012). Microscale patchiness leads to large and important intraspecific internal nutrient heterogeneity in phytoplankton. Aquatic Ecology, 46, 101–118.
Dorsey, J., Yentsch, C. M., Mayo, S., & McKenna, C. (1989). Rapid analytical technique for the assessment of cell metabolic activity in marine microalgae. Cytometry: the Journal of the International Society for Analytical Cytology, 10(5), 622–628.
Franklin, N. M., Stauber, J. L., & Lim, R. P. (2001). Development of flow cytometry-based algal bioassays for assessing toxicity of copper in natural waters. Environmental Toxicology and Chemistry: An International Journal, 20(1), 160–170.
Ipatova, V. I., Spirkina, N. E., & Dmitrieva, A. G. (2015). Resistance of microalgae to colloidal silver nanoparticles. Russian Journal of Plant Physiology, 62, 253–261.
Ipatova, V. I., Dmitrieva, A. G., Filenko, O. F., & Drozdenko, T. V. (2018). About some peculiarities of the physiological heterogeneity of the population of Scenedesmus quadricauda (Turp.) Breb. in the presence of low concentrations of metals. Toxicological Review, 2, 34–43.
Ivask, A., Juganson, K., Bondarenko, O., Mortimer, M., Aruoja, V., Kasemets, K., Blinova, I., Heinlaan, M., Slaveykova, V., & Kahru, A. (2014). Mechanisms of toxic action of Ag, ZnO and CuO nanoparticles to selected ecotoxicological test organisms and mammalian cells in vitro: A comparative review. Nanotoxicology, 8(sup1), 57–71.
Jamers, A., Blust, R., De Coen, W., Griffin, J. L., & Jones, O. A. (2013). Copper toxicity in the microalga Chlamydomonas reinhardtii: An integrated approach. BioMetals, 26, 731–740.
Jeffrey, S. W., Mantoura, R. F. C., & Wright, S. W. (1997). Phytoplankton pigments in oceanography: Guidelines to modern methods. Monographs on oceanographic methodology. UNESCO Publishing.
Kabanova, Yu. G. (1961). On the cultivation of marine planktonic diatoms and peridinium algae in laboratory conditions. Trudy IO AN SSSR, 47, 203–216. (In Russian).
Keller, A. A., McFerran, S., Lazareva, A., & Suh, S. (2013). Global life cycle releases of engineered nanomaterials. Journal of Nanoparticle Research, 15, 1–17.
Magdolenova, Z., Collins, A., Kumar, A., Dhawan, A., Stone, V., & Dusinska, M. (2014). Mechanisms of genotoxicity. A review of in vitro and in vivo studies with engineered nanoparticles. Nanotoxicology, 8(3), 233–278.
Murtey, M. D., & Ramasamy, P. (2016). Sample preparations for scanning electron microscopy–life sciences. Modern electron microscopy in physical and life sciences. IntechOpen, 161–185. https://doi.org/10.5772/61720
Mzozo, Z. B., Matcher, G. F., & Vine, N. G. (2019). Aspects of the feeding biology of the copepod Pseudodiaptomus hessei (Copepoda: Calanoida) under culture conditions. Aquaculture Research, 50(4), 1329–1337.
Navarro, E., Baun, A., Behra, R., Hartmann, N. B., Filser, J., Miao, A. J., Quigg, A., Peter, H., & Sigg, L. (2008a). Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology, 17, 372–386.
Navarro, E., Piccapietra, F., Wagner, B., Marconi, F., Kaegi, R., Odzak, N., Sigg, L., & Behra, R. (2008b). Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environmental Science & Technology, 42(23), 8959–8964.
Pogosyan, S. I., & Matorin, D. N. (2005). Variability in the condition of the photosynthetic system of the Black Sea phytoplankton. Oceanology, 45, 139–148.
Prado, R., Rioboo, C., Herrero, C., & Cid, Á. (2012). Screening acute cytotoxicity biomarkers using a microalga as test organism. Ecotoxicology and Environmental Safety, 86, 219–226.
Pulido-Reyes, G., Rodea-Palomares, I., Das, S., Sakthivel, T. S., Leganes, F., Rosal, R., Seal, S., & Fernández-Piñas, F. (2015). Untangling the biological effects of cerium oxide nanoparticles: The role of surface valence states. Scientific Reports, 5(1), 15613.
Schiavo, S., Oliviero, M., Miglietta, M., Rametta, G., & Manzo, S. (2016). Genotoxic and cytotoxic effects of ZnO nanoparticles for Dunaliella tertiolecta and comparison with SiO2 and TiO2 effects at population growth inhibition levels. Science of the Total Environment, 550, 619–627.
Sendra, M., Moreno-Garrido, I., Blasco, J., & Araújo, C. V. (2018). Effect of erythromycin and modulating effect of CeO2 NPs on the toxicity exerted by the antibiotic on the microalgae Chlamydomonas reinhardtii and Phaeodactylum tricornutum. Environmental Pollution, 242, 357–366.
Shoman, N., Solomonova, E., Akimov, A., Rylkova, O. A., & Meger, Y. (2022). Responses of Prorocentrum cordatum (Ostenfeld) Dodge, 1975 (Dinoflagellata) to copper nanoparticles and copper ions effect. Physiology and Molecular Biology of Plants, 28(8), 1625–1637.
Solomonova, E. S., & Mykhanov, V. S. (2011). Flow cytometry for the assessment of physiological active cells in batch cultures of Phaeodactylum tricornutum and Nitzschia specia. Marine Ecological Journal, 10, 67–72. (In Russian).
Solomonova, E. S., Shoman, NYu., Akimov, A. I., & Rylkova, O. A. (2022). Ecotoxicological aspects of the influence of ionic and nano copper on structural and functional characteristics of Dunaliella salina (Teod.). Russian Journal of Plant Physiology, 69(5), 97.
Stauber, J. L., & Florence, T. M. (1987). Mechanism of toxicity of ionic copper and copper complexes to algae. Marine Biology, 94, 511–519.
Tomas, C. R. (1997). Identifying marine phytoplankton. Academic press.
Traller, J. C., & Hildebrand, M. (2013). High throughput imaging to the diatom Cyclotella cryptica demonstrates substantial cell-to-cell variability in the rate and extent of triacylglycerol accumulation. Algal Research, 2(3), 244–252.
Vasilieva, S. G., Gorelova, O. A., Baulina, O. I., & Lobakova, E. S. (2022). Subcellular localization of manganese in two green microalgae species with different tolerance to elevated Mn concentrations. Russian Journal of Plant Physiology, 69(5), 94.
Wang, H., & Joseph, J. A. (1999). Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radical Biology and Medicine, 27(5–6), 612–616.
Wickman, G. R., Julian, L., Mardilovich, K., Schumacher, S., Munro, J., Rath, N., et al. (2013). Blebs produced by actin–myosin contraction during apoptosis release damage-associated molecular pattern proteins before secondary necrosis occurs. Cell Death & Differentiation, 20(10), 1293–1305.
Xiong, T., Dumat, C., Dappe, V., Vezin, H., Schreck, E., Shahid, M., Pierart, A., & Sobanska, S. (2017). Copper oxide nanoparticle foliar uptake, phytotoxicity, and consequences for sustainable urban agriculture. Environmental Science & Technology, 51(9), 5242–5251.
Xu, W., Tan, L., Zhao, T., Zhu, X., & Wang, J. (2020). Toxicity assessments of acrylamide in aquatic environment using two algae Nitzschia closterium and Scenedesmus quadricauda. Environmental Science and Pollution Research, 27, 20545–20553.
Yaakob, M. A., Mohamed, R. M. S. R., Al-Gheethi, A., Aswathnarayana Gokare, R., & Ambati, R. R. (2021). Influence of nitrogen and phosphorus on microalgal growth, biomass, lipid, and fatty acid production: An overview. Cells, 10(2), 393.
Zhang, C., Chen, X., Tan, L., & Wang, J. (2018). Combined toxicities of copper nanoparticles with carbon nanotubes on marine microalgae Skeletonema costatum. Environmental Science and Pollution Research, 25, 13127–13133.
Zhang, H., Chen, Z., & Huang, Q. (2020). Study of the toxicity of ZnO nanoparticles to Chlorella sorokiniana under the influence of phosphate: Spectroscopic quantification, photosynthetic efficiency and gene expression analysis. Environmental Science: Nano, 7(5), 1431–1443.
Funding
This work was supported by the A.O. Kovalevsky Institute of Biology of the Southern Seas of RAS (state projects No. 121041400077-1 “Functional, metabolic, and toxicological aspects of hydrobionts’ existence and their populations in habitats with different physical and chemical regime” and No. 121030300149-0 “Investigation of the mechanisms of controlling production processes in biotechnological complexes with the aim of developing the scientific foundations for the production of biologically active substances and technical products of marine genesis”).
The authors thank O.S. Alatartseva, leading engineer of the algae ecological physiology department of the A.O. Kovalevsky Institute of Biology of the Southern Seas (IBSS), for providing algae cultures, and V.N. Lishaev, head of the microscopy laboratory at IBSS, for help in working with the electron microscope.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shoman, N., Solomonova, E., Akimov, A. et al. Responses of Microalgae Isochrysis galbana Parke, 1949, on Copper Oxide Nanoparticles and Copper Ions Impact Under Short- and Long-term Cultivation. Water Air Soil Pollut 234, 382 (2023). https://doi.org/10.1007/s11270-023-06398-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06398-2