Abstract
The condensation of hydrazine, N-methylhydrazine, and N-phenylhydrazine with ethyl 4-chloro-2-oxo-1,2-dihydroquinoline-3-carboxylate derivatives has been investigated. As a result, 12 new antioxidant pyrazolo[4,3-c]quinolin-3,4-diones were obtained with good to high yields. When two cross-products could be possible, only one isomer bearing the methyl or the phenyl group at the N1 position is isolated and unequivocally characterized using 1D and 2D NMR techniques, FT-IR, and combustion analyses. DFT analysis of the reaction mechanism was carried out in the Pearson’s hard soft acid base framework, confirming the assigned structure to the observed pyrazolo[4,3-c]quinolin-3,4-diones. These calculations indicate a favored kinetic control for the synthesized pyrazolo[4,3-c]quinolin-3,4-diones compared to its possible regioisomer.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past two decades, functionalized pyrazoloquinolines have attracted much attention due to their considerable biological and pharmacological activities [1, 2]. Particularly, the pyrazolo[4,3-c]quinoline heterocyclic ring system is a very attractive scaffold in medicinal chemistry which has been incorporated in high-affinity benzodiazepine receptor ligands [3, 4], interleukin inhibitors [5], selective cyclooxygenase-2 (COX-2) inhibitors [6], phosphodiesterase 4 (PDE4) inhibitors [7], as well as anticancer [8] and anti-inflammatory agents [9]. Accordingly, several synthetic approaches have been reported, based on the annulation of the pyrazole ring onto a quinoline motif [10–12] or the quinoline ring onto a pyrazole scaffold [13–19].
Recently, Moyano et al. reported a new synthetic approach toward 2-aryl-2H-pyrazolo[4,3-c]quinolin-3-ones. They found that the reaction of arylhydrazines with ethyl 4-chloroquinolin-3-carboxylates in DMF at 130–140 °C, furnished only the N2 isomer of the corresponding pyrazolo[4,3-c]quinolin-3-one adduct, but when the condensation was carried out with benzylhydrazine, in the presence of sodium methoxide, a mixture of the N1 and N2 regioisomers was detected and conveniently characterized (Scheme 1; Eq. (1)) [20].

Based on this report and the recent synthetic developments using the related, diversely substituted ethyl 4-chloro-2-oxo-1,2-dihydroquinoline-3-carboxylate derivatives I [21–24], we describe in this paper the results we have obtained in the condensation of compounds of type I with hydrazine, N-methylhydrazine, and N-phenylhydrazine (Scheme 1; Eq. (2)). As a result, we have synthesized and characterized 12 pyrazolo[4,3-c]quinolin-3,4-diones 7a–7l, whose antioxidant properties are reported herein. In addition, in-depth NMR studies and mechanistic DFT-analysis have been carried out in order to shed light into the mechanism of this reaction, to justify the regioselective formation of the N1-substituted pyrazolo[4,3-c]quinolin-3,4-diones (isomer II-N1) versus the N2-substituted pyrazolo[4,3-c]quinolin-3,4-diones (isomer II-N2) (Scheme 1; Eq. (2)) in the reaction of ethyl 4-chloro-2-oxo-1,2-dihydroquinoline-3-carboxylate derivatives with N-methylhydrazine and N-phenylhydrazine.
Results and discussion
Chemistry
The key 4-chloro-2-oxo-1,2-dihydroquinoline-3-carboxylate intermediates 6 have been prepared as shown in Schemes 2 and 3. The first step in the synthetic sequence was the preparation of the two isatoic anhydrides 3 bearing a hydrogen atom or a chlorine atom at C5. Accordingly, while isatoic anhydride (3a) is commercially available, compound 3b was obtained from the corresponding 2-amino-6-chlorobenzoic acid (1) after reaction with triphosgene in dioxane at 0 °C [25].


Next, compound 3a was submitted to N-alkylation with benzyl bromide in the presence of di-iso-propylethylamine (DIPEA) as a base, in dimethylacetamide (DMA) as a solvent, to provide the benzylated isatoic anhydride 4a (Scheme 2) [26]. N-Methylisatoic anhydride (4b, Scheme 2) is commercially available, and used as received. In the next steps, these two intermediates were submitted to the same synthetic sequence starting the reaction with diethyl malonate in the presence of NaH in DMA, a process that afforded the expected quinolones 5a, 5b [21–24, 27] whose reaction with POCl3 provided the required chloride derivatives 6a, 6b (Scheme 2). Similarly, the reaction with diethyl malonate followed by treatment with POCl3 applied to precursor 3a, provided the required chloride 6c via compound 5c (Scheme 2).
Afterward, the isatoic anhydride 3b was submitted to the same synthetic procedures as shown for compound 3a, which afforded the expected key intermediates 6d–6f via anhydrides 4c, 4d and hydroxy-derivatives 5d–5f (Scheme 3) [21–24, 28]. All new compounds gave analytical and spectroscopic data in full agreement with their structures (see “Experimental” section).
Finally, the tricyclic pyrazolo[3,4-c]quinolin-3,4-diones 7a–7l were obtained by treatment of chlorides 6a–6f with hydrazine, N-methylhydrazine, and N-phenylhydrazine (Scheme 4), in yields ranging between 44 and 83 % (Table 1). As shown, only the N1-substituted (Me or Ph) pyrazolo[3,4-c]quinolin-3,4-dione regioisomers were isolated.

A complete elucidation of the structures was achieved by HMBC and ROESY 2D NMR experiments (Fig. 1). HMBC analysis of compound 7e showed correlations between hydrogen of methyl group of hydrazide and the C-9b at 138.74 ppm and three correlations between hydrogen of the N-methyl group of the quinoline ring (3.57 ppm), the carbonylic carbon signal (157.22 ppm) at the position 4, carbon C-5a (139.18 ppm), and carbon C-6 (115.86 ppm). In the ROESY experiment, two significant signals corresponding to interactions through space between protons of the methyl group of the hydrazide (4.13 ppm) and proton H-9 (8.17 ppm) were observed: the second interaction shows correlation between hydrogen of the N-methyl group (3.57 ppm) of the quinoline ring and proton H-6 (7.55 ppm). These findings allowed us to confirm the structure of compound 7e and the conclusion was also extended to the other derivatives 7.
Computational studies and reaction mechanism
Since the total electronic energy of isomers II-N1 is less negative than the other possible regioisomer II-N2, the elucidation of their regioselectivity cannot be studied on the basis of this global descriptor. In the HSAB framework, local descriptors such as Fukui functions f(r) and local chemical softness s(r) and electrophilicity (ω) are more appropriate and considered as the reactivity index. In this context, successful prediction criteria have been proposed on rigorous theoretical basis [29–31]. When applied to our molecules, all these elements provide a better understanding for the formation of a single product between the quinolinones 6 and hydrazine or derivatives.
The regioselectivity and reactivity of compounds 6 with hydrazine and its derivatives have been studied by calculating the local Fukui functions and then the corresponding softnesses and electrophilicity indexes of electrophilic and nucleophilic centers (f + and f −, then s + and s − respectively and ω + and ω −). In addition to these chemical indicators, the effect of solvent (i.e. ethoxyethanol) as a polarized continuum was taken into consideration since error can rise from reactivity difference of molecules in gas and liquid phases (Table 2).
By applying the site selectivity criteria to the reaction between compound 6b and methylhydrazine and between the four centers, there are two possible pathways ΔI and ΔII:
These calculations indicate a preferable mechanism ΔI (ΔI < ΔII) leading to the unique product 7b and further confirmed experimentally by HMBC and ROESY 2D NMR experiments. According to the DFT-based descriptors, the bond formation between the atoms concerns the ester carbon (from quinolinone) with the non-substituted nitrogen atom (from methylhydrazine) having antibonding molecular orbitals (MOs) and the carbon atom bonded to the chlorine atom (from quinolinone) with the substituted nitrogen atom (from methylhydrazine) having bonding MOs [32]. Moreover, the charges carried by these atoms show that the two nitrogen centers act as nucleophilic site and on the contrary, the two carbon atoms (from quinolinone) involved in the reaction are electrophilic sites. Therefore, a larger orbital overlap occurs explaining a single major product as confirmed experimentally.
The reaction path calculations allowed us to verify the connection between the reactant and the product via the transition structures. The resulting potential energy profile of the reaction is illustrated by Fig. 2. The energy profile for the synthesized pyrazolo[4,3-c]quinolin-3,4-diones 7a–7l compared to its possible regioisomer underlines a kinetic control of the reaction between selected quinolinone and hydrazine (and derivatives) since the more energetically stable isomer II-N2 is not obtained.
The preferable mechanism of the reaction between quinolone and hydrazine derivatives (Scheme 5) consists in the approach of hydrazine towards 6b near the ester function; this constitutes a transition state leading to an energy barrier. Then, a rearrangement occurs with the departure of ethanol (step 2) and finally a nucleophilic attack of the substituted nitrogen atom on the halogenated carbon atom (step 3). This step corresponds to a second energy transition state in gas phase which is found to disappear in 2-ethoxyethanol explaining why the solvent can affect the kinetic or thermodynamic control of the reaction. The departure of hydrochloric acid causes the reaction to evolve towards the final product (step 4).

Antioxidant activity study
Free radical scavenging is considered to be one of the major mechanisms by which antioxidants halt lipid peroxidation, thus mitigating any damages caused to the cell membrane lipids and mitochondrial machinery. In our quest to find potent antioxidants, we were then interested in assessing the antioxidant power of pyrazolo[4,3-c]quinolin-3,4-diones 7a–7l using the well-known 2,2-diphenyl-1-picryl-hydrazyl (DPPH) and hydroxyl radicals (standard assays) as probes to determine the radical scavenging activity (RSA) of the aforementioned compounds.
DPPH radical scavenging
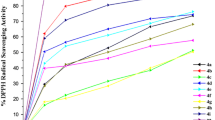
In a methanolic solution, the DPPH radical displays a deep purple color with a maximum absorption at 517 nm. This color vanishes gradually in the presence of various concentration of antioxidant in the medium (see Scheme 6). Thus, the DPPH radical can be quenched by the capture of a labile hydrogen atom or by electron donation from the antioxidant, probably via a free-radical attack on the DPPH molecule. This process provides then a blanched solution [33, 34]. RSA DPPH values of compound 7a–7l (Table 3) correspond to a percentage of the ratio of the decrease in optical density at 517 nm over the absorbance of a DPPH control solution which does not contain the compounds 7a–7l.

From the results gathered in Table 3, we can say that the pyrazolo[4,3-c]quinolin-3,4-diones 7a–7l scavenging effect on DPPH radicals increase was suitable for compounds 7a–7c (10.8–15.2 % at 50 µmol/dm3), but lower than RSA DPPH value found for ascorbic acid (37.9 % at 50 µmol/dm3) used as a reference. N-Substituted derivatives 7d–7g, 7i, and 7k have shown moderate activity while derivatives 7h and 7l presented the poorest activity compared to the standard one. Thus, in order to display a good antioxidant activity, the pyrazolo[4,3-c]quinolin-3,4-dione scaffold may embed the N–H moiety in the quinolone or pyrazole ring. Accordingly, compound 7a behaves as a better antioxidant with three N–H moieties in its structure.
Hydroxyl radical scavenging
The capacity of our compounds to scavenge the hydroxyl radical was evaluated using the benzoic acid method [35]. In brief, the benzoic acid is converted to salicylic acid or 4-hydroxybenzoic acid in the presence of the hydroxyl radical generated during the Fenton reaction. The fluorescence emission, measured at 407 nm with excitation wavelength set at 305 nm, decreases if there is any antioxidant in the medium. Potent antioxidant product impedes the hydroxylation of benzoic acid by providing hydrogen atom. The hydroxyl RSAs of the adducts 7a–7l are summarized in Table 3. We can conclude that all the products presented at 50 μmol/dm3 good hydroxyl radical scavenging activities ranging between 53.3 and 73.9 % compared to quercetol at 50 μmol/dm3 (88.9 %), used here as reference compound.
Conclusions
The spectroscopic (1D NMR 1H/13C and 2D NMR HMBC and ROESY) and the computational analyses (DFT) confirms that the reaction between ethyl 4-chloro-2-oxo-1,2-dihydroquinoline-3-carboxylate derivatives and the selected hydrazine derivatives yields a unique product, kinetically favored but disadvantaged in comparison to other possible regioisomers. Compared to conventional frontier molecular orbital description, any of the DFT-based descriptors predicted the correct regioselectivity in favor of the N1-II isomer. Our results showed also the important role played by 2-ethoxyethanol during this type of condensation. All new compounds gave interesting hydroxyl radical scavenging activities and slight capacity to inhibit DPPH radical. Based on these outcomes, compound 7a bearing three NH moieties was found to be the most balanced antioxidant. Further synthetic studies are underway to enhance the antioxidant potency of the pyrazolo[4,3-c]quinolin-3,4-dione backbone.
Experimental
All reactions were controlled by TLC using precoated silica gel aluminum plates (Macherey–Nagel) and revealed by UV light at 254 nm. Melting points were determined on a Köfler bench. Infrared spectra were recorded on a Shimadzu FTIR-8201 PC spectrometer in KBr (\(\overline{\nu }\) in cm−1). NMR spectra were recorded on Bruker AC 300, AC 250 spectrometers or on a Varian System-500 spectrometer. CDCl3 and DMSO-d 6 were used as deuterated solvents. Chemical shifts are reported in parts per millions relative to tetramethylsilane (TMS) signal and coupling constants (J) are given in Hertz (Hz). The following abbreviations are employed: s, singlet; br s, broad singlet; d, doublet; dd, doublet of doublets; t, triplet; q, quadruplet; m, multiplet. Microanalyses were performed at Service Central d’Analyses (CNRS, Vernaison, France). All reagents are of analytical grade pure and used without further purification. Isatoic anhydride (3a) and N-methylisatoic anhydride (4b) are commercially available. The compounds 5-chloroisatoic anhydride (3b) [25], N-benzylisatoic anhydride (4a) [26], 5-chloro-N-methylisatoic anhydride (4d) [21], ethyl 1-benzyl-4-hydroxy-2-oxo-1,2-dihydroquinoline-3-carboxylate (5a) [27], ethyl 4-hydroxy-1-methyl-2-oxo-1,2-dihydroquinoline-3-carboxylate (5b) [21], ethyl 4-hydroxy-2-oxo-1,2-dihydroquinoline-3-carboxylate (5c) [21], ethyl 5-chloro-4-hydroxy-1-methyl-2-oxo-1,2-dihydroquinoline-3-carboxylate (5e) [22], ethyl 5-chloro-4-hydroxy-2-oxo-1,2-dihydroquinoline-3-carboxylate (5f) [21], ethyl 1-benzyl-4-chloro-2-oxo-1,2-dihydroquinoline-3-carboxylate (6a) [23], ethyl 4-chloro-1-methyl-2-oxo-1,2-dihydroquinoline-3-carboxylate (6b) [21], and ethyl 4-chloro-2-oxo-1,2-dihydroquinoline-3-carboxylate (6c) [28] had been previously described.
N-Benzyl-5-chloroisatoic anhydride (4c, C15H10ClNO3)
5-Chloroisatoic anhydride (3b, 1 g, 5.06 mmol) was suspended in 10 cm3 DMA with 0.654 g DIPEA (5.06 mmol). The solution was stirred 10 min and then, 1.09 g benzyl bromide (6.07 mmol) was added and the mixture was heated for 3 h. After cooling to room temperature, 10 cm3 water was added and the solution was vigorously stirred for 30 min; the precipitate was filtered, washed with water and diethyl ether to give white product 4c (0.70 g, 48 %). M.p.: 115 °C; IR (KBr): \(\overline{\nu }\) = 1768, 1700, 1590 cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.87 (m, 2H), 7.44 (d, J = 8.2 Hz, 1H), 7.25–7.35 (m, 5H), 4.80 (s, 2H) ppm; 13C NMR (DMSO-d 6 , 75 MHz): δ = 156.15 (CO), 151.20 (CO), 139.42 (C8-a), 130.88 (C, benzyl), 129.11 (C5), 128.14 (C7), 126.13 (2CH, benzyl), 124.13 (2CH, benzyl), 123.77 (CH, benzyl), 123.28 (C6), 121.21 (C4-a), 112.44 (C8), 43.52 (CH2, benzyl) ppm.
Ethyl 1-benzyl-5-chloro-4-hydroxy-2-oxo-1,2-dihydroquinoline-3-carboxylate (5d, C19H16ClNO4)
To a solution of 1 g 5-chloro-N-benzylisatoic anhydride (4c, 3.47 mmol) in 10 cm3 DMF at 0 °C were added slowly 0.17 g sodium hydride (7.0 mmol) and 2.78 g diethyl malonate (17.6 mmol). The reaction was heated at 85 °C for 5 h. After cooling, water was added and the mixture obtained was acidified with conc. HCl. The resultant solid was filtered, washed with water, and dried to afford a yellow product 5d (0.78 g, 63 %). M.p.: 124 °C; IR (KBr): \(\overline{\nu }\) = 1672, 1630, 1570 cm−1; 1H ΝMR (300 MHz, DMSO-d 6 ): δ = 12.9 (s, 1H, OH), 7.53 (d, J = 7.3 Hz, 1H), 7.15–7.28 (m, 7H), 5.02 (s, 2 H), 4.16 (q, J = 7.0 Hz, 2H), 1.30 (t, J = 7.0 Hz, 3 H) ppm; 13C NMR (DMSO-d 6 , 75 MHz): δ = 162.45 (CO), 161.22 (CO), 139.45 (C8-a), 138.67 (C4), 135.67 (C, benzyl), 130.32 (C5), 127.14 (CH, benzyl), 126.68 (2×CH, benzyl), 126.32 (2×CH, benzyl), 124.33 (C7), 123.63 (C4-a), 123.27 (C6), 112.46 (C8), 102.33 (C3), 61.13 (CH2, ester), 46.28 (CH2, benzyl), 15.63 (CH3) ppm.
General procedure for preparation of 6d and 6e
The corresponding quinolines 5d, 5e suspended in POCl3 (2–6 cm3) were heated at 100 °C for 1 h. Then, the mixture was cooled to 0 °C and neutralized with water and NaOH (10 N). The resulting solid was filtered and dried.
Ethyl 1-benzyl-4,5-dichloro-2-oxo-1,2-dihydroquinoline-3-carboxylate (6d, C19H15Cl2NO3)
Following the general procedure, 0.50 g compound 5d (1.40 mmol) and 2 cm3 POCl3 gave white solid 6d (0.31 g, 58 %). M.p.: 115 °C; IR (KBr): \(\overline{\nu }\) = 1715, 1656, 1550 cm−1; 1H NMR (CDCl3, 500 MHz): δ = 7.40–7.24 (m, 6H), 7.23 (d, J = 7.5 Hz, 2H), 5.57 (s, 2H), 4.53 (q, J = 7.1 Hz, 2H), 1.46 (t, J = 7.1 Hz, 3H) ppm; 13C NMR (CDCl3, 125 MHz): δ = 164.25 (CO), 158.10 (CO), 141.52 (C8-a), 140.24 (C, benzyl), 135.51 (C5), 134.18 (C4), 132.04 (CH, benzyl), 130.13 (C4-a), 129.47 (2×CH, benzyl), 128.16 (C7), 128.02 (C6), 126.94 (2×CH, benzyl), 116.23 (C3), 115.44 (C8), 62.92 (CH2, ester), 47.82 (CH2, benzyl), 14.54 (CH3) ppm.
Ethyl 4,5-dichloro-1-methyl-2-oxo-1,2-dihydroquinoline-3-carboxylate (6e, C13H11Cl2NO3)
Following the general procedure, 1 g compound 5e (3.74 mmol) was suspended in 3 cm3 POCl3 and gave white product 6e (0.43 g, 40 %). M.p.: 106 °C; IR (KBr): \(\overline{\nu }\) = 1710, 1660, 1573 cm−1; 1H NMR (DMSO-d 6 , 500 MHz): δ = 7.70 (m, 2H), 7.51 (dd, J = 6.6, 2.1 Hz, 1H), 4.36 (q, J = 7.0 Hz, 2H), 1.31 (t, J = 7.0 Hz, 3H) ppm; 13C NMR (DMSO-d 6 , 125 MHz): δ = 163.18 (CO), 156.34 (CO), 141.51 (C8-a), 137.14 (C5), 132.50 (C7), 131.41 (C4), 129.13 (C4-a), 127.17 (C6), 115.71 (C8), 113.90 (C3), 61.81 (CH2), 30.74 (N-CH3) 13.80 (CH3 ester) ppm.
Ethyl 4,5-dichloro-2-oxo-1,2-dihydroquinoline-3-carboxylate (6f, C12H9Cl2NO3)
Compound 5f (0.8 g, 3 mmol) and 2 cm3 POCl3 were stirred at 110 °C for 6 h. After cooling, the solvent was concentrated. The residue was dissolved in a small amount of AcOEt and the mixture was poured into ice water followed by extraction with AcOEt. The extract was washed with 1 M NaOH, water, and brine, dried over MgSO4, and concentrated in vacuum. This mixture was then stirred with 0.27 g AcONa (3.3 mmol) in 2 cm3 AcOH at 120 °C for 20 h. The reaction mixture was then added to water, the precipitated solid was collected and washed with water to give the product 6f (0.52 g, 60 %) as a white solid. M.p.: 102–104 °C; IR (KBr): \(\overline{\nu }\) = 2995, 1735, 1615 cm−1; 1H NMR (CDCl3, 300 MHz): δ = 12.74 (s, 1H), 7.59 (t, J = 8.1 Hz, 1H), 7.46 (dd, J = 8.3, 0.8 Hz, 1H), 7.59 (dd, J = 7.8, 1.0 Hz, 1H), 4.34 (q, J = 7.1 Hz, 2H), 1.30 (t, J = 7.1 Hz, 3H) ppm; 13C NMR (DMSO-d 6 , 125 MHz): δ = 163.28 (CO), 156.69 (CO), 140.73 (C8-a), 138.42 (C5), 130.73 (C4), 132.45 (C7), 124.26 (C4-a), 126.27 (C6), 112.95 (C3), 116.27 (C8), 61.83 (CH2), 13.89 (CH3) ppm.
General procedure for preparation of compounds 7a–7l
The 4-choroquinolines were added to a solution of the corresponding hydrazine (5–7 equiv) in 5–25 cm3 ethoxyethanol. The solution was heated for 2–6 h and allowed cool to rt. The precipitate obtained was filtered, washed with diethyl ether, and dried to yield the desired compound.
1,2-Dihydro-3H-pyrazolo[4,3-c]quinoline-3,4(5H)-dione (7a, C10H7N3O2)
Following the general procedure, the reaction of 0.3 g compound 6c (1.19 mmol) and 0.6 cm3 hydrazine monohydrate (8.33 mmol) in 10 cm3 ethoxyethanol gave off-white compound 7a (0.19 g, 79 %). M.p.: >260 °C; IR (KBr): \(\overline{\nu }\) = 3320, 2990, 1651, 1585 cm−1; 1H NMR (DMSO-d 6 , 300 MHz): δ = 10.52 (br s, 1H, NH), 7.87 (d, J = 6.2 Hz, 1H), 7.09–7.32 (m, 3H) ppm; 13C NMR (DMSO-d 6 , 62.5 MHz): δ = 161.53 (CO), 160.36 (CO), 144.72 (C, C5-a), 138.49 (C, C9-b), 128.84 (CH, C7), 121.67 (CH, C9), 121.44 (CH, C8), 115.96 (2C, C6, C9-a), 95.22 (C, C3-a) ppm.
1-Methyl-1,2-dihydro-3H-pyrazolo[4,3-c]quinoline-3,4(5H)-dione (7b, C11H9N3O2)
Following the general procedure, the reaction of 0.3 g compound 6c (1.19 mmol) and 0.44 cm3 methylhydrazine (9.45 mmol) in 10 cm3 ethoxyethanol gave off-white compound 7b (0.12 g, 46 %). M.p.: >260 °C; IR (KBr): \(\overline{\nu }\) = 3330, 2995, 1675, 1590 cm−1; 1H NMR (DMSO-d 6 , 300 MHz): δ = 11.11 (br s, 1H, NH), 10.58 (br s, 1H, NH), 8.10 (d, J = 7.8 Hz, 1H), 7.48 (t, J = 7.4 Hz, 1H), 7.37 (m, 1H), 7.22 (d, J = 7.3 Hz, 1H), 4.11 (s, 3H) ppm; 13C NMR (DMSO-d 6 , 75 MHz): δ = 158.41 (CO), 157.69 (CO), 140.41 (C5-a), 139.02 (C9-b), 129.94 (C7), 123.44 (C9), 122.07 (C8), 116.63 (C6), 111.40 (C9-a), 98.89 (C3-a), 39.5 (CH3, overlapped with DMSO) ppm.
1-Phenyl-1,2-dihydro-3H-pyrazolo[4,3-c]quinoline-3,4(5H)-dione (7c, C16H11N3O2)
Following the general procedure, the reaction of 0.3 g compound 6c (1.19 mmol) and 0.82 cm3 phenylhydrazine (8.33 mmol) in 10 cm3 ethoxyethanol gave light brown compound 7c (0.24 g, 72 %). M.p.: >260 °C; IR (KBr): \(\overline{\nu }\) = 3327, 3015, 1666, 1588 cm−1; 1H NMR (DMSO-d 6 , 300 MHz): δ = 10.61 (br s, 1H, NH), 7.95–7.97 (m, 3H), 7.47 (m, 2H), 7.39 (m, 1H), 7.23 (m, 2H), 7.12 (m, 1H) ppm; 13C NMR (DMSO-d 6 , 75 MHz): δ = 161.44 (CO), 160.18 (CO), 147.32 (C, C5-a), 139.32 (C9-b), 135.12 (C, phenyl), 129.80 (C7), 129.22 (2×CH, phenyl), 122.21 (C9), 121.76 (C8), 121.47 (CH, phenyl), 121.39 (2×CH, phenyl), 116.21 (C6), 116.18 (C9-a), 107.09 (C3-a) ppm.
5-Methyl-1,2-dihydro-3H-pyrazolo[4,3-c]quinoline-3,4(5H)-dione (7d, C11H9N3O2)
Following the general procedure, the reaction of 0.3 g compound 6b (1.13 mmol) and 0.3 cm3 hydrazine (9.45 mmol) in 25 cm3 ethoxyethanol gave yellow compound 7d (0.16 g, 66 %). M.p.: >260 °C; IR (KBr): \(\overline{\nu }\) = 3328, 1651, 1585, 1554 cm−1; 1H NMR (DMSO-d 6 , 300 MHz): δ = 7.95 (dd, J = 1.5 Hz, 7.7 Hz, 1H), 7.45 (t, J = 7.0 Hz, 1H), 7.36 (d, J = 8.1 Hz, 1H), 7.18 (t, J = 7.2 Hz, 1H), 3.50 (s, 3H) ppm; 13C NMR (DMSO-d 6 , 75 MHz): δ = 161.18 (CO), 159.35 (CO), 143.57 (C, C5-a), 139.49 (C, C9-b), 129.49 (CH, C7), 122.16 (CH, C9), 121.81 (CH, C8), 115.79 (C, C6), 114.89 (CH, C9-a), 94.76 (C, C3-a), 28.48 (CH3) ppm.
1,5-Dimethyl-1,2-dihydro-3H-pyrazolo[4,3-c]quinoline-3,4(5H)-dione (7e, C12H11N3O2)
Following the general procedure, the reaction of 0.5 g compound 6b (1.88 mmol) and 0.5 cm3 methylhydrazine (9.55 mmol) in 20 cm3 ethoxyethanol gave yellow compound 7e (0.33 g, 77 %). M.p.: >260 °C; IR (KBr): \(\overline{\nu }\) = 3256, 1647, 1596, 1542 cm−1; 1H NMR (DMSO-d 6 , 500 MHz): δ = 8.17 (d, J = 8.0 Hz, 1H), 7.62 (t, J = 7.9 Hz, 1H) 7.55 (d, J = 8.5 Hz, 1H), 7.33 (t, J = 7.6 Hz, 1H), 4.13 (s, 3H), 3.57 (s, 3H) ppm; 13C NMR (DMSO-d 6 , 125 MHz): δ = 157.31 (CO), 157.22 (CO), 139.18 (C5-a), 138.74 (C9-b), 129.77 (C7), 123.30 (C9), 121.71 (C8), 115.86 (C6), 111.94 (C9-a), 97.90 (C3-a), 39.5 (CH3, overlapped with DMSO), 28.39 (CH3) ppm.
5-Methyl-1-phenyl-1,2-dihydro-3H-pyrazolo[4,3-c]quinoline-3,4(5H)-dione (7f, C17H13N3O2)
Following the general procedure, the reaction of 0.5 g compound 6b (1.88 mmol) and 1 cm3 phenylhydrazine (10.1 mmol) in 20 cm3 ethoxyethanol gave light brown compound 7f (0.094 g, 78 %). M.p.: 189 °C; IR (KBr): \(\overline{\nu }\) = 1627, 1593, 1569, 1542 cm−1; 1H NMR (DMSO-d 6 , 300 MHz): δ = 8.10 (d, J = 7.5 Hz, 1H), 7.83 (d, J = 7.8 Hz, 2H), 7.62–7.48 (m, 4H), 7.31 (m, 1H), 7.29 (t, J = 7.5 Hz, 1H), 3.57 (s, 3H) ppm; 13C NMR (DMSO-d 6 , 75 MHz): δ = 158.19 (2xCO), 146.53 (C, C5-a), 140.13 (C9-b), 138.10 (C, phenyl), 130.97 (C7), 129.53 (2×CH, phenyl), 127.29 (CH, phenyl), 122.91 (2×CH, phenyl), 122.74 (C9), 122.48 (C8), 116.23 (C6, C9-a), 96.30 (C3-a), 28.11 (CH3) ppm.
5-Benzyl-1,2-dihydro-3H-pyrazolo[4,3-c]quinoline-3,4(5H)-dione (7g, C17H13N3O2)
Following the general procedure, the reaction of 0.05 g compound 6a (0.15 mmol) and 25 mm3 hydrazine (0.78 mmol) in 5 cm3 ethoxyethanol gave yellow compound 7g (0.038 g, 83 %). M.p.: >260 °C; IR (KBr): \(\overline{\nu }\) = 1643, 1554 cm−1; 1H NMR (DMSO-d 6 , 250 MHz): δ = 13.00 (br s, 1H, NH), 8.06 (d, J = 7.0 Hz, 1H), 7.43 (t, J = 7.1 Hz, 1H), 7.10–7.33 (m, 7H), 5.49 (s, 2H) ppm; 13C NMR (DMSO-d 6 , 62.5 MHz): δ = 159.53 (CO), 158.43 (CO), 141.97 (C5-a), 138.47 (C9-b), 137.95 (C, benzyl), 130.32 (CH, benzyl), 128.99 (2×CH, benzyl), 127.28 (C7), 126.79 (2×CH, benzyl), 122.80 (C9), 122.34 (C8), 116.73 (C6), 112.31 (C9-a), 96.73 (C3-a), 44.14 (CH2, benzyl) ppm.
5-Benzyl-1-methyl-1,2-dihydro-3H-pyrazolo[4,3-c]quinoline-3,4(5H)-dione (7h, C18H15N3O2)
Following the general procedure, the reaction of 0.05 g compound 6a (0.15 mmol) and 39 mm3 methylhydrazine (0.75 mmol) in 5 cm3 ethoxyethanol gave yellow compound 7h (0.021 g, 44 %). M.p.: >260 °C; IR (KBr): \(\overline{\nu }\) = 3355, 1651, 1573 cm−1; 1H NMR (DMSO-d 6 , 300 MHz): δ = 12.55 (s, 1H, NH), 8.18 (d, J = 8.1 Hz, 1H), 7.47 (t, J = 7.5 Hz, 1H), 7.36 (d, J = 8.4 Hz, 1H), 7.26–7.21 (m, 3H), 7.21–7.21 (m, 3H), 5.51 (s, 2H), 4.16 (s, 3H) ppm; 13C NMR (DMSO-d 6 , 75 MHz): δ = 158.04 (2xCO), 139.42 (C5-a), 138.75 (C9-b), 137.77 (C, benzyl), 130.21 (CH, benzyl), 129.06 (2×CH, benzyl), 127.35 (C7), 126.78 (2×CH, benzyl), 124.12 (C9), 122.41 (C8), 116.95 (C6), 112.83 (C9-a), 98.16 (C3-a), 44.26 (CH2, benzyl), 39.5 (CH3, overlapped with DMSO) ppm.
5-Benzyl-1-phenyl-1,2-dihydro-3H-pyrazolo[4,3-c]quinoline-3,4(5H)-dione (7i, C23H17N3O2)
Following the general procedure, the reaction of 0.05 g compound 6a (0.15 mmol) and 74 mm3 phenylhydrazine (0.75 mmol) in 5 cm3 ethoxyethanol gave light brown compound 7i (0.030 g, 52 %). M.p.: >260 °C; IR (KBr): \(\overline{\nu }\) = 3200, 1667, 1580 cm−1; 1H NMR (DMSO-d 6 , 300 MHz): δ = 8.10 (d, J = 7.4 Hz, 1H), 7.96 (d, J = 7.8 Hz, 2H), 7.61–7.40 (m, 3H), 7.40–7.15 (m, 8H), 7.18–7.20 (m, 5H), 5.48 (s, 2H) ppm; 13C NMR (DMSO-d 6 , 75 MHz): δ = 160.09 (CO), 159.97 (CO), 146.22 (C5-a), 139.07 (C9-b), 138.07 (C, benzyl), 130.28 (C, phenyl), 129.43 (2×CH, benzyl), 129.33 (C7), 129.02 (2×CH, phenyl), 127.31 (CH, benzyl), 126.89 (2×CH, benzyl), 122.72 (CH, phenyl), 122.53 (2×CH, phenyl), 121.90 (C9), 121.85 (C8), 121.79 (C6), 116.73 (C9-a), 95.20 (C3-a), 43.95 (CH2, benzyl) ppm.
9-Chloro-1-methyl-1,2-dihydro-3H-pyrazolo[4,3-c]quinoline-3,4(5H)-dione (7j, C11H8ClN3O2)
Following the general procedure, the reaction of 0.05 g compound 6f (0.17 mmol) and 45 mm3 methylhydrazine (0.87 mmol) in 5 cm3 ethoxyethanol gave yellow compound 7j (0.031 g, 71 %). M.p.: >260 °C; IR (KBr): \(\overline{\nu }\) = 3002, 1680, 1590 cm−1; 1H NMR (DMSO-d 6 , 250 MHz): δ = 11.29 (br s, 1H), 7.46 (m, 1H), 7.33–7.26 (m, 2H + 1NH), 3.96 (s, 3H) ppm; 13C NMR (DMSO-d 6 , 62.5 MHz): δ = 158.94 (CO), 158.00 (CO), 141.71 (C5-a), 141.29 (C9-b), 130.82 (C9), 127.99 (C7), 124.24 (C9-a), 115.58 (C8), 110.42 (C6), 100.84 (C3-a), 44.07 (CH3) ppm.
9-Chloro-1,5-dimethyl-1,2-dihydro-3H-pyrazolo[4,3-c]quinoline-3,4(5H)-dione (7k, C12H10ClN3O2)
Following the general procedure, the reaction of 0.5 g compound 6e (0.17 mmol) and 45 mm3 methylhydrazine (0.87 mmol) in 5 cm3 ethoxyethanol gave yellow compound 7k (0.027 g, 61 %). M.p.: >260 °C; IR (KBr): \(\overline{\nu }\) = 3290, 1667, 1610 cm−1; 1H NMR (DMSO-d 6 , 300 MHz): δ = 10.80 (br s, 1H), 7.63–7.44 (m, 2H), 7.42 (d, J = 7.5 Hz, 1H), 3.93 (s, 3H), 3.55 (s, 3H) ppm; 13C NMR (DMSO-d 6 , 75 MHz): δ = 159.21 (CO), 157.52 (CO), 141.86 (C5-a), 140.84 (C9-b), 131.09 (C9), 128.55 (C7), 124.49 (C9-a), 115.27 (C8), 111.39 (C6), 100.41 (C3-a), 43.78 (CH3), 29.68 (CH3) ppm.
5-Benzyl-9-chloro-1-methyl-1,2-dihydro-3H-pyrazolo[4,3-c]quinoline-3,4(5H)-dione (7l, C18H14ClN3O2)
Following the general procedure, the reaction of 0.05 g compound 6d (0.14 mmol) and 36 mm3 methylhydrazine (0.70 mmol) in 5 cm3 ethoxyethanol gave yellow compound 7l (0.023 g, 51 %). M.p.: >260 °C; IR (KBr): \(\overline{\nu } n\) = 3300, 1675, 1580 cm−1; 1H NMR (DMSO-d 6 , 300 MHz): δ = 7.31–7.24 (m, 8H), 5.40 (s, 2H), 3.53 (s, 3H) ppm; 13C NMR (DMSO-d 6 , 75 MHz): δ = 161.27 (2xCO), 142.31 (C5-a), 140.36 (C9-b), 137.90 (C, benzyl), 129.90 (C9), 128.92 (CH, benzyl), 127.29 (2×CH, benzyl), 126.88 (2×CH, benzyl), 124.67 (C7), 123.96 (C9-a), 115.50 (C8), 114.88 (C6), 104.01 (C3-a), 44.32 (CH2, benzyl), 32.57 (CH3) ppm.
Computational studies
DFT calculations at the B3LYP level of theory using 6-311G** basis set were performed with the Gaussian09 package. Full geometry optimizations of reactants and products (in gas and liquid phases) were done at 298 K, for ground state as well as for transition state searches including vibrational analysis to locate stationary point and zero point energy (zpe). The effect of solvation in 2-ethoxyethanol as included in the code was carried out using PCM method [36, 37]. Transition state (TS) structures were determined by searching the synchronous transit-guided quasi-Newton (STQN) method (QST2 keyword) for first-order saddle points on the potential energy surface (PES) via frequency calculations. The Fukui function and local softness were computed from anionic and cationic forms of reactants with the same geometry as the neutral one using gross electronic population [38].
Hydroxyl radical scavenging activity assay
In a screw-capped test tube, 0.2 cm3 sodium benzoate (10 mmol), 0.2 cm3 FeSO4·7H2O (10 mmol), and EDTA (10 mmol) were added. Then a phosphate buffer (pH 7.4, 0.1 mol) was added to give a total volume of 1.8 cm3. A H2O2 solution (0.2 cm3, 10 mmol) was added, and the mixture was then incubated at 37 °C for 2 h. The fluorescence was measured at 407 nm emission and excitation 305 nm. RSA OH°% = Absorbance in the presence of sample divided by the absorbance in the absence of sample × 100. Mean values from three independent samples were calculated and showed for each compound a standard deviations less than 5 %. Quercetol was used as a positive control.
DPPH radical scavenging activity assay
For this assay the method of Hatano et al. [39] was used to evaluate the capacity of compounds to scavenge the “stable” free radical DPPH. Different concentrations of methanolic solutions (0.3 cm3) of the compounds were mixed with a DPPH methanolic solution (1.5 × 10−4 mol/dm3, 2.7 cm3). The solution was mixed vigorously and kept for 2 h in the dark, until stable absorption values were obtained. The quenching of the DPPH radical was determined by measuring the absorption at 517 nm. The RSA was calculated as a percentage of disappearance of DPPH purple color using the equation % \({\text{RSA}} = \left[ {\left( {A_{\text{DPPH}} - A_{\text{S}} } \right)/A_{\text{DPPH}} } \right] \times 100,\) where A DPPH is the absorbance of the DPPH solution and A S is the absorbance of the solution when the compound has been added at a given concentration. Mean values from three independent samples were calculated and showed for each compound a standard deviations less than 5 %. Ascorbic acid was used as a positive control.
References
Mekheimer RA, Ahmed EA, Sadek KU (2012) Tetrahedron 68:1637
Cin GT, Demirel S, Cakici A (2011) J Organomet Chem 696:613
Savini L, Chiasserini L, Pellerano C, Biggio G, Maciocco E, Serra M, Cinone N, Carrieri A, Altomare C, Carotti A (2001) Bioorg Med Chem 9:431
Carotti A, Altomare C, Savini L, Chiasserini L, Pellerano C, Mascia MP, Maciocco E, Busonero F, Mameli M, Biggio G, Sanna E (2003) Bioorg Med Chem 11:5259
Skotnicki JS, Gilman SC, Steinbaugh BA, Musser JH (1988) Pyrazolo[4,3-c]quinolines as anti inflammatories. US Patent 4,748,246, May 31, 1988; Chem Abstr 109:110425
Baruah B, Dasu K, Vaitilingam B, Vanguri A, Rao Casturi S, Rao Yeleswarapu K (2004) Bioorg Med Chem Lett 14:445
Crespo MI, Gràcia J, Puig C, Vega A, Bou J, Beleta J, Doménech T, Ryder H, Segarra V, Palacios JM (2000) Bioorg Med Chem Lett 10:2661
Wentland MP (1994) Preparation of 5-cyclopropyl-8-fluoro-7-(4-pyridyl)-3H-pyrazolo[4,3-c]quinolin-3-one topoisomerase-inhibiting anticancer agents. US Patent 5,334,595, Aug 02, 1994; Chem Abstr 121:230766
Suzuki F, Nakasato Y, Ohmori K, Tamura T, Hosoe H, Kubo K, Yoshitake I (1995) Preparation of pyrazolo quinolones as antiinflammatories and liver protective agents. Eur Patent 0476544, Mar 25, 1995; Chem Abstr 116:255609
Yokoyama N, Ritter B, Neubert AD (1982) J Med Chem 25:337
Ghotekar BK, Ghagare MG, Toche RB, Jachak MN (2010) Monatsh Chem 141:169
Ismaïli L, Refouvelet B, Robert JF (1999) J Heterocycl Chem 36:719
Truong AP, Aubele DL, Probst GD, Neitzel ML, Semko CM, Bowers S, Dressen D, Hom RK, Konradi AW, Sham HL, Garofalo AW, Keim PS, Wu J, Dappen MS, Wong K, Goldbach E, Quinn KP, Sauer J-M, Brigham EF, Wallace W, Nguyen L, Hemphill SS, Bova MP, Bard F, Yednock TA, Basi G (2009) Bioorg Med Chem Lett 19:4920
Beshore DC, DiPardo RM, Kuduk SD (2010) Tetrahedron Lett 51:970
Kalita PK, Baruah B, Bhuyan PJ (2006) Tetrahedron Lett 47:7779
Shawali AS (1993) Chem Rev 93:2731
Daou B, Soufiaoui M (1989) Tetrahedron 45:3351
Gál M, Fehér Ö, Tihanyi E, Horváth G, Jerkovich G, Argay G, Kálmán A (1980) Tetrahedron Lett 21:1567
Gál M, Fehér Ö, Tihanyi E, Horváth G, Jerkovich G (1982) Tetrahedron 38:2933
López Rivilli MJ, Moyano EL, Yranzo GI (2010) Tetrahedron Lett 51:478
Tomassoli I, Ismaili L, Pudlo M, de Los Ríos C, Soriano E, Colmena I, Gandía L, Rivas L, Samadi A, Marco-Contelles J, Refouvelet B (2011) Eur J Med Chem 46:1
Bjork A, Jonsson S, Fex T, Hedlund G (2000) Preparation of quinoline-3-carboxamides for diseases resulting from autoimmunity and pathol inflammation. US patent 6,077,851, Apr 22, 2000; Chem Abstr 131:322547
Hur W, Sun Z, Jiang T, Mason DE, Peters EC, Zhang DD, Luesch H, Schultz PG, Gray NS (2010) Chem Biol 17:537
Jansson K, Fristedt T, Olsson A, Svensson B, Jönsson S (2006) J Org Chem 71:1658
Tedesco R, Shaw AN, Bambal R, Chai D, Concha NO, Darcy MG, Dhanak D, Fitch DM, Gates A, Gerhardt WG, Halegoua DL, Han C, Hofmann GA, Johnston VK, Kaura AC, Liu N, Keenan RM, Lin-Goerke J, Sarisky RT, Wiggall KJ, Zimmerman MN, Duffy KJ (2006) J Med Chem 49:971
Beutner GL, Kuethe JT, Yasuda N (2007) J Org Chem 72:7058
Chai D, Colon M, Duffy J, Fitch DM, Tedesco R, Zimmerman, MN (2007) Preparation of N-[(4-hydroxy-2-oxo-1,2-dihydro-3-quinolinyl)carbonyl]glycine derivatives as prolyl hydroxylase inhibitors. PCT Int Appl WO 2007038571 A2, Apr 05, 2007; Chem Abstr 146:380309
Tomohiro O, Yuya O, Toshio T, Zenyu S, Sachio S, Yoshihiko S, Hiroko Y, Harumi H, Yukiko Y, Shigeru K, Maki M, Hideaki T, Atsuo B, Satoshi S (2012) Bioorg Med Chem 20:5496
Ponti A (2000) J Phys Chem A 104:8843
Parr RG, Szentpály L, Liu S (1999) J Am Chem Soc 121:1922
Parr RG, Yang RGPW (1989) Density-functional theory of atoms and molecules. Oxford University Press, Oxford
Fleming I (2010) Molecular orbitals and the structures of organic molecules. Wiley, London, p 69
Amarowicz R, Pegg RB, Rahimi-Moghaddam P, Barl B, Weil JA (2004) Food Chem 84:551
Siddhuraju P, Becker K (2007) Food Chem 101:10
Chung S-K, Osawa T, Kawakishi S (1997) Biosci Biotechnol Biochem 61:118
Khirade PW, Chaudhari A, Shinde JB, Helambe SN, Mehrotra SC (1999) J Chem Eng Data 44:879
Cossi M, Rega N, Scalmani G, Barone V (2003) J Comput Chem 24:669
Lee C, Yang W, Parr RG (1988) J Mol Struct (Theochem) 163:305
Hatano T, Kagawa H, Yasuhara T, Okuda T (1988) Chem Pharm Bull (Tokyo) 36:2090
Acknowledgments
The authors thank the Regional University Hospital of Besançon for support (France). Financial support from Regional Council of Franche-Comté (Besançon, France) is also acknowledged. Computations have been performed on the supercomputer facilities of the Mésocentre de calcul de Franche-Comté (Besançon, France).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tomassoli, I., Herlem, G., Picaud, F. et al. Synthesis, regioselectivity, and DFT analysis of new antioxidant pyrazolo[4,3-c]quinoline-3,4-diones. Monatsh Chem 147, 1069–1079 (2016). https://doi.org/10.1007/s00706-016-1660-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-016-1660-7