Abstract
During the past decade, there has been a paradigm shift in our understanding of the roles of intracellular lipid droplets (LDs). New genetic, biochemical and imaging technologies have underpinned these advances, which are revealing much new information about these dynamic organelles. This review takes a comparative approach by examining recent work on LDs across the whole range of biological organisms from archaea and bacteria, through yeast and Drosophila to mammals, including humans. LDs probably evolved originally in microorganisms as temporary stores of excess dietary lipid that was surplus to the immediate requirements of membrane formation/turnover. LDs then acquired roles as long-term carbon stores that enabled organisms to survive episodic lack of nutrients. In multicellular organisms, LDs went on to acquire numerous additional roles including cell- and organism-level lipid homeostasis, protein sequestration, membrane trafficking and signalling. Many pathogens of plants and animals subvert their host LD metabolism as part of their infection process. Finally, malfunctions in LDs and associated proteins are implicated in several degenerative diseases of modern humans, among the most serious of which is the increasingly prevalent constellation of pathologies, such as obesity and insulin resistance, which is associated with metabolic syndrome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The purpose of this article was to review recent progress in elucidating the origins, organization and functions of intracellular lipid droplets (LDs). Over the past few years, several articles have summarized various aspects of LD-related studies. Examples include: Martin and Parton (2005, 2006), Wältermann and Steinbüchel (2005), Fujimoto and Ohsaki (2006), Wolins et al. (2006a, b), Welte (2007, 2009), Ducharme and Bickel (2008), Fujimoto et al. (2008), Goodman (2008, 2009), Thiele and Spandl (2008), Walther and Farese (2009), Farese and Walther (2009), Guo et al. (2009), Murphy et al. (2009), Ohsaki et al. (2009), Olofsson et al. (2009), Zehmer et al. (2009b), Arrese and Soulages (2010), Beller et al. (2010b), Fujimoto and Parton (2010), Kalantari et al. (2010), Bozza et al. (2011), Kühnlein (2011), Greenberg et al. (2011) and Greenberg and Coleman (2011). Most of these articles focus on specific organisms and/or LD functions, and they are all recommended to readers interested in more detailed accounts of LDs in major groups such as prokaryotes, animals, plants and fungi. The present review is intended to complement these accounts and especially to draw together findings across all biological taxa in order to appreciate more fully both the similarities and differences in LD function in cells and organisms.
Many, and perhaps all, eukaryotic and bacterial cells contain varying amounts of cytosolic lipidic inclusions, and similar structures are also found in several types of archaea. These structures occur most commonly as spheroidal macromolecular assemblies of neutral lipid esters or lipid-based polymers that are normally bounded by a phospholipid monolayer membrane plus a variety of lipid-associated proteins. Cytosolic lipid inclusions in cells were originally described by Hanstein (1880), Altmann (1890), and Wilson (1896) and were originally called microsomes or liposomes. However, these terms were later appropriated by others to describe quite different bilayer-based lipid structures. Depending on the organism and scientific field, cytosolic neutral lipid inclusions have been variously referred to as lipid bodies, lipid droplets, adiposomes, granules, oleosomes, or oil bodies. Despite several attempts to arrive at a common terminology (Murphy 2001), several of the above names are still in widespread use in the literature. Although we originally favoured ‘lipid body’ (Murphy and Vance 1999; Murphy 2001), by far, the most common term in the current literature is ‘lipid droplet’ (Martin and Parton 2006). Therefore, this term, abbreviated as LD, is used in the present article.
During most of the twentieth century, intracellular LDs in multicellular organisms were almost universally regarded as specialized storage organelles that were largely limited to specific cell types such as adipocytes and steroidogenic cells in mammals; fat bodies in insects: and cotyledon, mesocarp or scutellar cells in plants. In most cases, these lipid deposits were believed to be relatively long-term carbon stores with slow rates of turnover. During the 1990s, however, evidence began to accumulate that some cytosolic LDs had more dynamic roles, e.g. as readily accessible sources of inflammatory mediators in leukocytes or steroid hormones in steroidogenic cells. In 1999, we attempted the first synthesis of knowledge about LD formation in both plants and animals (Murphy and Vance 1999). This was followed in 2001 by a more detailed review of LDs that stressed their roles as near-ubiquitous and highly dynamic organelles across the full range of biological organisms (Murphy 2001).
Over the past decade, technical advances in cell biology and the progress in genomics and proteomics have underpinned considerable advances in our understanding of the nature and function of intracellular LDs. The advent of cheap and rapid sequencing of the genomes of whole organisms and transcriptomes of specific cell types, coupled with the increasing ease of generating gene knockout or overexpression lines, has greatly extended or knowledge of LD composition and function. New developments in mass spectrometry have also enabled researchers to monitor lipid remodelling in living cells (de Kroon 2007).
However, some of the most important advances have come from the use of new imaging technologies. These have contributed greatly to our understanding of the real-time dynamics of LD behaviour in cells and in following such behaviour during processes such as inflammatory responses (Melo et al. 2011). Examples of such imaging techniques include: novel fixation methods for LDs in immunofluorescence microscopy DiDonato and Brasaemle 2003); vibrational imaging of LDs in live fibroblast cells (Nan et al. 2003); 1H NMR-visible lipid labels (Wright et al. 2003; Delikatny et al. 2011); third-harmonic generation microscopy (Débarre et al. 2005); fluorescent imaging (Kuerschner et al. 2008); multiplex CARS microscopy (Müller and Schins 2002; Rinia et al. 2008); quantitative electron microscopy (Cheng et al. 2009); freeze-fracture replica immunogold labelling (Robenek et al. 2011); stimulated Raman scattering microscopy (Bewersdorf et al. 2011); and confocal reflection microscopy (Gaspar and Szabad 2009). In the past few years, there have been particular advances in the use of live imaging systems including live microscopy (Digel et al. 2010; Somwar et al. 2011); vital staining in combination with fluorescence-activated cell sorting (Cooper et al. 2010); and time-lapse adaptive harmonic generation microscopy (Watanabe et al. 2010). The dynamic motion of cytosolic LDs as captured by live imaging has been reviewed by Welte (2009).
Studies employing these and other methods have now firmly established the roles of LDs in fundamental cellular processes such as the trafficking of lipids, proteins and entire membranes. Moreover, malfunctions in LDs are implicated in numerous human degenerative conditions including type 2 diabetes, Parkinson’s disease, some cancers and Alzheimer’s. LDs also play important roles in a number of serious infections including hepatitis, leprosy, Chlamydia, trypanosomiasis, Chagas disease and dengue fever. More recently, LDs have also been exploited for a wide range of biotechnological purposes including as carriers of pharmaceutical products (Bhatla et al. 2010; Bonsegna et al. 2011) or as renewable, carbon-neutral feedstocks for the manufacture of biodegradable polymers (Anderson and Dawes 1990; Grage et al. 2009; Quillaguamán et al. 2010) and biofuels (Kalscheuer et al. 2007a, b; Beopoulos et al. 2009; Kosa and Ragauskas 2011). In this review, a comparative approach will be adopted to survey recent findings about the nature, roles and exploitation of LDs across each of the major biological domains. The principal emphasis will be on the function rather than on the structures of LDs and their associated proteins. There have been several recent accounts of the structure and targeting mechanisms of LD proteins (Hickenbottom et al. 2004; Zehmer et al. 2008).
Prokaryotes
The cells of all biological organisms are bounded by lipid-based membranes that, except for some archaeal species, are based on lipid bilayer structures (Vereb et al. 2003). It is therefore interesting to pose the question: are all cells also capable of accumulating intracellular lipidic assemblies, and specifically cytosolic LDs? Prokaryotes are divided into bacteria and archaea, but which of these groups has the more ancient lineage is yet to be fully resolved. It is appealing to consider that early cells resembled modern archaea, many of which retain an ability to live in the kinds of extreme environments found on earth when life first evolved well over three billion years ago (Stetter 2006; Berg et al. 2010; Jarrell et al. 2011). However, this hypothesis has been challenged by suggestions that archaea may have evolved more recently and that bacteria are the more ancient taxon (Cavalier-Smith 2006). Archaeal membrane lipids are very different from those of bacteria and eukaryotes as they are made up of glycerol ether lipids rather than glycerol esters. Moreover, archaea do not synthesize fatty acyl esters, which are the most common constituents of LDs; instead, their lipids are based on isoprenoid chains. Despite these differences, however, recent evidence suggests that most bacteria and some (but not all) archaea can form various types of LDs (containing fluid acyl esters) or granules (containing semi-solid lipopolymers).
This begs the question: ‘when did intracellular lipid assemblies first arise?’ Despite the fundamental differences in their lipid compositions, many archaea and bacteria are able to accumulate LDs or granules of some description (Han et al. 2009; Jendrossek 2009; Rehm and Steinbüchel 1999). Unlike eukaryotes, however, only a minority of prokaryotes can accumulate triacylglycerol (TAG)-rich and/or wax ester-rich droplets. Instead, most lipid-accumulating bacterial and archaeal genera synthesize a range of polymeric lipids, of which the most common are polyhydroxyalkanoates (PHAs), such as polyhydroxybutyrate (PHB) or polyhydroxyvalerate (PHV). Other polymers synthesized by bacteria include polythioesters, which are sulphur analogs of PHAs (Lütke-Eversloh et al. 2001a, b; Tessmer et al. 2007). To date, all known LD-containing archaeal species accumulate PHAs as their exclusive storage lipid components, and current evidence suggests that the ability to accumulate TAG or wax esters has only arisen in bacterial lineages.
The accumulation of LDs or granules in prokaryotes is normally a facultative response to nutrient depletion. In cases where the organism is adapted for nutrient-rich habitats, little or no lipid accumulation is found. Examples of non-lipid-accumulating prokaryotes include lactobacilli, Enterobacteriaceae and methanogenic archaea (Wältermann and Steinbüchel 2005). Unlike in eukaryotes, LDs/granules in prokaryotes appear to act exclusively as energy stores, with the hyper-accumulation of lipid normally occurring in response to specific forms of nutrient limitation, most commonly a low carbon/nitrogen (C/N) ratio. Therefore, the accumulation of lipidic droplets in prokaryotes frequently marks the cessation of growth and division and the entry of cells into a quiescent phase.
PHA accumulators
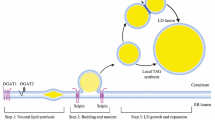
The most common class of intracellular lipid accumulated by prokaryotes is the PHAs. The monomers that make up PHAs are synthesized from acetyl-CoA via a short pathway, as shown in Fig. 1. For example, the assembly of PHB from acetyl-CoA involves three enzymes respectively encoded by the phaA, phaB and phaC genes, which in most bacteria are located in a single operon (Legat et al. 2010). The most important enzyme in this pathway is the PHA synthase that assembles monomers such as hydroxybutyrate or hydroxyvalerate into either homopolymers, such as PHB, or co-polymers, such as poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHB/V). A wide range of archaeal and bacterial taxa can accumulate PHAs via enzymes encoded by distinct, but clearly homologous, sets of phaA, phaB and phaC genes (Han et al. 2009, 2010a, b). For example, at least 15 genera of the salt-tolerant Haloarchaea can accumulate PHAs via variants of the type III PHA synthase found in bacteria (Quillaguamán et al. 2006, 2008; Legat et al. 2010; Han et al. 2010a, b). However, no archaeal taxa have so far been found to contain type I, II or IV phaC genes.
Polyhydroxyalkanoate metabolism in prokaryotes. In several groups of bacteria and archaea, PHAs can be synthesized via ß-oxidation from acyl substrates such as fatty acids or can be formed de novo from elongation of acetyl-CoA. Fatty acid-derived ß-hydroxyalkanoate monomers have chain lengths of C6 to C16, whilst monomers derived from acetyl-CoA tend to be much shorter, i.e. C4 to C5. Sequence similarity between bacterial and archaeal PHA synthases suggests that achaea acquired the ability to form PHA granules following horizontal transfer of a bacterial phaC gene and recruitment of an endogenous phasin analog (see text for further details)
The close similarity of archaeal and bacterial type III PHA synthase genes (Baliga et al. 2004; Bolhuis et al. 2006; Han et al. 2007b, 2010a; Lu et al. 2008; Quillaguamán et al. 2010) and the lack of other PHA gene types in archaea suggest that archaeal PHAs originated from the horizontal transfer of an ancestral type III gene from a bacterium (Kalia et al. 2007). Such a suggestion is in line with findings that modern archaea with non-extreme lifestyles have many other genes of diverse bacterial origins, indicating extensive horizontal acquisition of genes from bacteria over the course of their evolution (Galagan et al. 2002; Koonin 2003). During this time, the original bacterial type III PHA synthase gene has diverged into the distinctive type IIIA PHA synthase variants found in many extant archaea (Kalia et al. 2007; Quillaguamán et al. 2010). The date of archaeal acquisition of PHA synthase genes is unknown, but it has been suggested that it predated the Permian Era and hence occurred over 300 Mya (Legat et al. 2010). However, given that both groups of organisms have been around for over three billion years, the archaeal acquisition of PHA genes may be considerably more ancient.
The mechanism of PHA-accumulation in prokaryotes is of considerable interest in view of its potential biotechnological exploitation to produce biodegradable polymers as industrial feedstocks. Such polymers have biocompatible and thermoplastic features that make them suitable for use in medical implants and as substitutes for petrochemical-derived plastics (Grage et al. 2009; Quillaguamán et al. 2010). Large-scale culture of bioplastic-producing bacterial species, such as Ralstonia eutropha (also known in the literature as Alcaligenes eutropha, Wautersia eutropha or Cupriavidus necator), has been attempted, but high costs have meant that such products have so far only found small niche markets (Steinbüchel and Füchtenbusch 1998), although some progress in wider commercial manufacture has been made more recently (Kim and Dale 2005; Dias et al. 2006).
As an alternative to culturing PHA-producing bacteria, the transfer of PHA-related genes to annual or perennial crop plants could potentially result in larger scale bioplastic production at a significantly lower cost (Slater et al. 1999; Snell and Peoples 2002; Dalton et al. 2011). However, even here, several significant technical hurdles remain, one of the most challenging of which is the cost-efficient extraction of PHA granules from plant tissues such as leaves or seeds (Mooney 2009; Murphy 2010). Another promising approach is to develop archaeal species as industrial-scale PHA producers. In particular, several halophilic archaea have the advantages of utilizing much cheaper carbon sources (including waste materials), as well as having less strict sterilization requirements, plus easier and more efficient methods for PHA extraction (Lillo and Rodriguez-Valera 1990; Koller et al. 2007; Lu et al. 2008; Hezayen et al. 2009).
The number and the size of PHB granules in cells vary according to species and environmental conditions. Accumulation of PHAs in R. eutropha results in the formation of approximately 10 to 20 spheroidal cytosolic granules per cell, with diameters ranging from 240 to 500 nm that can amount to as much as 90% of the total cell dry weight (Anderson and Dawes 1990). In contrast, Azotobacter vinelandii can accumulate >40 granules per cell, with sizes of 500–1400 nm (Page et al. 1995). A typical 500-nm granule of PHA contains about 40,000 polymer molecules, each of which is made up of about 30,000 PHA monomers. These form a semi-solid lipidic core that is surrounded by a phospholipid monolayer into which several specific granule-binding proteins are embedded.
The major granule-binding proteins include two enzymes of PHA metabolism, namely PHA synthase (PhaC) and PHA depolymerase (PhaZ), a transcriptional repressor (PhaR) and at least four different low-molecular-weight structural proteins called phasins (PhaP). The expression of the major phasin gene, phaP1, is regulated by PhaR, which binds to its promoter and represses transcription. During permissive conditions for PHA accumulation, PhaR becomes bound to PHA granules, resulting in a reduced titre of free PhaR and the de-repression of phaP1 gene expression (Pötter et al. 2002, 2004). Genes encoding phaP1 homologs have so far only been detected in the ß-proteobacteria, although other proteins bound to PHA granules have also been found in different branches of the proteobacteria and in Gram-positive bacteria (Fukui et al. 2001; Vazquez et al. 1996). PHA granules are mobilized via the PHA depolymerase located on their surface (Kobayashi et al. 2003; Uchino et al. 2008), which is analogous to the roles of acyl lipases in eukaryotic LDs, as discussed below.
Phasins are by far the most abundant proteins on PHA granules and can form as much as 5% of the total cellular protein (Schultheiss et al. 2005). Phasins are non-catalytic structural proteins consisting of a granule-associated hydrophobic domain and a more polar cytosol-exposed domain that stabilize PHA granules and prevent their coalescence (Grage et al. 2009). Although all phasins characterized to date are small amphipathic proteins of 11–25 kDa, phasins from different bacterial genera have no sequence homology and appear to be phylogenetically unrelated (Hanley et al. 1999). This suggests that phasins are a diverse group of small polypeptides that, thanks to their amphipathic properties, have been recruited to serve as structural barriers around PHA granules on multiple occasions during bacterial evolution. The overexpression of endogenous phasin genes in R. eutropha resulted in the formation of many small PHA granules, whilst deletion of the phasin gene led to the accumulation of a single large granule of <2,000 nm (Tessmer et al. 2007). Expression of the R. eutropha H16 phasin PhaP1 in Rhodococcus opacus PD630 and Mycobacterium smegmatis mc2155 led to its targeting to TAG-rich LDs where the phasin also acted as an anchor to bind other proteins (Hänisch et al. 2006).
Phasins appear to be restricted to PHA-accumulating bacteria and are not present in any archaeal genome sequenced to date. However, it is possible that proteins with different annotations could carry out phasin-like roles in archaea. For example, in recombinant Escherichia coli (which cannot normally make PHAs) engineered to synthesize PHAs, but not expressing phasin genes, large amounts of the endogenous 16-kDa heat-shock protein, HspA, were formed and attached to PHA granules (Tessmer et al. 2007). This enabled recombinant E. coli cells to accumulate numerous small, stable PHA granules. However, when the phasin genes of R. eutropha (which can normally make PHAs) were deleted, there was no compensatory upregulation of Hsps and the overall amount of PHAs was severely reduced. These data demonstrate that in some species, but not all, HspA can functionally replace phasins as a stabilizer of PHA granules. One could speculate therefore that heat shock proteins or other structurally suitable small polypeptides might stabilize PHA granules in archaea in the absence of phasins. If archaea were to use such ad hoc proteins to stabilize their PHA granules, it may explain why there is such wide variation in PHB granule number and size, for example in some Halomonas spp. (Martinez-Canovas et al. 2004; Quesada et al. 2004). Whilst Halomonas boliviensis typically synthesizes one or two granules of 200–640 nm per cell, Halococcus morrhuae and Halococcus salifodinae make several smaller granules of 50–300 nm (Legat et al. 2010).
TAG/wax ester accumulators
Although the majority of bacteria, and many archaea, store carbon in the form of PHAs, a subset of bacteria, primarily nocardioform actinomycetes, streptomyces and some Gram-negative strains, are capable of storing carbon as LDs enriched in TAGs (Alvarez and Steinbüchel 2002; Wältermann and Steinbüchel 2005). The highest levels of TAG accumulation have been reported mainly in nocardioforms such as the genera Mycobacterium, Nocardia, Rhodococcus, Micromonospora, Dietzia and Gordonia and in some streptomycetes (Kosa and Ragauskas 2011). For example, in the well-studied Gram-positive, non-spore-forming actinomycete, R. opacus PD630, grown in low C/N media, LDs of 50- to 400-nm diameter may accumulate to form more than 75% of cellular dry weight (Alvarez et al. 1996), with a potential daily TAG production from organic wastes of almost 60 mg l−1 (Gouda 2008). Spherical wax ester-rich droplets of about 200-nm diameter have been reported in some Acinetobacter spp., although other species accumulated rectangular or rod-shaped wax ester structures (Wältermann and Steinbüchel 2005). Several TAG-accumulating cyanobacteria, including Dunaliella salina and Synechocystis spp, are currently being assessed for their potential to act as solar-powered sources of renewable hydrocarbons, especially in the context of the so-called third-generation biofuels (Murphy 2008; Sheehan 2009; Stephens et al. 2010).
Several phylogenetically related marine γ-proteobacteria can utilize petroleum hydrocarbons as energy sources and are therefore of great interest for their potential in dealing with oil spills (Harayama et al. 1999, 2004). Examples of these so-called hydrocarbonoclastic bacteria include the genera Alcanivorax, Cycloclasticus, Marinobacter, Neptunomonas, Oleiphilus, Oleispira and Thalassolituus (Kalscheuer et al. 2007a, b; Steinbüchel 2007). Whilst only present in low abundance in unpolluted water, species such as Alcanivorax borkumensis can multiply rapidly in oil-polluted water where they can eventually make up 80–90% of the entire microbial community by mass (Harayama et al. 1999; Kasai et al. 2002). However, this rapid population growth is followed by an equally dramatic crash once the hydrocarbons are used up. During the extended periods when suitable growth substrates are unavailable, hydrocarbonoclastic bacteria enter a dormant phase where they live off their accumulated LD reserves that consist of mixtures of TAGs and wax esters (WE; Kalscheuer et al. 2007a, b).
The biosynthesis of WE and/or TAG is catalyzed by a plasma membrane-associated multifunctional wax ester synthetase/diacylglycerol acyltransferase (WS/DGAT) that is found in many bacterial species (Kalscheuer 2010; Manilla-Pérez et al. 2010; Wältermann et al. 2005). The amino acid sequence of this bacterial enzyme is unrelated to that of any previously identified WS or DGAT in animals, fungi or plants (Kalscheuer and Steinbüchel 2003), which is consistent with its origin after the divergence of prokaryotic and eukaryotic lineages. Microscopic and biophysical evidence suggests that each WS/DGAT enzyme might give rise to a single microdroplet of about 60-nm diameter (Wältermann et al. 2005). These droplets may then coalesce into mature droplets of 300 nm in a process regulated by the protein, TadA. In support of this model, TadA-deficient cells accumulated 35% less TAG than wild-type cells, and TadA-overexpressing cells accumulated very large LDs. There are suggestions that there may be a further, as yet uncharacterized, WS/DGAT-independent TAG biosynthesis pathway in some bacteria. This follows observations that following a double knockout of WS/DGAT genes in A. borkumensis, cells were still capable of substantial TAG formation in LDs (Kalscheuer et al. 2007a, b).
It has been difficult to characterize genuine LD-associated proteins in TAG- or wax ester-accumulating prokaryotes due to the prevalence of nonspecific protein binding to LDs during their isolation. However, the product of the tadA (TAG accumulation-deficient) gene of R. opacus PD630 has recently been shown to be a droplet-associated protein (MacEachran et al. 2010). The TadA protein sequence is similar to the heparin-binding hemagglutinin (HbhA) family from the genus Mycobacterium. In the absence of tadA, TAG accumulation was decreased by 30-40%, whilst TadA in vitro was able to both bind heparin and to aggregate LDs. Therefore, TadA is hypothesized to mediate the aggregation of the tiny lipid microdroplets that bud off the plasma membrane and eventually coalesce to form larger mature cytosolic LDs. Prokaryotic cells tend to accumulate either PHAs or TAGs as their major lipidic energy store. However, a few actinomycetes such as Rhodococcus ruber, are capable of simultaneously synthesizing and accumulating similar amounts of TAGs and the co-polyester, PHB/V, from unrelated carbon sources such as glucose (Wältermann and Steinbüchel 2005).
The formation of LDs and the accumulation of TAGs are now known to play important roles in the metabolism of several pathogenic bacteria, such as Mycobacterium tuberculosis and Mycobacterium bovis (Garton et al. 2002; Daniel et al. 2004; D’Avila et al. 2006, 2007, 2008). In M. tuberculosis and Mycobacterium leprae, the TadA homolog, HbhA, acts as a virulence factor that promotes the spread of the pathogen during the early stages of infection (Pethe et al. 2001; de Lima et al. 2009). The pathogenesis of M. leprae, the causative agent of leprosy, is also linked to the proliferation of LDs enriched in eicosanoid precursors in a process involving Toll-like receptor organelles (Mattos et al. 2010). Interestingly, M. tuberculosis has been shown to accumulate TAG and store it in conspicuous inclusion bodies, similar to those seen for Rhodococcus (Garton et al. 2002, Garton et al. 2008; Deb et al. 2009). It is possible that much like TadA in Rhodococcus, HbhA in mycobacteria facilitates LD formation and maturation in addition to its predicted role in cytoadherence and dissemination. Once it is present in its host and encounters an immune response, M. tuberculosis begins to accumulate LDs following the induction of TAG synthase genes (Daniel et al. 2004). This enables the bacterium to enter a non-replicative, drug-resistant, dormancy-like state within the body of the host that can last for decades, during which time the now cryptic pathogen survives on its TAG reserves. The often crucial roles of LDs in several mammalian host–pathogen interactions are discussed further in “Mammals”.
Plants
All major groups of plants, from unicellular algae to the most complex angiosperms, are able to produce cytosolic LDs in at least some of their cells/tissues. As with animals, plant LDs used to be regarded as relatively inert carbon stores. In higher plants, the ability to accumulate cytosolic LDs was originally thought to be confined to specific tissues, such as oleogenic seeds or fruits. However, evidence is now emerging that demonstrates the presence of dynamic LDs in tissues that do not accumulate long-term lipid stores. In most cases, cytosolic LDs in plants accumulate TAGs, although at least one oilseed species, jojoba or Simmondsia chinensis, accumulates fluid wax esters instead (Ohlrogge et al. 1978). In addition to cytosolic LDs, some plant cells accumulate LD-like structures called plastoglobules in their plastid organelles. The lipidic phase of plastoglobules can include TAGs, sterol esters and various lipophilic pigments such as carotenoids. Although there have been fewer studies into plant LDs compared with their non-photosynthetic counterparts, a somewhat similar picture is emerging with regard to the mechanism of LD formation on specific domains of cytosolic endoplasmic reticulum (ER) or plastidial thylakoid membranes and the roles of LDs in several aspects of plant development and function. Some key aspects of the regulation of plant LD formation are summarized in Fig. 2.
Lipid droplet regulation in plants. Cytosolic LDs in plants are ER-derived with a TAG core often stabilized by an annulus of LD-binding proteins such as oleosins and caleosins. The presence or absence of these proteins affects the size and function of mature LDs. TAG accumulation in LDs is regulated by master switches such as the transcription factor, WRI1. Plastidial LDs (plastoglobules) may contain a variety of neutral lipids and often maintain intimate contact with the thylakoid membranes from which they are derived. The formation and turnover of plastoglobules are regulated by several physiological effectors, among the most important of which is the hormone, ABA
Algae
Algae range from simple unicellular organisms to comparatively complex multicellular species such as seaweeds. As with many other unicellular organisms, some algal species accumulate large numbers of cytosolic TAG-rich LDs as storage reserves in response to certain forms of nutrient limitation or abiotic stress (Murphy 2001; Wang et al. 2009). In some cases, these LDs can make up as much as 86% of cell dry weight. Oleogenic marine microalgae are of considerable biotechnological interest both for their ability to synthesize large amounts of high-value lipids and for their possible use as feedstocks for the production of renewable biofuels (Courchesne et al. 2009). Commercially useful lipids accumulated on algal cytosolic LDs or plastoglobules include long-chain polyunsaturates such as docosahexaenoic acid or pigments such as astaxanthin (Liu and Lin 2001). Other novel lipids include very long-chain polyunsaturated alkenones, alkenoates and alkenes (Eltgroth et al. 2005).
Even under normal growth conditions, smaller amounts of LD TAG may function as intermediates in membrane lipid biosynthesis, and perhaps as short-term stores of acyl chains that enable algae to respond to environmental changes that might require the rapid formation of additional membranes. In some cases, algal cells do not accumulate cytosolic LDs, but instead form plastidial lipid deposits termed plastoglobules. Plastoglobules are probably functionally equivalent to cytosolic LDs, but differ in three major respects. First, they are confined to the stroma, which is the major aqueous phase of plastid organelles; second, they can assume several different forms including rods, fibres and globules; and third, they are bounded by a specific family of proteins, variously termed plastoglobulins, plastid lipid-associated proteins and fibrillins. There is a more detailed discussion about these proteins in the section on higher plant plastoglobules below.
The elucidation of algal LDs and plastoglobules has benefited particularly from recent advances in cell biology and genomics. For example, BODIPY 505/515, a green lipophilic fluorescent dye, has been used as a vital stain for LDs in a wide range of live algal cells (Cooper et al. 2010). In addition, the analysis of several genome sequences has helped considerably to fill in gaps in our understanding of algal lipid metabolism (Khozin-Goldberg and Cohen 2011). All algae and terrestrial plants contain plastids, which are the major sites of de novo fatty acid biosynthesis. Newly synthesized saturated or monounsaturated fatty acids must be exported from plastids to the ER for subsequent desaturation, often followed by their re-import to form the photosynthetic membranes. It now seems unlikely that any part of this extensive constitutive trafficking in acyl lipids involves either plastoglobules or cytosolic LDs (Benning et al. 2006). Instead, there appears to be a combination of transporter proteins and vesicular mechanisms acting both in the cytosol and plastids that enables the formation and modification of membrane lipids (von Wettstein 2001: Andersson and Sandelius 2004).
Although algae often accumulate cytosolic LDs, the major class of exclusively LD-associated protein found in plants, namely oleosins, is only found in multicellular terrestrial plants. This implies that, as discussed in detail below, oleosins are not essential for LD formation per se, but may be part of the adaptive response of those plants that have colonized the land (Huang et al. 2009). In contrast to their lack of oleosins, however, algal genomes do encode another putative LD-associated protein, namely caleosins (Partridge and Murphy 2009). At present, it is unclear whether the products of the caleosin-like genes in algae such as Chlamydomonas reinhardtii and Auxenochlorella protothecoides are true LD-binding proteins or instead act as stress-inducible, membrane-bound enzymes as in some higher plants (see below). Interestingly, orthologs of algal and land plant caleosin genes have been found in many fungal genomes, whereas no such genes are present in any metazoan lineages. This implies that caleosin genes may have been transferred between basal algal and fungal species at some point in the remote past (Partridge and Murphy 2009).
A promising candidate for an authentic LD-associated structural protein in algae is the recently reported major lipid droplet protein (MLDP), a relatively hydrophobic 27-kDa polypeptide that was the most abundant constituent of the proteome of C. reinhardtii LDs (Moellering and Benning 2010), plus a possible homolog from Haematococcus pluvialis (Peled et al. 2011). Additional members of the C. reinhardtii LD proteome include predicted lipid metabolism enzymes and orthologs of various proteins also found associated with animal LD proteomes. Examples include predicted components of vesicular trafficking pathways, such as subunits of the COPI complex and its putative regulator, ARF1a, as well as other small Rab-type GTPases. This implies that the cytosolic LDs of even a simple unicellular alga like C. reinhardtii may have similar functional properties to the LDs of relatively complex metazoans such as Drosophila and mammals. The partial RNAi-mediated downregulation of MLDP (by <60%) resulted in an increase of about 40% in LD diameter, which is consistent with a structural role that may be analogous to that of perlipin, adipophilin and TIP47 (PAT)/Perilipin proteins in animals and the oleosins in land plants (Moellering and Benning 2010). Although possible orthologs of MLDP are present in other green algae such as Chlorella vulgaris and Volvox carteri, no orthologs were found in the genomes of diatoms, red algae or even in the green algal Ostreococcus spp. This implies that MLDP has arisen only in some green algal groups and was either always absent from or was subsequently lost from the lineage that produced land plants.
Land plants
The accumulation of cytosolic LDs in oleogenic tissues of terrestrial plants has been comprehensively reviewed previously (Murphy 2001, 2005; Herman 2008; He and Wu 2009; Baud and Lepiniec 2010), as has the accumulation of plastidial LDs, or plastoglobules, in many tissues (Bréhélin et al. 2007). Cytosolic TAG-rich LDs have also been detected in the leaf mesophyll cells of many angiosperms, although much of this literature is rarely cited, as discussed by Lersten et al. (2006). More recently, the case for leaves as sites of LD accumulation has been strengthened considerably. For example, Slocombe et al. (2009) noted that constitutive levels of TAG accumulation in leaves could be increased 10- to 20-fold following manipulation of fatty acid breakdown and lipid synthesis pathways. This indicates that, as in many other organisms, cytosolic LDs in leaves can act as a buffer to take up and/or release acyl moieties in order to maintain the optimal levels of these potentially disruptive metabolites in cells. Progress has also been made, thanks to technological advances such as quantitative electron microscopy (Neuberger et al. 2008), direct organelle mass spectrometry (Horn et al. 2011), and recombinant methods such as the creation of poly-oleosin fusion proteins (Scott et al. 2010) and the selective knockout of different oleosin isoforms (Schmidt and Herman 2009; Wu et al. 2010).
The physical mechanism of cytosolic LD formation in plant cells appears to be very similar to that of animals, namely a localized accumulation of TAG in specialized ER microdomains followed by the release of small LDs that mature to larger droplets under the control of specific proteins (Gidda et al. 2011). Other aspects of the roles of the ER as a multi-domain organelle in plants have been reviewed by Sparkes et al. (2009). There is probably a dynamic two-way flow of lipids between the ER and LDs in most plant cells whereby small numbers of LDs are constantly being formed and then recycled back to the ER. In order to increase the accumulation of LDs, their recycling must be prevented, and this may be one of the roles of the highly abundant LD-binding proteins such as oleosin, and possibly caleosin. When oleosin production in developing soybean seeds was suppressed using RNAi, the formation of LDs was severely disrupted (Schmidt and Herman 2009). Instead of producing normal mature LDs of about 1-μm diameter, many small 50-nm LDs were formed, some of which were recycled to the ER whilst others fused to create giant irregular LDs. The presence of similar giant LDs was also observed in an insertion mutant of Arabidopsis (Rodrigo et al. 2006). In both cases, reduction of oleosin formation led to cellular damage and decreased seed viability.
The accumulation of LDs in plant cells is regulated at the transcriptional level by a hierarchy of transcription factor proteins, of which one of the most important is WRINKLED1 (WRI1), a member of the APETALA2⁄ETHYLENE-RESPONSIVE ELEMENT BINDING (AP2⁄EREB) family (Cernac and Benning 2004; Cernac et al. 2006; Sanjaya et al. 2011). Several recent studies have shown that WRI1 is one of the key master switches that lead to TAG and cytosolic LD accumulation in higher plants (Baud et al. 2007, 2009; Maeo et al. 2009; Baud and Lepiniec 2010; Pouvreau et al. 2011; Tranbarger et al. 2011). Ectopic expression of WRI1 in tissues that do not normally accumulate large amounts of LDs, such as leaves, leads to the formation of numerous TAG-rich oleosin-bound LDs in a manner that is normally seen only in seeds (Liu et al. 2010; Shen et al. 2010).
It is now emerging that cytosolic LDs in many, but not all, plant cells are bounded by oleosins. Oleosins have an unusually hydrophobic central domain that mediates binding to LDs (Gohon et al. 2011; Li et al. 2002). Oleosins are the major protein associated with LDs in desiccation-tolerant seeds. In those mainly tropical oilseeds that do not undergo desiccation as a normal part of maturation, oleosins are much less abundant (Guilloteau et al. 2003), or may be absent (Murphy 2001). There are several reports that suggest roles for oleosins in the stabilization of LDs in relation to desiccation and freezing tolerance (Leprince et al. 1998; Shimada et al. 2008; Shimada and Nara-Nishimura 2010). However, even the presence of oleosins did not prevent freezing-induced LD fusion and consequent cell disruption in Cuphea spp. seeds enriched in TAGs containing crystallization-prone saturated acyl residues (Crane et al. 2003, 2006; Volk et al. 2006). It has been reported that oleosins are present in LD-enriched gametophytes and spores of the moss, Physcomitrella patens, but are absent in algae (Huang et al. 2009). This is consistent with the acquisition of oleosins by terrestrial plants as part of their adaptation to life on dry land. All the cytosolic LDs in P. patens cells appear to maintain physical continuity with the ER, whereas the LDs in the seeds of higher plants lose their connection with the ER following dehydration. The role of oleosins in preventing LD fusion is supported by the finding that oleosin-rich LDs in embryo, aleurone and scutellum cells of oat grains remained small, whereas oleosin-poor LDs in the endosperm cells of the same grains underwent fusion to create much larger structures (Heneen et al. 2008).
Recent findings suggest that the regulation of LD turnover in plants may have significant similarities with comparable processes in animals. As discussed in “Mammals”, Chanarin–Dorfman syndrome in humans is a neutral lipid disorder characterized by the hyper-accumulation of LDs in ectopic locations. This disease is caused by a malfunction in an α/ß-hydrolase-5 (also called CGI-58) that reduces the ability of cells to mobilize TAG in LDs. Disruption of a lipase-encoding CGI-58 homolog in Arabidopsis led to a similar pattern of LD accumulation in ectopic locations such as leaves (James et al. 2010). These findings are consistent with the notion that many, and maybe all, plant cells are able to accumulate small amounts of TAG in cytosolic LDs as part of constitutive acyl lipid trafficking (Murphy 2001; Wahlroos et al. 2003), and possibly as part of various stress responses (Coca and Segundo 2010). The small number of these rapidly turning over LDs may render them difficult to detect in normal non-lipid-accumulating cells. However, when their mobilization is blocked by a malfunctioning CGI-58-like lipase, LDs accumulate to relatively high levels. Similarly to yeast and mammal models, plants appear to have several lipin homologs, pah, that play important roles in membrane lipid homeostasis. However, there are no reports to date that plant PAH proteins are associated with LDs, although disruption of the pah gene did result in the proliferation of ER membranes, as also seen in yeast (Eastmond et al. 2010).
The involvement of LDs in stress responses has been illustrated by reports that an LD-associated AtCPK1 calcium-dependent protein kinase mediates pathogen resistance in Arabidopsis in various tissues, including roots (Coca and Segundo 2010). Interestingly, AtCPK1 regulates several Toll-like interleukin receptors, some of which can trigger immune responses in plants (Ausubel 2005). As noted in “Mammals”, bacterial infections in mammals can trigger cytosolic LD formation via Toll-like receptors (Pacheco et al. 2002; Mattos et al. 2010, 2011), which might imply a similar LD-mediated role in pathogen responses in plants and animals. As AtCPK1 can also co-localize with peroxisomes (Dammann et al. 2003), it may play a role in peroxisomal fatty acid ß-oxidation as well as in the release of acyl lipid-derived mediators involved in signalling processes associated with pathogen responses in an analogous manner to those observed in animals (Liang et al. 2003; Shah 2005; Shah and Chaturvedi 2008; Coca and Segundo 2010). Calcium-dependent protein kinases have also been reported on LDs in developing and germinating seeds of sandalwood, Santalum album (Anil et al. 2000, 2003), and may be more widespread components of cytosolic LDs in plants.
Mobilization of storage LDs in seeds after germination is a highly regulated process that must occur rapidly in order to supply sufficient energy and acyl chains to developing seedlings. As part of this process, the major LD proteins, oleosin and caleosin, undergo ubiquitination (Hsiao and Tzen 2011), which tags them for proteolytic degradation and thereby enables lipolytic enzymes such as TAG- and PL-specific lipases to access the TAG-rich core of the LDs (Vandana and Bhatla 2006; Quettier and Eastmond 2009; Rudolph et al. 2011). Analysis of the proteome of LDs from maize embryos undergoing post-germinative mobilization revealed two proteins similar to known membrane transport components from animals, namely karyopherin-beta-3 (Kap) and a stress-induced membrane pore protein (Tnani et al. 2011). Kap proteins transport molecules through pores of the nuclear envelope (Mosammaparast and Pemberton 2004), and Kap3 has been shown to be associated with LDs in animals (Cermelli et al. 2006). Human Kap3 interacts with a yeast apolipoproteinA-I (apoA-I), a secretion protein with a primary function in cholesterol transport (Chung et al. 2008). Seed LDs and oleosins are currently being used for various biotechnological applications including the production of recombinant human insulin in plants as well as in cosmetic formulations such as topical creams and lotions (Markley et al. 2006; Nykiforuk et al. 2006; Bhatla et al. 2010; Bonsegna et al. 2011).
Roots and meristems
There are several reports of cytosolic LDs in different cell types in roots (Murphy 2001). A common location is in young roots emerging from seeds shortly after germination, where LDs may be involved in the extensive lipid trafficking required to support the rapid expansion of this tissue. This is substantiated by reports that significant amounts of oleosins and LD-binding caleosin isoforms can be detected in the root tips of 2- to 3-day-old seedlings of rapeseed and Arabidopsis (Naested et al. 2000; Hernández-Pinzón et al. 2001). However, LDs may also play other roles in roots. For example, during their initial period of differentiation, root cap cells act as statocytes (gravity sensors). In root cap statocytes of cress, LDs appear to determine a preferential distribution of ER at the distal cell pole and may be one component of the positive orthogravitropic growth of roots (Hensel 1986). Caleosins are also found in young root tips where they may be located either on LDs or on the ER membrane (Hernández-Pinzón et al. 2001; Murphy et al. 2000; 2001). Cells from the shoot apical meristem in birch reportedly contain organelles similar to LDs, and the rearrangement of these structures may be involved in the breakage of bud cell dormancy (Rinne et al. 2002).
Cytosolic LDs may play important roles in meristematic development in plants, as discussed by van der Schoot and Rinne (2011). For example, the dormancy-release mechanism involves the production of numerous LDs, which appear in the cytosol of virtually all meristematic apical cells, but particularly in the RM/RZ (rib meristem/rib zone) and shoot apical meristem regions (Rinne et al. 2002). In the meristematic apex, LDs remain intact throughout the winter and assume peripheral positions where they associate with the plasma membrane and plasmodesmata (Sargent and Osborne 1980; Vigil et al. 1985; Pihakaski et al. 1987; Rinne et al. 2002), as also occurs in dehydrating seeds (Whitfield 1992; Cordova-Tellez and Burris 2002). This coincides with a restoration of the functionality of plasmodesmata, and it is suggested that LDs may play a direct role in this process (Rinne et al. 2002). These meristem LDs are TAG-enriched and bounded by specific proteins including oleosins, putative lipases and a putative 1,3-ß-d-glucanase. Abundant LDs are also found in several meristematic tissues in pine trees where they appear to function mainly as a winter energy reserve (Jordy 2004). This kind of lipid storage in overwintering tissues has also been found in other gymnosperms such as Douglas fir (Krasowski and Owens 1990) as well as in some deciduous tree species (Catesson 1964; Cragg and Willison 1980).
Cytosolic LDs are highly upregulated as part of the response of many plants to short day lengths. This may be an early downstream effect of photoperiod signalling at the ER where the ethylene receptor required for the timing of dormancy is located (Ruonala et al. 2006; Grefen et al. 2008) and where the LDs are formed. It has also been suggested that LDs may be involved in dormancy processes in root nodules (Gurusamy et al. 2000). The linkage between ethylene and LD induction is supported by the co-expression of genes for ethylene biosynthesis with an LD marker gene in aspen (Rinne et al. 2008). These and other data suggest that the ER and ER-derived LDs might play an important role in the processes that lead up to abscisic acid (ABA)-regulated dormancy processes. As well as inducing oleosin accumulation, ABA also regulates other LD-attached proteins such as some members of the ß-1,3-glucanase family (Leubner-Metzger and Meins 2000). These finding are consistent with earlier reports that similar LDs are produced during dormancy induction and function primarily as storage organelles that are stimulated to become mobilized by chilling during dormancy release and the subsequent resumption of growth (Rinne et al. 1998; Farrar and Evert 1997; Riding and Little 1984).
Floral tissues
The major lipid-accumulating organs of flowers are the anthers, which are responsible for the development and release of pollen grains. As with seeds, pollen grains are propagules that lead a brief independent existence before germinating on a compatible floral stigma. In plants that produce entomophilous (insect-borne) pollen grains, the tapetal cells of the anther accumulate large amounts of unusual cytosolic LDs (sometimes called tapetosomes). These 1- to 5-μm diameter LDs are composite structures made up of numerous small TAG-rich droplets interspersed with membranous vesicles and tubules (Hsieh and Huang 2005). Associated with these composite LD structures is a class of protein containing a domain with a striking similarity to oleosins that have been termed oleo-pollenins (Murphy 2005, 2006). These proteins are synthesized in tapetal cells and probably bind to tapetal LDs via their oleosin-like domains. However, when tapetal cells undergo apoptosis as part of pollen maturation, the oleosin domains are removed to leave a mature protein, pollenin, which is transferred to the outer wall of the pollen grains (Murphy and Ross 1998).
Pollenins are a diverse class of proteins made up of repeating motifs, often glycine-rich, that resemble structural proteins rather than enzymes. In Arabidopsis, pollenins are required for the rapid hydration of pollen grains that is needed for the successful fertilization of female flowers (Mayfield and Preuss 2000). The exact role(s) of pollenins have yet to be determined. They may be involved in pollen rehydration, possibly by facilitating the creation of water channels through the otherwise relatively impermeable lipidic extracellular pollen coat. However, there are also reports of the activation of a pollenin promoter following nematode infection (Karimi et al. 2002). The pollenin genes in some Brassicaceae are reportedly some of the most rapidly evolving genes yet identified (Schein et al. 2004). In addition to their extracellular LD-derived lipids and proteins, pollen grains in many plant species accumulate cytosolic LDs that are bounded by a group of pollen-specific oleosins that are very similar to those expressed in seeds (Kim et al. 2002). These LDs are rapidly mobilized after pollen germination on the female stigma, and the acyl groups contribute to the formation of the long pollen tube that enables haploid pollen nuclei to travel to the ovary and fertilize the female egg and polar nuclei (Murphy 2011).
Plastoglobules
Plants and algae contain an additional organelle that is not present in animals and fungi, namely the plastid. Plastids are the major sites of acyl lipid biosynthesis and the location of the most abundant plant membrane system, the photosynthetic thylakoids. Plastids also contain variable numbers of LDs that are conventionally termed plastoglobules (Bréhélin et al. 2007). As noted above for algae, the plastoglobules of land plants can assume a variety of shapes, including rods and fibres, but are most commonly spherical. They may contain a variety of neutral lipids including TAGs, sterol esters and lipophilic pigments. The colours of most flower petals and other plant tissues are often determined by the pigments contained in their plastoglobules. The major lipid-binding proteins in plastoglobules, the plastoglobulins, belong to a large group of homologous proteins found throughout oxygenic photosynthetic organisms from cyanobacteria to higher plants. This indicates that their origins may go back to the endosymbiotic bacterial precursors of plastids well over one billion years ago (Kaneko et al. 1996; Hernández-Pinzón et al. 1999; Katz et al. 1995; Pozueta-Romero et al. 1997; Vishnevetsky et al. 1996; Kim et al. 2001).
In addition to forming the major protein component of TAG/pigment-rich fibrils and globules in coloured chromoplasts, plastoglobulins are present in other plastid types such as elaioplasts and chloroplasts (Hernández-Pinzón et al. 1999; Ting et al. 1998; Pozueta-Romero et al. 1997). The plastoglobulins of elaioplasts are located on globular LDs that resemble those of chromoplasts, except that their lipid components are mainly sterol esters and fatty aldehydes (Hernández-Pinzón et al. 1999). In contrast, the plastoglobulins of chloroplasts are associated both with plastoglobules and thylakoid membranes (Pozueta-Romero et al. 1997; Pruvot et al. 1996a, b; Kessler et al. 1999). Plastids from Brassica rapa may contain up to three distinct plastoglobulin isoforms, each of which is associated with globules containing a different mixture of neutral lipids (Kim et al. 2001). Plastoglobulins have numerous functions in addition to their structural role of providing a stabilizing surface structure for plastoglobules (Deruere et al. 1994). For example, plastoglobulin gene expression is induced in response to environmental factors such as drought stress, wounding or application of exogenous ABA (Chen et al. 1998; Pruvot et al. 1996a).
Plastoglobulins probably have roles in the formation/disassembly/turnover of plastid membrane complexes (Chen et al. 1998) and in protection against stress-induced uncoupling (Simkin et al. 2007). A plastoglobulin homolog from potato is associated with photosystem II, which is one of the major multi-subunit pigment–protein complexes of thylakoid membranes (Monte et al. 1999). Antisense-mediated reduction of plastoglobulin accumulation in transgenic potato plants led to reduced photosynthetic efficiency and stunted growth, which demonstrates their important roles in plastid membranes and globules. It is likely that there are several classes of plastoglobulins in plants with varying locations in LDs and thylakoid membranes and with varying functions ranging from purely structural to more dynamic roles in protein trafficking and stress responses. The permanent structural coupling between plastoglobules and thylakoid membranes has been demonstrated by high-pressure freezing/freeze substitution methods combined with electron tomography (Austin et al. 2006). This study suggests that the neutral lipids in plastoglobule cores, including carotenoids, plastoquinone and tocopherols, are in a dynamic equilibrium with those located within thylakoid membranes.
As well as plastoglobulins, plastoglobules contain the enzyme tocopherol cyclase (VTE1), which extends across the surface monolayer into the interior of the globules. This enzyme catalyzes the penultimate step of tocopherol synthesis (Kanwischer et al. 2005). It has been shown that tocopherol cyclase activity is increased during oxidative stress, protecting thylakoid membranes and photosynthetic proteins from damage caused by reactive oxygen species (Porfirova et al. 2002; Kanwischer et al. 2005; Vidi et al. 2006). Substantial pools of some of the major lipophilic components of the photosynthetic pigment–protein complexes, such as phylloquinone, are located in plastoglobules (Lohmann et al. 2006). This implies that plastoglobules act as reservoirs to enable a rapid response to environmental conditions by either increasing or decreasing the amounts of such oxidation-prone compounds that in their active state are located adjacent to the vulnerable photosystem proteins.
The plastoglobule proteome also contains several other enzymes involved in lipid metabolism, including allene oxide synthase and a neoxanthin cleavage enzyme (NCED4/CDD4), plus several putative lipases, methyltransferases, steroid isomerases and four putative ABC1 kinases (Vidi et al. 2006; Ytterberg et al. 2006). During senescence, plastoglobules play a final role in the life cycle of the plastids of leaves (chloroplasts) by acting as temporary stores for thylakoid membrane lipids as these are broken down for eventual recycling back to the parent plant before leaf dehiscence. A similar chain of events occurs following the exposure of leaves to ozone, which is a frequent constituent of photochemical pollutants. During these processes, and under other stress conditions, plastoglobulin genes are upregulated via ABA-related hormonal signalling pathways (Pruvot et al. 1996a, b; Chen et al. 1998; Gillet et al. 1998; Manac’h and Kuntz 1999; Kim et al. 2001; Langenkamper et al. 2001; Laizet et al. 2004; Yang et al. 2006). In summary, as with other classes of LDs, plastoglobules have recently emerged as dynamic metabolic compartments that play key roles in a wide range of physiological processes in photosynthetic organisms. Finally, plastoglobules are being investigated as possible targeting sites for the more efficient expression of recombinant proteins (Vidi et al. 2007)
Caleosins
The caleosins are a group of calcium-binding proteins that are probably ubiquitous in multicellular plants, green algae and the true fungi (Naested et al. 2000; Murphy 2005; Partridge and Murphy 2009). Caleosin proteins are characterized by a single calcium-binding EF-hand motif, a putative membrane bilayer spanning domain, plus several potential phosphorylation and haem-binding sites. Structural studies with recombinant seed-specific caleosins indicate that the native proteins are able to bind calcium (Chen et al. 1999; Takahashi et al. 2000), phosphate (Purkrtova et al. 2007) and heme (Hanano et al. 2006). Caleosins appear to be highly flexible proteins that can dramatically alter their secondary structures in response to the polarity of the medium in which they are embedded (Purkrtova et al. 2007). Heterologous expression of plant LD-binding caleosin isoform in yeast led to the increased accumulation of cytosolic LDs (Froissard et al. 2009), indicating that these proteins might play a generic role in the stabilization of LDs and may also impede their turnover.
Caleosins are frequently described in the literature as LD-associated proteins that occur in storage tissues, such as developing or germinated seeds or caryopses (Liu et al. 2005; Murphy 2005; Toorop et al. 2005) and in somatic embryos (Che et al. 2006). Several caleosin isoforms are also found in the LD proteome (Frandsen et al. 2001; Katavic et al. 2006). However, more recent studies have revealed that although some caleosin isoforms can bind LDs, other isoforms are bilayer-associated enzymes that may be involved in stress responses. Although caleosins have a similar LD-binding proline-rich domain to oleosins, unlike the latter, they appear capable of binding to bilayer membranes as well as to LDs in a similar manner to many animal LD-binding proteins such as the PAT/Perilipin family. In Arabidopsis, even the LD-bound isoform, Clo-1, has a calcium-dependent heme oxygenase activity that is regulated by one or two conserved ferric-binding histidine residues (Hanano et al. 2006). This kind of peroxygenase activity may be involved in the formation of epoxy hydroxy alcohols from fatty acid hydroperoxides. These and other oxylipin metabolites play prominent roles in plant responses to a range of biotic and abiotic stresses, from drought tolerance to fungal infection. Similar oxylipins are involved in fungal spore development, and those produced in plants probably serve as antifungal compounds to deter the growth of competing fungal species (Tsitsigiannis and Keller 2007). Another Arabidopsis caleosin isoform, Clo-3, is involved in stomatal control, transpiration, drought tolerance and fungal resistance (Aubert et al. 2010), whilst Clo-4 can act as a negative regulator of ABA responses (Kim et al. 2011).
Protists and fungi
This section covers eukaryotes except for the animals and plants. It is mainly concerned with simple protists and with the large group of fungi that range from the unicellular yeasts to relatively complex and large multicellular organisms such as Basidiomycetes. Most or all of these organisms are able to accumulate cytosolic LDs, and one of the first indications that intracellular TAG pools might be actively involved in phospholipid metabolism came from studies of the ciliated protozoan, Tetrahymena pyriformis, as long ago as 1976 (Borowitz and Blum 1976). In this study, it was found that T. pyriformis contained a very labile TAG pool, separate from other endogenous TAG pools, and that both the glycerol backbone and the acyl groups of this labile pool served as precursors for membrane phospholipid biosynthesis. These early results already suggested the sort of intimate relationship between TAG and phospholipid metabolism that has subsequently been observed in many other organisms. For an up-to-date account of the metabolic regulation of TAG formation in heterotrophic microbes, see the review by Kosa and Ragauskas (2011).
Many protists and fungi act as parasites or pathogens, and the ability to accumulate cytosolic LDs is often a key part of their success as infectious agents. Moreover, several of these organisms are able to stimulate the formation of LDs in host cells that are then mobilized as energy sources by the parasite or pathogen. Some of the best studied organisms are the apicomplexan parasites of the Plasmodium and Toxoplasma genera (Vielemeyer et al. 2004; Coppens and Vielemeyer 2005; Coppens 2005). In the case of the malarial parasite, Plasmodium falciparum, an essential factor for the proliferation of the parasite within infected human erythrocytes is its ability to induce the accumulation and subsequent mobilization of large amounts of TAG (Palacpac et al. 2004). Whilst some of the derived acyl groups are transferred to the parasite and accumulate in its cytosol as TAGs, many are released into the infected erythrocyte as the parasite reaches the schizont stage. This sudden release of fatty acids may cause the membrane lysis that leads to cell rupture and the release of merozites (Palacpac et al. 2004). Therefore, in this case, host LDs appear to function not just as a nutrient source for the parasite but also as a cellulolytic mechanism to enable P. falciparum cells to escape from host cells and enter the next phase of their life cycle.
Other parasitic protozoans, such as Trypanosoma brucei (the cause of human African trypanosomiasis), accumulate large numbers of LDs during infection of their host. Unusually, a novel protein kinase (LDK) in T. brucei has been found to be an LD-binding protein, and its RNAi-mediated knockdown resulted in a greatly reduced abundance of LDs (Flaspohler et al. 2010). Whilst previous studies had shown some association of mitogen-activated protein kinases with leukocyte LDs (Yu et al. 1998), this was the first report of a kinase that could strongly bind to LDs and had an important role in their function. The exact role of LDK in regulating LD function remains to be determined, but it seems likely that it will involve the phosphorylation of proteins involved in LD formation/turnover.
There have been relatively few recent studies on the occurrence and function of cytosolic LDs in free-living protists, particularly in comparison with the much better characterized prokaryotes, animals and plants. However, it now appears likely that protists share a common mode of LD regulation with all other Unikonts. The Unikonts are a supergroup that includes Amoebozoa (e.g. slime moulds), Metazoa (multicellular animals), and Fungi (including secondarily reduced Microsporidia; Keeling et al. 2000; Keeling and Fast 2002; Lee et al. 2008; Koonin 2010). The emerging evidence of a common method of LD regulation comes mainly from comparative genomics, which has revealed the occurrence of PAT/perilipin-like genes in members of each of these very diverse groups of Unikont organisms. In much of the literature after 2000, the term ‘PAT proteins’ was commonly used, but this has now been superseded by Perilipin, as described in the box below.

Fungi
Cytosolic LDs can be found in the majority of fungal cells where their functions may vary according to the species, developmental stage and/or environmental conditions. For example, cytosolic LD formation commonly occurs during vegetative growth in saprophytic fungi, but LD numbers also increase markedly during the formation of resting and reproductive structures (Murphy 2005). The pathogenic fungus, Plasmodiophora brassicae, inserts itself into the cytosol of its Brassica plant host, whereupon it rapidly accumulates LDs in its own cytosol (Lösel and Sancholle 1996); these lipids are temporary carbon stores synthesized from precursors extracted from the host plant. An unusual function of fungal LDs is found in sporangiophores of the unicellular fungus, Phycomyces blakesleeanus, which contain aggregates of several dozen 1- to 2-μm diameter LDs, possibly tethered by microfilaments, which may play a role in gravity sensing (Schimek et al. 1999). Fungal LDs appear to arise from the ER in a similar manner to other eukaryotes (Schneider and Seaman 1977).
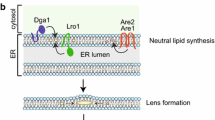
Some fungi, such as the ascomycete, Metarhizium anisopliae, contain a single PAT/Perilipin gene corresponding to Plin1, which encodes an LD-associated protein with a role in TAG storage (Wang and St Leger 2007). More recently, the strong coupling of LD formation and function with the ER has been investigated in more detail, and it has been shown that in cells that lack LDs, proteins normally associated with LDs are instead evenly distributed in the ER membrane (Jacquier et al. 2011). These studies show that transcriptional induction of the diacylglycerol (DAG) acyltransferase, Lro1, is sufficient to drive LD formation on ER membranes where nascent LDs become decorated by marker proteins. When LD formation is induced by the expression of a second DAG acyltransferase, Dga1, this enzyme moves from the ER membrane to LDs as they bud off and move into the cytosol. Photobleaching studies indicate that the movement of proteins from the ER to LDs is independent of temperature and energy, and thus not mediated by classical vesicular transport routes. In some cases, LD-localized proteins can relocate back to the ER, indicating that some continuity between the two organelles is maintained, even if only transiently, in a way that allows the two-way partitioning of proteins between the two compartments.
A novel aspect of LDs in some mycorrhizal fungi is their role as long-range transportable food reserves. Whilst most fungi translocate simple carbohydrates, in some species, such as Glomus intraradices and Glomus margarita, the majority of carbon is translocated as LDs that can comprise as much as 16% of the hyphal volume. Time-lapse micrographs show translocated LDs moving along specific tracks within the hyphal cells at speeds of up to 11 μm s−1 (Bago et al. 2002). It is calculated that for each of the principal fungal hyphae, as much as 1.3 μg h−1 TAG is transported in this way. The movement of the LDs along specific tracks, independently of cytoplasmic streaming, suggests an organized transport system, possibly via cytoskeletal elements, as found for some LDs in animal cells (Murphy 2001).
Many pathogenic fungi use specialized structures, termed appressoria, to break through the tough surfaces of their hosts. Lipid droplets appear to be crucial for appressorium function and especially in the production of the high turgor pressure that is required for virulence (Thines et al. 2000; Wang and St Leger 2007). In the rice blast fungus Magnaporthe grisea (Thines et al. 2000) and the insect pathogen M. anisopliae (Wang and St Leger 2007), lipid droplets originate in fungal spores and redistribute to the incipient appressorium. Whilst the underlying mechanism is unknown, several kinases are implicated (Thines et al. 2000). LDs also play roles in colonization and sexual development in other fungi, including the wheat pathogen, Fusarium graminearum (Guenther et al. 2009), and Aspergillus nidulans (Tsitsigiannis et al. 2004), which is a soil-dwelling fungus and an opportunistic human pathogen.
A. nidulans can produce either sexual or asexual spores according to environmentally determined hormone-like signals generated from oxylipins. A putative fatty acid dioxygenase involved in the biosynthesis of oxylipins from linoleic acid, PpoA, has been shown to be an LD protein (Tsitsigiannis et al. 2004). The deletion of this protein resulted in reduced oxylipin levels and a sixfold decrease in the ratio of asexual to sexual spores. This suggests that the formation of oxylipins may occur at the surface of cytosolic LDs, which are abundant in spore-producing fruiting bodies of most fungi. LDs also play a role in virulence in the human pathogen, Candida parapsilosis, as shown when the gene encoding the LD-associated Fat storage-Inducing Transmembrane (FIT2) protein was disrupted (Nguyen et al. 2011). Mutated cells showed greatly reduced TAG and LD accumulation, lower growth rates in nutrient-rich media and a much attenuated pathogenicity in murine infection models. In C. parapsilosis, the accumulation of LDs protects cells against glucotoxicity- or lipotoxicity-induced by exposure to elevated levels of glucose or fatty acids in growth media in a process that also involves acyl desaturases (Nguyen and Nosanchuk 2011).
The yeast model, Saccharomyces cerevisiae
Due to its ease of cultivation, the brewers’ yeast, Saccharomyces cerevisiae, is one of the best characterized model eukaryotes. Recent studies have demonstrated that this relatively simple organism shares many features of LD organization and function found in much more complex animals such as insects and mammals. Some yeast LDs contain only either TAGs or sterol esters (SEs; Ducharme and Bickel 2008; Horn et al. 2011), whilst others have a mixed composition (Czabany et al. 2008). Evidence from physical probes of LDs isolated from mutants unable to synthesize TAGs or sterol esters SEs suggests that these two lipid classes are partially segregated within the LD core, with thin shells of SEs forming concentric hollow spheres around an inner core composed principally of TAGs. Further evidence that LDs may not always contain a homogeneous core of mixed neutral lipids undergoing isotropic motion has come from the observation that they sometimes contain electron-dense material within their cores (Czabany et al. 2008). These so-called gnarls consist of tangles of elongated and curled tubules of diameter about 10–30 nm, and it was speculated that they might be lipid metabolites, such as free fatty acids, that had demixed or phase-separated from the isotropic TAG/SE-rich core. Similar observations relating to the possibly non-homogeneous structure of some LD cores have also been reported in mammalian systems (Robenek et al. 2004, 2005a, b; see also “Mammals”).
Analyses of the proteomes of yeast LDs and peroxisomes, as verified by microscopic immunodetection, have revealed the presence of numerous lipid metabolism and ER-related proteins (Athenstaedt et al. 1999; Binns et al. 2006). One notable absence is of any PAT/Perilipin homologs, in contrast to their presence in ascomycete fungi such as M. anisopliae. Among the most abundant LD proteins in yeast are three enzymes involved in the synthesis of its major neutral lipid component, ergosterol, namely sterol Δ24-methyltransferase, squalene epoxidase and lanosterol synthetase, as well as three enzymes of long-chain fatty acid activation. The yeast LD proteome also contains several peroxisomal and mitochondrial proteins as well as membrane-trafficking proteins, nuclear proteins, chaperones, enzymes and plasma membrane-associated proteins. The presence of peroxisomal proteins is consistent with the observed close physical association between LDs and peroxisomes in yeast, where the latter have been found to extend tubular projections called ‘pexopodia’ into adjacent LDs (Binns et al. 2006). The involvement of yeast LDs with ER function has been underscored by the reported association with the LD phospholipid monolayer of three proteins that are functionally linked to ER-associated degradation (ERAD) proteins, namely UBXD8 (Zehmer et al. 2009a), AUP1 and UBE2G2 (Spandl et al. 2011). However, another recent report suggests that LD formation is not essential for ERAD in yeast (Olzmann and Kopito 2011). Although the yeast genome does not have any orthologs of the abundant fugal and plant LD-binding protein, caleosin, the expression of a plant caleosin isoform in yeast cells led to the much increased accumulation of cytosolic LDs (Froissard et al. 2009). Therefore, the mere presence of this foreign protein caused yeast cells to overaccumulate LDs. The absence of caleosin genes in the yeasts, but their presence in the filamentous fungi and plants, may be due to the selective loss of these genes from yeasts at some point during their evolution.
Yeast cells are able to take up free fatty acids, which are activated to acyl-CoAs and either catabolized via ß-oxidation or converted to complex lipids. In the yeast, Yarrowia lipolytica, the synthesis of TAG and the formation of LDs are regulated in part by acyl-CoA oxidases (Mlícková et al. 2004a, b), although it is not known whether this applies to other species as well. If LD formation is blocked in yeasts, as in the S. cerevisiae quadruple mutant strain, dga1:lro1:are1:are2, exogenous fatty acids become toxic when they are taken up by cells (Lockshon et al. 2007; Petschnigg et al. 2009). This indicates that one of the constitutive functions of LDs is to maintain intracellular lipid homeostasis, and especially to minimize the risk of fatty acid toxicity by serving as a reservoir for the sequestration of excess acyl groups. However, unlike many animal cell lines, yeast cells are able to adapt to the presence of high levels of exogenous fatty acids even if LD formation is blocked (Connerth et al. 2010). In this case, it was observed that yeast was instead able to hyper-accumulate membrane lipids, mainly by proliferation of the ER network. Some exogenous fatty acids also altered the composition of LDs in yeast; hence, oleate feeding led to a 16:1 ratio of TAG/SE, whilst this ratio was near the wild-type value of 1:1 in the presence of palmitate (Connerth et al. 2010). This was probably due to the need to balance excessive membrane fluidity caused by high levels of oleate by suppressing SE formation so that relatively rigid free sterols could be incorporated into membranes. In this case, the LDs are playing an indirect role in the maintenance of optimal membrane fluidity in the face of environmental challenges caused by different types of exogenous lipid substrate.
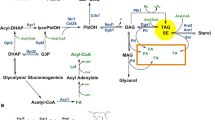
Further parallels between yeast and mammals are shown by the presence in yeast of homologs of LD-associated proteins involved in lipodystrophy in humans, such as seipin (Fei et al. 2008, 2011a) and lipin (Han et al. 2006; Adeyo et al. 2011). As discussed in “Mammals”, mutations in these genes in humans can result in defective adipogenesis with often severe clinical outcomes (Agarwal and Garg 2006). In yeast, a functional homolog of human seipin, Fld1p, regulates the LD size (Fei et al. 2008), which is consistent with its proposed role in humans in the assembly and maintenance of LDs (Binns et al. 2010). The yeast ortholog of lipin, Pah1p, is a phosphatidate phosphatase responsible for DAG formation. Whilst DAG produced by Pah1p is one source of TAG for LD formation, there are alternative pathways for TAG production in yeast (Murphy 2001). Deletion of Pah1p in yeast reduced but did not abolish TAG formation, and the cells responded by synthesizing more SE in order to maintain normal amounts of LDs and neutral lipids (Adeyo et al. 2011). In some cases, yeast Pah1 mutants also hyper-accumulated membranes of their ER-nuclear envelope networks (Han et al. 2006, 2007a; Santos-Rosa et al. 2005). However, when both Pah1p and the sterol acyltransferases Are1p and Are2p were deleted, no LDs were formed even though some TAG was still available. It is proposed that Pah1p-derived DAG plays a key role together with Are1p and Are2p in the formation of LDs on the ER membrane, as depicted in Fig. 3 (Adeyo et al. 2011).
Model for lipid droplet formation in yeast. In this theoretical model, sterol ester-enriched LDs are formed via the action of the enzyme, Are1/2p, which esterifies ER-located sterol precursors. Alternatively, TAG-enriched LDs are formed from PA, via DAG, by the acyltransferases, Pah1p and Nem1p. In both cases, the curvature of the outer bilayer leaflet of the ER that is required for directional budding of the nascent LD towards the cytosol may be facilitated by the localized accumulation of non-bilayer-forming lipid intermediates such as PA, DAG, and SE as well as the action of the transbilayer proteins in blocking lateral diffusion of lipid intermediates away from the site of LD formation. Most yeast LDs contain either SE or TAG cores (yellow) rather than a mixed composition. However, mixtures of SE and TAG are frequently found in LDs of higher animals and plants. Figure adapted from Adeyo et al. (2011)
The role of the seipin homolog in yeast, Fld1p, was confirmed by a recent screen of mutants that accumulate ‘supersized’ LDs, which are generally about 50-fold larger in volume than wild-type LDs (Fei et al. 2011b). In this study, ten mutant lines were found with ‘supersized’ LDs and the causal genetic lesions identified. One of the mutated genes encoded Fld1p, but another five genes (CDS1, INO2, INO4, CHO2 and OPI3) encoded known enzymes of phospholipid metabolism, whilst two genes (CKB1 and CKB2) encoded subunits of the casein kinase 2. A common feature of these mutants was their increased levels of phosphatidic acid that were shown to facilitate the coalescence of LDs into much larger structures that were more difficult for lipases to access. Fld1p is not an enzyme of lipid metabolism, and the mechanism by which it regulates LD size has yet to be determined, although its homolog in Drosophila may have a role in phosphatidic acid metabolism (Tian et al. 2011).
The overall process of LD turnover in yeast has clear similarities with that in mammals. This is seen by the presence of very similar enzymes of TAG breakdown and the conservation of mechanisms for lipolysis between yeast and mammals (Kurat et al. 2006, 2009). These observations are important because the hyper-accumulation of LDs and the proliferation of adipose tissue are key aspects involved in human obesity, and yeast could be a valuable model system for studying the cellular basis of this dysfunction. The process of LD lipolysis in yeast has been linked to cell cycle progression via a common Cdk1/cdc28 activation mechanism (Kurat et al. 2009), whilst a novel lipid hydrolase with esterase and TAG lipase activities has been shown to be involved in the overall cellular lipid homeostasis (Debelyy et al. 2011). Other studies have implicated LD dynamics and their interactions with peroxisomes and ER in the ageing process in yeast (Goldberg et al. 2009) in an analogous manner to that proposed for multicellular organisms, including mammals (Haemmerle et al. 2006).
The proposal from these and other studies is that LDs in yeast cells function as a hub in a regulatory network that modulates TAG and SE formation in the ER and fatty acid oxidation in peroxisomes. When yeast cells are exposed to a calorie-rich diet, it is proposed that the resultant accumulation of ethanol represses the peroxisomal oxidation of free fatty acids that originate from TAGs synthesized in the ER and deposited within LDs. The free fatty acids will then build up inside LDs to form the ‘gnarl’ structures discussed above. The presence of gnarls then initiates several negative feedback loops, resulting in the hyper-accumulation of TAGs in LDs and of DAGs and free fatty acids in the ER. As discussed by Goldberg et al. (2009), this can potentially lead to a cascade of metabolic and physiological events as follows: (1) loss of peroxisome function triggering necrosis; (2) lipoapoptosis induced by free fatty acids and DAG (a caspase- and mitochondria-independent form of programmed cell death); and (3) the triggering by DAG of a protein kinase C-dependent signal transduction network affecting multiple stress response- and longevity-related processes. Therefore, the ingestion of a calorie-rich diet by yeast cells may considerably shorten their life span due to LD-associated metabolic changes in a manner that has many analogies with similar processes in animals, including humans, where caloric restriction is correlated with increased longevity (Bordone and Guarente 2005; Feng et al. 2007; Puigserver and Kahn 2008).
As with several other efficient producers of LDs, some yeast species are being used for biotechnological purposes such as the breakdown and utilization of hydrophobic substrates (Kosa and Ragauskas 2011). Some species can accumulate lipids to as much as 70% of their biomass. One of the most promising of these lipogenic systems is Y. lipolytica whose potential role as a model for bio-oil production has recently been reviewed (Beopoulos et al. 2009). Other yeasts being used or investigated for commercial lipid production from organic wastes or simple low-cost substrates include Candida spp, Cryptococcus curvatus, Lipomyces starkeyi, Rhodotorula spp. and Trichosporon spp., whilst filamentous fungi also in use include Aspergillus oryzae, Mortierella spp. and Rhizopus spp. (Kosa and Ragauskas 2011). The most efficient commercial yeasts, C. curvatus and L. starkeyi, produce slightly higher lipid yields than the best bacterial systems, which are Rhodosporidium toruloides and R. opacus PD630 (see “Prokaryotes”).
Lower animals
All metazoans are able to accumulate cytosolic LDs containing TAG and/or SE in most or all of their cells. For example, LDs are ubiquitous organelles throughout the body of the nematode worm, Caenorhabditis elegans, although they are especially abundant in the fat-storing cells of the gut epithelium (Zhang et al. 2010b). As also described above for yeast, LDs in C. elegans play an important role in its longevity (Feng et al. 2007; Wang et al. 2008). Hence, mutants with impaired peroxisome function will hyper-accumulate LDs, leading to developmental abnormalities (Zhang et al. 2010a). As with the fungi, LD function in animals is tightly coupled with cycles of biosynthesis and lipolysis involving the ER and peroxisomes, with PAT/Perilipin proteins playing key roles in these processes. In recent years it has become apparent that not all LDs within a given cell are identical. For example, some LDs in the same cell may carry different proteins (Beller et al. 2010a; Wolins et al. 2006a, b; Beller et al. 2006; Ozeki et al. 2005), whilst some LDs in the same cell can also carry different lipid cargoes (Czabany et al. 2008).
The insect model, Drosophila melanogaster
The vast majority of research relating to invertebrate LDs has been done using the model fruit fly, Drosophila melanogaster. The importance of LDs in the overall function of Drosophila has been demonstrated by data from two RNAi screens that uncovered a large and diverse array of proteins and organelles that participate in LD physiology. It was found that as much as 1.5% of the expressed genome is implicated in LD function, totaling approx. 370 genes (Beller et al. 2008; Guo et al. 2008). The important and increasing contribution of the Drosophila model to wider LD research has been reviewed by Kühnlein (2011).
Early-stage Drosophila embryos were one of the first systems where it was shown that cytosolic LDs are able to move bidirectionally within cells in association with dynein, a motor protein that can move along microtubules whilst carrying a cargo (Gross et al. 2000). More recently, it has been shown that kinesin-1 also participates as a motor protein to drive LDs along microtubules (Shubeita et al. 2008). During Drosophila oogenesis, LDs and most other oocyte contents are first formed in nurse cells and then transferred through cytoplasmic bridges to the oocyte. This process involves actin-based cytoplasmic streaming (Gutzeit 1986) and active transport along microtubules (Gross et al. 2000). As nurse cell components arrive in the oocyte, they are thoroughly mixed via large-scale, microtubule motor-driven cytosolic streaming. Although most oocyte LDs are carried passively by the bulk flow of streaming, a subset moves actively and bidirectionally along microtubules (Gaspar and Szabad 2009). In early embryos, virtually all LDs move bidirectionally along radially organized microtubules. Embryos may be able to compensate for abnormal LD distribution since embryos with defects in various aspects of LD orientation appear to develop normally (Welte et al. 2005). In addition to dynein, Drosophila LDs bind the Klarsicht (Klar) protein, which, as its name suggests, was originally identified in photoreceptors where it functions as a nuclear envelope-located motor regulator. However, other isoforms of Klar are targeted to LDs in embryos, ovaries and several somatic tissues where they regulate the bidirectional motion of LDs via both dynein and kinesin-1 (Yu et al. 2011).
The most common neutral lipid in insect LDs is TAG, although SEs may be present in some tissues. As in nematodes, insect LDs appear to be ubiquitous throughout the body, but accumulate to very high levels in specialized storage tissues such as the fat body, as reviewed by Arrese and Soulages (2010). Despite its name, the insect fat body is a complex multifunctional organ involved in the coordination of growth with metamorphosis and reproduction. Its main function is to store and release compounds that play important roles in these processes. For example, in Aedes aegypti females, the fat body synthesizes vitellogenin in a transcriptionally mediated process that responds to the ingestion of a blood meal (Park et al. 2006). By far, the most common cells in fat bodies are the highly LD-enriched cells termed adipocytes. However, as they contain numerous small LDs, these insect cells are more similar to mammalian brown adipocytes rather than the far commoner white adipocytes where most mammalian storage lipid is deposited (see “Mammals”). Insects that fly for extended periods, such as during migrations, are able to use LDs directly to power their flight muscles. In such cases, LD TAG is converted into DAG and secreted into the hemolymph from where it is transported to flight muscles via the lipid carrier protein, lipophorin (Arrese and Soulages 2010).
The proteome of Drosophila LDs is similar to other animals in containing PAT/Perilipin family proteins, although only Plin1 and Plin2 are present, with Plin3–5 apparently restricted to vertebrates. The important roles of the PAT/Perilipin family proteins in Drosophila have been shown by abnormalities in LD formation and function that are found in mutants (Grönke et al. 2003; Teixeira et al. 2003). A role for Plin1 in LD homeostasis is suggested by the finding that it is downregulated in starvation (Grönke et al. 2005). More recently, it has been shown that the Plin1 homolog in Drosophila regulates LD structure and the access of other proteins to the LD surface (Beller et al. 2010a). In particular, some Plin1-deficient mutants had much larger LDs than wild-types, and in some cases, fat body cells were dominated by a single giant LD reminiscent of mammalian white adipocytes. These mutants were also characterized by an obesity phenotype that was clearly related to LD dysfunction and suggests that LD status can determine feeding behaviour, possibly via an adipokine or other signal from fat bodies (Grönke et al. 2007; Beller et al. 2010a). However, a knockout of both Plin1 and Plin2 only resulted in reduced fat body stores and reduced ability to mobilize these upon starvation. These results, and the fact that Plin homologs are absent from yeasts and C. elegans, show that Plin proteins are not essential for LD formation or survival of the organism, especially under favourable feeding conditions. However, they do appear to play key roles as potentiators of lipid metabolism and may have particular selective advantages under conditions of limited or varied access to food (Beller et al. 2010a).
Similar to fungi and mammals, Drosophila LDs contain homologs of the protein, Seipin, and knockout of the Seipin gene results in a much reduced accumulation of LDs in the fat body and their presence in ectopic locations such as salivary glands (Tian et al. 2011). The reduction in LD size and numbers was due to the lower rates of TAG formation rather than increased lipolysis. Genetic evidence suggests that aberrant forms of Seipin may cause a reduction in the activity of TAG biosynthetic enzymes, leading to lower levels of LD production. Another novel LD-associated protein recently found in Drosophila is the conserved metalloprotease, invadolysin (Cobbe et al. 2009). Invadolysin is a member of the M8 family of metzincin metalloproteinases (Gomis-Rüth 2003). Homologs of invadolysin are found in many different organisms and probably regulate the function of other proteins by cleaving them to generate active forms. In several protozoan parasites, invadolysin homologs are important in conferring virulence, whilst invadolysin mutants of Drosophila die in late larval stages (McHugh et al. 2004). Larvae of mutants in which invadolysin was undetectable had reduced fat bodies and lower TAG levels in a similar manner to that seen in PAT/Perilipin deficient mutants (Grönke et al. 2003; Teixeira et al. 2003).
Several studies of the Drosophila LD proteome at different developmental stages have shown the presence of expected components such as the PAT/Perilipin group, lipid metabolism enzymes and intracellular transport proteins, but also less predictable components such as storage proteins and histones (Beller et al. 2006; Cermelli et al. 2006). Further studies have demonstrated that these unexpected proteins have a genuine association with LDs in vivo. It is proposed that proteins such as histones are sequestered on LDs in embryonic cells to provide a readily accessible store that can be used at short notice. It may be necessary to sequester highly regulated proteins such as histones in this way in order to shield them from the efficient surveillance mechanisms that normally detect and destroy surplus, damaged or misplaced proteins (Gunjan and Verreault 2003). Similar roles for LDs as protein storage/recycling sites have been proposed in mammalian systems (Fujimoto and Parton 2010). In other cases, LDs may act as long-distance carriers of large protein cargoes along the cytoskeletal network. This mechanism may be particularly relevant during early embryo development when there is a great deal of directional movement of cellular components (Cermelli et al. 2006). The LD proteome from third-instar fat body cells did not include histones, but several storage proteins were present instead, as well as chaperones, including three heat shock proteins (Beller et al. 2006). In general, the Drosophila LD proteome is strikingly similar to that of mammals, which indicates both their evolutionarily conserved functions and the potential utility of Drosophila as a model to investigate LD malfunction in serious human pathologies such as metabolic syndrome and lipodystrophy.
An example of the utility of Drosophila as a model for studying LD function and regulation comes from studies that have elucidated the role of the coat protein I (COPI) complex as a regulator of lipid homeostasis (Beller et al. 2008). COPI and COPII vesicles are essential components of the trafficking system between the ER and Golgi (Lee et al. 2004). COPI vesicles mediate retrograde cargo transport from the Golgi back to the ER, including escaped ER-resident proteins. The anterograde counterpart, COPII, mediates the transport of proteins and lipids from the ER to the Golgi. If either COPI or COPII complexes are disrupted, the Golgi function is abolished, but only COPI is required for LD mobilization. In the absence of COPI function, LDs accumulate to abnormal levels. COPI components changed the LD protein coat composition, particularly by removing Plin3 and hence promoting lipase binding and the initiation of TAG hydrolysis. Following preliminary experiments in Drosophila, studies in mammalian cell lines demonstrated that COPI-mediated regulation of LD turnover is a general mechanism common to all animals (Beller et al. 2008).
Mammals
There have been numerous recent reviews focusing on mammalian LDs (Ducharme and Bickel 2008; Fujimoto et al. 2008; Goodman 2008; Thiele and Spandl 2008; Walther and Farese 2009; Bickel et al. 2009; Farese and Walther 2009; Guo et al. 2009; Murphy et al. 2009; Ohsaki et al. 2009; Olofsson et al. 2009; Zehmer et al. 2009b; Beller et al. 2010b; Bozza and Viola 2010; Fujimoto and Parton 2010; Kalantari et al. 2010; Greenberg et al. 2011; Hapala et al. 2011), plus several useful pictorial overviews of lipid trafficking in the mammalian cell (Krahmer et al. 2009; van Meer and de Kroon 2011). The roles of cytosolic LDs in the assembly and turnover of the various classes of secreted lipoproteins in mammals have been described elsewhere and will not be discussed further here (Olofsson and Boren 2005; Shelness and Ledford 2005; Ohsaki et al. 2006, 2008, 2009; Olofsson et al. 2009).
The emerging picture is that mammalian cells have the same basic LD system as other animal groups, such as the insects that are described above. However, in mammals, these basic LD mechanisms are supplemented by additional layers of complexity and functional redundancy that have been adaptive during the evolution of this group of relatively large and long-lived eukaryotes. For example, mammalian cells appear to have several distinct global mechanisms for the formation and turnover of LDs that are regulated by different effectors and/or are active in different tissues. Even at the metabolic level, mammals tend to have more forms of key enzymes, such as DAG acyltransferase (for LD formation; Harris et al. 2011) or lipases (for LD turnover; Osuga et al. 2000; Jenkins et al. 2004; Villena et al. 2004; Zimmermann et al. 2004; Quiroga and Lehner 2011). This means that multiple gene knockouts are frequently required to halt such processes. The LD population of individual mammalian cells can be heterogeneous, as demonstrated in isolated 3T3-L1 adipocytes which contained small LDs mainly loaded with Plin3/4, intermediate-sized LDs mainly loaded with Plin2 and large LDs mainly loaded with Plin1 (Wolins et al. 2005).
The composition and structure of the core of mature LDs in mammals has been the subject of some discussion in the literature. It was originally believed that all LDs had a relatively simple structure made up of an isotropic core of neutral lipid surrounded by a phospholipid monolayer and various coat proteins (Murphy and Vance 1999). However, there is increasing evidence that in some mammalian cells, normally soluble or amphipathic proteins (Dvorak et al. 1994; Bozza et al. 1997; Robenek et al. 2005a, b, 2006), and even entire ribosome-bound membranes (Wan et al. 2007), may sometimes partition into supposedly hydrophobic LD cores (Ohsaki et al. 2009). In particular, there have been several reports of the presence of caveolin-1 in LD cores, which is puzzling because caveolins are believed to be transferred to the LD surface either via lateral diffusion from the ER membrane or from the plasma membrane via the endocytic pathway (Fujimoto et al. 2001; Pol et al. 2001; Ostermeyer et al. 2001, 2004; Le Lay et al. 2006). Several lines of ultrastructural evidence from the group of Robenek et al. (2004, 2005a, b, 2011) are consistent with the presence of LD coat proteins, such as Plin1 and Plin2, as well as caveolin-1, within LD cores. Given recent evidence of the possible existence of distinct lipid domains, such as the fatty acid ‘gnarls’ found in yeast LD cores (Czabany et al. 2008), it is clear that the nature of LD cores in mammals and in other organisms requires further research.
Heterogeneity of mammalian LDs
The morphology and function of mammalian LDs tend to be more variable than those of other organisms. For example, mammals have two broad types of highly LD-enriched storage cells, namely white and brown adipocytes, which have very different LD structures and physiological functions. In mammals, cytosolic LDs are also prominent constituents of steroidogenic cells in the adrenal gland or reproductive organs (for steroid hormone synthesis), mammary gland epithelial cells (for milk fat synthesis), hepatocytes and enterocytes (for lipid metabolism and lipoprotein formation), and leukocytes (for synthesis of eicosenoid mediators; Murphy 2001). All of these roles are in addition to those of the constitutive LDs that are present in all cells and now known to have a dynamic involvement in lipid trafficking in all eukaryotes. More recently, the list of processes involving LDs in mammalian cells has expanded considerably, as has the number of proteins that are bound to or otherwise associated with these organelles (Beller et al. 2010b; Itabe 2010).
For example, multifunctional LDs are required in different cell types to store and release acyl groups that have been tailored to accommodate cell-specific requirements and functions (Wang and Sztalryd 2011). Such functions include rapid fatty acid storage/release from or into blood by white adipocytes (Ahmadian et al. 2010), mitochondrial fatty acid oxidation for thermal regulation by brown adipocytes (Ricquier 2010), fatty acid oxidation for long-term mobility demands by slow-twitch fibres in skeletal muscles (Moro et al. 2008), lipidation of very low-density lipoprotein production and mitochondrial ß-oxidation (Olofsson et al. 2009), milk production in mammary epithelial cells (Keenan and Mather 2006; Chong et al. 2011a, b) and surfactant production in type II alveolar pneumatocytes in the lung (Magra et al. 2006). Mammalian LDs have been implicated in acyl lipid turnover (Smirnova et al. 2006), cholesterol homeostasis (Naslavsky et al. 2007), hepatitis C infection (Miyanari et al. 2007), proteasomal and lysosomal degradation of apolipoprotein B (Ohsaki et al. 2006), and protein phosphorylation and GTP-mediated protein trafficking (Bartz et al. 2007c; Hommel et al. 2010). It is also now clear that brown adipocytes with their small and readily accessible LDs have roles in energy homeostasis in human adults, in addition to their well-known thermogenic roles in foetuses and neonates (Cypess and Kahn 2010).
As in other animals, the major group of LD-associated proteins in mammals is the PAT/Perilipin family, but the remainder of the mammalian LD proteome tends to be more variable and considerably larger than in non-mammals such as Drosophila (Hodges and Wu 2010). In addition, the LD proteome can vary significantly according to tissue, developmental stage and physiological status, and the list of novel LD-associated proteins only found in mammals continues to grow. Similar to other animals, the abundant LD-binding phosphoprotein, Plin1, has a major role in regulating LD size and access by lipases (Brasaemle 2007; Tansey et al. 2001), whilst Plin2/Adipophilin acts as the major LD coat protein that is especially important in the de novo formation of LDs and their maturation after release from the ER (Chang and Chan 2007; Bickel et al. 2009). In addition to these two basic members of the PAT/Perilipin family that are common to all animals, mammals have three additional members, Plin3-5, that act as regulators of LD formation/turnover (Wolins et al. 2006a, b) and may be capable of functionally substituting for Plin 1 (Sztalryd et al. 2006). A very basic overview of the regulation of LD formation/turnover in mammals is shown in Fig. 4.
Simplified overview of lipid droplet regulation in mammals. This is a brief summary of some of the major processes, components and organelles involved in the regulation of LD formation, maturation and turnover in mammalian cells. However, it should be stressed that these processes can vary considerably in different tissues and cell types, as well as being modified by numerous environmental factors including diet, stress, pathogen attack and ageing. The take-home message is that mammalian LD regulation is a highly complex process that plays a key role in cellular homeostasis and that its dysfunction is implicated in a host of pathological processes ranging from some forms of cancer to obesity and coronary heart disease. 1 Neutral lipid esters are released from the ER as μLDs in a process regulated by various proteins including caveolin2, seipin, cavin, etc. 2 In most cells, μLDs fuse to produce mature 0.5- to 2-μm LDs via a SNARE-mediated process. 3 In adipocytes, Cidea and Fsp1 stimulate small LDs to fuse further to giant 50- to 200-μm LDs. 4 In most cells, LDs are able to move along the cytoskeletal network via proteins such as Dynein and ERK2. 5 Mobilization of LDs is regulated via several hormones and protein factors such as Spartin
Mammals also have an additional protein family, the Cidea group (cell death-inducing DNA fragmentation factor-like effector), which has a similar function to some Plin isoforms. One member of the Cidea group, Fsp27, promotes the formation of the large unilocular LDs that are characteristic of white adipocytes, and knockouts of the fsp27 gene resulted in smaller LD size and increased rates of lipolysis (Puri and Czech 2008; Nishino et al. 2008). In general, Cidea-deficient mutants have similar lean phenotypes to Plin1 mutants and may be part of a redundant and therefore more resilient network of lipid regulation in mammals (Zhou et al. 2003; Christianson et al. 2010). In mice, the white adipocytes of Fsp27-deficient mutants also accumulated numerous small LDs and had elevated mitochondrial activity that is more characteristic of brown adipocytes (Toh et al. 2008). This implies that Fsp27 plays a role in promoting TAG biosynthesis and LD maturation directed towards the formation of large unilocular organelles that have a mainly storage function. In the absence of Fsp27 function, the default pathway, even in white adipocytes, appears to accumulate smaller LDs that can be more rapidly mobilized. In humans, Fsp27 is also an LD-bound protein that is associated with insulin sensitivity (Puri et al. 2007, 2008). Analysis of the proteome of LDs from rat hepatocytes revealed a novel protein termed ‘Associated with LD protein 1’, or ALD1 (Turró et al. 2006). As with many other LD proteins, ALD1 was initially found on the ER, but then became associated with nascent LDs where it appeared to be considerably more mobile than other LD protein components such as Plin2. The precise function of ALD1 has yet to be determined, but as the corresponding gene is only expressed in liver and kidney tissues, it may regulate specific forms of hepatic and nephritic LD behaviour that are distinct from LD behaviour in adipocytes.
Some additional roles of mammalian LDs
In addition to their well-known roles in neutral lipid biosynthesis, mammalian LDs can act as a second site (in addition to the ER) for the biosynthesis of the major membrane phospholipid, phosphatidylcholine (PC; Moessinger et al. 2011). Several other proteins that regulate various aspects of LD formation have recently been identified. These include PLD1 and ERK2 (Andersson et al. 2006), the BARS protein (Bartz et al. 2007b), Fsp27 (Puri et al. 2007), Prp19p (Cho et al. 2007) and FIT1/2 (Kadereit et al. 2008). Numerous lines of evidence point to an interaction of LDs with other subcellular compartments (Olofsson et al. 2008). Examples include the ER (Wu et al. 2000), ribosomes (Wan et al. 2007), endosomes (Liu et al. 2007), mitochondria (Jägerström et al. 2009) and peroxisomes (Binns et al. 2006). LDs also function as transient components of the membrane trafficking system, as short-term metabolic reservoirs for membrane acyl groups (Bartz et al. 2007a; Zehmer et al. 2009b), in signal transduction (Granneman and Moore 2008) and as rapidly turning over sites of protein storage and trafficking (Hodges and Wu 2010). Lipidomic studies are also consistent with roles for LDs in the trafficking of both phospholipids and ether lipids (Wu et al. 2000; Bartz et al. 2007a). As with Drosophila LDs (see “Lower animals”), mammalian LDs appear to act as storage sites for various hydrophobic proteins that may disrupt cell function if allowed to aggregate freely in the cytosol (Fujimoto and Parton 2010). Examples include apolipoprotein B-100 (Ohsaki et al. 2006, 2008) and α-synuclein (Cole et al. 2002; Webb et al. 2003).
The role of LDs in a range of caveolin-mediated intracellular transport processes has been demonstrated, and caveolins are a prominent and dynamic constituent of the mammalian LD proteome (Ostermeyer et al. 2001, 2004; Pol et al. 2001; Liu et al. 2004; Robenek et al. 2004; Martin and Parton 2005; Le Lay et al. 2009). Caveolins are named after caveolae, which are caveolin-enriched lipid raft-like structures found as 50- to 100-nm disk-shaped pits, or invaginations, in the plasma membrane of many mammalian cells. Caveolae give rise to a class of non-clathrin-coated vesicles that are involved in receptor-mediated endocytosis, cholesterol transport, TAG synthesis and signal transduction (Oh et al. 1998; Fujimoto et al. 2000, 2004; Nabi and Le 2003; Öst et al. 2005). Caveolin 2 is also associated with lipid rafts and ER-associated LDs that bind σ-1 receptors; these receptors in turn bind lipidic neurosteroids and psychotropic drugs, including neuroleptics and cocaine, which also demonstrates the trafficking role of LDs (Hayashi and Su 2003). Caveolin trafficking is regulated by cholesterol and fatty acids and involves the Golgi, plasma membrane and LDs (Pol et al. 2005). Clearly, caveolins have a more complex and wider ranging role beyond acting as components of caveolae, and LDs are intimately involved in at least some of these additional functions (Parton and Simons 2007). Other proteins implicated in the trafficking of the aqueous-cored vesicles that are sometimes associated with LDs include numerous small G-proteins, EHD2, α-SNAP and Sec22 (Brasaemle et al. 2004; Liu et al. 2004), stomatin, which is additionally associated with lipid rafts and transport vesicles (Umlauf et al. 2004), and cavins, which are essential for the formation of caveolae as well as being implicated in LD function in mice (Liu et al. 2008), humans (Hayashi et al. 2009; Rajab et al. 2010) and cultured adipocytes (Aboulaich et al. 2006).
In addition to conventional TAG- or SE-rich LDs, some mammalian cells accumulate more specialized neutral lipids. For example, pigmented epithelial cells in the mammalian retina and hepatic stellate cells both contain retinyl ester-enriched LDs termed retinosomes (Blaner et al. 2009; Orban et al. 2011). These esters are mobilized to replenish the visual chromophore, 11-cis-retinal, and their storage ensures proper visual function despite fluctuations in dietary vitamin A intake. Retinosomes also contain TAG, SE and typical LD-binding proteins such as Plin1-3 and Rab proteins such as CGI-58, whilst their formation is regulated by the enzyme lecithin:retinol acyltransferase (Orban et al. 2011).
Because mammalian LDs are dynamic and rapidly turning over organelles, the process of lipolysis in highly regulated via several different and overlapping signalling networks. It was originally believed that lipolysis of LD core lipids was catalyzed by a single enzyme, hormone-sensitive lipase (HSL). However, HSL knockouts were still capable of some lipolysis in white adipocytes (Osuga et al. 2000). In 2004, three laboratories independently characterized a novel TAG lipase, variously termed adipose triglyceride lipase (Zimmermann et al. 2004), desnutrin (Villena et al. 2004) and phospholipase A2ζ (Jenkins et al. 2004). Recent studies may provide evidence for yet more neutral lipid hydrolases (reviewed in Quiroga and Lehner 2011). These include some members of the carboxylesterase [Ces (mouse) or CES (human)] family [mouse Ces3/triglyceride hydrolase (TGH); its human ortholog CES1/CEH/TGH; mouse CesML1/TGH2; mouse Ces1/Es-x and Es22/egasyn; and arylacetamide deacetylase (AADA or AADAC) plus an AADA homolog KIAA1363, also named neutral cholesteryl ester hydrolase 1 (NCEH1) or arylacetamide deacetylase-like 1 (AADACL1)]. Puzzlingly, however, these enzymes are ER-associated glycoproteins with lumen-facing active sites, which is unexpected if their physiological substrates really are cytosolic LDs; more research is clearly needed to clarify their function. The process of lipolysis in adipocytes is also mediated by the GTPase, Rab18, which is recruited to the LD surface following insulin induction of a phosphatidylinositol 3-kinase (Pulido et al. 2011)
In mice, LD formation and lipolysis are both regulated by the ADP ribosylation factor (ARF)-like GTPase, ARFRP1, which is a molecular switch involved in Golgi function (Kahn et al. 2006). The deletion of this GTPase results in the activation of adipocyte TAG lipase and the disruption of LD maturation, leading to the virtual abolition of TAG accumulation in both white and brown adipocytes (Hommel et al. 2010). In addition to direct lipase-mediated breakdown, animal LDs can be degraded after incorporation, together with lysosomes, into so-called autolysosomes (Dong and Czaja 2011). The roles of lipases and other LD-associated proteins in LD mobilization as investigated using transgenic mice have recently been reviewed (Girousse and Langin 2011). Another example of global transcription factors regulating LD formation is the induction of the dehydrogenase/reductase, Dhrs3, by the stress-responsive transcription factor, p53 (Deisenroth et al. 2011). In addition to its roles in stress, p53 is now known to regulate cell metabolism under non-stressed conditions, and one of these roles is to induce Dhrs3 synthesis and accumulation at ER domains where TAG or retinol are being synthesized, which leads to the budding of Dhrs3-bound LDs.
In mammals, the enzymes that catalyze phosphatidic acid phosphatase activity (which produces DAG) are encoded by a family of genes originally named lipins. Because they control the cellular concentration of the bioactive lipids, phosphatidic acid and DAG, lipins are key regulatory enzymes of lipid metabolism and many signalling pathways. A role in neutral lipid homeostasis has been shown by the effects of lipin-1 overexpression in mice leading to obesity, whilst lipin-1 knockouts lack adipose tissue (Péterfy et al. 2001; Phan and Reue 2005). Lipin proteins are associated with several subcellular locations and can rapidly move within the cell where they may also act as transcriptional regulatory proteins in the nucleus (Gropler et al. 2009; Harris and Finck 2011). It has recently been reported that in human macrophages, lipin-1 is an LD-bound protein that regulates the activation of cytosolic group IVA phospholipase A2α, which is a pro-inflammatory enzyme that is involved in the release of LD-located arachidonic acid in order to form eicosanoid signalling mediators (Valdearcos et al. 2011). The wider roles of leukocyte LDs in inflammatory responses are reviewed by Bozza et al. (2009b, 2011), Bozza and Viola (2010) and Melo et al. (2011), whilst the nature of lipid signalling in disease has been reviewed by Wymann and Schneiter (2008).
The toxicity of some lipophilic environmental pollutants may involve LDs. For example, combustion-derived hydrocarbons, including carcinogenic components of cigarette smoke, are taken up into human bronchoepithelial cells and alveolar macrophages where they become localized on cytosolic LDs (Murphy et al. 2008). These toxins can also be taken up by adipocytes where the LDs could potentially act as long-term stores of carcinogenic toxins. If such accumulated toxins were released en masse once the LDs are mobilized, they could potentially have localized oncogenic effects.
Roles of LDs in disease
It is evident that mammalian cells have developed precise homeostatic mechanisms to regulate lipid uptake, synthesis, storage and usage (Horton et al. 2002; Coleman et al. 2000). In recent years, a great deal of mammalian lipid research has focused on metabolic dysfunctions whereby either too much or too little lipid is stored in cytosolic LDs (Agarwal and Garg 2006). In humans, too little LD production results in lipodystrophy, which is a relatively rare and normally genetically related condition (Vigouroux et al. 2011). The most severe form of human lipodystrophy is Berardinelli–Seip congenital lipodystrophy, which is caused by mutations either in a TAG biosynthesis gene or in the LD-binding protein, seipin (Magré et al. 2001; Agarwal et al. 2002; Payne et al. 2008; Chen et al. 2009; Garg and Agarwal 2009). The study of seipin-defective mutations in humans and in various model organisms has revealed much useful information about the role of this protein (Fei et al. 2011a, b). For example, seipin deficiency leads to altered ∆9 fatty acid desaturase function as well as alterations in LD morphology (Boutet et al. 2009; Szymanski et al. 2007). It has been suggested that seipin may play a role in LD assembly by forming a collar at the ER–LD interface or by preventing LDs from being released into the ER lumen instead of the cytosol. However, further evidence is required to verify such a structural role because apparently normal cytosolic LDs were still observed in yeast and mammalian cells in the absence of Fld1p/seipin (Binns et al. 2010). Another type of lipodystrophy, autosomal dominant partial lipodystrophy, is caused by Plin1 deficiency, leading to a failure to accumulate LDs in white adipocytes (Gandotra et al. 2011). The human phenotype in this case is similar to that of Plin1 knockouts in some animal models (Grönke et al. 2007; Beller et al. 2010a).
In contrast to lipodystrophies where there is too little LD accumulation, the overproduction and ectopic appearance of LDs is associated with one of the most common metabolic malfunctions in human populations in the twenty-first century, namely obesity. Obesity (i.e. body mass index >30) affects 20–40% of the adult population of developed countries, and its prevalence has increased three to fourfold since the 1980s (Canoy and Buchan 2007). In terms of overall lipid mass, the bulk of LDs in normal adult mammals is present in white adipose tissue. White adipocytes differentiate from mesenchymal stem cells to preadipocytes and then to mature adipocytes, which are dominated by a single, giant unilocular LD that occupies over 90% of the cytosol (Rosen and MacDougald 2006). Obesity is characterized by the hyper-accumulation of LD-loaded white adipocytes and their ectopic proliferation in non-adipose tissues. Obesity-related lipid dysfunctions are implicated in several serious clinical conditions including metabolic syndrome, type 2 diabetes, hypertension, cardiovascular disease, osteoarthritis and cancer. For example, the LD-binding proteins, Plin2 and Plin5, are involved in ectopic LD accumulation in the skeletal muscle of both rats and humans (Minnaard et al. 2009), whilst Plin2 is also involved in diabetes (Mishra et al. 2004). The more general roles of LD dysfunction in some of these disease conditions have been reviewed by Le Lay and Dugail (2009), Meex et al. (2009) and Greenberg et al. (2011).
In order to understand the nature of obesity and to develop possible treatments, it is important to elucidate the molecular mechanisms of white adipocyte differentiation and the cellular dynamics (biogenesis, size, distribution and interaction with other organelles) of their cytosolic LDs. Equally, it is important to understand why some non-adipocyte cells, such as hepatocytes, cardiomyocytes and skeletal myocytes, appear to enter an adipocyte-like developmental programme and hyper-accumulate LDs. Such ectopic formation of LDs in non-adipocyte cells is correlated with metabolic syndrome and insulin resistance in most individuals in a process often termed ‘lipotoxicity’ (Unger 2002; van Herpen and Schrauwen-Hinderling 2008; Unger and Scherer 2010). Also, the hyper-accumulation of cholesterol ester-laden LDs in foam cells is a key stage in the development of arterial plaques and atherosclerosis. Indeed, some LD-associated proteins, such as Plin2, may have direct roles in atherogenesis (Larigauderie et al. 2004; Buers et al. 2011). Interestingly, however, there are a few exceptions to the rule that the presence of ectopic LDs lead to disease. For example, in the ‘athlete’s paradox’, highly trained elite athletes are able to accumulate high levels of TAG-rich LDs in skeletal muscle without developing dysfunctions such as insulin sensitivity (Goodpaster et al. 2001; Russell 2004).
Abnormalities in the function of LDs and/or LD-associated proteins have been found in several degenerative conditions including Parkinson’s and Altzheimer’s diseases (Bozza and Viola 2010). For example, the normally soluble Parkinson’s disease protein, σ-synuclein, becomes bound to LDs in affected patients, suggesting that relocation to LDs may be an early and important stage in the onset of this disease (Cole et al. 2002; Scherzer and Feany 2004). Another protein, called Nir2, which is essential for the proper function of the retina, can, upon phosphorylation, become relocated to LDs (Litvak et al. 2002). The resulting disruption of intracellular lipid trafficking may be involved in the degeneration of retinal function. The hereditary spastic paraplegias are a heterogeneous group of neurodegenerative disorders characterized by progressive lower limb spastic paralysis that are caused by mutations in a wide range of genes. One of these mutations that is also the cause of Troyer syndrome occurs in the gene encoding the recently characterized LD-associated protein, spartin. In normal individuals, spartin recruits and activates the ubiquitin ligase, AlP4, onto LDs, where it mediates their turnover by promoting ubiquitination of the major resident LD protein, Plin2 (Hooper et al. 2010). When spartin is absent or defective as in Troyer syndrome, LDs tend to accumulate to abnormal levels (Alberts and Rotin 2010). For some as yet unknown reason, in this case, the resulting LD accumulation specifically affects neurons and leads to the progressive degeneration of motor neurons. In mice, a mutation of the AUP1 gene results in a lethal neuromuscular disorder, and the gene product, ancient ubiquitous protein 1 (AUP1), is mostly bound to LDs where it binds E2 ubiquitin conjugases (Spandl et al. 2011). This demonstrates another direct molecular link between LDs and the ubiquitination machinery in mammalian cells.
Several forms of degenerative liver disease involve hepatic steatosis, or the hyper-accumulation of LDs in hepatocytes. In many cases, hepatic steatosis is induced by chronic alcohol intake (French 1989), and in a mouse model, enlarged Plin1-coated LDs were selectively induced by a high alcohol diet (Orlicky et al. 2011). Hepatic steatosis is also found in about half of all individuals who are chronically infected with the hepatitis C virus (Castera et al. 2005; Boulant et al. 2006, 2007, 2008). Although lipid accumulation was long thought of as a mere by-product of viral infection, recent work suggests that the viral life cycle is closely connected with liver lipid metabolism (Herker and Ott 2011). The major cause of LD accumulation in hepatocytes infected with the hepatitis C virus appears to be the action of one of the viral core proteins, which mimics the role of some of the endogenous Plin proteins of the host by directing the formation of LDs from the ER and then stabilizing the LDs by shielding them from lipolysis (Boulant et al. 2006; Roingeard et al. 2008). Recent work suggests that the LD production system of liver cells is essential for hepatitis C virus replication (Herker et al. 2010). The interferon-induced antiviral protein, viperin, which is induced by hepatitis C virus infection, is targeted to ER and LDs, possibly via an α-helical domain that competes for binding sites with the viral core protein (Hinson and Cresswell 2009). Other viral proteins are also able to subvert the LD trafficking system of their host cells in order to escape from the ER lumen (Ploegh 2007), whilst the dengue virus capsid protein hijacks LDs for viral particle formation (Samsa et al. 2009). Also, several human adenoviruses have been shown to act as stimulators of adipogenesis, both in vivo and in vitro (Whigham et al. 2005).
Abnormal functioning of LDs is associated with several forms of cancer (Menendez and Lupu 2007). A regular feature of some early stages of cancerous transformations is the appearance of putative microdomains of TAG within the lipid bilayer of cell membranes (Murphy 2001). Whilst aspects of these microdomains and their oncogenic significance remain controversial, they may have roles in abnormal LD formation and could be useful diagnostic tools for early-stage cancer detection (Khandelia et al. 2010; Delikatny et al. 2011). In neoplastic colon cancer cells, LDs proliferate and act as reservoirs of cyclooxygenase-2 and sites of prostaglandin-E2 synthesis (Accioly et al. 2008). In contrast, during the early stages of renal cell carcinoma, Plin2 (adipophilin) expression is upregulated, but then becomes gradually downregulated during subsequent stages of tumorigenesis and metastasis, with corresponding alterations in LD function (Yao et al. 2007). Finally, the major LD protein, Plin3 (TIP47), is involved in apoptotic and the differentiation processes of human epithelial cervical carcinoma cells (Than et al. 2003). In some forms of cancer, such as pancreatic and gastric cancers, and in some chronic infections, such as tuberculosis and AIDS, patients suffer from a progressive wasting condition called cachexia, which involves symptoms such as kidney and pulmonary disease and heart failure. Cachexia is the immediate cause of death in an estimated 15% of all cancer patients and appears to be the consequence of excessive lipase activity whereby LDs in adipocytes are broken down, resulting in the dramatic depletion in adipocyte size (Das et al. 2011). This is followed by muscle wasting due to fat and overall tissue loss that may be a secondary effect of lipid breakdown products such as cytokines, although this has yet to be tested experimentally (Arner 2011). The role of LDs in modulating the wide range of physiological and pathological stresses perceived by the ER network has been reviewed by Hapala et al. (2011).
Interactions of pathogens with LDs
As outlined in “Prokaryotes” and “Protists and fungi”, the LDs of several pathogens that affect mammals are known to play important roles in their respective infection processes. However, as with the viruses discussed above, numerous microbial pathogens are also able to subvert the LD systems of their hosts for various purposes ranging from simply providing the pathogen with a food source to more sophisticated manipulations such as the induction of lipid mediators or the induction of specific sets of genes (Bozza et al. 2009a). Among the many examples of eukaryotic pathogens/parasites that disrupt LDs are the malarial agent, P. falciparum, which induces LD synthesis, storage and trafficking during its intraerythrocytic stages (Palacpac et al. 2004; Vielemeyer et al. 2004). The pathogen, Trypanosoma cruzi, which causes Chagas disease, induces LD formation in host macrophages and the generation of eicosenoids (Melo et al. 2003). Infections by several opportunist human pathogens that disrupt LD systems have become more common due the greater numbers of immunocompromised individuals with chronic HIV-1 infections. For example, the dimorphic fungus, Histoplasma capsulatum, causes a broad spectrum of diseases that can progress to life-threatening systemic infections. This fungus induces LD formation in host leukocytes by means of a secreted cell wall ß-glucan that interacts with host cell CD18, TLR2 and Dectin-1 receptors, resulting in the generation of the signalling molecule, leukotriene B4 (Sorgi et al. 2009).
Several bacterial pathogens are also able to subvert the LD and lipid trafficking systems of their host cells. For example, many bacterial pathogens find it difficult to gain access to intracellular iron stores, but M. tuberculosis has evolved a strategy to accomplish this using host cell LDs as carriers (Luo et al. 2005). It is possible that mammalian LDs are normally involved in iron mobilization, in which case all the pathogen needs to do is to redirect iron-laden LDs towards the phagosome compartment in which it resides. The obligate intracellular pathogen Chlamydia trachomatis, is able to redirect endogenous cytosolic LDs within the cells of its host into the lumen of the parasitophorous vacuole (Cocchiaro et al. 2008). This is achieved by the secretion of Lda3 proteins from the pathogen into the host cell where they tag and capture cytosolic LDs that are then delivered to the inclusion lumen, from where they can be directly accessed by the pathogen for lipolysis. The related bacterium and cause of leprosy, M. leprae, is able to induce LD formation in macrophages via Toll-like receptors in order to generate eicosanoids required for pathogenesis (Mattos et al. 2010), whilst in host Schwann cells, the induction of LD formation may play a role in the survival of bacteria within phagosomes (Mattos et al. 2011).
One of the mechanisms of LD induction in macrophages during mycobacterial infections is via the activation of the ligand-dependent transcription factor, peroxisome proliferator-activated receptor γ (PPARγ; Almeida et al. 2009). This results in the proliferation of LDs and a reduced capacity for macrophages to attack the pathogen. In contrast, exposure to non-pathogenic M. smegmatis failed to trigger PPARγ expression in macrophages. On the other hand, if macrophage LD accumulation is decreased, the macrophages have an enhanced ability to kill infecting mycobacteria. This suggests that LDs play an important, albeit negative, role in the mounting of a successful anti-pathogen response in mammalian macrophages. PPARγ also plays a central role in adipocyte differentiation where it is regulated by various lipidic metabolites (Goto et al. 2011). Several other PPAR variants are involved in various aspects of normal LD function, such as PPAR∂ as a sensor of very low-density lipoproteins in macrophages (Chawla et al. 2003) and PPARα as an inducer of TAG and Plin2 protein formation in hepatocytes (Edvardsson et al. 2006).
In summary, mammalian cytosolic LDs have now emerged as integral components that are involved in an ever-growing list of subcellular processes in both health and disease. The correct functioning of LDs is crucial to many aspects of constitutive cellular homeostasis, and they often also play specialized roles in particular tissues or organs, ranging from the retina to the lung. However, one of the most interesting aspects of LD studies in recent years relates to the central roles that these organelles appear to play in several serious disease and degenerative conditions in humans, including those involved in major public health issues such as obesity/metabolic syndrome and cardiovascular disease. As discussed in previous sections, research in non-mammalian model systems has greatly improved our understanding of LD behaviour under a range of normal and pathological conditions. In the future, it will be important for mammalian LD researchers to be aware of and exploit the immense potential of these model systems to underpin further advances in LD-related studies in humans.
Mechanisms for the biogenesis and maturation of LDs
Many aspects of fundamental cellular processes, such as cell division and protein synthesis, are highly conserved in biological organisms, and this probably applies to such a widespread and important phenomenon as the formation and maturation of LDs. The physical processes involved in LD formation and release from cell membranes, and the continuing exchange of components between mature LDs and bilayer membranes, have yet to be explained in detail. In particular, there are very few thermodynamically rigorous models to account for how rapidly diffusing neutral lipid molecules such as TAGs and SEs are constrained to form swellings within membrane bilayers (Zanghellini et al. 2010a, b). Nor are there satisfactory explanations of how nascent droplets are able to bud off from or reattach to intact bilayer membranes. Although most textbooks and reviews (including those of the author) tend to depict the accumulation of TAGs as occurring in bulges or blisters within the ER bilayer, such structures are unlikely to arise spontaneously in a conventional planar bilayer membrane. This is because: (1) newly formed TAG/SE molecules would tend to disperse rapidly within the lateral plane of the bilayer and (2) the tight curvature around a small TAG/SE blister is thermodynamically unfavourable for most bilayer lipids, and especially the major ER lipid, PC. Therefore, how is it possible for localized TAG/SE inclusions to form inside a phospholipid bilayer? There are several possible mechanisms that might operate in LD-forming organisms, two of which are highlighted here.
Firstly, nascent TAG/SE molecules may be constrained from lateral diffusion by physical barriers, such as transmembrane proteins that can effectively pen in the TAGs so that they forced to form a bulge in the bilayer (see Fig. 5). Lipid rafts provide a mechanism to generate lateral heterogeneity in membranes (Jacobson et al. 2007), and specific transmembrane proteins could be recruited into localized lipid raft domains that are enriched in TAG/SE biosynthetic enzymes. It is known that LD formation occurs on membrane microdomains in both prokaryotes and eukaryotes, and these are probably related to lipid rafts (Browman et al. 2007). For example, some lipid raft proteins with characteristic SPFH (stomatin/prohibitin/flotillin/HflK) domains, such as Flotillin-1 and Flotillin-2 and stomatin, co-localize on various cellular membranes and on LDs (Rajendran et al. 2007). In particular, Flotillin-1 appears to specifically associate with nascent LDs, and it has been proposed that it could induce vesiculation at the ER or plasma membrane following loading of the bilayer with neutral lipids. Flotillin-1 has recently been described as a determinant of a clathrin-independent endocytic pathway in mammalian cells, which implicates this protein in vesicular trafficking (Glebov et al. 2006), whilst its expression in insect cells can induce the formation of caveolae-like vesicles (Volonte et al. 1999).
Model of lipid droplet formation on the ER membrane. a LD formation occurs in discrete ER microdomains that are enriched in enzymes of neutral lipid ester (e.g. TAG) and non-planar bilayer lipid (e.g. PE) biosynthesis, plus LD coat proteins such as Plin or oleosin. Lateral diffusion of the TAG and PE products is physically restricted by a ring of transbilayer proteins around their site of formation. b The accumulating TAG (yellow rectangles) within the ER bilayer is forced into a cytosol-facing bulge that is stabilized at its concave neck by localized domains enriched in PE (black wedges) and by coat proteins that are recruited into the bulge, possibly thanks to the favourable free energy transition conferred by moving from a monolayer (pink rectangles) to a bilayer (pink wedges) environment. c As more TAG enters the bulge and its neck is increasingly constrained, it begins to assume a spherical shape. The final excision of the neck to release a nascent μLD may be facilitated by a SNARE complex, as shown in Fig. 7
Secondly, the increased membrane curvature around growing TAG/SE blisters can be stabilized by phospholipid demixing (Mukherjee and Maxfield 2004; Zanghellini et al. 2010a, b; van Meer et al. 2008; van Meer and de Kroon 2011). Simulations using grained molecular dynamics have shown that lipid bilayers can accommodate 17-nm diameter TAG-rich domains, and these may be the precursors of LDs as well as providing diagnostic evidence of early malignancy in certain cancers (Khandelia et al. 2010). The demixing of bilayer phospholipids can be generated by the selective biosynthesis of cone-shaped lipids in TAG/SE-rich regions of the ER. Such lipids have headgroups with relatively small cross-sectional areas and larger acyl chains. The importance of conical lipids in the stabilization of curved bilayer regions has been demonstrated for plant membrane systems (Murphy 1982). Two of the most common conical lipids capable of stabilizing regions of highly negative membrane curvature are phosphatidylethanolamine (PE) and DAG (Mukherjee and Maxfield 2004; Dowhan et al. 2008). In addition, signalling events can lead to the localized accumulation of highly charged lipids such as phosphorylated phosphoinositides or phosphatidic acid (PA) and lyso-PA on one bilayer leaflet (Jenkins and Frohman 2005). Such lipids can themselves induce curvature and/or recruit specific proteins to such localized domains that then influence curvature. Current concepts of the energetic implications of membrane curvature and the roles of lipids and proteins in generating such curvature are based on the theory of membrane bending elasticity as proposed originally by Helfrich (1973), and discussed and extended more recently by Zimmerberg and Kozlov (2006).
Recently, it has been shown that in mammalian systems, PE may play a key role in stabilizing the tightly curved TAG blisters and micro-LDs required for LD formation (Hörl et al. 2011). Although the most common lipid component of the ER membrane is PC, during LD formation, there is an upregulation of enzymes involved in the conversion of PC to PE (Vance and Vance 2008). The localized high concentration of PE in the vicinity of TAG formation may stabilize TAG blisters within the ER bilayer and may also promote their evagination as micro-LDs. These results are consistent with the observation in plants that plastid LDs, or plastoglobules, are associated with the tightly curved margins of thylakoid membranes (Austin et al. 2006). We have previously shown that thylakoid margins are enriched in and stabilized by cone-shaped monogalactolipids (Murphy and Woodrow 1983). As shown in Fig. 5, the directionality of LD release could be promoted by the selective accumulation of PE on the cytosolic monolayer of TAG/SE-producing ER domains. Providing the TAG/SE and PE molecules in such domains were constrained from lateral diffusion within the bilayer, as discussed above, the presence of localized PE clusters could facilitate an outward, cytosol-facing bulge in the ER that could then be stabilized by the recruitment of structural LD-binding proteins such as Plin2 (in Unikonts) or oleosin (in plants).
This process would result in the formation of a large cytosol-facing bulge enriched in neutral lipids and enclosed/stabilized by a phospholipid/protein coat. It is possible that in some cells, these LD bulges stay attached to the ER via a narrow neck stabilized by lipids such as PE and specialized proteins such as SNAREs. This would enable their contents to remain in dynamic equilibrium with metabolite pools within the ER. For example, cytosolic LDs in the moss, P. patens, appear to maintain physical continuity with the ER, whereas the LDs in the seeds of higher plants lose their connection with the ER as they mature (Huang et al. 2009). As shown in Fig. 6 and reported by us previously (Piffanelli et al. 1997), there are very close associations between LDs and ER membranes in certain plant cells, and in many cases, LDs appear to be partially or completely encircled by closely appressed ER sheets or tubules. Similar findings have been reported from other plant and animal systems (Fisher et al. 1968; Jensen et al. 1968; Wetzel and Jensen 1992; Targett-Adams et al. 2003; Robenek et al. 2005a, b; Bozza et al. 2007). In the analogous process of plastoglobule formation in plant plastids, there is evidence that at least some plastoglobules remain in direct contact with the thylakoid membranes from which they were formed (Austin et al. 2006).
ER–lipid droplet associations in plants. Cytosolic lipid droplets in developing microspores in the plant, Brassica napus. Note the close association between the lipid droplets and the ER membrane system, which in many cases appears to enfold the droplets. Similar ER–LD associations are found in animals and other eukaryotes. Bar, 0.5 μm
The process of LD formation is separate from TAG biosynthesis and requires the presence of additional proteins. The best known of these are LD coat proteins such as Plin2, oleosin or phasins, but other ER-resident proteins are now known to be essential to LD formation and release. One example is the family of fat-inducing transcript (FIT) proteins found in most, and perhaps all, Unikonts (Kadereit et al. 2008). Confusingly, these FIT proteins are completely unrelated to the similarly named fungal LD proteins where FIT stands for fat storage-inducing transmembrane (Nguyen et al. 2011). We (Abell et al. 1997; Sarmiento et al. 1997) and others (Abell et al. 2002, 2004) have previously suggested that LD-associated proteins might facilitate LD formation by assuming a lower energy conformation on a budding LD monolayer compared to their conformation on or within the cytosolic face of the ER bilayer. This would provide a thermodynamic driver for the partitioning of such proteins onto the LD surface and could facilitate the formation of a cytosol-facing bulge in the ER bilayer. Such a tightly curved bulge in the outer face of the ER bilayer would be additionally stabilized by the demixing of conical phospholipids, as discussed above. Meanwhile, the lateral escape of neutral lipids from the incipient LD would be further constrained by the presence of transmembrane proteins, possibly including seipin (Binns et al. 2010), which could form an ever-diminishing collar around the neck of the LD (see Fig. 5).
Whilst some LDs remain physically and/or functionally connected with the ER, others (perhaps the majority in most cells) may be released into the cytosol as microdroplets (μLD) of about 50- to 60-nm diameter, although a recent theoretical model predicts that the earliest nascent μLDs may be as small as 12 nm in diameter (Zanghellini et al. 2010a, b). It seems likely that a similar process occurs during PHA granule formation in prokaryotes, although in this case the granules bud off from the plasma membrane (Wältermann and Steinbüchel 2005). These various types of μLD probably then undergo fusion via highly regulated processes in order to produce the small LDs (sLD) of about 500- to 2,000-nm diameter, as found in the mature LD populations of most cell types in most organisms. In mammalian cells, it was observed that spontaneous fusion of LDs was relatively slow under normal growth conditions, but was promoted by several pharmacological fusogenic agents (Murphy et al. 2010).
When two spheres fuse, the surface area/volume ratio of the new larger sphere is smaller than the sum of the two original spheres (see Fig. 7). As the internal volume of fused LD spheres is conserved, some of the surface PL and/or protein components (about 20%) must be removed rapidly in order to stabilize each newly enlarged LD (Ohsaki et al. 2009; Murphy et al. 2010). It is estimated that the surface PL content of a mature adipocyte giant unilocular LD (gLD) is only one tenth of the PL content of all the μLDs that originally fused to produce it (Zanghellini et al. 2010a, b). During LD growth, membrane curvature decreases substantially, and, as expected if PE tends to stabilize μLDs, LD maturation has been found to be correlated with the conversion of PE back to PC (Hörl et al. 2011). Whilst many models of LD maturation involve fusion of smaller LDs and the consequent generation of surplus phospholipids and coat proteins (Boström et al. 2005, 2007), other models posit the growth of LDs via the gradual accretion of neutral lipids, as occurs in caveolae (Uittenbogaard et al. 2002; Kuerschner et al. 2008; Yen et al. 2008; Cheng et al. 2009). In contrast with LD fusion, the accretion mechanism would require the recruitment of additional PLs and LD coat proteins in order to stabilize the growing LDs (Fujimoto et al. 2008).
Model for lipid droplet fusion in mammals. a In this model, two equal-sized lipid droplets become tethered together via a SNARE fusion complex consisting of a four-helix bundle formed between Syntaxin-5, SNAP23 and VAMP4. b The SNARE complex forces the two lipid monolayers together until they fuse, which enables the neutral lipid cores to combine into a single larger droplet. The resulting droplet will be about 26% larger in diameter than each original droplet and will have a 20% excess of phospholipid/protein surface area that will require removal or recycling. See text for further details. Adapted from Olofsson et al. (2009)
In most mammalian cells, the accumulation of a small number of sLDs appears to be the default mechanism. However, in a very small number of cell types, most notably white adipocytes, small numbers of sLDs are not the mature state. Instead, sLDs continue to fuse with each other until the cell is almost entirely filled with a single giant unilocular LD. This process is mediated by LD maturation genes, such as fsp27, and lesions in such genes can result in the failure of sLDs to fuse into gLD in white adipocytes (Le Lay and Dugail 2009). In contrast, the default LD maturation pathway in some cells in Drosophila appears to be the formation of a unilocular gLD, but this is normally prevented by the action of Plin1 to promote lipolysis (Beller et al. 2010a). Therefore, Plin1 deficiency in Drosophila often leads to a unilocular LD phenotype, whereas in humans, it results in a complete failure to accumulate LDs in white adipocytes (Gandotra et al. 2011). In addition to regulatory proteins such as Fsp27, LD fusion in adipocytes involves microtubule and SNARE proteins, and the latter are implicated in insulin sensitivity (Boström et al. 2005, 2007). These proteins, which also play roles in vesicle budding and fusion, probably facilitate the fusion of LDs. In mammals, LD fusion may also be promoted by the recruitment by Arf1-type GTPases of phospholipase D, resulting in the generation of localized accumulations of the non-planar bilayer lipid, PA (Nakamura et al. 2005; Andersson et al. 2006; Walther and Farese 2009).
The importance of LD fusion can be appreciated from the calculation that it requires about 1,000 μLDs to fuse in order to produce a typical mature unilocular LD in a white adipocyte. It has been proposed that LD fusion occurs via a simplified version of SNARE-mediated bilayer vesicle fusion (Boström et al. 2007 et al.; Olofsson et al. 2009). According to this model, two small LDs approach and are tethered together via a SNARE complex that consists of a four-helix bundle between Syntaxin-5, SNAP23 and VAMP4. As shown in Fig. 7, this SNARE complex pulls the two adjacent monolayers towards each other, eventually forcing them to fuse into a single monolayer that now encloses the combined contents of both original LDs. Following LD fusion, the four-helix bundle is recognized by α-SNAP, which, together with the ATPase, NSF, unwraps the bundle, thus enabling new fusions to be initiated. It is likely that additional proteins, such as members of the Plin family, then either stabilize the new enlarged LD or facilitate its continued fusion to create even larger LDs. As noted above, individual mammalian cells can contain several size classes of LD, each stabilized and regulated by different groups of Plin proteins. It is likely that these LD size populations are dynamic, shifting towards larger LD sizes when lipid storage is favoured and to smaller sizes when LD turnover and mobilization are required. The disruption of this dynamic process appears to be a central event in the interrelated pathologies of metabolic syndrome, obesity and insulin sensitivity.
Concluding remarks
The past decade has witnessed a transformation in our knowledge of the roles of LDs in cellular function across the full range of biological organisms. We are beginning to uncover the true extent of the function of LDs in highly dynamic short-term processes such as signalling, as well as in longer-term processes such as energy storage. In the higher multicellular eukaryotes, LDs also have roles in developmental processes, environmental responses, pathogenesis and ageing that are often manifest at the whole organism level. In particular, LD dysfunction in humans is now implicated in some of the most serious contemporary public health concerns, including obesity, insulin-resistant diabetes and coronary heart disease. In the coming years, the elucidation of the precise roles of LDs in health and disease in a range of model organisms, from yeast to mice, will doubtless greatly extend our understanding of human LD dysfunction and hopefully will enable new methods of prevention and treatment to be devised.
There has also been much progress in the harnessing of LDs as feedstocks for the production of renewable biofuels, e.g. in microalgae, and a broad range of renewable and biodegradable industrial materials, including biopolymers and oleochemicals. Finally, the fats and oils derived from plant and animal LDs provide the second most important source of dietary calories to human societies across the world. The task of increasing global food supplies has become increasingly urgent in this era of food insecurity and climate change. In the future, the emerging ability to engineer ectopic LD formation in little-used crop tissues, such as leaves and roots, has the potential to greatly increase our supply of lipids that could be used for food, fuel and chemical production. In short, the study of LDs has now emerged fully from its previous ‘Cinderella’ status to become one of the central themes of modern cell biology. Moreover, continuing advances in analytic techniques and genomics give the prospect of continued advances in this exciting area for many years to come.
Abbreviations
- ABA:
-
Abscisic acid
- DAG:
-
Diacylglycerol
- ER:
-
Endoplasmic reticulum
- LD:
-
Lipid droplet
- MLDP:
-
Myocardial lipid droplet protein
- PA:
-
Phosphatidic acid
- PAT:
-
Perlipin, adipophilin and TIP47 (now termed Plin)
- PC:
-
Phosphatidylcholine
- PE:
-
Phosphatidylethanolamine
- PHA:
-
Polyhydroxyalkanoate
- PHB:
-
Polyhydroxybutyrate
- PHB/V:
-
Co-polymer of hydroxybutyrate and hydroxyvalerate
- Plin:
-
Perilipin
- PPAR:
-
Peroxisome proliferator-activated nuclear receptor
- TAG:
-
Triacylglycerol
- TIP47:
-
Tail-interacting 47-kDa protein (or Plin3)
- WS:
-
Wax ester synthase
- WS/DGAT:
-
Wax ester synthetase/diacylglycerol acyltransferase
References
Abell BM, Holbrook LA, Abenes M, Murphy DJ, Hills MJ, Moloney MM (1997) Role of the proline knot motif in oleosin endoplasmic reticulum topology and oil body targeting. Plant Cell 9:1481–1493
Abell BM, High S, Moloney MM (2002) Membrane protein topology of oleosin is constrained by its long hydrophobic domain. J Biol Chem 277:8602–8610
Abell BM, Hahn M, Holbrook LA, Moloney MM (2004) Membrane topology and sequence requirements for oil body targeting of oleosin. Plant J 37:461–470
Aboulaich N, Ortegren U, Vener AV, Stralfors P (2006) Association and insulin regulated translocation of hormone-sensitive lipase with ptrf. Biochem Biophys Res Commun 350:657–661
Accioly MT, Pacheco P, Maya-Monteiro CM, Carrossini N, Robbs BK, Oliveira SS, Kaufmann C, Morgado-Diaz JA, Bozza PT, Viola JP (2008) Lipid bodies are reservoirs of cyclooxygenase-2 and sites of prostaglandin-E2 synthesis in colon cancer cells. Cancer Res 68:1732–1740
Adeyo O, Horn PJ, Lee SK, Binns DD, Chandrahas A, Chapman KD, Goodman JM (2011) The yeast lipin orthologue Pah1p is important for biogenesis of lipid droplets. J Cell Biol 192:1043–1055
Agarwal AK, Garg A (2006) Genetic disorders of adipose tissue development, differentiation, and death. Annu Rev Genomic Hum Genet 7:175–199
Agarwal AK, Arioglu E, De Almeida S, Akkoc N, Taylor SI, Bowcock AM, Barnes RI, Garg A (2002) AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat Genet 31:21–23
Ahmadian M, Wang Y, Sul HS (2010) Lipolysis in adipocytes. Int J Biochem Cell Biol 42:555–559
Alberts P, Rotin D (2010) Regulation of lipid droplet turnover by ubiquitin ligases. BMC Biol 8:94
Almeida PE, Silva AR, Maya-Monteiro CM, Töröcsik D, D’Avila H, Dezsö B, Magalhaes KG, Castro-Faria-Neto HC, Nagy L, Bozza PT (2009) Mycobacterium bovis bacillus Calmette–Guérin infection induces TLR2-dependent peroxisome proliferator-activated receptor gamma expression and activation: functions in inflammation, lipid metabolism, and pathogenesis. J Immunol 183:1337–1345
Altmann R (1890) Die Elementarorganisem und ihre Beziehungen zu den Zellen. Veit, Leipzig
Alvarez HM, Steinbüchel A (2002) Triacylglycerols in prokaryotic microorganisms. Appl Microbiol Biotechnol 60:367–376
Alvarez HM, Mayer F, Fabritius D, Steinbüchel A (1996) Formation of intracytoplasmic lipid inclusions by Rhodococcus opacus strain PD630. Arch Microbiol 165:377–386
Anderson AJ, Dawes EA (1990) Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev 54:450–472
Andersson MX, Sandelius AS (2004) A chloroplast-localized vesicular transporter system: a bioinformatics approach. BMC Genomic 5:40
Andersson L, Bostrom P, Ericson J, Rutberg M, Magnusson B, Marchesan D, Ruiz M, Asp L, Huang P, Frohman MA, Borén J, Olofsson SO (2006) PLD1 and ERK2 regulate cytosolic lipid droplet formation. J Cell Sci 119:2246–2257
Anil VS, Harmon AC, Sankara Rao K (2000) Spatio-temporal accumulation and activity of calcium-dependent protein kinases during embryogenesis, seed development, and germination in sandalwood. Plant Physiol 122:1035–1043
Anil VS, Harmon AC, Sankara Rao K (2003) Ca2+-dependent protein kinase in oil bodies: its biochemical characterization and significance. Plant Cell Physiol 44:367–376
Arner P (2011) Lipases in cachexia. Science 333:163–164
Arrese EL, Soulages JL (2010) Insect fat body: energy, metabolism, and regulation. Annu Rev Entomol 55:207–225
Athenstaedt K, Zweytick D, Jandrositz A, Kohlwein SD, Daum G (1999) Identification and characterization of major lipid particle proteins of the yeast Saccharomyces cerevisiae. J Bacteriol 181:6441–6448
Aubert Y, Vile D, Pervent M, Aldon D, Ranty B, Simonneau T, Vavasseur A, Galaud JP (2010) RD20, a stress-inducible caleosin, participates in stomatal control, transpiration and drought tolerance in Arabidopsis thaliana. Plant Cell Physiol 51:1975–1987
Austin JR, Frost E, Vidi PA, Kessler F, Staehelin LA (2006) Plastoglobules are lipoprotein subcompartments of the chloroplast that are permanently coupled to thylakoid membranes and contain biosynthetic enzymes. Plant Cell 18:1693–1703
Ausubel FM (2005) Are innate immune signalling pathways in plants and animals conserved? Nature Immunol 6:973–979
Bago B, Zipfel W, Williams RM, Jun J, Arreola R, Lammers PJ, Pfeffer PE, Shachar-Hill Y (2002) Translocation and utilization of fungal storage lipid in the arbuscular mycorrhizal symbiosis. Plant Physiol 128:108–124
Baliga NS, Bonneau R, Facciotti MT, Pan M, Glusman G, Deutsch EW, Shannon P, Chiu Y, Weng RS, Gan RR, Hung P, Date SV, Marcotte E, Hood L, Ng WV (2004) Genome sequence of Haloarcula marismortui: a halophilic archaeon from the Dead Sea. Genome Res 14:2221–2234
Bartz R, Li WH, Venables B, Zehmer JK, Roth MR, Welti R, Anderson RG, Liu P, Chapman KD (2007a) Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J Lipid Res 48:837–847
Bartz R, Seemann J, Zehmer JK, Serrero G, Chapman KD, Anderson RG, Liu P (2007b) Evidence that mono-ADP-ribosylation of CtBP1/BARS regulates lipid storage. Mol Biol Cell 18:3015–3025
Bartz R, Zehmer JK, Zhu M, Chen Y, Serrero G, Zhao Y, Liu P (2007c) Dynamic activity of lipid droplets: protein phosphorylation and GTP-mediated protein translocation. J Proteome Res 6:3256–3265
Baud S, Lepiniec L (2010) Physiological and developmental regulation of seed oil production. Prog Lipid Res 49:235–249
Baud S, Mendoza MS, To A, Harscoet E, Lepiniec L, Dubreucq B (2007) WRINKLED1 specifies the regulatory action of LEAFY COTYLEDON2 towards fatty acid metabolism during seed maturation in Arabidopsis. Plant J 50:825–838
Baud S, Wuilleme S, To A, Rochat C, Lepiniec L (2009) Role of WRINKLED1 in the transcriptional regulation of glycolytic and fatty acid biosynthetic genes in Arabidopsis. Plant J 60:933–947
Beller M, Riedel D, Jansch L, Dieterich G, Wehland J, Jäckle H, Kühnlein RP (2006) Characterization of the Drosophila lipid droplet subproteome. Mol Cell Proteomics 5:1082–1094
Beller M, Sztalryd C, Southall N, Bell M, Jäckle H, Auld DS, Oliver B (2008) COPI complex is a regulator of lipid homeostasis. PLoS Biol 6:2530–2549
Beller M, Bulankina AV, Hsiao HH, Urlaub H, Jäckle H, Kühnlein RP (2010a) PERILIPIN-dependent control of lipid droplet structure and fat storage in Drosophila. Cell Metab 12:521–532
Beller B, Thiel K, Thul PJ, Jäckle H (2010b) Lipid droplets: a dynamic organelle moves into focus. FEBS Lett 584:2176–2182
Benning C, Xu C, Awai K (2006) Non-vesicular and vesicular lipid trafficking involving plastids. Curr Opin Plant Biol 9:241–247
Beopoulos A, Cescut J, Haddouche R, Uribelarrea JL, Molina-Jouve C, Nicauda JM (2009) Yarrowia lipolytica as a model for bio-oil production. Prog Lipid Res 48:375–387
Berg IA, Kockelkorn D, Ramos-Vera WH, Say RF, Zarzycki J, Hügler M, Alber BE, Fuchs G (2010) Autotrophic carbon fixation in archaea. Nat Rev Microbiol 8:447–460
Bewersdorf J, Farese RV, Walther TC (2011) A new way to look at fat. Nat Meth 8:132–133
Bhatla SC, Kaushik V, Yadav MK (2010) Use of oil bodies and oleosins in recombinant protein production and other biotechnological applications. Biotechnol Adv 28:293–300
Bickel PE, Tansey JT, Welte MA (2009) PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim Biophys Acta 1791:419–440
Binns D, Januszewski T, Chen Y, Hill J, Markin VS, Zhao Y, Gilpin C, Chapman KD, Anderson RGW, Goodman JM (2006) An intimate collaboration between peroxisomes and lipid bodies. J Cell Biol 173:719–731
Binns D, Lee S, Hilton CL, Jiang QX, Goodman JM (2010) Seipin is a discrete homooligomer. Biochemistry 49:10747–10755
Blaner WS, O’Byrne SM, Wongsiriroj N, Kluwe J, D’Ambrosio DM, Jiang H, Schwabe RF, Hillman EM, Piantedosi R, Libien J (2009) Hepatic stellate cell lipid droplets: a specialized lipid droplet for retinoid storage. Biochim Biophys Acta 1791:467–473
Bolhuis H, Palm P, Wende A, Falb M, Rampp M, Rodriguez-Valera F, Pfeiffer F, Oesterhelt D (2006) The genome of the square archaeon Haloquadratum walsbyi: life at the limits of water activity. BMC Genomics 7:169
Bonsegna S, Bettini S, Pagano R, Zacheo A, Vergaro V, Giovinazzo G, Caminati G, Leporatti S, Valli L, Santino A (2011) Plant oil bodies: novel carriers to deliver lipophilic molecules. Appl Biochem Biotechnol 163:792–802
Bordone L, Guarente L (2005) Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol 6:298–305
Borowitz MJ, Blum JJ (1976) Triacylglycerol turnover in Tetrahymena pyriformis. Relation to phospholipid synthesis. Biochim Biophys Acta 424:114–124
Boström P, Rutberg M, Ericsson J, Holmdahl P, Andersson L, Frohman MA, Boren J, Olofsson SO (2005) Cytosolic lipid droplets increase in size by microtubule-dependent complex formation. Arterioscler Thromb Vasc Biol 25:1945–1951
Boström P, Andersson L, Rutberg M, Perman J, Lidberg U, Johansson BR, Fernandez-Rodriguez J, Ericson J, Nilsson T, Borén J, Olofsson SO (2007) SNARE proteins mediate fusion between cytosolic lipid droplets and are implicated in insulin sensitivity. Nat Cell Biol 9:1286–1293
Boulant S, Montserret R, Hope RG, Ratinier M, Targett-Adams P, Lavergne JP, Penin F, McLauclan J (2006) Structural determinants that target the hepatitis C virus core protein to lipid droplets. J Biol Chem 281:22236–22247
Boulant S, Targett-Adams P, McLauchlan J (2007) Disrupting the association of hepatitis C virus core protein with lipid droplets correlates with a loss in production of infectious virus. J Gen Virol 88:2204–2213
Boulant S, Douglas MW, Moody L, Budkowska A, Targett-Adams P, McLauchlan J (2008) Hepatitis C virus core protein induces lipid droplet redistribution in a microtubule- and dynein-dependent manner. Traffic 9:1268–1282
Boutet E, El Mourabit H, Prot M, Nemani M, Khallouf E, Colard O, Maurice M, Durand-Schneider AM, Chrétien Y, Grès S, Wolf C, Saulnier-Blache JS, Capeau J, Magré J (2009) Seipin deficiency alters fatty acid Delta9 desaturation and lipid droplet formation in Berardinelli–Seip congenital lipodystrophy. Biochimie 91:796–803
Bozza PT, Viola JPB (2010) Lipid droplets in inflammation and cancer. Prostaglandin Leukot Essent Fatty Acids 82:243–250
Bozza PT, Yu W, Penrose JF, Morgan ES, Dvorak AM, Weller PF (1997) Eosinophil lipid bodies: specific, inducible intracellular sites for enhanced eicosanoid formation. J Exp Med 186:909–920
Bozza PT, Melo RC, Bandeira-Melo C (2007) Leukocyte lipid bodies regulation and function: contribution to allergy and host defense. Pharmacol Ther 113:30–49
Bozza PT, D’Avila H, Almeida PE, Magalhães KG, Molinaro R, Almeida CA, Maya-Monteiro CM (2009a) Lipid droplets in host–pathogen interactions. Clin Lipidol 4:791–807
Bozza PT, Magalhães KG, Weller PF (2009b) Leukocyte lipid bodies—biogenesis and functions in inflammation. Biochim Biophys Acta 1791:540–551
Bozza PT, Bakker-Abreu I, Navarro-Xavier RA, Bandeira-Melo C (2011) Lipid body function in eicosanoid synthesis: an update. Prostaglandins Leukot Essent Fatty Acids. doi:10.1016/j.plefa.2011.04.020
Brasaemle DL (2007) The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res 48:2547–2559
Brasaemle DL, Barber T, Wolins NE, Serrero G, Blanchette-Mackie EJ, Londos C (1997) Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res 38:2249–2263
Brasaemle DL, Dolios G, Shapiro L, Wang R (2004) Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J Biol Chem 279:46835–46842
Bréhélin C, Kessler F, van Wijk KJ (2007) Plastoglobules: versatile lipoprotein particles in plastids. Trends Plant Sci 12:260–266
Browman DT, Hoegg MB, Robbins SM (2007) The SPFH domain-containing proteins: more than lipid raft markers. Trends Cell Biol 17:394–402
Buers I, Hofnagel O, Ruebel A, Severs NJ, Robenek H (2011) Lipid droplet associated proteins: an emerging role in atherogenesis. Histol Histopathol 26:631–642
Canoy D, Buchan I (2007) Challenges in obesity epidemiology. Obes Rev 8:1–11
Castera L, Chouteau P, Hezode C, Zafrani ES, Dhumeaux D, Pawlotsky JM (2005) Hepatitis C virus-induced hepatocellular steatosis. Am J Gastroent 100:711–715
Catesson AM (1964) Origine, fonctionnement, et variation cytologiques saisonnières du cambium. Ann Sci Nat 5:368–375
Cavalier-Smith T (2006) Rooting the tree of life by transition analyses. Biol Direct 1:19
Cermelli S, Guo Y, Gross SP, Welte MA (2006) The lipid-droplet proteome reveals that droplets are a protein-storage depot. Curr Biol 16:1783–1795
Cernac A, Benning C (2004) WRINKLED1 encodes an AP2 ⁄ EREB domain protein involved in the control of storage compound biosynthesis in Arabidopsis. Plant J 40:575–585
Cernac A, Andre C, Hoffmann-Benning S, Benning C (2006) WRI1 is required for seed germination and seedling establishment. Plant Physiol 141:745–757
Chang BH, Chan L (2007) Emerging role of lipid droplet protein ADFP in health and disease. Am J Physiol Gastrointest Liver Physiol 292:1465–1468
Chawla A, Lee CH, Barak Y, He W, Rosenfeld J, Liao D, Han J, Kang H, Evans RM (2003) PPAR∂ is a very low-density lipoprotein sensor in macrophages. Proc Natl Acad Sci USA 100:1268–1273
Che P, Love TM, Frame BR, Wang K, Carriquiry AL, Howell SH (2006) Gene expression patterns during somatic embryo development and germination in maize Hi II callus cultures. Plant Mol Biol 62:1–14
Chen H, Klein A, Xiang M, Backhaus RA, Kuntz M (1998) Drought and wound induced expression in leaves of a gene encoding a chromoplast carotenoid-associated protein. Plant J 14:317–326
Chen JC, Tsai CC, Tzen JT (1999) Cloning and secondary structure analysis of caleosin, a unique calcium-binding protein in oil bodies of plant seeds. Plant Cell Physiol 40:1079–1086
Chen W, Yechoor VK, Chang BH, Li MV, March KL, Chan L (2009) The human lipodystrophy gene product Berardinelli–Seip congenital lipodystrophy 2/seipin plays a key role in adipocyte differentiation. Endocrinology 150:4552–4561
Cheng J, Fujita A, Ohsaki Y, Suzuki M, Shinohara Y, Fujimoto T (2009) Quantitative electron microscopy shows uniform incorporation of triglycerides into existing lipid droplets. Histochem Cell Biol 132:281–291
Cho SY, Shin ES, Park PJ, Shin DW, Chang HK, Kim D, Lee HH, Lee JH, Kim SH, Song MJ, Chang IS, Lee OS, Lee TR (2007) Identification of mouse Prp19p as a lipid droplet-associated protein and its possible involvement in the biogenesis of lipid droplets. J Biol Chem 282:2456–2465
Chong BM, Reigan P, Mayle-Combs KD, Orlicky DJ, McManaman JL (2011a) Determinants of adipophilin function in milk lipid formation and secretion. Trend Endocrinol Metab 22:211–217
Chong BM, Russell TD, Schaack J, Orlicky DJ, Reigan P, Ladinsky M, McManaman JL (2011b) The adipophilin C terminus is a self-folding membrane-binding domain that is important for milk lipid secretion. J Biol Chem 286:23254–23265
Christianson JL, Boutet E, Puri V, Chawla A, Czech MP (2010) Identification of the lipid droplet targeting domain of the Cidea protein. J Lipid Res 51:3455–3462
Chung KM, Cha SS, Jang SK (2008) A novel function of karyopherin beta3 associated with apolipoprotein A-I secretion. Mol Cells 26:291–298
Cobbe N, Marshall KM, Rao SG, Chang CW, Di Cara F, Duca E, Vass S, Kassan A, Heck MMS (2009) The conserved metalloprotease invadolysin localizes to the surface of lipid droplets. J Cell Sci 122:3414–3423
Coca M, San Segundo B (2010) AtCPK1 calcium-dependent protein kinase mediates pathogen resistance in Arabidopsis. Plant J 63:526–540
Cocchiaro JL, Kumar Y, Fischer ER, Hackstadt T, Valdivia RH (2008) Cytoplasmic lipid droplets are translocated into the lumen of the Chlamydia trachomatis parasitophorous vacuole. Proc Natl Acad Sci USA 105:9379–9384
Cole NB, Murphy DD, Grider T, Rueter S, Brasaemle D, Nussbaum RL (2002) Lipid droplet binding and oligomerization properties of the Parkinson’s disease protein σ-synuclein. J Biol Chem 277:6344–6352
Coleman RA, Lewin TM, Muoio DM (2000) Physiological and nutritional regulation of enzymes of triacylglycerol synthesis. Annu Rev Nutr 20:77–103
Connerth M, Tibor Czabany T, Wagner A, Zellnig G, Leitner E, Steyrer E, Daum G (2010) Oleate inhibits steryl ester synthesis and causes liposensitivity in yeast. J Biol Chem 285:26832–26841
Cooper MS, Hardin WR, Petersen TW, Cattolico RA (2010) Visualizing “green oil” in live algal cells. J Biosci Bioeng 109:198–201
Coppens I (2005) Contribution of host lipids to Toxoplasma pathogenesis. Cell Microbiol 8:1–9
Coppens I, Vielemeyer O (2005) Insights into unique physiological features of neutral lipids in Apicomplexa: from storage to potential mediation in parasite metabolic activities. J Parasitol 35:597–615
Cordova-Tellez L, Burris JS (2002) Alignment of lipid bodies along the plasma membrane during the acquisition of desiccation tolerance in maize seed. Crop Sci 42:1982–1988
Courchesne NMD, Parisien A, Wang B, Lan CQ (2009) Enhancement of lipid production using biochemical, genetic and transcription factor engineering approaches. J Biotechnol 141:31–41
Cragg FJ, Willison JHM (1980) The ultrastructure of quiescent buds of Tilia europaea. Can J Bot 58:1804–1813
Crane J, Miller A, Van Roekel JW, Walters C (2003) Triacylglycerols determine the unusual storage physiology of Cuphea seed. Planta 217:699–708
Crane J, Kovach D, Gardner C, Walters C (2006) Triacylglycerol phase and ‘intermediate’ seed storage physiology: a study of Cuphea carthagenensis. Planta 223:1081–1089
Cypess AM, Kahn CR (2010) The role and importance of brown adipose tissue in energy homeostasis. Curr Opin Pediatr 22:478–484
Czabany T, Wagner A, Zweytick D, Lohner K, Leitner E, Ingolic E, Daum G (2008) Structural and biochemical properties of lipid particles from the yeast Saccharomyces cerevisiae. J Biol Chem 283:17065–17074
D’Avila H, Melo RC, Parreira GG, Werneck-Barroso E, Castro-Faria-Neto HC, Bozza PT (2006) Mycobacterium bovis bacillus Calmette–Guérin induces TLR2-mediated formation of lipid bodies: intracellular domains for eicosanoid synthesis in vivo. J Immunol 176:3087–3097
D’Avila H, Almeida PE, Roque NR, Castro-Faria-Neto HC, Bozza PT (2007) Toll-like receptor-2-mediated C-C chemokine receptor 3 and eotaxin-driven eosinophil influx induced by Mycobacterium bovis BCG pleurisy. Infect Immun 75:1507–1511
D’Avila H, Maya-Monteiro CM, Bozza PT (2008) Lipid bodies in innate immune response to bacterial and parasite infections. Int Immunopharmacol 8:1308–1315
Dalen KT, Dahl T, Holter E, Arntsen B, Londos C, Sztalryd C, Nebb HI (2007) LSDP5 is a PAT protein specifically expressed in fatty acid oxidizing tissues. Biochim Biophys Acta 1771:210–212
Dalton DA, Ma C, Shrestha S, Kitin P, Strauss SH (2011) Trade-offs between biomass growth and inducible biosynthesis of polyhydroxybutyrate in transgenic poplar. Plant Biotechnol J 9:759–767
Dammann C, Ichida A, Hong B, Romanowsky SM, Hrabak EM, Harmon AC, Pickard BG, Harper JF (2003) Subcellular targeting of nine calcium-dependent protein kinase isoforms from Arabidopsis. Plant Physiol 132:1840–1848
Daniel J, Deb C, Dubey VS, Sirakova TD, Abomoelak B, Morbidoni HR, Kolattukudy P (2004) Induction of a novel class of diacylglycerol acyltransferases and triacylglycerol accumulation in Mycobacterium tuberculosis as it goes into a dormancy-like state in culture. J Bacteriol 186:5017–5030
Das SK, Eder S, Schauer S, Diwoky C, Temmel H, Guertl B, Gorkiewicz G, Tamilarasan KP, Kumari P, Trauner M, Zimmermann R, Vesely P, Haemmerle G, Zechner R, Hoefler G (2011) Adipose triglyceride lipase contributes to cancer-associated cachexia. Science 333:233–238
de Kroon AI (2007) Metabolism of phosphatidylcholine and its implications for lipid acyl chain composition in Saccharomyces cerevisiae. Biochim Biophys Acta 1771:343–352
de Lima CS, Marques MA, Debrie AS, Almeida EC, Silva CA, Brennan PJ, Sarno EN, Menozzi FD, Pessolani MC (2009) Heparin-binding hemagglutinin (HBHA) of Mycobacterium leprae is expressed during infection and enhances bacterial adherence to epithelial cells. FEMS Microbiol Lett 292:162–169
Deb C, Lee CM, Dubey VS, Daniel J, Abomoelak B, Sirakova TD, Pawar S, Rogers L, Kolattukudy PE (2009) A novel in vitro multiple- stress dormancy model for Mycobacterium tuberculosis generates a lipid-loaded, drug-tolerant, dormant pathogen. PLoS One 4:e6077
Débarre D, Supatto W, Pena AM, Fabre A, Tordjmann T, Combettes L, Schanne-Klein MC, Beaurepaire E (2005) Imaging the distribution and transport dynamics of lipid bodies in unstained cells and tissues using third-harmonic generation microscopy. Nat Method 3:47–53
Debelyy MO, Thoms S, Connerth M, Daum G, Erdmann R (2011) Involvement of the yeast hydrolase Ldh1p in lipid homeostasis. Eukaryot Cell 10:776–781
Deisenroth C, Itahana Y, Tollini L, Jin A, Zhang Y (2011) p53 inducible Dhrs3 is an endoplasmic reticulum protein associated with lipid droplet accumulation. J Biol Chem 286:28343–28356
Delikatny EJ, Chawla S, Leung DJ, Poptani H (2011) MR-visible lipids and the tumor microenvironment. NMR Biomed 24:592–611
Deruere J, Romer S, D'Harlingue A, Backhaus RA, Kuntz M, Camara B (1994) Fibril assembly and carotenoid over accumulation in chromoplasts: a model for supramolecular lipoprotein structures. Plant Cell 6:119–133
Dias JML, Lemos PC, Serafim LS, Oliveira C, Eiroa M, Albuquerque MGE, Ramos AM, Oliveira R, Reis MAM (2006) Recent advances in polyhydroxyalkanoate production by mixed aerobic cultures: from the substrate to the final product. Macromol Biosci 6:885–906
DiDonato D, Brasaemle DL (2003) Fixation methods for the study of lipid droplets by immunofluorescence microscopy. J Histchem Cytochem 51:773–780
Digel M, Ehehalt R, Füllekrug J (2010) Lipid droplets lighting up: insights from live microscopy. FEBS Lett 584:2168–2175
Dong H, Czaja MJ (2011) Regulation of lipid droplets by autophagy. Trend Endocrinol Metab 22:234–240
Dowhan W, Bogdanov M, Mileykovskaya E (2008) Functional roles of lipids in membranes. In: Vance DE, Vance JE (eds) Biochemistry of lipids, lipoproteins and membranes, 5th edn. Elsevier, Amsterdam, pp 1–37
Ducharme NA, Bickel PE (2008) Lipid droplets in lipogenesis and lipolysis. Endocrinol 149:942–949
Dvorak AM, Morgan ES, Tzizik DM, Weller PF (1994) Prostaglandin endoperoxide synthase (cyclooxygenase): ultrastructural localization to nonmembrane-bound cytoplasmic lipid bodies in human eosinophils and 3T3 fibroblasts. Int Arch Allergy Immunol 105:245–250
Eastmond PJ, Quettier AL, Kroon JTM, Craddock C, Adams N, Slabas AR (2010) Phosphatidic acid phosphohydrolase 1 and 2 regulate phospholipid synthesis at the endoplasmic reticulum in Arabidopsis. Plant Cell 22:2796–2811
Edvardsson U, Ljungberg A, Linden D, William-Olsson L, Peilot-Sjogren H, Ahnmark A, Oscarsson J (2006) PPARα activation increases triglyceride mass and adipose differentiation-related protein in hepatocytes. J Lipid Res 47:329–340
Eltgroth ML, Watwood RL, Wolfe GV (2005) Production and cellular localization of neutral long-chain lipids in the haptophyte algae Isochrysis galbana and Emiliana huxleyi. J Phycol 41:1000–1009
Farese RV, Walther TC (2009) Lipid droplets finally get a little R-E-S-P-E-C-T. Cell 139:855–860
Farrar JJ, Evert RF (1997) Seasonal changes in the ultrastructure of the vascular cambium of Robinia pseudoacacia. Trees 11:191–202
Fei W, Shui G, Gaeta B, Du X, Kuerschner L, Li P, Brown AJ, Wenk MR, Parton RG, Yang H (2008) Fld1p, a functional homologue of human seipin, regulates the size of lipid droplets in yeast. J Cell Biol 180:473–482
Fei W, Du X, Yang H (2011a) Seipin, adipogenesis and lipid droplets. Trend Endocrinol Metab 22:204–210
Fei W, Shui G, Zhang Y, Krahmer N, Ferguson C, Kapterian TS, Lin RC, Dawes IW, Brown AJ, Li P, Huang X, Parton RG, Wenk MR, Walther TC, Yang H (2011b) A role for phosphatidic acid in the formation of “supersized” lipid droplets. PLoS Genet 7:e1002201
Feng H, Ren M, Chen L, Rubin CS (2007) Properties, regulation and in vivo functions of a novel protein kinase D: C. elegans DKF-2 links diacylglycerol second messenger to the regulation of stress responses and lifespan. J Biol Chem 282:31273–31288
Fisher DB, Jensen WA, Ashton ME (1968) Histochemical studies of pollen: storage pockets in the endoplasmic reticulum (ER). Histochem 13:169–182
Flaspohler JA, Jensen BC, Saveria T, Kifer CT, Parsons M (2010) A novel protein kinase localized to lipid droplets is required for droplet biogenesis in trypanosomes. Eukaryot Cell 9:1702–1710
Frandsen GI, Mundy J, Tzen JTC (2001) Oil bodies and their associated proteins, oleosin and caleosin. Physiol Plant 112:301–307
French SW (1989) Biochemical basis for alcohol-induced liver injury. Clin Biochem 22:41–49
Froissard M, D’andréa S, Boulard C, Chardot T (2009) Heterologous expression of AtClo1, a plant oil body protein, induces lipid accumulation in yeast. FEMS Yeast Res 9:428–438
Fujimoto T, Ohsaki Y (2006) Cytoplasmic lipid droplets: rediscovery of an old structure as a unique platform. Ann NY Acad Sci 1086:104–115
Fujimoto T, Parton RG (2010) Not just fat: the structure and function of the lipid droplet. Cold Spring Harb Perspect Biol 3:a004838
Fujimoto T, Kogo H, Nomura R, Une T (2000) Isoforms of caveolin 1 and caveolar structure. J Cell Sci 113:3509–3517
Fujimoto T, Kogo H, Ishiguro K, Tauchi K, Nomura R (2001) Caveolin-2 is targeted to lipid droplets, a new “membrane domain” in the cell. J Cell Biol 152:1079–1085
Fujimoto Y, Itabe H, Sakai J, Makita M, Noda J, Mori M, Higashi Y, Kojima S, Takano T (2004) Identification of major proteins in the lipid droplet-enriched fraction isolated from the human hepatocyte cell line HuH7. Biochim Biophys Acta 1644:47–59
Fujimoto T, Ohsaki Y, Cheng J, Suzuki M, Shinohara Y (2008) Lipid droplets: a classic organelle with new outfits. Histochem Cell Biol 130:263–279
Fukui T, Kichise T, Iwata T, Doi Y (2001) Characterization of 13 kDa granule-associated protein in Aeromonas caviae and biosynthesis of polyhydroxyalkanoates with altered molar composition by recombinant bacteria. Biomacromol 2:148–153
Galagan JE, Nusbaum C, Roy A, Endrizzi MG, Macdonald P, FitzHugh W, Calvo S, Engels R, Smirnov S, Atnoor D, Brown A, Allen N, Naylor J, Stange-Thomann N, DeArellano K, Johnson R, Linton L, McEwan P, McKernan K, Talamas J, Tirrell A, Ye W, Zimmer A, Barber RD, Cann I, Graham DE, Grahame DA, Guss AM, Hedderich R, Ingram-Smith C, Kuettner HC, Krzycki JA, Leigh JA, Li W, Liu J, Mukhopadhyay B, Reeve JN, Smith K, Springer TA, Umayam LA, White O, White RH, Conway de Macario E, Ferry JG, Jarrell KF, Jing H, Macario AJ, Paulsen I, Pritchett M, Sowers KR, Swanson RV, Zinder SH, Lander E, Metcalf WW, Birren B (2002) The genome of M.acetivorans reveals extensive metabolic and physiological diversity. Genome Res 12:532–542
Gandotra S, Le Dour C, Bottomley W, Cervera P, Giral P, Reznik Y, Charpentier G, Auclair M, Delépine M, Barroso I, Semple RK, Lathrop M, Lascols O, Capeau J, O'Rahilly S, Magré J, Savage DB, Vigouroux C (2011) Perilipin deficiency and autosomal dominant partial lipodystrophy. N Engl J Med 364:740–748
Garg A, Agarwal AK (2009) Lipodystrophies: disorders of adipose tissue biology. Biochim Biophys Acta 1791:507–513
Garton NJ, Christensen H, Minnikin DE, Adegbola RA, Barer MR (2002) Intracellular lipophilic inclusions of mycobacteria in vitro and in sputum. Microbiol 148:2951–2958
Garton NJ, Waddell SJ, Sherratt AL, Lee SM, Smith RJ, Senner C, Hinds J, Rajakumar K, Adegbola RA, Besra GS, Butcher PD, Barer MR (2008) Cytological and transcript analyses reveal fat and lazy persister-like bacilli in tuberculous sputum. PLoS Med 5:e75
Gaspar I, Szabad J (2009) In vivo analysis of MT-based vesicle transport by confocal reflection microscopy. Cell Motil Cytoskelet 66:68–79
Gidda SK, Shockey JM, Falcone M, Kim PK, Rothstein SJ, Andrews DW, Dyer JM, Mullen RT (2011) Hydrophobic-domain-dependent protein–protein interactions mediate the localization of GPAT enzymes to ER subdomains. Traffic 12:452–472
Gillet B, Beyly A, Peltier G, Rey P (1998) Molecular characterization of CDSP 34, a chloroplastic protein induced by water deficit in Solanum tuberosum L. plants, and regulation of CDSP 34 expression by ABA and high illumination. Plant J 16:257–262
Girousse A, Langin D (2011) Adipocyte lipases and lipid droplet-associated proteins: insight from transgenic mouse models. Int J Obesity. doi:10.1038/ijo.2011.113
Glebov OO, Bright NA, Nichols BJ (2006) Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat Cell Biol 8:46–54
Gohon Y, Vindigni JD, Pallier A, Wien F, Celia H, Giuliani A, Tribet C, Chardot T, Briozzo P (2011) High water solubility and fold in amphipols of proteins with large hydrophobic regions: oleosins and caleosin from seed lipid bodies. Biochim Biophys Acta 1808:706–716
Goldberg AA, Bourque SD, Kyryakov P, Boukh-Viner T, Gregg C, Beach A, Burstein MT, Machkalyan G, Richard V, Rampersad S, Titorenko VI (2009) A novel function of lipid droplets in regulating longevity. Biochem Soc Trans 37:1050–1055
Gomis-Rüth FX (2003) Structural aspects of the metzincin clan of metalloendopeptidases. Mol Biotechnol 24:157–202
Goodman JM (2008) The gregarious lipid droplet. J Biol Chem 283:28005–28009
Goodman JM (2009) Demonstrated and inferred metabolism associated with cytosolic lipid droplets. J Lipid Res 50:2148–2156
Goodpaster BH, He J, Watkins S, Kelley DE (2001) Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 86:5755–5761
Goto T, Nagai H, Egawa K, Kim YI, Kato S, Taimatsu A, Sakamoto T, Ebisu S, Hohsaka T, Miyagawa H, Murakami S, Takahashi N, Kawada T (2011) Farnesyl pyrophosphate regulates adipocyte functions as an endogenous PPARγ agonist. Biochem J 483:111–119
Gouda MK (2008) Single cell oil production by Gordonia sp. DG using agro-industrial wastes. World J Microbiol Biotechnol 24:1703–1711
Grage K, Jahns AC, Parlane N, Palanisamy R, Rasiah IA, Atwood JA, Rehm BHA (2009) Bacterial polyhydroxyalkanoate granules: biogenesis, structure, and potential use as nano-/micro-beads in biotechnological and biomedical applications. Biomacromol 10:660–669
Granneman JG, Moore HP (2008) Location, location: protein trafficking and lipolysis in adipocytes. Trend Endocrinol Metab 19:3–9
Greenberg AS, Coleman RA (2011) Expanding roles for lipid droplets. Trend Endocrinol Metab 22:195–196
Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C (1991) Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem 266:11341–11346
Greenberg AS, Coleman RA, Kraemer FB, McManaman JL, Obin MS, Puri V, Yan QW, Miyoshi H, Mashek DG (2011) The role of lipid droplets in metabolic disease in rodents and humans. J Clin Investig 121:2102–2110
Grefen C, Städele K, Ruzicka KL, Obrdlik P, Harter K, Horák J (2008) Subcellular localization and in vivo interactions of the Arabidopsis thaliana ethylene receptor family members. Mol Plant 1:308–320
Grönke S, Beller M, Fellert S, Ramakrishnan H, Jäckle H, Kühnlein RP (2003) Control of fat storage by a Drosophila PAT domain protein. Curr Biol 13:603–606
Grönke S, Mildner A, Fellert S, Tennagels N, Petry S, Müller G, Jäckle H, Kühnlein RP (2005) Brummer lipase is an evolutionary conserved fat storage regulator in Drosophila. Cell Metab 1:323–330
Grönke S, Müller G, Hirsch J, Fellert S, Andreou A, Haase T, Jäckle H, Kühnlein RP (2007) Dual lipolytic control of body fat storage and mobilization in Drosophila. PLoS Biol 5:e137
Gropler MC, Harris TE, Hall AM, Wolins NE, Gross RW, Han X, Chen Z, Finck BN (2009) Lipin 2 is a liver-enriched phosphatidate phosphohydrolase enzyme that is dynamically regulated by fasting and obesity in mice. J Biol Chem 284:6763–67720
Gross SP, Welte MA, Block SM, Wieschaus EF (2000) Dynein-mediated cargo transport in vivo: a switch controls travel distance. J Cell Biol 148:945–956
Guenther JC, Hallen-Adams HE, Bücking H, Shachar-Hill Y, Trail F (2009) Triacylglyceride metabolism by Fusarium graminearum during colonization and sexual development on wheat. Mol Plant Path Inter 22:1492–1503
Guilloteau M, Laloi M, Blais D, Crouzillat D, Mc Carthy J (2003) Oil bodies in Theobroma cacao seeds: cloning and characterization of cDNA encoding the 15.8 and 16.9 kDa oleosins. Plant Sci 164:597–606
Gunjan A, Verreault A (2003) A Rad53 kinase-dependent surveillance mechanism that regulates histone protein levels in S. cerevisiae. Cell 115:537–549
Guo Y, Walther TC, Rao M, Stuurman N, Goshima G, Terayama K, Wong JS, Vale RD, Walter P, Farese RV (2008) Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature 453:657–661
Guo Y, Cordes KR, Farese RV, Walther TC (2009) Lipid droplets at a glance. J Cell Sci 122:749–752
Gurusamy C, Davis PJ, Bal AK (2000) Seasonal changes in perennial nodules of beach pea (Lathyrus maratimus [L] Bigel.) with special reference to oleosomes. Int J Plant Sci 161:631–638
Gutzeit HO (1986) The role of microfilaments in cytoplasmic streaming in Drosophila follicles. J Cell Sci 80:159–169
Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspor M, Hoefler G, Zechner R (2006) Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 312:734–737
Han GS, Wu WI, Carman GM (2006) The Saccharomyces cerevisiae lipin homolog is a Mg2+-dependent phosphatidate phosphatase enzyme. J Biol Chem 281:9210–9218
Han GS, Siniossoglou S, Carman GM (2007a) The cellular functions of the yeast lipin homolog PAH1p are dependent on its phosphatidate phosphatase activity. J Biol Chem 282:37026–37035
Han J, Lu Q, Zhou L, Zhou J, Xiang H (2007b) Molecular characterization of the phaECHm genes, required for biosynthesis of poly(3-hydroxybutyrate) in the extremely halophilic archaeon Haloarcula marismortui. Appl Environ Microbiol 73:6058–6065
Han J, Lu Q, Zhou L, Liu H, Xiang H (2009) Identification of the polyhydroxyalkanoate (PHA)-specific acetoacetyl coenzyme A reductase among multiple FabG paralogs in Haloarcula hispanica and reconstruction of the PHA biosynthetic pathway in Haloferax volcanii. Appl Environ Microbiol 75:6168–6175
Han J, Hou J, Liu H, Cai S, Feng B, Zhou J, Xiang H (2010a) Wide distribution among halophilic Archaea of a novel polyhydroxyalkanoate synthase subtype with homology to bacterial type III synthases. Appl Environ Microbiol 76:7811–7819
Han J, Li M, Hou J, Wu L, Zhou J, Xiang H (2010b) Comparison of four phaC genes from Haloferax mediterranei and their function in different PHBV copolymer biosyntheses in Haloarcula hispanica. Saline Syst 6:9
Hanano A, Burcklen M, Flenet M, Ivancich A, Louwagie M, Garin J, Blée E (2006) Plant seed peroxygenase is an original heme-oxygenase with an EF-hand calcium binding motif. J Biol Chem 281:33140–33151
Hänisch J, Wältermann M, Robenek H, Steinbüchel A (2006) The Ralstonia eutropha H16 phasin PhaP1 is targeted to intracellular triacylglycerol inclusions in Rhodococcus opacus PD630 and Mycobacterium smegmatis mc2155, and provides an anchor to target other proteins. Microbiol 152:3271–3280
Hanley SZ, Pappin DJ, Rahman D, White AJ, Elborough KM, Slabas AR (1999) Re-evaluation of the primary structure of Ralstonia eutropha phasin and implications for polyhydroxyalkanoic acid granule binding. FEBS Lett 447:99–105
Hanstein J (1880) Ueber die Gestaltungsvorgange in den Zellkerne bei der Theilung der Zellen. Botan Abhandl Morphol Physiol Bonn 4:2
Hapala I, Marza E, Ferreira T (2011) Is fat so bad? Modulation of endoplasmic reticulum stress by lipid droplet formation. Biol Cell 103:271–285
Harayama S, Kishira H, Kasai Y, Shutsubo K (1999) Petroleum biodegradation in marine environments. J Mol Microbiol Biotechnol 1:63–70
Harayama S, Kasai Y, Hara A (2004) Microbial communities in oil-contaminated seawater. Curr Opin Biotechnol 15:205–214
Harris TE, Finck BN (2011) Dual function lipin proteins and glycerolipid metabolism. Trend Endocrinol Metab 22:226–233
Harris CA, Haas JT, Streeper RS, Stone SJ, Kumari M, Yang K, Han X, Brownell N, Gross RW, Zechner R, Farese RV (2011) DGAT enzymes are required for triacylglycerol synthesis and lipid droplets in adipocytes. J Lipid Res 52:657–667
Hayashi T, Su TP (2003) Sigma-1 receptors (sigma(1) binding sites) form raft-like microdomains and target lipid droplets on the endoplasmic reticulum: roles in endoplasmic reticulum lipid compartmentalization and export. J Pharmacol Exp Ther 306:718–725
Hayashi YK, Matsuda C, Ogawa M, Goto K, Tominaga K, Mitsuhashi S, Park YE, Nonaka I, Hino-Fukuyo N, Haginoya K, Sugano H, Nishino I (2009) Human ptrf mutations cause secondary deficiency of caveolins resulting in muscular dystrophy with generalized lipodystrophy. J Clin Invest 119:2623–2633
He YQ, Wu Y (2009) Oil body biogenesis during Brassica napus embryogenesis. J Integr Plant Biol 51:792–799
Helfrich W (1973) Elastic properties of lipid bilayers: theory and possible experiments. Z Naturforsch C 28:693–703
Heneen WK, Karlsson G, Brismar K, Gummeson PO, Marttila S, Leonova S, Carlsson AS, Bafor M, Banas A, Mattsson B, Debski H, Stymne S (2008) Fusion of oil bodies in endosperm of oat grains. Planta 228:589–599
Hensel W (1986) Cytodifferentiation of polar plant cells. Planta 169:293–303
Herker E, Ott M (2011) Unique ties between hepatitis C virus replication and intracellular lipids. Trend Endocrinol Metab 22:241–248
Herker E, Harris C, Hernandez C, Carpentier A, Kaehlcke K, Rosenberg AR, Farese RV Jr, Ott M (2010) Efficient hepatitis C virus particle formation requires diacylglycerol acyltransferase-1. Nat Meth 16:1295–1298
Herman E (2008) Endoplasmic reticulum bodies: solving the insoluble. Curr Opin Plant Biol 11:672–679
Hernández-Pinzón I, Ross JHE, Barnes KA, Damant AP, Murphy DJ (1999) Composition and role of tapetal lipid bodies in the biogenesis of the pollen coat of Brassica napus. Planta 208:588–598
Hernández-Pinzón I, Patel K, Murphy DJ (2001) The novel calcium-binding protein, caleosin, has distinct endoplasmic reticulum and lipid-body associated isoforms. Plant Physiol Biochem 39:1–9
Hezayen FF, Gutierrez CM, Steinbüchel A, Tindall BJ, Rehm BH (2009) Halopiger aswanensis sp. nov., a polymer-producing, extremely halophilic archaeon isolated from hypersaline soil. Int J Syst Evol Microbiol 60:633–637
Hickenbottom SJ, Kimmel AR, Londos C, Hurley JH (2004) Structure of a lipid droplet protein: the PAT family member TIP47. Structure 12:1199–1207
Hinson ER, Cresswell P (2009) The antiviral protein, viperin, localizes to lipid droplets via its N-terminal amphipathic α -helix. Proc Natl Acad Sci USA 106:20452–20457
Hodges BDM, Wu CC (2010) Proteomic insights into an expanded cellular role for cytoplasmic lipid droplets. J Lipid Res 51:262–273
Hommel A, Hesse D, Volker W, Jaschke A, Moser M, Engel T, Blüher M, Zahn C, Chadt A, Ruschke K, Vogel H, Kluge R, Robenek H, Joost HJ, Schürmann A (2010) The ARF-like GTPase ARFRP1 is essential for lipid droplet growth and is involved in the regulation of lipolysis. Mol Cell Biol 30:1231–1242
Hooper C, Puttamadappa SS, Loring Z, Shekhtman A, Bakowska JC (2010) Spartin activates atrophin-1-interacting protein 4 (AIP4) E3 ubiquitin ligase and promotes ubiquitination of adipophilin on lipid droplets. BMC Biol 8:72
Hörl G, Wagner A, Cole LK, Malli R, Reicher H, Kotzbeck P, Köfeler H, Höfler G, Frank S, Bogner-Strauss JG, Sattler W, Vance DE, Steyrer E (2011) Sequential synthesis and methylation of phosphatidylethanolamine promote lipid droplet biosynthesis and stability in tissue culture and in vivo. J Biol Chem 285:17338–17350
Horn PJ, Ledbetter NR, James CN, Hoffman WD, Case CR, Verbeck GF, Chapman KD (2011) Visualization of lipid droplet composition by direct organelle mass spectrometry. J Biol Chem 286:3298–3306
Horton JD, Goldstein JL, Brown MS (2002) SREBPs: transcriptional mediators of lipid homeostasis. Cold Spring Harb Symp Quant Biol 67:491–498
Hsiao ESL, Tzen JTC (2011) Ubiquitination of oleosin-H and caleosin in sesame oil bodies after seed germination. Plant Physiol Biochem 49:77–81
Hsieh K, Huang A (2005) Lipid-rich tapetosomes in Brassica tapetum are composed of oleosin-coated oil droplets and vesicles, both assembled in and then detached from the endoplasmic reticulum. Plant J 43:889–899
Huang CY, Chung CI, Lin YC, Hsing YC, Huang AHC (2009) Oil bodies and oleosins in Physcomitrella possess characteristics representative of early trends in evolution. Plant Physiol 150:1192–1203
Itabe H (2010) Intracellular lipid droplet-associated proteins: unique members and their biological functions. Foreword. Biol Pharm Bull 33:341–341
Jacobson K, Mouritsen OG, Anderson RGW (2007) Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol 9:7–14
Jacquier N, Choudhary V, Mari M, Toulmay A, Reggiori F, Schneiter R (2011) Lipid droplets are functionally connected to the endoplasmic reticulum in Saccharomyces cerevisiae. J Cell Sci 124:2424–2437
Jägerström S, Polesie S, Wickström Y, Johansson BR, Schröder HD, Hojlund K, Boström P (2009) Lipid droplets interact with mitochondria using SNAP23. Cell Biol Int 33:934–940
James CN, Horn PJ, Case CR, Gidda SK, Zhang D, Mullen RT, Dyer JM, Anderson RG, Chapman KD (2010) Disruption of the Arabidopsis CGI-58 homologue produces Chanarin–Dorfman-like lipid droplet accumulation in plants. Proc Natl Acad Sci USA 107:17833–17838
Jarrell KF, Walters AD, Bochiwal C, Borgia JM, Dickinson T, Chong JPJ (2011) Major players on the microbial stage: why archaea are important. Microbiol 157:919–936
Jendrossek D (2009) Polyhydroxyalkanoate granules are complex subcellular organelles (carbonosomes). J Bacteriol 191:3195–3202
Jenkins GM, Frohman MA (2005) Phospholipase D: a lipid centric review. Cell Mol Life Sci 62:2305–2316
Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW (2004) Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J Biol Chem 279:48968–48975
Jensen WA, Fisher DB, Ashton ME (1968) Cotton embryogenesis: the pollen cytoplasm. Planta 81:206–228
Jordy MN (2004) Seasonal variation of organogenetic activity and reserves allocation in the shoot apex of Pinus pinaster Ait. Ann Bot 93:25–37
Kadereit B, Kumar P, Wang WJ, Miranda D, Snapp EL, Severina N, Torregroza I, Evans T, Silver DL (2008) Evolutionarily conserved gene family important for fat storage. Proc Natl Acad Sci USA 105:94–99
Kahn RA, Cherfils J, Elias M, Lovering RC, Munro S, Schümann A (2006) Nomenclature for the human Arf family of GTP-binding proteins: ARF, ARL, and SAR proteins. J Cell Biol 172:645–650
Kalantari F, Bergeron JJ, Nilsson T (2010) Biogenesis of lipid droplets—how cells get fatter. Mol Memb Biol 27:462–468
Kalia VC, Lal S, Cheema S (2007) Insight in to the phylogeny of polyhydroxyalkanoate biosynthesis: horizontal gene transfer. Gene 389:19–26
Kalscheuer R (2010) Genetics of wax ester and triacylglycerol biosynthesis in bacteria. In: Timmis KN (ed) Handbook of hydrocarbon and lipid microbiology. Springer, Berlin
Kalscheuer R, Steinbüchel A (2003) A novel bifunctional wax ester synthase/acyl-CoA: diacylglycerol acyltransferase mediates wax ester and triacylglycerol biosynthesis in 653 Acinetobacter calcoaceticus ADP1. J Biol Chem 278:8075–8082
Kalscheuer R, Stöveken T, Malkus U, Reichelt R, Golyshin PN, Sabirova JS, Ferrer M, Timmis KN, Steinbüchel A (2007a) Analysis of storage lipid accumulation in Alcanivorax borkumensis: Evidence for alternative triacylglycerol biosynthesis routes in bacteria. J Bacteriol 189:918–928
Kalscheuer R, Stölting T, Steinbüchel A (2007b) Microdiesel: Escherichia coli engineered for fuel production. Microbiol 152:2529–2536
Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, Matsuno A, Muraki A, Nakazaki N, Naruo K, Okumura S, Shimpo S, Takeuchi C, Wada T, Watanabe A, Yamada M, Yasuda M, Tabata S (1996) Sequence analysis of the genome of the unicellular Cyanobacterium sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res 3:109–136
Kanwischer M, Porfirova S, Bergmuller E, Dormann P (2005) Alterations in tocopherol cyclase activity in transgenic and mutant plants of Arabidopsis affect tocopherol content, tocopherol composition, and oxidative stress. Plant Physiol 137:713–723
Karimi M, de Oliviera Manes CL, van Montagu M, Gheysen G (2002) Activation of a pollenin promoter upon nematode infection. J Nemat 34:75–79
Kasai Y, Kishira H, Sasaki T, Syutsubo WK, Harayama S (2002) Predominant growth of Alcanivorax strains in oil-contaminated and nutrient-supplemented sea water. Environ Microbiol 4:141–147
Katavic V, Agrawal GK, Hajduch M, Harris SL, Thelen JJ (2006) Protein and lipid composition analysis of oil bodies from two Brassica napus cultivars. Proteomics 16:4586–4598
Katz A, Jimenez C, Pick U (1995) Isolation and characterization of a protein associated with carotene globules in the alga Dunaliella bardawil. Plant Physiol 108:1657–1664
Keeling PJ, Fast NM (2002) Microsporidia: biology and evolution of highly reduced intracellular parasites. Annu Rev Microbiol 56:93–116
Keeling PJ, Luker MA, Palmer JD (2000) Evidence from beta-tubulin phylogeny that microsporidia evolved from within the fungi. Mol Biol Evol 17:23–31
Keenan TW, Mather IH (2006) Intracellular origin of milk fat globules and the nature of the milk fat globule membrane. In: Fox PF, McSweeney PLH (eds) Advanced Dairy Chemistry, vol 2, lipids. Springer, New York, pp 137–171
Kessler F, Schnell D, Blobel G (1999) Identification of proteins associated with plastoglobules isolated from pea (Pisum sativum L.) chloroplasts. Planta 208:107–113
Khandelia H, Duelund L, Pakkanen KI, Ipsen JH (2010) Triglyceride blisters in lipid bilayers: implications for lipid droplet biogenesis and the mobile lipid signal in cancer cell membranes. PLoS One 5:e12811
Khozin-Goldberg I, Cohen Z (2011) Unraveling algal lipid metabolism: recent advances in gene identification. Biochimie 93:91e100
Kim S, Dale B (2005) Life cycle assessment study of biopolymers (polyhydroxyalkanoates)-derived from no-tilled corn. Int J Life Cycle Anal 10:200–210
Kim HU, Wu SSH, Ratnayake C, Huang AHC (2001) Brassica rapa has three genes that encode proteins associated with different neutral lipids in plastids of specific tissues. Plant Physiol 126:330–341
Kim HU, Hsei K, Ratnayake C, Huang AHC (2002) A novel group of oleosins is present inside the pollen of Arabidopsis. J Biol Chem 277:22677–22684
Kim YY, Jung KW, Yoo KS, Jeung JU, Shin JS (2011) A stress-responsive caleosin-like protein, AtCLO4, acts as a negative regulator of ABA responses in Arabidopsis. Plant Cell Physiol 52:874–884
Kimmel AR, Brasaemle DL, McAndrews-Hill M, Sztalryd C, Londos C (2010) Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J Lipid Res 51:468–471
Kobayashi T, Shiraki M, Abe T, Sugiyama A, Saito T (2003) Purification and properties of an intracellular 3-hydroxybutyrate oligomer hydrolase (PhaZ2) in Ralstonia eutropha H16 and its identification as a novel intracellular poly(3-hydroxybutyrate) depolymerase. J Bacteriol 185:3485–3490
Koller M, Hesse P, Bona R, Kutschera C, Atlić A, Braunegg G (2007) Potential of various archae- and eubacterial strains as industrial polyhydroxyalkanoate producers from whey. Macromol Biosci 7:218–226
Koonin EV (2003) Horizontal gene transfer: the path to maturity. Mol Microbiol 50:725–727
Koonin EV (2010) The origin and early evolution of eukaryotes in the light of phylogenomics. Genome Biology 11:209
Kosa M, Ragauskas AJ (2011) Lipids from heterotrophic microbes: advances in metabolism research. Trends Biotechnol 29:53–61
Krahmer N, Guo Y, Farese RV, Walther TC (2009) SnapShot: lipid droplets. Cell 139:1024–1024
Krasowski MJ, Owens JN (1990) Seasonal changes in the apical zonation and ultrastructure of coastal Douglas fir seedlings (Pseudotsuga menziesii). Am J Bot 77:245–260
Kuerschner L, Moessinger C, Thiele C (2008) Imaging of lipid biosynthesis: how a neutral lipid enters lipid droplets. Traffic 9:338–352
Kühnlein RP (2011) The contribution of the Drosophila model to lipid droplet research. Prog Lipid Res 50:348–356
Kurat CF, Natter K, Petschnigg J, Wolinski H, Scheuringer K, Scholz H, Zimmermann R, Leber R, Zechner R, Kohlwein SD (2006) Obese yeast: triglyceride lipolysis is functionally conserved from mammals to yeast. J Biol Chem 281:491–500
Kurat C, Wolinski H, Petschnigg J, Kaluarachchi S, Andrews B, Natter K, Kohlwein S (2009) Cdk1/cdc28-dependent activation of the major triacylglycerol lipase tgl4 in yeast links lipolysis to cell-cycle progression. Mol Cell 33:53–63
Laizet Y, Pontier D, Mache R, Kuntz M (2004) Subfamily organization and phylogenetic origin of genes encoding plastid lipid-associated proteins of the fibrillin type. J Genome Sci Tech 3:19–28
Langenkamper G, Manac'h N, Broin M, Cuiné S, Becuwe N, Kuntz M, Rey P (2001) Accumulation of plastid lipid- associated proteins (fibrillin/CDSP34) upon oxidative stress, ageing and biotic stress in Solanaceae and in response to drought in other species. J Exp Bot 52:1545–1554
Larigauderie G, Furman C, Jaye M, Lasselin C, Copin C, Fruchart JC, Castro G, Rouis M (2004) Adipophilin enhances lipid accumulation and prevents lipid efflux from THP-1 macrophages: potential role in atherogenesis. Arterioscler Thromb Vasc Biol 24:504–510
Le Lay S, Dugail I (2009) Connecting lipid droplet biology and the metabolic syndrome. Prog Lipid Res 48:191–195
Le Lay S, Hajduch E, Lindsay MR, Le Liepvre X, Thiele C, Ferre P, Parton RG, Kurzchalia T, Simons K, Dugail I (2006) Cholesterol-induced caveolin targeting to lipid droplets in adipocytes: a role for caveolar endocytosis. Traffic 7:549–561
Le Lay S, Blouin CM, Hajduch E, Dugail I (2009) Filling up adipocytes with lipids. Lessons from caveolin-1 deficiency. Biochim Biophys Acta 1791:514–518
Lee MC, Miller EA, Goldberg J, Orci L, Schekman R (2004) Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol 20:87–123
Lee SC, Corradi N, Byrnes EJ 3rd, Torres-Martinez S, Dietrich FS, Keeling PJ, Heitman J (2008) Microsporidia evolved from ancestral sexual fungi. Curr Biol 18:1675–1679
Legat A, Gruber C, Zangger K, Wanner G, Stan-Lotter H (2010) Identification of polyhydroxyalkanoates in Halococcus and other haloarchaeal species. Appl Microbiol Biotechnol 87:1119–1127
Leprince O, van Aelst AC, Pritchard HW, Murphy DJ (1998) Oleosins prevent oil-body coalescence during seed imbibition as suggested by a low-temperature scanning electron microscope study of desiccation-tolerant and -sensitive oilseeds. Planta 204:109–119
Lersten NR, Czlapinski AR, Curtis JD, Freckmann R, Horner HT (2006) Oil bodies in leaf mesophyll cells of angiosperms: overview and a selected survey. Am J Bot 93:1731–1739
Leubner-Metzger G, Meins F (2000) Sense transformation reveals a novel role for class I ß-1,3-glucanase in tobacco seed germination. Plant J 23:215–221
Li M, Murphy DJ, Lee KH, Wilson R, Smith LJ, Clark DC, Sung JY (2002) Purification and structural characterization of the central hydrophobic domain of oleosin. J Biol Chem 277:37888–37895
Liang H, Yao N, Song JT, Luo S, Lu H, Greenberg JT (2003) Ceramides modulate programmed cell death in plants. Genes Dev 17:2636–2641
Lillo JG, Rodriguez-Valera F (1990) Effects of culture conditions on poly(β-hydroxybutyric acid) production by Haloferax mediterranei. Appl Environ Microbiol 56:2517–2521
Litvak V, Shaul YD, Shulewitz M, Amarilio R, Carmon S, Lev S (2002) Targeting of Nir2 to lipid droplets is regulated by a specific threonine residue within its PI-transfer domain. Curr Biol 12:1513–1518
Liu CP, Lin LP (2001) Ultrastructural study and lipid formation of Isochrysis sp. CCMP1324. Bot Bull Acad Sin 42:207–214
Liu P, Ying Y, Zhao Y, Mundy DI, Zhu M, Anderson RG (2004) Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. J Biol Chem 279:3787–3792
Liu H, Hedley P, Cardle L, Wright KM, Hein I, Marshall D, Waugh R (2005) Characterisation and functional analysis of two barley caleosins expressed during barley caryopsis development. Planta 221:513–522
Liu P, Bartz R, Zehmer J, Ying Y, Zhu M, Serrero G, Anderson R (2007) Rab-regulated interaction of early endosomes with lipid droplets. Biochim Biophys Acta 1773:784–793
Liu L, Brown D, McKee M, Lebrasseur NK, Yang D, Albrecht KH, Ravid K, Pilch PF (2008) Deletion of cavin/ptrf causes global loss of caveolae, dyslipidemia, and glucose intolerance. Cell Metab 8:310–317
Liu J, Hua W, Zhan G, Wei F, Wang X, Liu G, Wang H (2010) Increasing seed mass and oil content in transgenic Arabidopsis by the overexpression of wri1-like gene from Brassica napus. Plant Physiol Biochem 48:9–15
Lockshon D, Surface LE, Kerr EO, Kaeberlein M, Kennedy BK (2007) The sensitivity of yeast mutants to oleic acid implicates the peroxisome and other processes in membrane function. Genetics 175:77–91
Lohmann A, Schöttler MA, Bréhélin C, Kessler F, Bock R, Cahoon EB, Dörmann P (2006) Deficiency in phylloquinone (vitamin K1) methylation affects prenyl quinone distribution, photosystem I abundance, and anthocyanin accumulation in the Arabidopsis AtmenG mutant. J Biol Chem 281:40461–40472
Lösel DM, Sancholle M (1996) Fungal lipids. In: Prasad R, Ghannoum MA (eds) Lipids of pathogenic fungi. CRC, Boca Raton, pp 27–62
Lu Q, Han J, Zhou L, Zhou J, Xiang H (2008) Genetic and biochemical characterization of the poly(3-hydroxybutyrate-co-3-hydroxyvalerate) synthase in Haloferax mediterranei. J Bacteriol 190:4173–4180
Luo M, Fadeev EA, Groves J (2005) Mycobactin-mediated iron acquisition within macrophages. Nat Chem Biol 1:149–153
Lütke-Eversloh T, Bergander K, Luftmann H, Steinbüchel A (2001a) Biosynthesis of a new class of biopolymer: bacterial synthesis of a sulfur-containing polymer with thioester linkages. Microbiology 147:11–19
Lütke-Eversloh T, Bergander K, Luftmann H, Steinbüchel A (2001b) Biosynthesis of poly(3hydroxybutyrate-co-3-mercaptobutyrate) as a sulfur analogue to poly(3-hydroxybutyrate) (PHB). Biomacromolecules 2:1061–1065
MacEachran DP, Prophete ME, Sinskey AJ (2010) The Rhodococcus opacus PD630 heparin-binding hemagglutinin homolog TadA mediates lipid body formation. App Env Microbiol 76:7217–7225
Maeo K, Tokuda T, Ayame A, Mitsui N, Kawai T, Tsukagoshi H, Ishiguro S, Nakamura K (2009) An AP2-type transcription factor, WRINKLED1, of Arabidopsis thaliana binds to the AW-box sequence conserved among proximal upstream regions of genes involved in fatty acid synthesis. Plant J 60:476–487
Magra AL, Mertz PS, Torday JS, Londos C (2006) Role of adipose differentiation-related protein in lung surfactant production: a reassessment. J Lipid Res 47:2367–2373
Magré J, Delepine M, Khallouf E, Gedde-Dahl T Jr, Van Maldergem L, Sobel E, Papp J, Meier M, Megarbane A, Bachy A, Verloes A, d’Abronzo FH, Seemanova E, Assan R, Baudic N, Bourut C, Czernichow P, Huet F, Grigorescu F, de Kerdanet M, Lacombe D, Labrune P, Lanza M, Loret H, Matsuda F, Navarro J, Nivelon-Chevalier A, Polak M, Robert JJ, Tric P, Tubiana-Rufi N, Vigouroux C, Weissenbach J, Savasta S, Maassen JA, Trygstad O, Bogalho P, Freitas P, Medina JL, Bonnicci F, JoVe BI, Loyson G, Panz VR, Raal FJ, O’Rahilly S, Stephenson T, Kahn CR, Lathrop M, Capeau J (2001) Identification of the gene altered in Berardinelli–Seip congenital lipodystrophy on chromosome 11q13. Nat Genet 28:365–370
Manac’h N, Kuntz M (1999) Stress induction of a nuclear gene encoding for a plastid protein is mediated by photo-oxidative events. Plant Physiol Biochem 37:859–868
Manilla-Pérez E, Lange AB, Hetzler S, Wältermann M, Kalscheuer R, Steinbüchel A (2010) Isolation and characterization of a mutant of the marine bacterium Alcanivorax borkumensis SK2 defective in lipid biosynthesis. Appl Env Microbiol 76:2884–2894
Markley N, Nykiforuk CL, Boothe JG, Moloney MM (2006) Producing proteins using transgenic oilbody-oleosin technology. BioPharm Int 19:34–57
Martin S, Parton RG (2005) Caveolin, cholesterol, and lipid bodies. Sem Cell Devel Biol 16:163–174
Martin S, Parton RG (2006) Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol 7:373–378
Martinez-Canovas MJ, Quesada E, Llamas I, Bejar V (2004) Halomonas ventosae sp. nov., a moderately halophilic, denitrifying, exopolysaccharide-producing bacterium. Int J Syst Evol Microbiol 54:733–737
Mattos KA, D'Avila H, Rodrigues LS, Oliveira VG, Sarno EN, Atella GC, Pereira GM, Bozza PT, Pessolani MC (2010) Lipid droplet formation in leprosy: Toll-like receptor-regulated organelles involved in eicosanoid formation and Mycobacterium leprae pathogenesis. J Leukoc Biol 87:371–384
Mattos KA, Lara FA, Oliveira VGC, Rodrigues LS, D’Avila H, Melo RCN, Manso PPA, Sarno EN, Bozza PT, Pessolani MCV (2011) Modulation of lipid droplets by Mycobacterium leprae in Schwann cells: a putative mechanism for host lipid acquisition and bacterial survival in phagosomes. Cell Microbiol 13:259–273
Mayfield JA, Preuss D (2000) Rapid initiation of Arabidopsis pollination requires the oleosin-domain protein GRP17. Nat Cell Biol 2:128–130
McHugh B, Krause SA, Yu B, Deans AM, Heasman S, McLaughlin P, Heck MM (2004) Invadolysin: a novel, conserved metalloprotease links mitotic structural rearrangements with cell migration. J Cell Biol 167:673–686
Meex RC, Schrauwen P, Hesselink MK (2009) Modulation of myocellular fat stores: lipid droplet dynamics in health and disease. Am J Physiol Regul Integr Comp Physiol 297:913–924
Melo RC, D’Avila H, Fabrino DL, Almeida PE, Bozza PT (2003) Macrophage lipid body induction by Chagas disease in vivo: putative intracellular domains for eicosanoid formation during infection. Tissue Cell 35:59–67
Melo RCN, D'Avila H, Wan HC, Bozza PT, Dvorak AM, Weller PF (2011) Lipid bodies in inflammatory cells: structure, function, and current imaging techniques. J Histochem Cytochem 59:540–556
Menendez JA, Lupu R (2007) Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 7:763–777
Minnaard R, Schrauwen P, Schaart G, Jorgensen JA, Lenaers E, Mensink M, Hesselink MKC (2009) Adipocyte differentiation-related protein and OXPAT in rat and human skeletal muscle: involvement in lipid accumulation and type 2 diabetes mellitus. J Clin Endocrinol Metab 94:4077–4085
Mishra R, Eminacipator SN, Miller C, Kern T, Simonson MS (2004) Adipose differentiation related protein and regulators of lipid homeostasis identified by gene expression profiling in murine db/db diabetic kidney. Am J Physiol Renal Physiol 286:913–921
Miura S, Gan JW, Brzostowski J, Parisi MJ, Schultz CJ, Londos C, Oliver B, Kimmel AR (2002) Functional conservation for lipid storage droplet association among perilipin, ADRP, and TIP47 (PAT)-related proteins in mammals, Drosophila, and Dictyostelium. J Biol Chem 277:32253–32257
Miyanari Y, Atsuzawa K, Usuda N, Watashi K, Hishiki T, Zayas M, Bartenschlager R, Wakita T, Hijikata M, Shimotohno K (2007) The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol 9:1089–1097
Mlícková K, Luo Y, D’Andrea S, Pec P, Chardot T, Nicaud J (2004a) Acyl-CoA oxidase, a key step for lipid accumulation in the yeast Yarrowia lipolytica. J Mol Catal B: Enzym 28:81–85
Mlícková K, Roux E, Athenstaedt K, d’Andrea S, Daum G, Chardot T, Nicaud JM (2004b) Lipid accumulation, lipid body formation, and acyl coenzyme A oxidases of the yeast Yarrowia lipolytica. Appl Environ Microbiol 70:3918–3924
Moellering ER, Benning C (2010) RNA interference silencing of a major lipid droplet protein affects lipid droplet size in Chlamydomonas reinhardtii. Eukaryot Cell 9:97–106
Moessinger C, Kuerschner L, Spandl J, Shevchenko A, Thiele C (2011) Human lysophosphatidylcholine acyltransferase 1 and 2 are located to lipid droplets, where they catalyze the formation of phosphatidylcholine. J Biol Chem 286:21330–21339
Monte E, Ludevid D, Prat S (1999) Leaf C40.4: a carotenoid-associated protein involved in the modulation of photosynthetic efficiency? Plant J 19:399–410
Mooney BP (2009) The second green revolution? Production of plant-based biodegradable plastics. Biochem J 418:219–232
Moro C, Bajpeyi S, Smith SR (2008) Determinants of intramyocellular triglyceride turnover: implications for insulin sensitivity. Am J Physiol Endocrinol Metab 294:E203–E213
Mosammaparast N, Pemberton LF (2004) Karyopherins: from nuclear-transport mediators to nuclear-function regulators. Trends Cell Biol 14:547–556
Mukherjee S, Maxfield FR (2004) Membrane domains. Ann Rev Cell Dev Biol 20:839–866
Müller M, Schins JM (2002) Imaging the thermodynamic state of lipid membranes with multiplex CARS microscopy. J Phys Chem B 106:3715–3723
Murphy DJ (1982) The importance of non-planar bilayer regions in photosynthetic membranes and their stabilisation by galactolipids. FEBS Lett 150:19–26
Murphy DJ (2001) Biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog Lipid Res 40:325–438
Murphy DJ (2005) Lipid-associated proteins. In: Murphy DJ (ed) Plant Lipids: biology, utilisation and manipulation. Blackwell, Oxford
Murphy DJ (2006) The extracellular pollen coat in members of the Brassicaceae: composition, biosynthesis, and functions in pollination. Protoplasma 228:31–39
Murphy DJ (2008) Future prospects for biofuels. Chem Today 26:44–48
Murphy DJ (2010) Manipulation of oil crops for industrial applications. In: Singh BP (ed) Industrial crops and uses. CABI, UK, pp 183–206
Murphy DJ (2011) Plants, biotechnology and agriculture. CABI, UK
Murphy DJ, Ross JHE (1998) Biosynthesis, targeting and processing of oleosin-like proteins, which are major pollen coat components in Brassica napus. Plant J 13:1–16
Murphy DJ, Vance J (1999) Mechanisms of lipid body formation. Trends Biochem Sci 24:109–115
Murphy DJ, Woodrow IE (1983) Lateral heterogeneity in the distribution of acyl lipids and proteins in photosynthetic membranes. Biochim Biophys Acta 725:104–112
Murphy DJ, Hernendez-Pinzon I, Patel K, Hope RG, McLauchlan J (2000) New insights into the mechanisms of lipid-body biogenesis in plants and other organisms. Biochem Soc Trans 28:710–711
Murphy DJ, Hernandez-Pinzon I, Patel K (2001) Roles of lipid bodies and lipid-body proteins in seeds and other tissues. J Plant Physiol 158:471–478
Murphy G, Rouse RL, Polk WW, Henk WG, Barker SA, Boudreaux MJ, Floyd ZE, Penn AL (2008) Combustion-derived hydrocarbons localize to lipid droplets in respiratory cells. Am J Respir Cell Mol Biol 38:532–540
Murphy S, Martin S, Parton RG (2009) Lipid droplet–organelle interactions: sharing the fats. Biochim Biophys Acta 1791:441–447
Murphy S, Martin S, Parton RG (2010) Quantitative analysis of lipid droplet fusion: inefficient steady state fusion but rapid stimulation by chemical fusogens. PLoS One 5:e15030
Nabi IR, Le PU (2003) Caveolae/raft-dependent endocytosis. J Cell Biol 161:673–677
Naested H, Frandsen GI, Jauh GY, Hernandez-Pinzon I, Nielsen HB, Murphy DJ, Rogers JC, Mundy J (2000) Caleosins: Ca2+-binding proteins associated with lipid bodies. J Plant Mol Biol 44:463–476
Nakamura N, Banno Y, Tamiya-Koizumi K (2005) Arf1-dependent PLD1 is localized to oleic acid-induced lipid droplets in NIH3T3 cells. Biochem Biophys Res Commun 335:117–123
Nan X, Cheng JX, Xie XS (2003) Vibrational imaging of lipid droplets in live fibroblast cells with coherent anti-Stokes Raman scattering microscopy. J Lipid Res 44:2202–2208
Naslavsky N, Rahajeng J, Rapaport D, Horowitz M, Caplan S (2007) EHD1 regulates cholesterol homeostasis and lipid droplet storage. Biochem Biophys Res Commun 357:792–799
Neuberger T, Sreenivasulu N, Rokitta M, Rolletschek H, Göbel C, Rutten T, Radchuk Feussner I, Wobus U, Jakob P, Webb A, Ljudmilla Borisjuk L (2008) Quantitative imaging of oil storage in developing crop seeds. Plant Biotech J 6:31–45
Nguyen LN, Nosanchuk JD (2011) Lipid droplet formation protects against gluco/lipotoxicity in Candida parapsilosis: an essential role of fatty acid desaturase Ole1. Cell Cycle 10(18):3159–3167
Nguyen LN, Hamari Z, Kadereit B, Trofa D, Agovino M, Martinez LR, Gacser A, Silver DL, Nosanchuk JD (2011) Candida parapsilosis fat storage-inducing transmembrane (FIT) protein 2 regulates lipid droplet formation and impacts virulence. Microb Infect 13:663–672
Nishino N, Tamori Y, Tateya S, Kawaguchi T, Shibakusa T, Mizunoya W, Inoue K, Kitazawa R, Kitazawa S, Matsuki Y, Hiramatsu R, Masubuchi S, Omachi A, Kimura K, Saito M, Amo T, Ohta S, Yamaguchi T, Osumi T, Cheng J, Fujimoto T, Nakao H, Nakao K, Aiba A, Okamura H, Fushiki T, Kasuga M (2008) FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J Clin Invest 118:2808–2821
Nykiforuk CL, Boothe JG, Murray EW, Keon RG, Goren HJ, Markley NA, Moloney MM (2006) Transgenic expression and recovery of biologically active recombinant human insulin from Arabidopsis thaliana seeds. Plant Biotechnol J 4:77–85
Oh P, McIntosh DP, Schnitzer JE (1998) Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J Cell Biol 14:101–114
Ohlrogge JN, Pollard MR, Stumpf PK (1978) Studies on biosynthesis of waxes by developing jojoba seed tissue. Lipids 13:203–210
Ohsaki Y, Cheng J, Fujita A, Tokumoto T, Fujimoto T (2006) Cytoplasmic lipid droplets are sites of convergence of proteasomal and autophagic degradation of apolipoprotein B. Mol Biol Cell 17:2674–2683
Ohsaki Y, Cheng J, Suzuki M, Fujita A, Fujimoto T (2008) Lipid droplets are arrested in the ER membrane by tight binding of lipidated apolipoprotein B-100. J Cell Sci 121:2415–2422
Ohsaki Y, Cheng J, Suzuki M, Shinohara Y, Fujita A, Fujimoto T (2009) Biogenesis of cytoplasmic lipid droplets: from the lipid ester globule in the membrane to the visible structure. Biochim Biophys Acta 1791:399–407
Olofsson SO, Boren J (2005) Apolipoprotein B: a clinically important apolipoprotein which assembles atherogenic lipoproteins and promotes the development of atherosclerosis. J Intern Med 258:395–410
Olofsson SO, Boström P, Andersson L, Rutberg M, Levin M, Perman J, Boren J (2008) Triglyceride containing lipid droplets and lipid droplet-associated proteins. Curr Opin Lipidol 19:441–447
Olofsson SO, Boström P, Andersson L, Rutberg M, Perman J, Borén J (2009) Lipid droplets as dynamic organelles connecting storage and efflux of lipids. Biochim Biophys Acta 1791:448–458
Olzmann JA, Kopito RR (2011) Lipid droplet formation is dispensable for endoplasmic reticulum-associated degradation. J Biol Chem 286:27872–27874
Orban T, Palczewska G, Palczewski K (2011) Retinyl ester storage particles (retinosomes) from the retinal pigmented epithelium resemble lipid droplets in other tissues. J Biol Chem 286:17248–17258
Orlicky DJ, Roede JR, Bales E, Greenwood C, Greenberg A, Petersen D, McManaman JL (2011) Chronic ethanol consumption in mice alters hepatocyte lipid droplet properties. Alcohol Clin Exp Res 35:1020–1033
Öst A, Ortegren U, Gustavsson J, Nystrom FH, Stralfors P (2005) Triacylglycerol is synthesized in a specific subclass of caveolae in primary adipocytes. J Biol Chem 280:5–8
Ostermeyer AG, Paci JM, Zeng Y, Lublin DM, Munro S, Brown DA (2001) Accumulation of caveolin in the endoplasmic reticulum redirects the protein to lipid storage droplets. J Cell Biol 152:1071–1078
Ostermeyer AG, Ramcharan LT, Zeng Y, Lublin DM, Brown DA (2004) Role of the hydrophobic domain in targeting caveolin-1 to lipid droplets. J Cell Biol 164:69–78
Osuga J, Ishibashi S, Oka T, Yagyu H, Tozawa R, Fujimoto A, Shionoiri F, Yahagi N, Kraemer FB, Tsutsumi O, Yamada N (2000) Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Proc Natl Acad Sci USA 97:787–792
Ozeki S, Cheng J, Tauchi-Sato K, Hatano N, Taniguchi H, Fujimoto T (2005) Rab18 localizes to lipid droplets and induces their close apposition to the endoplasmic reticulum-derived membrane. J Cell Sci 118:2601–2611
Pacheco P, Bozza FA, Gomes RN, Bozza M, Weller PF, Faria-Neto HC, Bozza PT (2002) Lipopolysaccharide-induced leukocyte lipid body formation in vivo: innate immunity elicited intracellular loci involved in eicosanoid metabolism. J Immunol 169:6498–6506
Page WJ, Sherburne R, D’Elia L, Graham LL (1995) Poly(-hydroxybutyrate) extrusion from pleomorphic cells of Azotobacter vinelandii UWD. Can J Microbiol 41:22–31
Palacpac NMQ, Hiramine Y, Mi-ichi F, Torii M, Kita K, Hiramatsu R, Horii T, Mitamura T (2004) Developmental-stage-specific triacylglycerol biosynthesis, degradation and trafficking as lipid bodies in Plasmodium falciparum-infected erythrocytes. J Cell Sci 117:1469–1480
Park JH, Attardo GM, Hansen IA, Raikhel AS (2006) GATA factor translation is the final downstream step in the amino acid/target-of-rapamycin-mediated vitellogenin gene expression in the anautogenous mosquito Aedes aegypti. J Biol Chem 281:11167–11176
Parton RG, Simons K (2007) The multiple faces of caveoli. Nat Rev Mol Cell Biol 8:185–194
Partridge M, Murphy DJ (2009) Roles of a membrane-bound caleosin and putative peroxygenase in biotic and abiotic stress responses in Arabidopsis. Plant Physiol Biochem 47:796–806
Payne VA, Grimsey N, Tuthill A, Virtue S, Gray SL, Dalla Nora E, Semple RK, O’Rahilly S, Rochford JJ (2008) The human lipodystrophy gene BSCL2/seipin may be essential for normal adipocyte differentiation. Diabetes 57:2055–2060
Peled E, Leu S, Zarka A, Weiss M, Pick U, Khozin-Goldberg I, Boussiba S (2011) Isolation of a novel oil globule protein from the green alga Haematococcus pluvialis (Chlorophyceae). Lipids 46:851–861
Péterfy M, Phan J, Xu P, Reue K (2001) Lipodystrophy in the fld mouse results from mutation of a new gene encoding a nuclear protein, lipin. Nat Genet 27:121–124
Pethe K, Alonso S, Biet F, Delogu G, Brennan MJ, Locht C, Menozzi FD (2001) The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination. Nature 412:190–194
Petschnigg J, Wolinski H, Kolb D, Zellnig G, Kurat CF, Natter K, Kohlwein SD (2009) Good fat, essential cellular requirements for triacylglycerol synthesis to maintain membrane homeostasis in yeast. J Biol Chem 284:30981–30993
Phan J, Reue K (2005) Lipin, a lipodystrophy and obesity gene. Cell Metab 1:73–83
Piffanelli P, Ross JHE, Murphy DJ (1997) Intra and extracellular lipid composition and associated gene expression patterns during pollen development in Brassica napus. Plant J 11:549–562
Pihakaski K, Pihakaski S, Karunen P, Kallio P (1987) Seasonal changes in leaf lipids of Diapensia lapponica, with special reference to storage lipid bodies. Nord J Bot 7:281–292
Ploegh HL (2007) A lipid-based model for the creation of an escape hatch from the endoplasmic reticulum. Nature 448:435–438
Pol A, Luetterforst R, Lindsay M, Heino S, Ikonen E, Parton RG (2001) A caveolin dominant negative mutant associates with lipid bodies and induces intracellular cholesterol imbalance. J Cell Biol 152:1057–1070
Pol A, Martin S, Fernandez MA, Ingelmo-Torres M, Ferguson C, Enrich C, Parton RG (2005) Cholesterol and fatty acids regulate dynamic caveolin trafficking through the Golgi complex and between the cell surface and lipid bodies. Mol Biol Cell 16:2091–2105
Porfirova S, Bergmuller E, Tropf S, Lemke R, Dormann P (2002) Isolation of an Arabidopsis mutant lacking vitamin E and identification of a cyclase essential for all tocopherol biosynthesis. Proc Natl Acad Sci USA 99:12495–12500
Pötter M, Madkour MH, Mayer F, Steinbüüchel A (2002) Regulation of phasin expression and polyhydroxyalkanoate (PHA) granule formation in Ralstonia eutropha H16. Microbiol 148:2413–2426
Pötter M, Müller H, Reinecke F, Wieczorek R, Fricke F, Bowien B, Friedrich B, Steinbüchel A (2004) The complex structure of polyhydroxybutyrate (PHB) granules: four orthologous and paralogous phasins occur in Ralstonia eutropha. Microbiol 150:2301–2311
Pouvreau B, Baud S, Vernoud V, Morin V, Py C, Gendrot G, Pichon JP, Rouster J, Paul W, Rogowsky PM (2011) Duplicate maize Wrinkled1 transcription factors activate target genes involved in seed oil biosynthesis. Plant Physiol 156:674–686
Pozueta-Romero J, Rafia F, Houlne G, Cheniclet C, Carde JP, Schantz ML, Schantz R (1997) A ubiquitous plant housekeeping gene, PAP, encodes a major protein component of bell pepper chromoplasts. Plant Physiol 115:1185–1194
Pruvot G, Cuine S, Peltier G, Rey P (1996a) Characterization of a novel drought-induced 34-kDa protein located in the thylakoids of Solanum tuberosum L. plants. Planta 198:471–479
Pruvot G, Massimino J, Peltier G, Rey P (1996b) Effects of low temperature, high salinity and exogenous ABA on the synthesis of two chloroplastic drought-induced proteins in Solanum tuberosum. Physiol Plant 97:123–131
Puigserver P, Kahn CR (2008) Mammalian metabolism in aging. In: Guarente LP, Partridge L, Wallace DC (eds) Molecular biology of aging. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 545–574
Pulido MR, Diaz-Ruiz A, Jimenez-Gomez Y, Garcia-Navarro S, Gracia-Navarro F, Tinahones F, Lopez-Miranda J, Fruhbeck G, Vazquez-Martinez R, Malagon MM (2011) Rab18 dynamics in adipocytes in relation to lipogenesis, lipolysis and obesity. PLoS One 6:10.1371
Puri V, Czech MP (2008) Lipid droplets: FSP27 knockout enhances their sizzle. J Clin Invest 118:2693–2696
Puri V, Konda S, Ranjit S, Aouadi M, Chawla A, Chouinard M, Chakladar A, Czech MP (2007) Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J Biol Chem 282:34213–34218
Puri V, Ranjit S, Konda S, Nicoloro SM, Straubhaar J, Chawla A, Chouinard M, Lin C, Burkart A, Corvera S, Perugi RA, Czech MP (2008) Cidea is associated with lipid droplets and insulin sensitivity in humans. Proc Natl Acad Sci USA 105:7833–7838
Purkrtova Z, d'Andrea S, Jolivet P, Lipovova P, Kralova B, Kodicek M, Chardot T (2007) Structural properties of caleosin: a MS and CD study. Arch Biochem Biophys 464:335–343
Quesada E, Béjar V, Ferrer MR, Calvo C, Llamas I, Martínez-Checa F, Arias S, Ruiz-García C, Páez R, Martínez-Cánovas J, Del Moral A (2004) Moderately halophilic, exopolysaccharide-producing bacteria. In: Ventosa A (ed) Halophilic microorganisms. Springer, Berlin, pp 297–314
Quettier AL, Eastmond PJ (2009) Storage oil hydrolysis during early seedling growth. Plant Physiol Biochem 47:485–490
Quillaguamán J, Delgado O, Mattiasson B, Hatti-Kaul R (2006) Poly(ß-hydroxybutyrate) production by a moderate halophile, Halomonas boliviensis LC1. Enz Microbial Technol 38:148–154
Quillaguamán J, Doan-Van T, Guzmán H, Guzmán D, Martin J, Everest A, Hatti-Kaul R (2008) Poly(ß-hydroxybutyrate) production by Halomonas boliviensis in fed-batch culture. Appl Microbiol Biotechnol 78:227–232
Quillaguamán J, Guzmán H, Doan-Van T, Hatti-Kaul R (2010) Synthesis and production of polyhydroxyalkanoates by halophiles: current potential and future prospects. Appl Microbiol Biotechnol 85:1687–1696
Quiroga AD, Lehner R (2011) Role of endoplasmic reticulum neutral lipid hydrolases. Trends Endocrinol Metab 22:218–225
Rajab A, Straub V, McCann LJ, Seelow D, Varon R, Barresi R, Schulze A, Lucke B, Lutzkendorf S, Karbasiyan M, Bachmann S, Spuler S, Schuelke M (2010) Fatal cardiac arrhythmia and long-qt syndrome in a new form of congenital generalized lipodystrophy with muscle rippling (cgl4) due to ptrf-cavin mutations. PLoS Genet 6:e1000874
Rajendran L, Le Lay S, Illges H (2007) Raft association and lipid droplet targeting of flotillins are independent of caveolin. Biol Chem 388:307–314
Rehm BH, Steinbüchel A (1999) Biochemical and genetic analysis of PHA synthases and other proteins required for PHA synthesis. Int J Biol Macromol 25:3–19
Ricquier D (2010) Biology of brown adipose tissue: view from the chair. Int J Obesity 34:S3–S6
Riding RT, Little CHA (1984) Anatomy and histochemistry of Abies balsamea cambial zone cells during the onset and breaking of dormancy. Can J Bot 62:2570–2579
Rinia HA, Burger KNJ, Bonn M, Müller M (2008) Label-free cellular imaging of lipid composition and packing of individual lipid droplets using multiplex CARS microscopy. Biophys J 95:4908–4914
Rinne PLH, Welling A, Kaikuranta P (1998) Onset of freezing tolerance in birch (Betula pubescens Ehrh) involves LEA proteins and osmoregulation and is impaired in an ABA-deficient genotype. Plant Cell Environ 21:601–611
Rinne PLH, Kaikuranta PM, van der Schoot C (2002) The shoot apical meristem restores its symplasmic organization during chilling-induced release from dormancy. Plant J 26:249–264
Rinne PLH, Ruonala R, Ripel L, van der Schoot C (2008) Dormancy release-proteins in the shoot apical meristem of populus. Physiol Plant S133:P06–P047
Robenek MJ, Severs NJ, Schlattmann K, Plenz G, Zimmer K-P, Troyer D, Robenek H (2004) Lipids partition caveolin-1 from ER membranes into lipid droplets: updating the model of lipid droplet biogenesis. FASEB J 18:866–868
Robenek H, Robenek MJ, Buers I, Lorkowski S, Hofnagel O, Troyer D, Severs NJ (2005a) Lipid droplets gain PAT family proteins by interaction with specialized plasma membrane domains. J Biol Chem 280:26330–26338
Robenek H, Robenek MJ, Troyer D (2005b) PAT family proteins pervade lipid droplet cores. J Lipid Res 46:1331–1338
Robenek H, Hofnagel O, Buers I, Robenek MJ, Troyer D, Severs NJ (2006) Adipophilin-enriched domains in the ER membrane are sites of lipid droplet biogenesis. J Cell Sci 119:4215–4224
Robenek H, Buers I, Robenek MJ, Hofnagel O, Ruebel A, Troyer D, Severs NJ (2011) Topography of lipid droplet-associated proteins: insights from freeze-fracture replica immunogold labeling. J Lipids Article 2011:ID 409371
Rodrigo MP, Findlay K, Lopez-Villalobos A, Yeung EC, Nykiforuk CL, Moloney MM (2006) The accumulation of oleosins determines the size of seed oilbodies in Arabidopsis. Plant Cell 18:1961–1974
Roingeard P, Hourioux C, Blanchard E, Prensier G (2008) Hepatitis C virus budding at lipid droplet-associated ER membrane visualized by 3D electron microscopy. Histochem Cell Biol 13:561–566
Rosen ED, MacDougald OA (2006) Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 7:885–896
Rudolph M, Schlereth A, Körner M, Feussner K, Berndt E, Melzer M, Hornung E, Feussner I (2011) The lipoxygenase dependent oxygenation of lipid body membranes is promoted by a patatin-type phospholipase in cucumber cotyledons. J Exp Bot 62:749–760
Ruonala R, Rinne PLH, Baghour M, Moritz T, Tuominen H, Kangasjärvi J (2006) Transitions in the functioning of the shoot apical meristem in birch (Betula pendula) involves ethylene. Plant J 46:628–640
Russell AP (2004) Lipotoxicity: the obese and endurance-trained paradox. Int J Obes Relat Metab Disord 28:S66–S71
Samsa MM, Mondotte JA, Iglesias NG, Assuncao-Miranda I, Barbosa-Lima G, Da Poian AT, Bozza PT, Gamarnik AV (2009) Dengue virus capsid protein usurps lipid droplets for viral particle formation. PLoS Pathog 5:e1000632
Sanjaya DTP, Weise SE, Benning C (2011) Increasing the energy density of vegetative tissues by diverting carbon from starch to oil biosynthesis in transgenic Arabidopsis. Plant Biotech J 9:874–883
Santos-Rosa H, Leung J, Grimsey N, Peak-Chew S, Siniossoglou S (2005) The yeast lipin Smp2 couples phospholipid biosynthesis to nuclear membrane growth. EMBO J 24:1931–1941
Sargent JA, Osborne DJ (1980) A comparative study of the fine structure of coleorhiza and root cells during the early hours of germination of rye embryos. Protoplasma 104:91–103
Sarmiento C, Ross JH, Herman E, Murphy DJ (1997) Expression and subcellular targeting of a soybean oleosin in transgenic rapeseed. Implications for the mechanism of oil-body formation in seeds. Plant J 11:783–796
Schein M, Yang Z, Mitchell-Olds T, Schmid K (2004) Rapid evolution of a pollen-specific oleosin-like gene family from Arabidopsis thaliana and closely related species. Mol Biol Evol 21:659–669
Scherzer CR, Feany MB (2004) Yeast genetics targets lipids in Parkinson’s disease. Trends Genet 20:273–277
Schimek C, Eibel P, Grolig F, Horie T, Ootaki T, Galland P (1999) Gravitropism in Phycomyces: a role for sedimenting protein crystals and floating lipid globules. Planta 210:132–142
Schmidt MA, Herman EM (2009) Suppression of soybean oleosin produces micro-oil bodies that aggregate into oil body/ER complexes. Mol Plant 1:910–924
Schneider EF, Seaman WL (1977) Ontogeny of lipid bodies in the endoplasmic reticulum of Fusarium sulphureum. Can J Microbiol 23:190–196
Schultheiss D, Handrick R, Jendrossek D, Hanzlik M, Schüler D (2005) The presumptive magnetosome protein Mms16 is a poly(3-hydroxybutyrate) granule-bound protein (phasin) in Magnetospirillum gryphiswaldense. J Bacteriol 187:2416–2425
Scott RW, Winichayakul S, Roldan M, Cookson R, Willingham M, Castle M, Pueschel R, Peng CC, Tzen JTC, Roberts NJ (2010) Elevation of oil body integrity and emulsion stability by polyoleosins, multiple oleosin units joined in tandem head-to-tail fusions. Plant Biotech J 8:912–927
Shah J (2005) Lipid, lipases and lipid-modifying enzymes in plant disease resistance. Ann Rev Phytopathol 43:229–260
Shah J, Chaturvedi R (2008) Lipid signals in plant–pathogen interactions. Ann Plant Rev 34:292–333
Sheehan J (2009) Engineering direct conversion of CO2 to biofuel. Nat Biotechnol 27:1128–1129
Shelness GS, Ledford AS (2005) Evolution and mechanism of apolipoprotein B-containing lipoprotein assembly. Curr Opin Lipidol 16:325–332
Shen B, Allen WB, Zheng P, Li C, Glassman K, Ranch J, Nubel D, Tarczynski MC (2010) Expression of ZmLEC1 and ZmWRI1 increases seed oil production in maize. Plant Physiol 153:980–987
Shimada TL, Nara-Nishimura I (2010) Oil-body-membrane proteins and their physiological functions in plants. Biol Pharm Bull 33:360–363
Shimada TL, Shimada T, Takahashi H, Fukao Y, Hara-Nishimura I (2008) A novel role for oleosins in freezing tolerance of oilseeds in Arabidopsis thaliana. Plant J 55:798–809
Shubeita GT, Tran SL, Xu J, Vershinin M, Cermelli S, Cotton SL, Welte MA, Gross SP (2008) Consequences of motor copy number on the intracellular transport of kinesin-1-driven lipid droplets. Cell 135:1098–1107
Simkin AJ, Gaffé J, Alcaraz JP, Carde JP, Bramley PM, Fraser PD, Kuntz M (2007) Fibrillin influence on plastid ultrastructure and pigment content in tomato fruit. Phytochem 68:1545–1556
Slater S, Mitsky TA, Houmiel KL, Hao M, Reiser SE, Taylor NB, Tran M, Valentin HE, Rodriguez DJ, Stone DA, Padgette SR, Kishore G, Gruys KJ (1999) Metabolic engineering of Arabidopsis and Brassica for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer production. Nat Biotechnol 17:1011–1016
Slocombe SP, Cornah J, Pinfield-Wells H, Soady K, Zhang Q, Gilday A, Dyer JM, Graham IA (2009) Oil accumulation in leaves directed by modification of fatty acid breakdown and lipid synthesis pathways. Plant Biotech J 7:694–703
Smirnova E, Goldberg EB, Makarova KS, Lin L, Brown WJ, Jackson CL (2006) ATGL has a key role in lipid droplet/adiposome degradation in mammalian cells. EMBO Rep 7:106–113
Snell KD, Peoples OP (2002) Polyhydroxyalkanoate polymers and their production in transgenic plants. Metabol Eng 4:29–40
Somwar R, Roberts CT, Varlamov O (2011) Live-cell imaging demonstrates rapid cargo exchange between lipid droplets in adipocytes. FEBS Lett 585:1946–1950
Sorgi CA, Secatto A, Fontanari C, Turato WM, Belangér C, de Medeiros AI, Kashima S, Marleau S, Covas DT, Bozza PT, Faccioli LH (2009) Histoplasma capsulatum cell wall ß-glucan induces lipid body formation through CD18, TLR2, and Dectin-1 receptors: correlation with leukotriene B4 generation and role in HIV-1 infection. J Immunol 182:4025–4035
Spandl J, Lohmann D, Kuerschner L, Moessinger C, Thiele C (2011) Ancient ubiquitous protein 1 (AUP1) localizes to lipid droplets and binds the E2 ubiquitin conjugase G2 (Ube2g2) via its G2 binding region. J Biol Chem 286:5599–5606
Sparkes IA, Frigerio L, Tolley N, Hawes C (2009) The plant endoplasmic reticulum: a cell-wide web. Biochem J 423:145–155
Steinbüchel A (2007) Analysis of storage lipid accumulation in Alcanivorax borkumensis: evidence for alternative triacylglycerol biosynthesis routes in bacteria. J Bacteriol 189:918–928
Steinbüchel A, Füchtenbusch B (1998) Bacterial and other biological systems for polyester production. Trends Biotechnol 16:419–427
Stephens E, Ross IL, Mussgnug JH, Wagner LD, Borowitzka MA, Posten C, Kruse O, Hankame B (2010) Future prospects of microalgal biofuel production systems. Trends Plant Sci 15:554–564
Stetter KO (2006) Hyperthermophiles in the history of life. Philos Trans R Soc Lond B Biol Sci 361:1837–1842
Sztalryd C, Bell M, Lu X, Mertz P, Hickenbottom S, Chang BH, Chan L, Kimmel AR, Londos C (2006) Functional compensation for adipose differentiation-related protein (ADFP) by TIP47 in an adfp nullembryonic cell line. J Biol Chem 281:34341–34348
Szymanski KM, Binns D, Bartz R, Grishin NV, Li WP, Agarwal AK, Garg A, Anderson RG, Goodman JM (2007) The lipodystrophy protein seipin is found at endoplasmic reticulum lipid droplet junctions and is important for droplet morphology. Proc Natl Acad Sci USA 104:20890–20895
Takahashi S, Katagiri T, Yamaguchi-Shinozaki K, Shinozaki K (2000) An Arabidopsis gene encoding a Ca2+-binding protein is induced by abscisic acid during dehydration. Plant Cell Physiol 41:898–903
Tansey JT, Sztalryd C, Gruia-Gray J, Roush DL, Zee JV, Gavrilova O, Reitman ML, Deng CX, Li C, Kimmel AR, Londos C (2001) Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci USA 98:6494–6499
Targett-Adams P, Chambers D, Gledhill S, Hope RG, Johannes JF, Girod A, McLauchlan J (2003) Live cell analysis and targeting of the lipid droplet-binding adipocyte differentiation-related protein. J Biol Chem 278:15998–16007
Teixeira L, Rabouille C, Rorth P, Ephrussi A, Vanzo NF (2003) Drosophila Perilipin/ADRP homologue Lsd2 regulates lipid metabolism. Mech Dev 120:1071–1081
Tessmer N, König S, Malkus U, Reichelt R, Pötter M, Steinbüchel A (2007) Heat-shock protein HspA mimics the function of phasins sensu strictu in recombinant strains of Escherichia coli accumulating polythioesters or polyhydroxyalkanoates. Microbiol 153:366–374
Than NG, Sumegi B, Bellyei S, Berki T, Szekeres G, Janaky T, Szigeti A, Bohn H, Than GN (2003) Lipid droplet and milk lipid globule membrane associated placental protein 17b (PP17b) is involved in apoptotic and differentiation processes of human epithelial cervical carcinoma cells. Eur J Biochem 270:1176–1188
Thiele C, Spandl J (2008) Cell biology of lipid droplets. Curr Opin Cell Biol 20:378–385
Thines E, Weber RW, Talbot NJ (2000) MAP kinase and protein kinase A-dependent mobilization of triacylglycerol and glycogen during appressorium turgor generation by Magnaporthe grisea. Plant Cell 12:1703–1718
Tian Y, Bi J, Shui G, Liu Z, Xiang Y, Liu Y, Wenk MR, Yang H, Huang X (2011) Tissue-autonomous function of Drosophila seipin in preventing ectopic lipid droplet formation. PLoS Genet 7:e1001364
Ting JTL, Wu SSH, Ratnayake C, Huang AHC (1998) Constituents of the tapetosomes and elaioplasts in Brassica campestris and their degradation and retention during microsporogenesis. Plant J 16:541–551
Tnani H, López I, Jouenne T, Vicient CM (2011) Protein composition analysis of oil bodies from maize embryos during germination. J Plant Physiol 168:510–513
Toh SY, Gong J, Du G, Li JZ, Yang S, Ye J, Yao H, Zhang Y, Xue B, Li Q, Yang H, Wen Z, Li P (2008) Up-regulation of mitochondrial activity and acquirement of brown adipose tissue-like property in the white adipose tissue of fsp27 deficient mice. PLoS One 3:e2890
Toorop PE, Barroco RM, Engler G, Groot SP, Hilhorst HW (2005) Differentially expressed genes associated with dormancy or germination of Arabidopsis thaliana seeds. Planta 221:637–647
Tranbarger TT, Dussert S, Joët T, Argout X, Summo M, Champion A, Cros D, Omore A, Nouy B, Morcillo F (2011) Regulatory mechanisms underlying oil palm fruit mesocarp maturation, ripening and functional specialization in lipid and carotenoid metabolism. Plant Physiol 156:564–584
Tsitsigiannis D, Keller NP (2007) Oxylipins as developmental and host–fungal communication signals. Trends Microbiol 16:109–117
Tsitsigiannis DI, Zarnowski R, Keller NP (2004) The lipid body protein, PpoA, coordinates sexual and asexual sporulation in Aspergillus nidulans. J Biol Chem 279:11344–11353
Turró S, Ingelmo-Torres M, Estanyol JM, Tebar F, Fernandez MA, Albor CV, Gaus K, Grewal T, Enrich C, Pol A (2006) Identification and characterization of associated with lipid droplet protein 1: a novel membrane-associated protein that resides on hepatic lipid droplets. Traffic 7:1254–1269
Uchino K, Saito T, Jendrossek D (2008) Poly(3-hydroxybutyrate) (PHB) depolymerase PhaZa1 is involved in mobilization of accumulated PHB in Ralstonia eutropha H16. Appl Environ Microbiol 74:1058–1063
Uittenbogaard A, Everson WV, Matveev SV, Smart EJ (2002) Cholesteryl ester is transported from caveolae to internal membranes as part of a caveolin–annexin II lipid–protein complex. J Biol Chem 277:4925–4931
Umlauf E, Csaszar E, Moertelmaier M, Schuetz GJ, Parton RG, Prohaska R (2004) Association of stomatin with lipid bodies. J Biol Chem 279:23699–23709
Unger RH (2002) Lipotoxic diseases. Annu Rev Med 53:319–336
Unger RH, Scherer PE (2010) Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends Endocrinol Metab 21:345–352
Valdearcos M, Esquinas E, Meana C, Gil-de-Gómez L, Guijas C, Balsinde J, Balboa MA (2011) Subcellular localization and role of Lipin-1 in human macrophages. J Immunol 186:6004–6013
Van der Schoot C, Rinne PLH (2011) Dormancy cycling at the shoot apical meristem: transitioning between self-organization and self-arrest. Plant Sci 180:120–131
Van Herpen NA, Schrauwen-Hinderling VB (2008) Lipid accumulation in non- adipose tissue and lipotoxicity. Physiol Behav 94:231–241
Van Meer G, de Kroon AIMP (2011) Lipid map of the mammalian cell. J Cell Sci 124:5–8
Van Meer G, Voelker DG, Feignenson GW (2008) Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 9:112–124
Vance DE, Vance JE (2008) Phospholipid biosynthesis in eukaryotes. In: Vance DE, Vance JE (eds) Biochemistry of lipids, lipoproteins and membranes, 5th edn. Elsevier, Amsterdam, pp 213–244
Vandana S, Bhatla SC (2006) Evidence for the probable oil body association of a thiol-protease, leading to oleosin degradation in sunflower seedling cotyledons. Plant Physiol Biochem 44:714–723
Vazquez GJ, Wieczoreck R, Steinbüchel A, Mendez BS (1996) Poly-(3-hydroxybutyrate) granules in Bacillus megaterium. Isolation and analysis of associated proteins. Rev Argent Microbiol 28:118–122
Vereb G, Szöllösi J, Matkó J, Nagy P, Farkas T, Vıgh L, Mátyus L, Waldmann TA, Damjanovich S (2003) Dynamic, yet structured: the cell membrane three decades after the Singer–Nicolson model. Proc Natl Acad Sci USA 100:8053–8058
Vidi PA, Kanwischer M, Baginsky S, Austin JR, Csucs G, Dörmann P, Kessler F, Bréhélin C (2006) Tocopherol cyclase (VTE1) localization and vitamin E accumulation in chloroplast plastoglobule lipoprotein particles. J Biol Chem 281:11225–11234
Vidi PA, Kessler F, Bréhélin C (2007) Plastoglobules: a new address for targeting recombinant proteins in the chloroplast. BMC Biotechnol 7:4
Vielemeyer O, McIntosh MT, Joiner KA, Coppens I (2004) Neutral lipid synthesis and storage in the intraerythrocytic stages of Plasmodium falciparum. Mol Biochem Parasitol 135:197–209
Vigil EL, Steere RL, Wergin WP, Christiansen MN (1985) Tissue preparation and fine structure of radicle apex from cotton seeds. Protoplasma 129:168–177
Vigouroux C, Caron-Debarle M, Le Dour C, Magré J, Capeau J (2011) Molecular mechanisms of human lipodystrophies: from adipocyte lipid droplet to oxidative stress and lipotoxicity. Int J Biochem Cell Biol 43:862–876
Villena JA, Roy S, Sarkadi-Nagy E, Kim KH, Sul HS (2004) Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J Biol Chem 279:47066–47075
Vishnevetsky M, Ovadis M, Itzhaki H, Levy M, Libal Weksler Y, Adam Z, Vainstein A (1996) Molecular cloning of a carotenoid-associated protein from Cucumis sativa corollas: homologous genes involved in carotenoid biosynthesis. Plant J 10:1111–1118
Volk GM, Crane J, Caspersen AM, Hill LM, Gardner C, Walters C (2006) Massive cellular disruption occurs during early imbibition of Cuphea seeds containing crystallized triacylglycerols. Planta 224:1415–1426
Volonte D, Galbiati F, Li S, Nishiyama K, Okamoto T, Lisanti MP (1999) Flotillins/cavatellins are differentially expressed in cells and tissues and form a hetero-oligomeric complex with caveolins in vivo. Characterization and epitope mapping of a novel flotillin-1 monoclonal antibody probe. J Biol Chem 274:12702–12709
Von Wettstein D (2001) Discovery of a protein required for photosynthetic membrane assembly. Proc Natl Acad Sci USA 98:3633–3635
Wahlroos T, Soukka J, Denesyuk A, Wahlroos R, Korpela T, Kilby NJ (2003) Oleosin expression and trafficking during oil body biogenesis in tobacco leaf cells. Genesis 35:125–132
Wältermann M, Steinbüchel A (2005) Neutral lipid bodies in prokaryotes: recent insights into structure, formation, and relationship to eukaryotic lipid depots. J Bacteriol 187:3607–3619
Wältermann M, Hinz A, Robenek H, Troyer D, Reichelt R, Malkus U, Galla HJ, Kalscheuer R, Stoveken T, von Landenberg P, Steinbüchel A (2005) Mechanism of lipid-body formation in prokaryotes: how bacteria fatten up. Mol Microbiol 55:750–763
Walther TC, Farese RV (2009) The life of lipid droplets. Biochim Biophys Acta 1791:459–466
Wan HC, Melo RC, Jin Z, Dvorak AM, Weller PF (2007) Roles and origins of leukocyte lipid bodies: proteomic and ultrastructural studies. FASEB J 21:167–178
Wang C, St Leger RJ (2007) The Metarhizium anisopliae perilipin homolog MPL1 regulates lipid metabolism, appressorial turgor pressure, and virulence. J Biol Chem 282:21110–21115
Wang H, Sztalryd C (2011) Oxidative tissue: perilipin 5 links storage with the furnace. Trends Endocrinol Metab 22:197–203
Wang MC, O’Rourke EJ, Ruvkun G (2008) Fat metabolism links germline stem cells and longevity in C. elegans. Science 322:957–960
Wang ZT, Ullrich N, Joo S, Waffenschmidt S, Goodenough U (2009) Algal lipid bodies: stress induction, purification, and biochemical characterization in wild-type and starchless Chlamydomonas reinhardtii. Eukaryotic Cell 8:1856–1868
Watanabe T, Thayil A, Jesacher A, Grieve K, Debarre D, Wilson T, Booth M, Srinivas S (2010) Characterisation of the dynamic behaviour of lipid droplets in the early mouse embryo using adaptive harmonic generation microscopy. BMC Cell Biol 11:38
Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC (2003) α-synuclein is degraded by both autophagy and the proteasome. J Biol Chem 278:25009–25013
Welte MA (2007) Proteins under new management: lipid droplets deliver. Trends Cell Biol 17:363–369
Welte MA (2009) Fat on the move: intracellular motion of lipid droplets. Biochem Soc Trans 37:991–996
Welte MA, Cermelli S, Griner J, Viera A, Guo Y, Kim DH, Gindhart JG, Gross SP (2005) Regulation of lipid-droplet transport by the Perilipin homologue LSD2. Curr Biol 15:1266–1275
Wetzel CLR, Jensen WA (1992) Studies of pollen maturation in cotton: the storage reserve accumulation phase. Sexual Plant Reprod 5:117–127
Whigham LD, Israel BA, Atkinson RL (2005) Adipogenic potential of multiple human adenoviruses in vivo and in vitro. Am J Physiol Regul Integr Comp Physiol 290:190–194
Whitfield HV (1992) The synthesis and utilisation of very-long chain fatty acids by developing seeds of nasturtium and other oilseeds. PhD thesis, University of East Anglia, Norwich, UK
Wilson E (1896) The cell in development and inheritance. Macmillan, New York
Wolins N, Quaynor B, Skinner J, Schoenfish M, Tzekov A, Bickel P (2005) S3-12, adipophilin, and TIP47 package lipid in adipocytes. J Biol Chem 280:19146–19155
Wolins NE, Brasaemle DL, Bickel PE (2006a) A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett 580:5484–5491
Wolins NE, Quaynor BK, Skinner JR, Tzekov A, Croce MA, Gropler MC, Varma V, Yao-Borengasser A, Rasouli N, Kern PA, Finck BN, Bickel PE (2006b) OXPAT/PAT-1 is a PPAR-induced lipid droplet protein that promotes fatty acid utilization. Diabetes 55:3418–3428
Wright LC, Djordjevic JT, Schibeci SD, Himmelreich U, Muljadi N, Williamson P, Lynch GW (2003) Detergent-resistant membrane fractions contribute to the total 1H NMR-visible lipid signal in cells. Eur J Biochem 270:2091–2100
Wu CC, Howell KE, Neville MC, Yates JR, McManaman JL (2000) Proteomics reveal a link between the endoplasmic reticulum and lipid secretory mechanisms in mammary epithelial cells. Electrophoresis 21:3470–3482
Wu YY, Chou YR, Wang CS, Tseng TH, Chen LJ, Tzen JT (2010) Different effects on triacylglycerol packaging to oil bodies in transgenic rice seeds by specifically eliminating one of their two oleosin isoforms. Plant Physiol Biochem 48:81–89
Wymann MP, Schneiter R (2008) Lipid signalling in disease. Nature Rev Mol Cell Biol 9:162–176
Yang Y, Sulpice R, Himmelbach A, Meinhard M, Christmann A, Grill E (2006) Fibrillin expression is regulated by abscisic acid response regulators and is involved in abscisic acid-mediated photoprotection. Proc Natl Acad Sci USA 103:6061–6066
Yao M, Huang Y, Shioi K, Hattori K, Murakami T, Nakaigawa N, Kishida T, Nagashima Y, Kubota Y (2007) Expression of adipose differentiation-related protein: a predictor of cancer-specific survival in clear cell renal carcinoma. Clin Cancer Res 13:152–160
Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV (2008) Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res 49:2283–2301
Ytterberg AJ, Peltier J, van Wijk KJ (2006) Protein profiling of plastoglobules in chloroplasts and chromoplasts. A surprising site for differential accumulation of metabolic enzymes. Plant Physiol 140:984–997
Yu W, Bozza PT, Tzizik DM, Gray JP, Cassara J, Dvorak AM, Weller PF (1998) Co-compartmentalization of MAP kinases and cytosolic phospholipase A2 at cytoplasmic arachidonate-rich lipid bodies. Am J Pathol 152:759–769
Yu YV, Li Z, Rizzo NP, Einstein J, Welte MA (2011) Targeting the motor regulator Klar to lipid droplets. BMC Cell Biol 12:9
Zanghellini J, Ruckerbauer D, Wodlei F, von Grunberg HH, Jungreuthmayer C (2010a) Phospholipid demixing: molecular interpretation of lipid droplet biogenesis. Advan Planar Lipid Bilayers Liposomes 11:1–28
Zanghellini J, Wodlei F, von Grunberg HH (2010b) Phospholipid demixing and the birth of a lipid droplet. J Theor Biol 264:952–961
Zehmer JK, Bartz R, Liu P, Anderson RG (2008) Identification of a novel N-terminal hydrophobic sequence that targets proteins to lipid droplets. J Cell Sci 121:1852–1860
Zehmer JK, Bartz R, Bisel B, Liu P, Seemann J, Anderson RG (2009a) Targeting sequences of UBXD8 and AAM-B reveal that the ER has a direct role in the emergence and regression of lipid droplets. J Cell Sci 122:3694–3702
Zehmer JK, Huang Y, Peng G, Anderson RG, Liu P (2009b) A role for lipid droplets in inter-membrane lipid traffic. Proteomics 9:914–921
Zhang SO, Box AC, Xu N, Le Men J, Yu J, Guo F, Trimble R, Mak HY (2010a) Genetic and dietary regulation of lipid droplet expansion in Caenorhabditis elegans. Proc Natl Acad Sci USA 107:4640–4645
Zhang SO, Trimble R, Guo F, Mak HY (2010b) Lipid droplets as ubiquitous fat storage organelles in C. elegans. BMC Cell Biol 11:96
Zhou Z, Yon Toh S, Chen Z, Guo K, Ng CP, Ponniah S, Lin SC, Hong W, Li P (2003) Cidea-deficient mice have lean phenotype and are resistant to obesity. Nat Genet 35:49–56
Zimmerberg J, Kozlov MM (2006) How proteins produce membrane curvature. Nat Rev Mol Cell Biol 7:9–19
Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R (2004) Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306:1383–1386
Acknowledgements
I am grateful to the many colleagues who kindly sent me copies of recent publications, including many pre-publication manuscripts.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: David Robinson
Rights and permissions
About this article
Cite this article
Murphy, D.J. The dynamic roles of intracellular lipid droplets: from archaea to mammals. Protoplasma 249, 541–585 (2012). https://doi.org/10.1007/s00709-011-0329-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-011-0329-7