Abstract
The reaction of 2-[bis(methylsulfanyl)methylidene]malononitrile with 1H-1,2,4-triazol-3-amine in N,N-dimethylformamide in the presence of anhydrous potassium carbonate led to the formation of 7-imino-5-(methylsulfanyl)-1,7-dihydro[1,2,4]triazolo[1,5-a]pyrimidine-6-carbonitrile. The latter was then reacted with some nitrogen and carbon nucleophiles such as substituted anilines and active methylene compounds to afford the corresponding 5-substituted derivatives. The structure of the synthesized compounds was confirmed by IR, NMR, and mass spectra and elemental analyses. Furthermore, their potential as antioxidant agents was evaluated using DPPH and hydroxyl radical scavenging assays.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Multicomponent reactions (MCRs) are classical examples of synthetic efficiency and reaction design for diversity-oriented synthesis in organic chemistry [1–3]. The simplest definition of MCR is that it is a one-pot reaction wherein two or more reactants rearrange into a single complex structure with functional diversity, without adding any external reagents or changing reaction conditions throughout the progress of the reaction [4, 5]. For the past few decades, significant research has been devoted both at academic and industrial scale toward the development of new heterocyclic compounds in a one-pot synthetic protocol [4–7]. Usually, MCRs follow a one-pot multistep process and a complex mechanistic pathway where all the steps are reversible and the last step is irreversible [8].
However, the major challenge in the commercialization of novel MCRs and elementary heterocyclic synthesis is to master and gather expertise toward uncommon combinations and arrangements under similar reaction conditions [4–6, 8]. The most advantageous feature of MCRs is that it can be extended into various promising synthetic protocols which can further be developed into novel targeted structural motifs [9]. These new lead molecules can be used as active agents for various applications, e.g., in the field of catalysis and medicinal and biological applications. MCRs have also been known to produce a wide range of structurally and stereochemically different heterocyclic compounds along with their polyheterocyclic derivatives, which have been further evaluated as potential biologically active compounds [9–11]. Without a doubt, most of these potential biologically active heterocyclic compounds are used as scaffolds in the pharmaceutical and drug industries [9, 10, 12]. However, the development of new types of MCRs for the discovery of new pairs of functional groups to access new scaffolds is still in flourishing demand to produce new drug-like molecules.
Over the years, it has been observed that triazolo[1,5-a]pyrimidinedione as a versatile compound holds a significant position in the field of synthetic and organic chemistry [13]. The fact that triazolo[1,5-a]pyrimidine can form stable coordination complexes and possesses a high binding capacity for metal ions has led to increased attention to the development of novel triazolo[1,5-a]pyrimidinedione derivatives [14–16]. In particular, due to their diverse agricultural, pharmacological, and biological properties, such compounds have attracted attention of a large number of organic chemists [17]. Their biological properties include antitumor [13], analgesic [18], anticancer [19], antifungal [15], antibiotic [20], and antileishmanial activities [21]. Compounds based on the [1,2,4]triazolo[1,5-a]pyrimidine core have recently been reported as phosphodiesterase inhibitors [22] for the treatment of Alzheimer’s disease [23, 24], antimalarial agents [25], CB2 cannabinoid receptor inverse agonists [26], and hypnotic agents [27]. Owing to their beneficial biological applications, the use of [1,2,4]triazolo[1,5-a]pyrimidine in multicomponent reactions for the development of new heterocyclic compounds can definitely provide further opportunities. However, these compounds have not been tested for their antioxidant activity.
In the present article we report the synthesis of 7-imino-5-(methylsulfanyl)-1,7-dihydro[1,2,4]triazolo[1,5-a]pyrimidine-6-carbonitrile and its derivatives in a facile and cost efficient one-pot route and their antioxidant activity.
RESULTS AND DISCUSSION
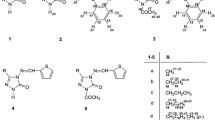
Initially, we focused on the synthesis of 7-imino-5-(methylsulfanyl)-1,7-dihydro[1,2,4]triazolo[1,5-a]pyrimidine-6-carbonitrile (3) as the parent compound. It was prepared by reacting 2-{bis(methylsulfanyl)methylidene]malononitrile (1) and 1H-1,2,4-triazol-3-amine (2) on heating in DMF in the presence of anhydrous potassium carbonate (Scheme 1). The synthetic details are given in Experimental.
Molecule 3 possesses a replaceable methylsulfanyl group at the 5-position, which is activated due to electron-withdrawing effects of the N1 atom and cyano group on C6. Therefore, it can be presumed that compound 3 is a potential precursor for the synthesis of 5-substituted derivatives. In fact, 5-substituted derivatives of 3 were obtained by reacting it with different nucleophiles such as active methylene compounds and substituted anilines. Accordingly, the reactions of 3 with diethyl malonate, ethyl acetoacetate, ethyl cyanoacetate, and malononitrile in dimethylformamide in the presence of anhydrous potassium carbonate afforded the corresponding 5-substituted derivatives 4a–4d as shown in Scheme 2.
Under similar experimental conditions, compound 3 reacted with substituted anilines to produce 5-(R-anilino)-7-imino-1,7-dihydro[1,2,4]triazolo[1,5-a]pyrimidine-6-carbonitriles 5a–5j (Scheme 3).
A tentative mechanism was proposed for the formation of parent compound 3 (Scheme 4). Initially, nucleophilic addition of 1H-1,2,4-triazol-3-amine (2) to 2-[bis(methylsulfanyl)methylidene]malononitrile (1) gives zwitterionic intermediate A [28] which is stabilized via elimination of methanethiolate anion and deprotonation followed by proton migration to produce intermediates B and C, respectively. Finally, intramolecular Michael addition between the NH and C≡N groups of C leads to the formation of triazolopyrimidine 3 [29]. In the next stage, nucleophilic substitution of the 5-methylsulfanyl group in molecule 3 yields final compounds 4 and 5.
Antioxidant activity. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay. The antioxidant properties of newly synthesized compounds 4a–4d and 5a–5d were evaluated by the DPPH radical scavenging assay [17] using 1 mM ascorbic acid as the reference compound. Reaction mixtures were prepared by adding a solution of individual compound 4a–4d or 5a–5d in absolute ethanol to an equal volume of a 0.1 mM solution of DPPH radical. The mixture was incubated at room temperature for 20 min, and the absorbance at λ 517 nm was measured with a UV-Vis spectrophotometer. Compounds 5c and 5b showed the weakest activities by scavenging 60.2 and 40.6% of DPPH, respectively (Table 1).
Hydroxyl radical scavenging assay. The well-known Fenton reaction was used for measuring the OH radical scavenging activity [30]. In a typical Fenton reaction, 60 μL of FeCl2 (1 mM), 90 μL of 1,10-phenanthroline (1 mM), 2.4 mL of phosphate buffer (pH 7.8), and 150 μL of 0.17 M H2O2 were mixed together along with 1.5 mL of 1 mM solution of compound 3–5. After incubation at room temperature for 5 min, the absorbance at λ 560 nm was recorded. Ascorbic acid (1 mM) was used as reference compound. The results are shown in Table 1. Ascorbic acid as the reference compound showed a scavenging activity of 89.5%. Compound 3 showed OH radical scavenging activity of 45.6%. Compounds 4a, 4b, 4d, 5a, 5c, and 5d showed good OH radical scavenging activities. The highest OH radical scavenging activity was observed for compounds 4a and 4b, 84.0 and 82.2%, respectively. Compounds 4d and 5d were slightly less active (80.2 and 78.2%, respectively), and the activities of 4c, 5a, and 5c were estimated at 62.9, 69.5, and 66.6%, respectively. The lowest OH radical scavenging activity was found for compound 5b (45.2%). Based on the performance results of both radical scavenging assays, it can be said that newly synthesized compounds 4a–4d and 5a–5d showed significant antioxidant activity. It can also be said that quantitative difference in the antioxidant properties of the newly synthesized compounds were influenced by the nature of substituents attached to the parent compound.
In summary, a new series of substituted 7-imino[1,2,4]triazolo[1,5-a]pyrimidine-6-carbonitrile derivatives were synthesized using an efficient, green, and easy protocol. The products could be easily isolated by simple work-up techniques in a less time-consuming manner. Moreover, the proposed protocol is believed to be cost-economic, and it provided high yields under mild conditions. Most of the newly synthesized compounds showed promising antioxidant potential in DPPH and OH radical scavenging assays.
EXPERIMENTAL
All chemicals of reagent grade were supplied by Sigma–Aldrich (India) and were used without further purification. The melting points were determined using open capillary tubes and are uncorrected. The IR spectra were recorded in KBr on a Perkin Elmer FT-IR spectrometer. The mass spectra (electrospray ionization) were run on a Shimadzu 2010EV LC/MS instrument. The 1H and 13C NMR spectra were recorded at 400 and 100 MHz, respectively, using CDCl3 as solvent and tetramethylsilane as an internal standard. The progress of reactions and the purity of the isolated compounds were monitored by TLC on UV-active silica gel plate (Merck).
7-Imino-5-(methylsulfanyl)-1,7-dihydro[1,2,4]triazolo[1,5-a]pyrimidine-6-carbonitrile (3). A mixture of 1H-1,2,4-triazol-3-amine (2, 1.68 g, 20 mmol), 2-[bis(methylsulfanyl)methylidene]malononitrile (1, 3.40 g, 20 mmol), and anhydrous potassium carbonate (1.38 g, 1 equiv) in 30 mL of DMF was refluxed with continuous stirring for 4–5 h. After completion of the reaction (TLC), the mixture was cooled, and the solid product was collected by filtration, washed with water, and recrystallized from ethanol. Yield 3.26 g (80%), mp 203–205°C. IR spectrum, ν, cm–1: 3347 (NH), 2228 (C≡N). 1H NMR spectrum, δ, ppm: 9.20 br.s (1H, NH), 7.48 s (1H, CH), 4.08 br.s (1H, NH), 2.80 s (3H, SCH3). 13C NMR spectrum, δC, ppm: 164.8, 158.8, 157.8, 146.9, 115.7, 81.6, 15.07. Mass spectrum: m/z 205 (Irel 100%) [M + 1]. Found, %: C 46.62; H 3.24; N 33.92; S 14.60. C7H6N6S. Calculated, %: C 40.77; H 2.93; N 40.75; S 15.55.
General procedure for the synthesis of triazolopyrimidines 4a–4d and 5a–5j. A mixture of 1 mmol of compound 3, 1 mmol of the corresponding CH acid or substituted aniline, and 1 equiv of anhydrous potassium carbonate in DMF was heated with continuous stirring for 4–5.5 h. After completion of the reaction (TLC), the mixture was cooled, and the solid product was collected by filtration, washed with water, and recrystallized from ethanol. If necessary, the product was additionally purified using a short silica gel column. The IR, 1H and 13C NMR, and mass spectra of 4a–4d and 5a–5j were in well agreement with the assigned structures and consistent with literature data for structurally related compounds [16, 31–43].
Diethyl 2-(6-cyano-7-imino-1,7-dihydro[1,2,4]triazolo[1,5-a]pyrimidin-5-yl)malonate (4a). Yield 0.238 g (74%), mp 185–187°C. IR spectrum, ν, cm–1: 3341 (NH), 3137 (C=NH), 2223 (C≡N), 1721 (C=O), 1154 (C-O); 1H NMR spectrum, δ, ppm: 9.24 br.s (1H, NH), 7.56 s (1H, 2-H), 4.13 m (4H, OCH2), 4.05 br.s (1H, NH), 3.84 s (1H, CH), 1.31 t (6H, CH3). 13C NMR spectrum, δC, ppm: 167.3, 166.0, 155.8, 146.7, 115.8, 91.7, 61.4, 60.0, 14.1. Mass spectrum: m/z 318 (Irel 100%) [M + 1]. Found, %: C 49.09; H 4.38; N 26.48. C13H14N6O4. Calculated, %: C 49.06; H 4.43; N 26.40.
Ethyl 2-(6-cyano-7-imino-1,7-dihydro[1,2,4]triazolo[1,5-a]pyrimidin-5-yl)-3-oxobutanoate (4b). Yield 0.207 g (72%), mp 190–192°C. IR spectrum, ν, cm–1: 3352 (NH), 3124 (=N–H), 2218 (C≡N), 1725 (C=O), 1143 (C–O). 1H NMR spectrum, δ, ppm: 9.12 br.s (1H, NH), 7.50 s (1H, 2-H), 4.21 m (2H, OCH2), 4.17 br.s (1H, NH), 3.87 s (1H, CH), 2.18 s (3H, COCH3), 1.25 t (3H, CH3). 13C NMR spectrum, δC, ppm: 202.4, 167.5, 166.2, 158.8, 146.7, 115.8, 91.7, 61.4, 57.8, 28.2, 14.1. Mass spectrum: m/z 288 (Irel 100%) [M + 1]. Found, %: C 50.09; H 4.25; N 29.20. C12H12N6O3. Calculated, %: C 50.00; H 4.20; N 29.15 .
Ethyl 2-cyano-2-(6-cyano-7-imino-1,7-dihydro[1,2,4]triazolo[1,5-a]pyrimidin-5-yl)acetate (4c). Yield 0.205 g (76%), mp 186–188°C. IR spectrum, ν, cm–1: 3343 (NH), 3131 (=N–H), 2219 (C≡N), 1724 (C=O), 1145 (C–O). 1H NMR spectrum, δ, ppm: 9.04 br.s (1H, NH), 7.54 s (1H, 2-H), 4.18 m (2H, OCH2), 4.12 br.s (1H, NH), 4.01 s (1H, CH), 1.29 t (3H, CH3). 13C NMR spectrum, δC, ppm: 171.2, 167.3, 158.9, 146.7, 115.8, 91.7, 63.8, 32.8, 14.1. Mass spectrum: m/z 271 (Irel 100%) [M + 1]. Found, %: C 48.65; H 3.32; N 36.24; O 11.77. C11H9N7O2. Calculated, %: C 48.71; H 3.34; N 36.15.
2-(6-Cyano-7-imino-1,7-dihydro[1,2,4]triazolo[1,5-a]pyrimidin-5-yl)malononitrile (4d). Yield 0.174 g (78%), mp 180–182°C. IR spectrum, ν, cm–1: 3335 (NH), 3118 (=N–H), 2226 (C≡N). 1H NMR spectrum, δ, ppm: 9.21 s (1H, NH), 7.50 d (1H, 2-H), 4.18 s (1H, CH), 4.15 br.s (1H, NH). 13C NMR spectrum, δC, ppm: 167.35, 158.9, 158.8, 146.8, 115.8, 114.9, 91.7, 17.5. Mass spectrum: m/z 224 (Irel 100%) [M + 1]. Found, %: C 48.05; H 1.65; N 49.73. C9H4N8. Calculated, %: C 48.22; H 1.80; N 49.98.
5-(4-Bromoanilino)-7-imino-1,7-dihydro[1,2,4]triazolo[1,5-a]pyrimidine-6-carbonitrile (5a). Yield 0.246 g (75%), mp 208–210°C. IR spectrum, ν, cm–1: 3326 (NH), 3114 (=N–H), 2219 (C≡N), 779 (C–Br). 1H NMR spectrum, δ, ppm: 10.59 s (1H, NH), 9.07 s (1H, =NH), 7.58 s (1H, 2-H), 7.31 d (2H, Harom), 6.68 d (2H, Harom), 4.08 br.s (1H, NH). 13C NMR spectrum, δC, ppm: 174.7, 159.7, 158.3, 146.8, 138.1, 120.7, 116.6, 69.7. Mass spectrum: m/z 329 (Irel 100%) [M + 1]. Found, %: C 43.60; H 2.47; Br 24.28; N 29.68. C12H8BrN7. Calculated, %: C 43.66; H 2.44; Br 24.20; N 29.70.
7-Imino-5-(4-nitroanilino)-1,7-dihydro[1,2,4]triazolo[1,5-a]pyrimidine-6-carbonitrile (5b). Yield 0.230 g (78%), mp 210–212°C. IR spectrum, ν, cm–1: 3318 (NH), 3104 (=N–H), 2220 (C≡N), 1328 (NO2), 1158 (NO2). 1H NMR spectrum, δ, ppm: 10.50 s (1H, NH), 9.10 s (1H, =NH), 8.18–6.68 m (4H, Harom), 4.01 br.s (1H, NH). 13C NMR spectrum, δC, ppm: 174.2, 159.1, 158.5, 146.8, 137.9, 124.5, 114.6, 69.1. Mass spectrum: m/z 296 (Irel 100%) [M + 1]. Found, %: C 48.42; H 2.58; N 37.65. C12H8N8O2. Calculated, %: C 48.65; H 2.72; N 37.82.
7-Imino-5-(4-methoxyanilino)-1,7-dihydro[1,2,4]triazolo[1,5-a]pyrimidine-6-carbonitrile (5c). Yield 0.207 g (74%), mp 214–216°C. IR spectrum, ν, cm–1: 3337 (NH), 3121 (=N–H), 2215 (C≡N), 1178 (C–O). 1H NMR spectrum, δC, ppm: 10.41 s (1H, NH), 9.12 s (1H, =NH), 7.51 s (1H, 2-H), 6.71 d (2H, Harom), 6.23 d (2H, Harom), 4.19 br.s (1H, NH), 3.83 s (3H, OMe). 13C NMR spectrum, δC, ppm: 174.2, 158.3, 153.8, 146.2, 131.1, 127.3, 115.8, 115.2, 69.2, 55.6. Mass spectrum: m/z 281 (Irel 100%) [M + 1]. Found, %: C 55.47; H 3.93; N 34.82. C13H11N7O. Calculated, %: C 55.51; H 3.94; N 34.86.
5-(4-Chloroanilino)-7-imino-1,7-dihydro[1,2,4]triazolo[1,5-a]pyrimidine-6-carbonitrile (5d). Yield 0.199 g (70%), mp 219–221°C. IR spectrum, ν, cm–1: 3317 (NH), 3145 (=N–H), 2229 (C≡N), 812 (C–Cl). 1H NMR spectrum, δ, ppm: 10.72 s (1H, NH), 9.13 s (1H, =NH), 7.43 s (1H, 2-H), 7.38 d (2H, Harom), 6.61 d (2H, Harom), 4.02 br.s (1H, NH). 13C NMR spectrum, δC, ppm: 174.1, 158.8, 146.6, 137.1, 129.4, 127.3, 120.7, 115.6, 69.1. Mass spectrum: m/z 285 (Irel 100%) [M + 1]. Found, %: C 50.43; H 2.83; Cl 12.42; N 34.31. C12H8ClN7. Calculated, %: C 50.45; H 2.82; Cl 12.41; N 34.32.
5-(4-Fluoroanilino)-7-imino-1,7-dihydro[1,2,4]triazolo[1,5-a]pyrimidine-6-carbonitrile (5e). Yield 0.201 g (75%), mp 218–220°C. IR spectrum, ν, cm–1: 3310 (NH), 3142 (=N–H), 2227 (C≡N), 690 (C–F). 1H NMR spectrum, δ, ppm: 10.61 s (1H, NH), 9.01 s (1H, =NH), 7.51 s (1H, 2-H), 6.93 d (2H, Harom), 6.48 d (2H, Harom), 4.17 br.s (1H, NH). 13C NMR spectrum, δC, ppm: 174.3, 158.4, 157.7, 146.2, 134.7, 120.8, 116.3, 115.5, 69.7. Mass spectrum: m/z 269 (Irel 100%) [M + 1]. Found, %: C 53.57; H 2.97; F 7.08; N 36.42. C12H8FN7. Calculated, %: C 53.53; H 2.99; F 7.06; N 36.42.
7-Imino-5-(4-methylanilino)-1,7-dihydro[1,2,4]triazolo[1,5-a]pyrimidine-6-carbonitrile (5f). Yield 0.190 g (72%), mp 224–226°C. IR spectrum, ν, cm–1: 3317 (NH), 3132 (=N–H), 2987 (CH3), 2212 (C≡N). 1H NMR spectrum, δ, ppm: 10.66 s (1H, NH), 9.07 s (1H, =NH), 7.52 s (1H, 2-H), 6.98 d (2H, Harom), 6.36 d (2H, Harom), 4.06 br.s (1H, NH), 1.24 s (3H, CH3). 13C NMR spectrum, δC, ppm: 174.1, 158.3, 146.7, 136.7, 131.5, 129.8, 116.4, 115.8, 69.7, 21.3. Mass spectrum: m/z 265 (Irel 100%) [M + 1]. Found, %: C 58.82; H 4.17; N 36.97. C13H11N7. Calculated, %: C 58.86; H 4.18; N 36.96.
5-(2,4-Dichloroanilino)-7-imino-1,7-dihydro[1,2,4]triazolo[1,5-a]pyrimidine-6-carbonitrile (5g). Yield 0.223 g (70%), mp 214–216°C. IR spectrum, ν, cm–1: 3305 (NH), 3149 (=N–H), 2233 (C≡N), 809 (C–Cl). 1H NMR spectrum, δC, ppm: 10.59 s (1H, NH), 9.09 s (1H, =NH), 8.04 s (1H, Harom), 7.50 s (1H, 2-H), 7.29–7.21 m (2H, Harom), 4.04 br.s (1H, NH). 13C NMR spectrum, δC, ppm: 174.3, 158.8, 146.7, 141.3, 131.2, 125.7, 123.6, 122.6, 121.9, 115.8, 69.3. Mass spectrum: m/z 319 (Irel 100%) [M + 1]. Found, %: C 45.07; H 2.22; Cl 22.16; N 30.67. C12H7Cl2N7. Calculated, %: C 45.02; H 2.20; Cl 22.15; N 30.63.
7-Imino-5-(3-nitroanilino)-1,7-dihydro[1,2,4]triazolo[1,5-a]pyrimidine-6-carbonitrile (5h). Yield 0.201 g (68%), mp 208–210°C. IR spectrum, ν, cm–1: 3320 (NH), 3112 (=N–H), 2220 (C≡N), 1322 (NO2), 1152 (NO2). 1H NMR spectrum, δ, ppm: 10.42 s (1H, NH), 9.14 s (1H, =NH), 7.62 d (1H, Harom), 7.56 s (1H, Harom), 7.50 s (1H, 2-H), 7.46 m (1H, Harom), 6.82 d (1H, Harom), 4.11 br.s (1H, NH). 13C NMR spectrum, δC, ppm: 174.4, 158.9, 148.5, 146.8, 145.3, 130.4, 122.8, 115.6, 113.8, 109.2, 69.5. Mass spectrum: m/z 296 (Irel 100%) [M + 1]. Found, %: C 48.58; H 2.76; N 37.86. C12H8N8O2. Calculated, %: C 48.65; H 2.72; N 37.82.
5-(3-Bromoanilino)-7-imino-1,7-dihydro[1,2,4]triazolo[1,5-a]pyrimidine-6-carbonitrile (5i). Yield 0.180 g (55%), mp 210–212°C. IR spectrum, ν, cm–1: 3337 (NH), 3121 (=N–H), 2215 (C≡N), 772 (C–Br). 1H NMR spectrum, δ, ppm: 10.61 s (1H, NH), 9.11 s (1H, =NH), 7.51 s (1H, 2-H), 6.96–6.91 m (2H, Harom), 6.69 s (1H, Harom), 6.37 m (1H, Harom), 4.13 br.s (1H, NH). 13C NMR spectrum, δC, ppm: 174.2, 158.8, 146.8, 130.1, 123.7, 121.7, 115.6, 69.2. Mass spectrum: m/z 329 (Irel 100%) [M + 1]. Found, %: C 43.63; H 2.47; Br 24.28; N 29.71. C12H8BrN7. Calculated, %: C 43.66; H 2.44; Br 24.20; N 29.70.
5-(3-Chloroanilino)-7-imino-1,7-dihydro[1,2,4]triazolo[1,5-a]pyrimidine-6-carbonitrile (5j). Yield 0.176 g (62%), mp 219–221°C. IR spectrum, ν, cm–1: 3328 (NH), 3132 (=N–H), 2228 (C≡N), 805 (C–Cl). 1H NMR spectrum, δ, ppm: 10.63 s (1H, NH), 9.21 s (1H, =NH), 7.51 s (1H, 2-H), 7.17 s (1H, Harom), 6.86–6.79 m (2H, Harom), 6.31 s (1H, Harom), 4.08 br.s (1H, NH). 13C NMR spectrum, δC, ppm: 174.1, 158.7, 146.2, 145.6, 135.2, 130.8, 122.8, 116.5, 115.4, 114.2, 69.7. Mass spectrum: m/z 285 (Irel 100%) [M + 1]. Found, %: C 50.49; H 2.82; Cl 12.47; N 34.28. C12H8ClN7. Calculated, %: C 50.45; H 2.82; Cl 12.41; N 34.32.
REFERENCES
Toure, B.B. and Hall, D.G., Chem. Rev., 2009, vol. 109, p. 4439. https://doi.org/10.1021/cr800296p
Multicomponent Reactions, Zhu, J. and Bienaymé, H., Eds., Weinheim: Wiley, 2005. https://doi.org/10.1002/3527605118
Atar, A.B., Kang, J., and Jadhav, A.H., New J. Chem., 2020, vol. 44, p. 3241. https://doi.org/10.1039/C9NJ05738B
Dömling, A., Chem. Rev., 2006, vol. 106, p. 17. https://doi.org/10.1021/cr0505728
Shaaban, M.R. and Elwahy, A.H.M., Curr. Org. Synth., 2014, vol. 11, p. 471. https://doi.org/10.2174/15701794113106660076
D’Souza, D.M. and Müller, T.J.J., Chem. Soc. Rev., 2007, vol. 36, p. 1095. https://doi.org/10.1039/B608235C
Mathapati, S.R., Jadhav, A.H., Swami, M.B., and Dawle, J.K., Lett. Org. Chem., 2019, vol. 16, p. 740. https://doi.org/10.2174/1570178616666181211094040
Dömling, A., Wang, W., and Wang, K., Chem. Rev., 2012, vol. 112, p. 3083. https://doi.org/10.1021/cr100233r
Sunderhaus, J.D. and Martin, S.F., Chem. Eur. J., 2009, vol. 15, p. 1300. https://doi.org/10.1002/chem.200802140
Nair, V., Rajesh, C., Vinod, A.U., Bindu, S., Sreekanth, A.R., Mathen, J.S., and Balagopal, L., Acc. Chem. Res., 2003, vol. 36, p. 899. https://doi.org/10.1021/ar020258p
Mathapati, S.R., Prasad, D., Atar, A.B., Nagaraja, B.M., and Jadhav, A.H., Mater. Today: Proc., 2019, vol. 9, p. 661. https://doi.org/10.1016/j.matpr.2018.10.390
Jiang, B., Rajale, T., Wever, W., Tu, S., and Li, G., Chem. Asian J., 2010, vol. 5, p. 2318. https://doi.org/10.1002/asia.201000310
Zhao, X.-L., Zhao, Y.-F., Guo, S.-C., Song, H.-S., Wang, D., and Gong, P., Molecules, 2007, vol. 12, p. 1136. https://doi.org/10.3390/12051136
Navarro, J.A.R., Salas, J.M., Romero, M.A., Vilaplana, R., Gonzalez-Vílchez, F., and Faure, R., J. Med. Chem., 1998, vol. 41, p. 332. https://doi.org/10.1021/jm970358e
Chen, Q., Zhu, X.-L., Jiang, L.-L., Liu, Z.-M., and Yang, G.-F., Eur. J. Med. Chem., 2008, vol. 43, p. 595. https://doi.org/10.1016/j.ejmech.2007.04.021
Bhosale, V.N., Khansole, G.S., and Angulwar, J.A., World J. Pharm. Pharm. Sci., 2016, vol. 5, no. 5, p. 1434.
Molyneux, P. and Songklanakarin, J., Sci. Technol., 2004, vol. 26, p. 211.
Hour, M.-J., Huang, L.-J., Kuo, S.-C., Xia, Y., Bastow, K., Nakanishi, Y., Hamel, E., and Lee, K.-H., J. Med. Chem., 2000, vol. 43, p. 4479. https://doi.org/10.1021/jm000151c
Holla, B.S., Poojary, K.N., Rao, B.S., and Shivananda, M.K., Eur. J. Med. Chem., 2002, vol. 37, p. 511. https://doi.org/10.1016/S0223-5234(02)01358-2
Cei, M., Pardelli, R., Sani, S., and Mumoli, N., Clin. Exp. Med., 2014, vol. 14, p. 77. https://doi.org/10.1007/s10238-012-0219-0
Süleymanoğlu, N., Ünver, Y., Ustabaş, R., Direkel, Ş., and Alpaslan, G., J. Mol. Struct., 2017, vol. 1144, p. 80. https://doi.org/10.1016/j.molstruc.2017.05.017
Gomez, L., Massari, M.E., Vickers, T., Freestone, G., Vernier, W., Ly, K., Xu, R., McCarrick, M., Marrone, T., and Metz, M., J. Med. Chem., 2017, vol. 60, p. 2037. https://doi.org/10.1021/acs.jmedchem.6b01793
Kumar, J., Meena, P., Singh, A., Jameel, E., Maqbool, M., Mobashir, M., Shandilya, A., Tiwari, M., Hoda, N., and Jayaram, B., Eur. J. Med. Chem., 2016, vol. 119, p. 260. https://doi.org/10.1016/j.ejmech.2016.04.053
Kovalevich, J., Cornec, A.-S., Yao, Y., James, M., Crowe, A., Lee, V.M.-Y., Trojanowski, J.Q., Smith, A.B., Ballatore, C., and Brunden, K.R., J. Pharmacol. Exp. Ther., 2016, vol. 357, p. 432. https://doi.org/10.1124/jpet.115.231175
Phillips, M.A., White, K.L., Kokkonda, S., Deng, X., White, J., El Mazouni, F., Marsh, K., Tomchick, D.R., Manjalanagara, K., and Rudra, K.R., ACS Infect. Dis. 2016, vol. 2, p. 945. https://doi.org/10.1021/acsinfecdis.6b00144
Tabrizi, M.A., Baraldi, P.G., Ruggiero, E., Saponaro, G., Baraldi, S., Poli, G., Tuccinardi, T., Ravani, A., Vincenzi, F., and Borea, P.A., Eur. J. Med. Chem., 2016, vol. 113, p. 11. https://doi.org/10.1016/j.ejmech.2016.02.032
Faizi, M., Dabirian, S., Tajali, H., Ahmadi, F., Zavareh, E.R., Shahhosseini, S., and Tabatabai, S.A., Bioorg. Med. Chem. 2015, vol. 23, p. 480. https://doi.org/10.1016/j.bmc.2014.12.016
Singh, M., Fatma, S., Ankit, P., Singh, S.B., and Singh, J., Tetrahedron Lett., 2014, vol. 55, p. 525. https://doi.org/10.1016/j.tetlet.2013.11.090
Alluri, H., Gonthina, H., Ganta, R.K., Ch, M., and Rao, B.V., Pharma Chem., 2015, vol. 7, no. 10, p. 515.
Khansole, G.S., Prasad, D., Angulwar, J.A., Atar, A.B., Nagaraja, B.M., Jadhav, A.H., and Bhosale, V.N., Mater. Today: Proc., 2019, vol. 9, p. 653. https://doi.org/10.1016/j.matpr.2018.10.389
Vartale, S.P., Halikar, N.K., Pawar, Y.D., and Tawde, K.V., Arab. J. Chem., 2016, vol. 9, p. S1117. https://doi.org/10.1016/j.arabjc.2011.12.007
Danagulyan, G.G., Boyakhchyan, A.P., Danagulyan, A.G., and Panosyan, H.A., Chem. Heterocycl. Compd., 2011, vol. 47, p. 321. https://doi.org/10.1007/s10593-011-0760-x
Wendt, M.D., Kunzer, A., Henry, R.F., Cross, J., and Pagano, T.G., Tetrahedron Lett., 2007, vol. 48, p. 6360. https://doi.org/10.1016/j.tetlet.2007.07.039
Abdel‐Rahman, H.M., El‐Koussi, N.A., and Hassan, H.Y., Arch. Pharm., 2009, vol. 342, p. 94. https://doi.org/10.1002/ardp.200800113
Ranjbar-Karimi, R., Beiki-Shoraki, K., and Amiri, A., Monatsh. Chem., 2010, vol. 141, p. 1101. https://doi.org/10.1007/s00706-010-0371-8
Bhosale, V.N., Khansole, G.S., Angulwar, J.A., and Choudhare, S.S., World J. Pharm. Res., 2015, vol. 5, no. 2, p. 1261.
Hassan, A.Y., Sarg, M.T., Bayoumi, A.H., and El‐ Deeb, M.A., J. Heterocycl. Chem., 2018, vol. 55, p. 1450. https://doi.org/10.1002/jhet.3184
Bhosale, V.N., Choudhare, S.S., Khansole, G.S., and Wadwale, N.B., Pharma Chem., 2017, vol. 9, no. 5, p. 66.
Khansole, G.S., Angulwar, J.A., and Bhosale, V.N., Indian J. Heterocycl. Chem., 2018, vol. 28, p. 267.
Hassaneen, H.M.E., Hassaneen, H.M., Khiry, S.F.M., and Pagni, R.M., Z. Naturforsch., Teil B, 2008, vol. 63, p. 217. https://doi.org/10.1515/znb-2008-0216
Karoui, A., Allouche, F., Deghrigue, M., Agrebi, A., Bouraoui, A., and Chabchoub, F., Med. Chem. Res. 2014, vol. 23, p. 1591. https://doi.org/10.1007/s00044-013-0742-x
Lipson, V.V, Karnozhitskaya, T.M., Desenko, S.M., Shishkina, S.V, Shishkin, O.E., and Musatov, V.I., Russ. J. Org. Chem., 2007, vol. 43, p. 249. https://doi.org/10.1134/S1070428007020169
Erkin, A.V. and Krutikov, V.I., Russ. J. Gen. Chem., 2009, vol. 79, p. 1204. https://doi.org/10.1134/S1070363209060309
ACKNOWLEDGMENTS
The authors duly acknowledge the Principal, Yeshwant Mahavidyalaya, Nanded, for providing laboratory facilities. V.N.B. is thankful to SRTMU, Nanded, for sanctioning a grant of MRP (APDS/Univ. MRP/Sci. and Tech. Chemistry/2019-20/2819). The authors also express their gratitude to the Director, CSIR-IICT Hyderabad, Vishnu Chemicals Pvt. Ltd., Hyderabad, and Punjab University, Chandigarh, for providing help with the analysis of spectral data.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare the absence of conflict of interest.
Rights and permissions
About this article
Cite this article
Wadwale, N.B., Prasad, D., Jadhav, A.H. et al. Synthetic Development and Assessment of Antioxidant Activity of Imino[1,2,4]triazolo[1,5-a]pyrimidine-6-carbonitrile and Its Derivatives. Russ J Org Chem 57, 2031–2038 (2021). https://doi.org/10.1134/S1070428021120204
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428021120204