Abstract
The electrochemical oxidation of butylated hydroxyanisole (BHA), a synthetic phenolic antioxidant, has been studied by differential pulse voltammetry (DPV) at multiwalled carbon nanotube (MWCNT)-modified platinum sensor in 0.1 M phosphate buffer (pH 4) as supporting electrolyte. The developed sensor showed catalytic activity and stability for BHA oxidation. Linear calibration graph was obtained in the concentration range of 1 × 10−6–1 × 10−7 M, and the detection limit was 9.49 × 10−8 M. Effect of common interfering ions have been investigated in simulated mixtures containing high levels of interfering ions, and the sensor was found to be tolerant against these ions. This method has been proved to be effective and successfully applied for the determination of BHA in commercially available vegetable oil and mayonnaise samples, providing a promising and convenient method to monitor the superscale use of phenolic antioxidant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Butylated hydroxyanisole (BHA) is a commonly used phenolic preservative in commercial products such as vitamin tablets, edible oils, confectionary, food packing, animal feed, cosmetics, and junk food. BHA is added to food products to improve their stability, especially to prevent rancidity in products containing lipids or fats, and display high chemical activity for suppressing chain initiation or breaking chain propagation of the peroxidation of unsaturated fatty acids (Xiu et al. 2009). Since it has the ability to remain active even at high temperatures while cooking or baking, BHA is exclusively used in food products (Richard Prabakar and Sriman Narayanan 2006).
Although they are powerful in protecting product quality in food distribution, excess antioxidants added to food might produce toxicities or mutagenicities and, thus, endanger the health of people (Williams 1993, 1994). In most countries, the content of phenolic antioxidants in processed food is strictly limited. So, there are still genuine needs to establish an effective and convenient qualitation and quantitation method for analytically monitoring the proper use of prohibited antioxidants and the superscale use of permitted antioxidants (Richard Prabakar and Sriman Narayanan 2006).
Many methods for determining BHA have been reported based on liquid and gas chromatography (Guo et al. 2006; Perrin and Meyer 2002; Saad et al. 2007), spectrophotometry (Capitan-Vallvey et al. 2004), flow injection and HPLC with amperometric detection (Luque et al. 1999; Riber et al. 2000; Ruiz et al. 1999) and micellar electrokinetic chromatography (Delgado-Zamarreno et al. 2007; Guan et al. 2006). These methods are found to be complicated, time-consuming, and unsuitable for trace level detection.
Doubtlessly, HPLC is the most widely used method for the determination of phenolic antioxidants in technical and pharmaceutical products, as well as in food. Besides, various electroanalytical techniques were proposed for the determination of common antioxidants as an alternative to HPLC (Michalkiewicz et al. 2004). However, an additional and common practical problem which occurs during the electrochemical oxidation of phenols is the formation of non-conducting polymeric films on the bare electrode surface (Gatrell and Kirk 1990, 1993; Wang et al. 1998), followed by loss of the oxidation response. This problem becomes particularly severe as the concentration of phenolic compound increases. In consequence of this, voltammetry at bare platinum (Litescu et al. 2001; Mc Bridge and Evans 1973; Rifkin and Evans 1976) or glassy carbon electrodes (Ni et al. 2000; Yanez-Sedeno et al. 1991) is relatively seldom employed in this area. In order to overcome the electrode fouling, numerous voltammetric determinations of phenolic antioxidants were performed with modified electrodes (De la Fuente et al. 1997, 1999; Garcia and Ortiz 1998) that protect the surface from poisoning.
Chemically modified electrodes (CMEs) improve the sensitivity and selectivity of electrochemical analysis by improving the kinetics of the reaction via an electrocatalytic process at the CME surface (Richard Prabakar and Sriman Narayanan 2006). Owing to their unique structural, mechanical, and electronic properties, carbon nanotubes (CNTs), especially multiwalled carbon nanotube (MWCNT), have recognized as attractive candidates for the realization of numerous electrochemical applications (Issac and Girish Kumar 2009; Lonappan and Girish Kumar 2011; Lonappan et al. 2011). The special structure and subtle electronic properties suggest that CNT may have the ability to promote electron transfer when it is used as electrode material.
In continuation to our work in food analysis (Thomas et al. 2012; Vikraman et al. 2013), we have investigated the electrochemical activity of BHA in phosphate-buffered solutions at MWCNT-modified platinum electrodes (Pt electrodes). The practical use of the method is demonstrated by determining the concentration of BHA in commercial vegetable oil (coconut oil) and mayonnaise samples and comparing the obtained results with standard liquid chromatography (LC)-UV method.
Materials and Methods
Apparatus
Voltammetric measurements were performed with a PC-controlled electrochemical workstation (CH Instruments, USA). A three-electrode cell system was used. MWCNT-modified Pt electrode was the working electrode, while Ag/AgCl electrode and platinum wire were the reference and auxiliary electrodes, respectively. A glassy carbon electrode and gold electrode were also used for comparative purposes. Fourier transform infrared (FTIR) spectra of MWCNT were recorded on JASCO 4100 spectrometer using KBr discs. LC-UV method was performed with WATERS 2489 instrument (Milford, USA). SEM images were obtained on a JOEL 6390 LV. pH measurements were performed on a Metrohm pH metre equipped with Ag/AgCl glass combination electrode.
Reagents and Solutions
All reagents used were of analytical grade. BHA was purchased from Spectrochem Pvt. Ltd, Mumbai, India. Disodium hydrogen orthophosphate anhydrous was purchased from S D Fine-Chem Limited, Mumbai, India, and sodium dihydrogen phosphate monohydrate GR from Merck Limited, Mumbai, India. MWCNT (6-20 nm diameter and 1-5 μm length) and Nafion were purchased from Sigma-Aldrich Co., St. Louis, MO, USA. For application studies, the oil used was a premium quality 100 % pure edible coconut oil, and the mayonnaise used was a product of Fruitoman’s food product, India. Stock solution of BHA was prepared by dissolving the compound in pure methanol. Standard solution was then prepared from this stock solution by serial dilution as required. Phosphate-buffered solution of 0.1 M (pH 4) was used as supporting electrolyte. All solutions were prepared using ultrapure Millipore water with a resistivity greater than 18 MΩcm. For segmentation and carboxylation, the MWCNT was refluxed in concentrated HNO3 for 4 h.
Fabrication and Characterization of Working Electrode
Pt electrode with dimensions 8-cm length and 0.6-cm diameter, acquired from CH Instruments (USA), was used for sensor fabrication. Prior to modification, the electrode was mechanically polished with alumina slurries on flat pads, followed by rinsing with ultrapure water and sonicated successively in ethanol and water for 3 min each. The polished electrode was subjected to an electrochemical cleaning with 0.05 M sulphuric acid, after which it was rinsed with water and allowed to air dry.
Acid treatment of MWCNT was carried out as described in literature (Lonappan et al. 2011; Tsang et al. 1994). The presence of carboxy and carboxylate groups on the surface of acid-treated MWCNT were confirmed by peaks at 1,703 and 1,564 cm−1 in the FTIR spectrum.
Five milligrams of acid-treated MWCNT was dispersed in 13 % Nafion–water solution (2 mL water + 300 μL Nafion) with the aid of ultrasonicate agitation to form a black homogeneous suspension. Three microlitres of the dark solution was dropped onto the surface of cleaned Pt electrode and dried at room temperature. The modified electrode was stable up to 8–10 measurements, and the lifetime of the electrode was up to 7 days under room temperature.
The microscopic areas of the bare Pt electrode and MWCNT-modified Pt electrode were obtained by cyclic voltammetry using 2 mM K3Fe (CN)6 as a redox probe. The voltammograms were recorded at different scan rates. For a reversible process, the relationship between the peak current (i p) and scan rate (ν) is given by the Randles–Sevicick equation (Randles 1948).
where i p refers to the peak current; A, the surface area of the electrode; C, concentration of K3Fe (CN)6; and ν, the scan rate. For 2 × 10−3 M K3Fe (CN)6, the number of electron transferred is n = 1 and the diffusion coefficient is D R = 7.6 × 10−6 cm2 s−1 (Adams 1969). Thus, from the slope of i p versus ν1/2 plot, the effective surface areas of MWCNT-modified Pt electrode can be calculated.
Analytical Procedure
Ten millilitres of 0.1 M phosphate-buffered solution (PBS), a suitable volume of BHA to obtain a concentration of 1 × 10−3 M, was added to an electrochemical cell, and then, the three-electrode system was installed. The solution was then stirred for 30 s to reduce the variation between each measurement. Differential pulse voltammogram was recorded between 200 and 800 mV at a scan rate of 20 mVs−1. Pulse amplitude of 50 mV, pulse period of 0.5 s and pulse width of 0.2 s were used. The potential step was 4 mV. The analytical signal for the oxidation of BHA was measured at a potential of 340 mV. Since DPV gave a well-defined oxidation peak with a lower potential and higher peak current compared to CV and SWV, DPV approach was selected for all the experiments.
Procedure for Determination of BHA in Foods
Treatment of Vegetable Oil Samples
Five grams of a vegetable oil sample was placed into an Erlenmeyer flask (with a screwcap), and 5.0 mL of pure methanol was added. After agitating with the use of a laboratory shaker for 5 min, the mixture was transferred to a 25-mL centrifuging tube and centrifuged at 3,000 rpm for 5 min. The methanol extract was transferred into a 25-mL flask after allowing to set for 2 min. The above extraction procedure was repeated, and all the methanol extracts were collected and transferred into the same flask. The solution was then diluted to the mark with methanol (Ni et al. 2000). One-millilitre aliquot of this sample solution was transferred to the electrochemical cell already containing 9 mL of the supporting electrolyte and was analyzed by voltammetry.
Treatment of Mayonnaise Sample
Mayonnaise samples were purchased at local stores and were treated according to the literature (Luque et al. 1999). A sample of about 1.0 g was dissolved in 1 mL ethanol in a large test tube. After shaking for 5 min, the mixture was centrifuged at 5,000 rpm. This extraction procedure was repeated twice. The extracts were collected and diluted to 5 mL with ethanol. A 500 μL aliquot was transferred to the electrochemical cell already containing 9.5 mL of the supporting electrolyte where BHA was determined by the standard addition method.
Results and Discussions
Electrochemical Behaviour of BHA
The electrochemical behaviour of BHA was studied using various electrodes which include glassy carbon electrode (GCE), gold electrode (GE), and Pt electrode. Bare and MWCNT-modified GCEs and GE did not give reliable results for BHA determination. For both the electrodes, the oxidation peak potential for BHA was high and also the difference in potential between bare and modified electrodes was not satisfactory. Hence, Pt electrode was used for the study. At bare Pt electrode, the electrochemical oxidation of BHA occurred at a potential of 520 mV, but under the same condition, a well-defined and sensitive oxidation peak appeared at 340 mV on MWCNT-modified Pt electrode in 0.1 M PBS (pH 4). Compared with the bare Pt electrode, there was also an enhancement of oxidation peak current for BHA from 8.5 to 25 μA at MWCNT-modified Pt electrode. The anodic peak generated for the BHA electrooxidation process may be due to the oxidation of the hydroxyl functional group present in the BHA structure. Herein, the oxidation peak obtained is attributed to the two-electron oxidation of BHA. The mechanism of the anodic oxidation of BHA is well known and is presented on Fig 1. (Ceballos et al. 2006; De la Fuente et al. 1999; Medeiros et al. 2010). The obtained results confirm this process.
In the reverse sweep of cyclic voltammetry of BHA, no reduction peak was observed indicating an irreversible electrochemical process. Figure 2 displays the comparison of DPV of 1 × 10−3 M BHA in PBS (pH 4) at bare Pt electrode (curve a) and MWCNT-modified Pt electrode (curve b). From the comparison of curves a and b, it is clear that the oxidation peak currents of BHA have obviously increased and that the peak potentials have considerably decreased from 520 to 340 mV at the MWCNT-modified Pt electrode. This can be attributed to the special properties of MWCNT such as large surface area, strong absorptive ability and subtle electronic properties.
Surface Area Study
As explained in “Fabrication and Characterization of Working Electrode” section, the active surface area of the electrode can be calculated using Randles–Sevicick equation. Thus, from the slope of i p versus ν1/2 plot, the microscopic area of MWCNT-modified Pt electrode was calculated to be 2.8695 cm2, which was about two times greater than that of bare Pt electrode (1.3672 cm2). This is strong evidence for the successful and effective modification of Pt electrode using MWCNT. Overlay of cyclic voltammograms of bare and MWCNT/Pt electrodes in K3Fe(CN)6 is shown in Fig. 3a, b, respectively.
Further evidence for the modification of Pt electrode was obtained from the surface morphology studies using SEM. SEM images of both bare and MWCNT-modified Pt electrodes are shown in Fig. 4.
Effect of Supporting Electrolyte and pH
The choice of supporting electrolyte can affect the thermodynamics and kinetics of electrochemical processes, as well as mass transfer within the cell. Different electrolytes of 0.1 M concentration (phosphate buffer, acetate buffer, nitric acid, hydrochloric acid, sulphuric acid, perchloric acid, NaCl and NaOH) were tested as supporting electrolytes for BHA oxidation by DPV, and the results are shown in Table 1. The oxidation peak obtained was best defined in 0.1 M PBS. Hence, PBS was chosen as the experimental medium.
The effect of pH on the anodic peak of BHA at MWCNT-modified Pt electrode was investigated by DPV. The pH range was studied from 3 to 9. Figure 5 depicts the effect of pH on the oxidation peak current of 1 × 10−3 M BHA. The maximal peak current was obtained at pH 4. Therefore, pH 4 was fixed as optimal pH.
Effect of the Amount of MWCNT–Nafion Dispersion
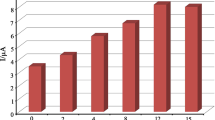
Nafion is a hydrophobic surfactant which can form a perfect thin film on Pt electrode surface. Much Nafion is unhelpful for electron transfer and mass transportation of BHA. Therefore, the effect of the amount of MWCNT–Nafion dispersion (5 mg MWCNT in 2 mL water + 300 μL Nafion) on the oxidation current of BHA was examined (Fig. 6). When the volume of MWCNT–Nafion dispersion was increased from 1 to 3 μL, the oxidation peak current of BHA increased sharply. On increasing the amount of MWCNT suspension, the electrode surface area increases, showing higher accumulation efficiency for BHA. So, the oxidation peak current shows remarkable enhancement. However, the peak current conversely decreased on continuing increasing the amount of MWCNT–Nafion suspension from 4 to 8 μL. This may be due to the insulating property of Nafion at higher concentrations (Yi et al. 2001). Additionally, the time for evaporating water undoubtedly increases when the amount of MWCNT–Nafion dispersion is too much. For above-mentioned reasons, the volume of modifier on the electrode surface was kept at 3 μL in the present study.
Effect of Scan Rate
The effect of scan rate on the oxidation peak current of 1 × 10−3 BHA was studied by DPV (Fig. 7a). The anodic peak current showed a linear relationship with scan rate in the range 30–140 mVs−1. The plot of peak current versus square root of scan rate (ν1/2) is linear over the whole range of scan rate studied (Fig. 7b), which indicates that it is a typical diffusion-controlled current system (Ni et al. 2000). Moreover, the scan rate increased the peak potential shifted to more positive value and also became slightly positive with increase in concentration of BHA. This indicates the irreversible character of the oxidation process.
More information about the mechanism of the oxidation process under study may be obtained from the number of electrons (n a) involved in the reaction. The number of electrons (n a) involved in the reaction can be calculated from the scan rate study using the Laviron’s equation (Laviron 1974).
The relationship between the peak potential E p and scan rate (ν) was examined. It was found that E p varies linearly with ln (ν). The number of electrons (n a) involved in the reaction can be calculated from the slope of the plot according to the relation b=RT / αn aF, where α is the transfer coefficient and its value is assumed to be 0.5. For the irreversible oxidation of BHA, n a value was calculated to be around 2. It indicates that the oxidation of BHA involves two electrons per molecule.
Analytical Properties
Reproducibility
The reproducibility of the peak potential and peak current was estimated by making seven replicate measurements of a standard BHA solution (1 × 10−3 M), under the selected optimum conditions. The relative standard deviation (RSD) of the peak potential was 3.1 %, and the peak current was 3.4 %, suggesting that this method possesses good reproducibility. The electrode surface after each determination was regenerated by repetitive cycling in the supporting electrolyte.
Interference
Selectivity generally defined as the absence of interfering peaks in the potential range studied was investigated. The possible interfering species examined are listed in Table 2. A 100-fold concentration of sodium sulphite, citric acid, NaCl, CH3COOH and EDTA had no influence on 1 × 10−3 M BHA signals. Also, a 5-fold molar excess of other antioxidants such as BHT, propyl gallate and TBHQ did not influence BHA determination. However, ascorbic acid interferes severely.
Linear Range and Limit of Detection
The relationship between the oxidation peak current and concentration of BHA was studied by DPV under optimized conditions (Fig. 8a), and the calibration graph is presented in Fig. 8b. The oxidation peak current, i p, has a linear relationship with the concentration in the range of 1 × 10−6 to 1 × 10−7 M. The detection limit of BHA was 9.49 × 10−8 M, which was much lower than that of the reported voltammetric works (Table 3).
Application
In order to ascertain its potential applications, this newly developed method was employed to detect BHA in commercially available vegetable oil and mayonnaise samples according to the procedure described in the “Materials and Methods” section. Table 4 shows the content of BHA in spiked vegetable oil and mayonnaise samples, obtained by the standard addition method using MWCNT-modified Pt electrode. Each sample undergoes six parallel measurements, and the RSD is below 3 %. Table 4 also compares the BHA concentration determined in the analyzed food products employing the proposed method and standard LC-UV method. By analyzing the results obtained, one can conclude that the proposed electrochemical sensor is reliable for the determination of BHA in spiked samples. Also, from Table 4, it is obvious that no matrix effect was observed in the determination of BHA in spiked samples.
Conclusion
A voltammetric sensor for the determination of BHA has been developed based on MWCNT-modified Pt electrode. The MWCNT film significantly improved the electron transfer, decreased the oxidation potential and enhanced the oxidation peak current of BHA. The obtained results allow concluding that DPV along with MWCNT-modified Pt electrode can be used for the quantitative determination of BHA in vegetable oil (coconut oil) and mayonnaise samples. Very low detection limit was obtained, and the value is lower than the ones previously reported in the literature using voltammetric methods. The concentration values obtained for BHA are similar to those obtained using reference LC-UV method. The simplicity, sensitivity and low analytical cost demonstrate its analytical utility as a sensor for the determination of BHA. Furthermore, the use of less toxic reagents, ease of fabrication and regeneration of electrode surface make it more advantage over the standard method for BHA determination.
References
Adams RN (1969) Electrochemistry at solid electrodes. Marcel-Dekker, New York
Agui ML, Reviejo AJ, Yanez-Sedeno P, Pingarron JM (1995) Analytical applications of cylindrical carbon fiber microelectrodes. Simultaneous voltammetric determination of phenolic antioxidants in food. Anal Chem 67:2195–2200
Capitan-Vallvey LF, Valencia MC, Nicolas EA (2004) Solid-phase ultraviolet absorbance spectrophotometric multisensor for the simultaneous determination of butylated hydroxytoluene and co-existing antioxidants. Anal Chim Acta 503:179–186
Ceballos CD, Zón MA, Fernández H (2006) Using square wave voltammetry on ultramicroelectrodes to determine synthetic antioxidants in vegetable oils. J Chem Educ 83:1349–1352
De la Fuente C, Acuna JA, Vazquez MD, Tascon LM, Gomez MI, Sanchez Batanero P (1997) Preparation of a polypyrrole electrode modified with a nickel phthalocyanine complex. Application to the determination of an antioxidant (propylgallate) in foods. Talanta 44:685–695
De la Fuente C, Acuna JA, Vazquez MD, Tascon LM, Batanero PS (1999) Voltammetric determination of the phenolic antioxidants 3-tert-butyl-4-hydroxyanisole and tert-butylhydroquinone at a polypyrrole electrode modified with a nickel phthalocyanine complex. Talanta 49:441–452
Delgado-Zamarreno MM, Gonzalez-Maza I, Sanchez-Perez A, Martinez RC (2007) Analysis of synthetic phenolic antioxidants in edible oils by micellar electrokinetic capillary chromatography. Food Chem 100:1722–1727
Garcia CD, Ortiz PI (1998) Determination of tert-butylhydroxytoluene by flow injection analysis at polymer modified glassy carbon electrodes. Electroanalysis 10:832–835
Gatrell M, Kirk DW (1990) The electrochemical oxidation of aqueous phenol at a glassy carbon electrode. Can J Chem Eng 68:997–1003
Gatrell M, Kirk DW (1993) A study of electrode passivation during aqueous phenol electrolysis. J Electrochem Soc 140:903–911
Guan YQ, Chu QC, Fu L, Wu T, Ye JN (2006) Determination of phenolic antioxidants by micellar electrokinetic capillary chromatography with electrochemical detection. Food Chem 94:157–162
Guo L, Xie MY, Yan AP, Wan YQ, Wu YM (2006) Simultaneous determination of five synthetic antioxidants in edible vegetable oil by GC–MS. Anal Bioanal Chem 386:1881–1887
Issac S, Girish Kumar K (2009) Voltammetric determination of sulfamethoxazole at a multiwalled carbon nanotube modified glassy carbon sensor and its application studies. Drug Test Anal 1:350–354
Laviron E (1974) Surface linear potential sweep voltammetry equation of the peaks for a reversible reaction when interactions between the adsorbed molecules are taken into account. J Electroanal Chem 52:395–402
Litescu S, Radu GL, Diaconu M (2001) antioxidative power evaluation of some phenolic antioxidants—electroanalytical approach. Electroanalysis 13:804–806
Lonappan L, Girish Kumar K (2011) Carbon nanotube based sensor for voltammetric determination of pyridine-2-aldoxime methochloride. Sens Lett 9:541–545
Lonappan L, Issac S, Joseph R, Thomas D, Girish Kumar K (2011) Electrochemical studies of TAM using multiwalled carbon nanotube modified glassy carbon sensor. Micro Nano Lett 6:867–870
Luque M, Rios A, Valcarcel M (1999) A poly(vinyl choloride) graphite composite electrode for flow-injection amperometric determination of antioxidants. Anal Chim Acta 395:217–223
Mc Bridge HD, Evans DH (1973) Rapid voltammetric method for the estimation of tocopherols and antioxidants in oils and fats. Anal Chem 45:446–449
Medeiros RA, Rocha-Filho RC, Fatibello- Filho O (2010) Simultaneous voltammetric determination of phenolic antioxidants in food using a boron-doped diamond electrode. Food Chem 123:886–891
Michalkiewicz S, Mechanik M, Malyszko J (2004) Voltammetric study of some synthetic antioxidants on platinum microelectrodes in acetic acid medium. Electroanalysis 16:588–595
Ni Y, Wang L, Kokot S (2000) Voltammetric determination of butylated hydroxyanisole, butylated hydroxytoluene, propyl gallate and tert-butylhydroquinone by use of chemometric approaches. Anal Chim Acta 412:185–193
Perrin C, Meyer L (2002) Quantification of synthetic phenolic antioxidants in dry foods by reversed-phase HPLC with photodiode array detection. Food Chem 77:93–100
Randles JEB (1948) Cathode ray polarograph. Trans Faraday Soc 44:322–327
Riber J, Fuente C, Vazquez MD, Tascon ML, Batanero PS (2000) Electrochemical study of antioxidants at a polypyrrole electrode modified by a nickel phthalocyanine complex. Application to their HPLC separation and to their FIA system detections. Talanta 52:241–252
Richard Prabakar SJ, Sriman Narayanan S (2006) Surface modification of amine-functionalised graphite for preparation of cobalt hexacyanoferrate (CoHCF)-modified electrode: an amperometric sensor for determination of butylated hydroxyanisole (BHA). Anal Bioanal Chem 386:2107–2115
Rifkin SC, Evans DH (1976) Analytical evaluation of differential pulse voltammetry at stationary electrodes using computer-based instrumentation. Anal Chem 48:2174–2179
Ruiz MA, Garcia-Moreno E, Barbas C, Pingarron JM (1999) Determination of phenolic antioxidants by HPLC with amperometric detection at a nickel phthalocyanine polymer modified electrode. Electroanalysis 11:470–474
Saad B, Sing YY, Nawi MA, Hashim N, Ali ASM, Saleh MI (2007) Determination of synthetic phenolic antioxidants in food items using reversed-phase HPLC. Food Chem 105:389–394
Thomas D, Rajith L, Lonappan L, Issac S, Girish Kumar K (2012) Sensitive determination of nitrite in food samples. Food Anal Methods 5:752–758
Tsang SC, Chen YK, Harris PJE, Green MLH (1994) A simple chemical method of opening and filling carbon nanotubes. Nature 372:159–161
Vikraman AE, Rasheed Z, Rajith L, Lonappan LA, Girish Kumar K (2013) MWCNT-modified gold electrode sensor for the determination of propyl gallate in vegetable oils. Food Anal Methods 6:775–780
Wang J, Jiang M, Lu F (1998) Electrochemical quartz crystal microbalance investigation of surface fouling due to phenol oxidation. J Electroanal Chem 444:127–132
Williams GM (1993) Inhibition of chemical-induced experimental cancer of synthetic phenolic antioxidants. In: Williams GM, Sies H, Baker GT III, Erdmann JW Jr, Henry CJ (eds) Antioxidants: chemical, physiological, nutritional and toxicological aspects. Princeton Scientific Press, Princeton, pp 202–208
Williams GM (1994) Interventive prophylaxis of liver cancer. Eur J Cancer Prev 3:89–99
Xiu LQ, Chao J, Yan-Yan S (2009) Analysis of synthetic antioxidants and preservatives in edible vegetable oil by HPLC/TOF-MS. Food Chem 113:692–700
Yanez-Sedeno P, Pingarron JM, DÌez LMP (1991) Determination of tert-butylhydroxyanisole and tert-butylhydroxytoluene by flow injection with amperometric detection. Anal Chim Acta 252:153–159
Yi H, Wu K, Hu S, Cui D (2001) Talanta 55:1205
Acknowledgments
The authors are grateful to the University Grants Commission (UGC), Council of Scientific and Industrial Research (CSIR), and Defence Research and Development Organisation (DRDO), India, for financial assistance.
Compliance with Ethics Requirements
Krishnapillai Girish Kumar has received research grants from the Defence Research and Development Organisation (DRDO), India.
Conflict of Interest
Zafna Rasheed declares that she has no conflict of interest. Anuja Elevathoor Vikraman declares that she has no conflict of interest. Divya Thomas declares that she has no conflict of interest. Jesny Siri Jagan declares that she has no conflict of interest. This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rasheed, Z., Vikraman, A.E., Thomas, D. et al. Carbon-Nanotube-Based Sensor for the Determination of Butylated Hydroxyanisole in Food Samples. Food Anal. Methods 8, 213–221 (2015). https://doi.org/10.1007/s12161-014-9894-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-014-9894-7