Abstract
A novel method for voltammetric determination of 4,4′-methylenebis(2,6-di-tert-butylphenol) antioxidant was developed employing linear sweep voltammetry and gold working electrode. Different supporting electrolytes were applied during development of this methodology and 0.05 mol dm−3 H2SO4 containing ethanol with 6.6 % toluene was selected as the suitable medium to ensure a sufficient solubility of the analyte. In this work, the electrochemical behavior of the mentioned antioxidant was investigated and different voltammetric techniques were compared. The developed method based on linear sweep voltammetry was purposed for determination of the antioxidant in base oil. It was found that this matrix does not allow the direct voltammetric determination of the antioxidant, thus, the sample pretreatment, namely the extraction with ethanol, was necessary. The proposed electroanalytical method enabled the determination of antioxidants in model and real samples with satisfactory results and provides good prospects for practical analysis.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Typical lubricating oils contain various additive compounds, such as corrosion inhibitors, dispersants, antioxidants, and extreme-pressure agents. The quality of oil is negatively affected by oxidation processes which are related with the presence of oxygen, higher temperatures, and pressures [1, 2]. Antioxidants are used for retardation of the oxidative degradation process of fuels and lubricating oils [3].
The most commonly used synthetic antioxidants are butylated phenols and polyphenols (butylated hydroxytoluene—BHT, butylated hydroxyanisole—BHA, tert-butylhydroquinone—TBHQ, propyl gallate—PG, and pyrogallol—PA) [4–6]. Various instrumental techniques allowing the precise determination of these antioxidants, including spectrophotometry [7–9], liquid chromatography [4, 10–12] for example in combination with electrochemical detection [13–15] or gas chromatography [16–19], were already described.
Electrochemical methods have recently been used for the determination of a wide spectrum of synthetic antioxidants in different types of samples, especially food products [20–25]. Only few authors [26–35] have published papers on the determination in petroleum product samples. Goulart et al. [26] presented a novel method for determination of TBHQ in soybean biodiesel by differential pulse voltammetry on a glassy carbon electrode (GCE). Two different supporting electrolytes, a solution of 10 mmol dm−3 KNO3 with 30 % acetonitrile and 30 % ethanol (pH 1.5) were investigated. The determination of TBHQ in soybean biodiesel samples was possible after previous liquid–liquid extraction. The accuracy and applicability of the method was demonstrated by analysis of a commercial biodiesel sample. GCEs were employed also in the work [27]. Tormin et al. developed a method for a direct amperometric determination of TBHQ in biodiesel. An aliquot of biodiesel was dosed into the electrochemical cell containing an appropriate electrolyte (75 % v/v ethanol–water solution with 0.05 mol dm−3 HClO4) and then the multiple-pulse amperometry was applied. In next paper [28], authors reported application of batch injection analysis with amperometric detection for determination of BHA in biodiesel using a boron-doped diamond electrode. The antioxidant BHA was determined also in paper [29]. Authors proposed voltammetric technique based on the use of screen-printed electrodes and supporting electrolyte containing Britton–Robinson buffer (0.04 mol dm−3), methanol (2 %), and the cationic surfactant cetyltrimethylammonium bromide for analysis BHA in mixture with TBHQ. The developed electrochemical technique can be used for simultaneous detection of these antioxidants and it can be employed for direct determination of the antioxidants in biodiesel samples without any pretreatment.
Araujo et al. [30, 31] studied phenolic antioxidant TBHQ in biodiesel. In the paper [30], it was developed an electroanalytical method for determination of TBHQ based on the enhancement effect of cetyltrimethylammonium bromide in Britton–Robinson buffer with pH 6.5. Samples of soybean biodiesel were analyzed using linear sweep voltammetry (LSV) at a carbon paste electrode. The method provided a sensitive alternative for TBHQ detection and the analysis could be realized directly in biodiesel samples without any pretreatment only after simple dilution with methanol. In the following paper [31], the method for determination of TBHQ in presence of surfactant Triton X-100 was investigated. Authors successfully developed methodology for ascertainment of TBHQ in soybean biodiesel using square wave voltammetry at a hanging mercury drop electrode.
In our previous papers [32–35], voltammetric behavior of different synthetic phenolic antioxidants was investigated using LSV at a gold disc electrode in supporting electrolyte containing isopropanol and 0.1 mol dm−3 H2SO4. Our proposed methods allow determination of the antioxidants in biodiesel samples without any pretreatment.
In this work, we focused on development of sensitive voltammetric method for determination of the antioxidant 4,4′-methylenebis(2,6-di-tert-butylphenol) (MBP) based on its electro-oxidation at a gold disc electrode. MBP is phenolic antioxidant for engine oil and industrial lubricants. It is highly effective additive for suppressing the formation of acidic and insoluble products of oil oxidation [36, 37]. Based on the literature survey, this antioxidant was yet not determined by any analytical method and our paper brings an interesting approach in this issue.
Results and discussion
Choice of the suitable supporting electrolyte for the voltammetric determination of antioxidant MBP
The acidic medium proved to be the most suitable for determination of analyzed antioxidant MBP. It is caused mainly by enhanced reactivity of phenols with other components of the sample, which leads to decreasing of their concentration in the alkaline medium. Furthermore, it was necessary to ensure the good solubility of the analyzed substance in the used supporting electrolyte. This was carried out by the presence of a suitable organic solvent [38]. At the beginning of the measurements, LSV method was used for investigation of MBP electrochemical behavior in various supporting electrolytes. Concentration of MBP in the cell was increased from 12.98 to 101.57 μg cm−3. At first, the determination of MBP was tested in medium containing isopropanol and 0.2 mol dm−3 H2SO4. Using this supporting electrolyte, peaks of anodic oxidation were obtained at potentials from 1.0 to 1.55 V, but it was impossible to make their correct evaluation due to their shape and wide. From this reason, the value of the peak height (I p ) could not be precisely identified. The next examined organic solvent was acetonitrile in combination with 0.2 mol dm−3 H2SO4. In this solution, some peaks belonging to anodic oxidation of MBP were obtained. In this case, it was also problem with evaluation of peak height of MBP, but in this case, owing to the effect of the background. It was necessary to differentiate signals using subtraction of the baseline (blank or supporting electrolyte) for correct evaluation of the peak height. The peaks after this step are clearly distinguishable and easily evaluable. In addition, it was found that the dependence I = f(c) is nonlinear. Therefore, ethanol was investigated as the next solvent but the same results as in case of acetonitrile were obtained.
It was found out that MBP is very soluble in organic solvents—toluene and ethanol, but insoluble in water. From this reason, the effect of the amount of toluene on the MBP peaks recorded in the supporting electrolyte containing ethanol and sulfuric acid was examined. H2SO4 with concentration 0.05 mol dm−3 was chosen from range 0.05–1.5 mol dm−3, because the antioxidant MBP is insoluble in water and the higher amount of aqueous solution of H2SO4 (1:1 v/v) decreased the solubility of MBP. It was examined the amount of toluene in range from 6.6 to 33.0 % in the supporting electrolyte (15 cm3). With increasing of toluene amount, the peaks of anodic oxidation of MBP were unchanged, but the curve of supporting electrolyte shifted to lower potentials. This shift did not affect the peaks of MBP. Figure 1 documents the peaks of anodic oxidation of MBP in solution of ethanol with 6.6 % toluene. As can be seen in Fig. 1a, peaks of MBP anodic oxidation were obtained after addition of toluene into the supporting electrolyte and the dependence I = f(c) was linear due the increasing of MBP solubility in the supporting electrolyte.
Anodic linear sweep voltammograms of MBP (a) before and (b) after subtraction baseline. Experimental conditions: LSV method, supporting electrolyte: ethanol containing 0.05 mol dm−3 H2SO4 and 6.6 % toluene, E in = +0.4 (V), E fin = +1.3 (V), v = 40 mV s−1, MBP in concentration range from 12.98 to 101.57 (μg cm−3)
Considering all of the above-mentioned information, 0.05 mol dm−3 of H2SO4 containing ethanol with 6.6 % toluene was selected as the suitable medium in this work. The problem with accurate determination of MBP peak height was solved by baseline subtraction, which is illustrated by Fig. 1b, where the peaks of anodic oxidation of MBP recorded using LSV after the subtraction are documented. It is obvious, that the peak height is linearly proportional to the concentration, which also resulted from statistical evaluation [39] of voltammograms. This dependence could be described by the followed equation, I p = (0.015) c MBP + 0.011 (where I p is in μA and c MBP in μg cm−3). Limit of detection (LOD) and limit of quantification (LOQ) were calculated and are 0.94 and 3.13 μg cm−3, respectively.
The recovery of MBP determination was tested by repeated determination of solution with concentration 13.24 μg cm−3. The obtained results shown that the error of the individual determinations was in range from −6.9 to +9.3 %. The average (13.31 μg cm−3) for n = 10 was within 95 % interval of reliability (lower limit: 12.85 μg cm−3; upper limit: 13.77 μg cm−3) and differs by +0.5 % from the real value. The standard deviation is 0.643 μg cm−3.
Comparison of the proposed method with other voltammetric techniques
In this work, the antioxidant determination was performed using different voltammetric methods, as presented in Fig. 2. Concentration of MBP ranged from 13.24 to 103.54 μg cm−3. The first method used for determination of MBP was square wave voltammetry (SWV) (see Fig. 2a). Figure 2b presents the curves of anodic oxidation of MBP using differential pulse voltammetry (DPV). The last investigated method was LSV (Fig. 1). The maximum peak potentials (E p ) for antioxidant under study with concentration 65.34 μg cm−3 using SWV, DPV, and LSV were registered at 0.94, 0.89, and 0.97 V, respectively. The optimization of the experimental parameters that affect the SWV and DPV responses was carried out. The optimum values obtained for SWV were: pulse amplitude 30 mV, pulse period 80 ms, and scan rate 40 mV s−1. Following parameters were chosen for DPV: pulse amplitude 30 mV, pulse length 60 ms, scan rate 40 mV s−1, and scan step 5 mV. The regression equations for different voltammetric method were obtained from statistical analysis (Table 1). According to the obtained results in Table 1, the electroanalytical procedure developed using LSV yielded the best value of correlation coefficient. The method LSV was chosen for next analyses.
Voltammograms of MBP recorded using (a) SWV and (b) DPV. Experimental conditions: for SWV: E in = +0.4 (V), E fin = +1.2 (V), pulse amplitude 30 mV, pulse period 80 ms, v = 40 (mV s−1); for DPV: E in = +0.4 (V), E fin = +1.1 (V), pulse amplitude 30 (mV), pulse length 60 (ms), v = 40 (mV s−1), scan step 5 (mV); supporting electrolyte: EtOH containing 0.05 (mol dm−3) H2SO4 and 6.6 % TLN, MBP in concentration range from 13.24 to 103.54 (μg cm−3)
Investigation of the electrochemical behavior in the proposed supporting electrolyte
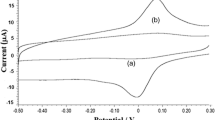
Voltammetric techniques (especially cyclic voltammetry) are the most convenient ones for clarifying the redox behavior of the organic compounds. Therefore, the electrochemical behavior of MBP on a gold disc electrode was studied by cyclic voltammetry (CV). The analysis was performed in the proposed supporting electrolyte containing ethanol with 6.6 % toluene and 0.05 mol dm−3 H2SO4. The influence of various scan rates was studied at first. This parameter was changed from 10 to 125 mV s−1 for two different concentrations 1.29 × 10−3 and 6.46 × 10−4 mol dm−3. Cyclic voltammogram of 6.46 × 10−4 mol dm−3 MBP recorded at 10 mV s−1 is presented in Fig. 3. As it was found, the peak potential values at higher rates slightly shift to higher potentials, from 0.90 to 1.01 V, indicating kinetically controlled electrode reaction. On the reverse sweep, no distinct wave was observed, showing that MBP antioxidant is irreversibly oxidized under the used conditions. From the dependence of the first peak height on the square root of the scan rate, a linear dependence was obtained and could be described by equation: I p = 0.083v 1/2 − 0.018, where I p is in μA and v 1/2 in (mV s−1)1/2. The regression coefficient was 0.9911. Due the low value of intercept in the equation, it could be concluded that the electrode process is driven by diffusion. For next analyses, a polarization rate of 40 mV s−1 was chosen, because the peak height recorded at this scan rate was well defined. Next, cyclic voltammograms of MBP showed a similar electrochemical behavior to gallate derivatives, as it was reported in the paper [40]. As seen in the MBP cyclic voltammograms (Fig. 3), a second oxidation peak is observed at more positive potentials. In addition, this second peak showed a similar behavior but the quantification was rather imprecise because the resolution of the peak was very poor.
Determination of MBP in model samples of base oil
Because of this antioxidant is used to prevent oxidation, the voltammetric determination of MBP in oils by oxidation was examined. For application of the proposed method, oil samples were treated as described in the section Experimental subsection Samples. Model samples of oils were at first extracted using ethanol. Appropriate volumes, according to the actual MBP concentration in samples, were placed into the electrochemical cell with the electrolyte and analyzed. Figure 4a shows peaks of MBP anodic oxidation in the extract of model base oil sample obtained at a gold disc electrode in the suggested supporting electrolyte. The standard addition method was used. The curve 1 represents record of the ethanolic extract of oil spiked with antioxidant: 0.77 g MBP being expressed per kilogram of oil. The curves 2 and 3 belong to voltammograms measured after addition of 0.1 cm3 27.1 μg cm−3 MBP. It was confirmed that interferences from the matrix of oil extract affected peaks of anodic oxidation, especially the second peak could not be observed at all. On the other hand, the first peak was affected only slightly. It was also found that the height of the first peak was not possible to determine accurate. This effect is especially apparent for very low concentration of MBP in sample, where bigger volume of the sample has to be taken for analyses (see Fig. 4a). In this case, the problem with determining of peak height was identified but it could be solved by mathematical approach, which was ensured by the software of the used analyzer providing various mathematic operations. Our employed approach is depicted in Fig. 4b. The voltammetric curves were derived and then the peaks were clearly distinguishable. Such an approach was used for all analyses of spiked oil samples, where the height peaks of anodic oxidation was not very well defined. Three different concentrations of MBP were prepared (0.5, 0.1, and 0.05 %, respectively). These concentration levels correspond to real contents of MBP in oils because the initial concentration of MBP in oil is about 0.5 % and it decreases with time. Results of spiked oil samples analyses are presented in Table 2. The results obtained experimentally are in good agreement with the values declared concentration in model samples of oil. Excellent recovery values were obtained, associated with low values of RSD, corroborating the accuracy and precision of the voltammetric method.
Anodic linear sweep voltammograms of MBP determination (a) before and (b) after mathematical correction. Experimental conditions: LSV method, supporting electrolyte: EtOH containing 0.05 (mol dm−3) H2SO4 and 6.6 % TLN, E in = +0.4 (V), E fin = +1.3 (V), v = 40 (mV s−1). Curve 1 sample of oil [c MBP = 0.77 (g kg−1)]; curves 2, 3 additions of MBP standard solution with concentration of 27.1 (μg cm−3) (volume 0.1 cm3); curves 1′,2′,3′ the same as 1, 2, 3 but after mathematical correction
Conclusion
In this paper, a simple and rapid voltammetric method for analysis of MBP antioxidant was proposed and tested on model samples of oil. The gold disc electrode was successfully applied for analyses of samples containing the studied phenolic antioxidant. First, a suitable supporting electrolyte for the analysis was found as 0.05 mol dm−3 of H2SO4 containing ethanol with 6.6 % toluene. This medium ensured the sufficient solubility of analyte. Very low detection and quantification limit was obtained for MBP determination using linear sweep voltammetry: LOD 0.94 μg cm−3 and LOQ 3.13 μg cm−3.
In suggested supporting electrolyte, recovery percentages ranged from 93 to 100 %. Moreover, these recovery percentages obtained for antioxidant under study show clearly that the extraction procedure was efficient. The method appears to be convenient for monitoring of the antioxidant content in oils at the trace concentration level as the detection capabilities are sufficient and, in fact, even extraordinary for such a type of analyte.
In conclusion, the presented method seems to be convenient and fully recommendable for routine analysis in laboratories of refineries and similar industrial service units.
Experimental
All used chemicals were of analytical reagent grade and originated from Penta or Sigma-Aldrich. Stock solution of MBP (4 g dm−3) was prepared by dissolving appropriate amount of MBP (Sigma-Aldrich, CAS: 118-82-1) in 96 % (v/v) ethanol. Supporting electrolytes for voltammetric determination of MBP were based on diluted H2SO4 (Penta) mixed with ethanol (Penta, Czech Republic) or acetonitrile (AcN; Penta, Czech Republic) and toluene (TLN; Penta, Czech Republic).
The antioxidant was analyzed in base oil from refinery Paramo, Czech Republic. The base oil is fully comparable with similar oils produced worldwide, which find their use in wide variety of automotive and industrial oils. The oil samples were extracted with 96 % EtOH. Anhydrous sodium sulfate, Na2SO4 (Sigma-Aldrich, CAS: 239313), was used to remove the rest of the oils from the extract.
Apparatus and accessories
Voltammetric analyses of MBP were performed using an electrochemical analyzer (model “EP 100VA”, HSC Servis Bratislava, Slovak Republic) in three-electrode cell which was composed of the gold disc (diameter 2 mm, HSC Servis Bratislava, Slovak Republic) as the working electrode, Ag|AgCl|3 mol dm−3 KCl as the reference, and the Pt plate (3 × 5 mm) as the counter electrode (both from Monokrystaly, Turnov, Czech Republic).
Procedure
The supporting electrolyte of choice contained H2SO4, and ethanol with toluene, when the typical volume was 15 cm3 and the exact composition is specified in Sect. “Result and discussion”; always, in association with a given experiment. The anodic oxidation of antioxidant MBP was performed in LSV mode, with these experimental parameters: current range ±4 μA, initial potential (E in ) +0.4 V, final potential (E fin ) +1.3 V, and scan rate (v) 40 mV s−1.
Samples
Spiked motor oil samples were prepared by dissolving a known amount of antioxidant MBP in pure oil. Model samples were processed as follows: the latter had to be extracted with 96 % EtOH (according to the procedure described in Ref. [34]), where taking 1–2 g of oil, accompanied yet by thoroughly sonification (for 10 min). Anhydrous sodium sulfate Na2SO4 was used to remove the residual of oils from the extracts because even small quantities of oil caused contamination of the electrode surface. After achieving the separation of the suspension formed, the upper organic layer was separated and filtered and then analyzed immediately.
References
Gresham RM, Totten GE (2008) Lubrication and maintenance of industrial machinery. Taylor and Francis Group, Florida
Rudnick LR (2009) Lubricant—chemistry and application. Taylor and Francis Group, Florida
Mukhopadhyay AK (2006) Antioxidants—natural and synthetic. Amani International Publishers, Kiel
Bruzzoniti MC, Maina R, Tumiatti V (2015) J Liq Chromatogr Relat Technol 38:15
Karavalakis G, Hilari D, Givalou L, Karonis D, Stournas S (2011) Energy 36:369
Santos AL, Takeuchi RM, Munoz RAA, Angnes L, Stradiotto NR (2014) Electroanal 26:233
Capitan-Vallvey LF, Valencia MC, Nicolas EA (2004) Anal Chim Acta 503:179
Versari A, Parpinello GP, Scazzina F, Del Rio D (2010) Food Control 21:786
Tabart J, Kevers C, Pincemail J, Defraigne JO, Dommes J (2010) Food Chem 120:607
Li XL, Meng DL, Zhao J, Yang YL (2014) Chin Chem Lett 25:1198
Chen M, Hu XJ, Tai ZG, Qin H, Tang HN, Liu MS, Yang YL (2013) Food Anal Methods 6:28
Bahmaei M, Peyman H (2012) Ital J Food Sci 24:55
Plaza M, Kariuki J, Turner C (2014) J Agr Food Chem 62:409
Bayram B, Ozcelik B, Schultheiss G, Frank J, Rimbach G (2013) Food Chem 138:1663
Skrinjar M, Kolar MH, Jelsek N, Hras AR, Bezjak M, Knez Z (2007) J Food Compos Anal 20:539
Ding MZ, Zou JK (2012) Food Chem 131:1051
Choong YM, Lin HJ (2001) J Food Drug Anal 9:20
Yang MH, Lin HJ, Choong YM (2002) Food Res Int 35:627
Lin HJ, Wang ML, Choong YM, Chen CW, Hwang BS, Tsai SL, Chung LC, Yang MH (2003) J Food Drug Anal 11:141
Rasheed Z, Vikraman AE, Thomas D, Jagan JS, Kumar KG (2015) Food Anal Methods 8:213
Ervin EM, Kariuki JK (2014) Int J Electrochem Sci 9:6235
Ziyatdinova G, Saveliev AA, Evtugyn GA, Budnikov HC (2014) Electrochim Acta 137:114
Ziyatdinova G, Gainetdinova A, Morozov M, Budnikov H, Grazhulene S, Redkin A (2012) J Solid State Electrochem 16:127
Robledo SN, Zon MA, Ceballos CD, Fernandez H (2011) Food Chem 127:1361
Medeiros RA, Rocha RC, Fatibello O (2010) Food Chem 123:886
Goulart LA, Teixeira ARL, Ramalho DA, Terezo AJ, Castilho M (2014) Fuel 115:126
Tormin TF, Gimenes DT, Silva LG, Ruggiero R, Richter EM, Ferreira VS, Munos RAA (2010) Talanta 82:1599
Tormin TF, Gimenes DT, Richter EM, Munos RAA (2011) Talanta 85:1274
Caramit RP, Freitas AGA, Souza JBG, Araujo TA, Viana LH, Trindade MAG, Ferreira VS (2013) Fuel 105:306
Araujo TA, Barbosa AMJ, Viana LH, Ferreira VS (2010) Fuel 90:707
Araujo TA, Barbosa AMJ, Viana LH, Ferreira VS (2010) Colloid Surf B 79:409
Tomášková M, Chýlková J, Jehlička V, Navrátil T, Švancara I, Šelešovská R (2014) Fuel 123:107
Tomášková M, Chýlková J, Navrátil T, Šelešovská R (2014) Energ Fuel 28:4731
Tomášková M, Chýlková J, Jehlička V, Navrátil T, Šelešovská R (2013) Sci Pap Univ Pardubice A 19:155
Chýlková J, Tomášková M, Mikysek T, Šelešovská R, Jehlička J (2012) Electroanal 24:1374
Quinchia LA, Delgado MA, Valencia C, Franco JM, Gallegos C (2011) J Agric Food Chem 59:12917
Boyle TJ, Steele LAM, Yonemoto DT (2012) J Coord Chem 65:487
Tomášková M, Chýlková J, Mikysek T, Jehlička V (2015) Determination of hindered phenolic antioxidant in oils using linear sweep voltammetry with a gold disc electrode. In: Navratil T, Fojta M, Schwarzova K (eds) XXXVth Modern Electrochemical Methods. BestServis, Jetrichovice, p 250
Jehlička V (2015) Media4u Magazine 12:41
Gunckel S, Santander P, Cordano G, Ferreira J, Munoz S, Nunez-Vergara LJ, Squella JA (1998) Chem-Biol Interact 114:45
Acknowledgments
Financial support was provided by The Ministry of Education, Youth and Sports of the Czech Republic (Project No. CZ.1.07/2.3.00/30.0021 “Strengthening of Research and Development Teams at the University of Pardubice”).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tomášková, M., Chýlková, J., Mikysek, T. et al. Voltammetric determination of antioxidant 4,4′-methylenebis(2,6-di-tert-butylphenol) in lubricating oils using gold disc electrode. Monatsh Chem 147, 231–237 (2016). https://doi.org/10.1007/s00706-015-1562-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-015-1562-0