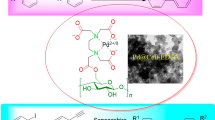
Abstract
Finely powdered diethylaminoethyl cellulose is prepared through a bottom–up synthetic method using NaOH/urea aqueous solution as solvent. Phosphotungstic acid anchored on the powdered diethylaminoethyl cellulose can be used as a biopolymer-supported solid acidic catalyst for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones from isatoic anhydride, amines, and aldehydes. The catalyst could be easily recovered by simple filtration and reused several times with minor decreases in the reaction yields.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, nearly all polymeric materials still depend on petroleum-derived monomers. Within both the scientific and industrial communities, one of the main challenges in polymer manufacture is the replacement of fossil resources as raw material by renewable resources such as biomass [1, 2]. Cellulose is the most extensively distributed renewable, biodegradable, nontoxic, non-edible, and low cost natural polymer, an almost inexhaustible raw material which has been exploited for thousands of years in the manufacture of a wide spectrum of products and materials in daily life [3]. Utilization of cellulose as new source for polymer industry is certainly benefited for environmental protection and sustainable development [4].
The use of functionalized cellulose as biomass-derived polymeric supports provides new opportunities for the design of more eco-friendly catalysts serve in greener catalysis. For example, carboxymethyl cellulose has been used as a functional support for cinchonine which acted as a recyclable catalyst for asymmetric Michael reaction [5]. Shaabani group reported the synthesis and characterization of ethylenediamine-functionalized cellulose as a novel bio-support for palladium nanoparticles, which was found to be a highly efficient heterogeneous catalyst for the Heck and Sonogashira couplings in aqueous media [6].
Diethylaminoethyl cellulose (DEAEC) is a kind of functionalized cellulose routinely used in ion exchange chromatography. As a kind of anion exchange fiber, DEAEC is widely used in the separation and purification of polysaccharides, peptides, nucleotides, and viruses [7–9], etc. However, its application as a support for the catalytic applications has not been explored.
Theoretically, DEAEC can be applied as a support for polyprotic acids due to its basic nature. In the design of heterogeneous supported catalysts, powdered supports were recognized to be superior to their bulk counterparts (e.g., fibrous ones) [10, 11]. Unfortunately, finely powdered DEAEC is commercially unavailable. Moreover, no literature was found in the preparation of powdered DEAEC from commercially available fibrous products. Powdered cellulose could be obtained from two routes: top–down process and bottom–up process. Top–down process routinely needs the aid of mechanical force. Mechanical treatment of cellulose is a simple and cheap method [12, 13], but the micronization efficiency is relatively low. Therefore, we decided to develop a bottom–up process (namely dissolution–regeneration method) to prepare powdered DEAEC.
Cellulose is difficult to dissolve in conventional solvents because of the strong hydrogen bonding and partially crystalline structure. Only a few solvents are suitable for cellulose dissolution. The most important solvents NaOH-CS2 (viscose process) and N-methylmorpholine N-oxide monohydrate (Lyocell process) have serious drawbacks such as hazardous byproducts. Some binary mixtures (e.g., LiCl/DMA) and ionic liquids could directly dissolve cellulose [14, 15], but they always meet inevitable problems such as environmental impact, high costs, and difficulty in solvent recovery [16, 17]. Compared with above-mentioned solvent system, NaOH/urea aqueous solution is a kind of more economical and environmental-friendly solvent for rapid cellulose dissolution accompanied with essentially nontoxic byproducts [18–22]. In continuation of our research on catalysts from biorenewable resources [23], we explored a bottom–up synthetic method to obtain finely powdered DEAEC using NaOH/urea aqueous solution as cellulose solvent.
In the family of heterocyclic compounds, 2,3-dihydroquinazolin-4(1H)-ones have attracted much attention as they exhibit wide range of pharmacological and biological activities [24–26]. The most convenient route to 2,3-dihydroquinazolin-4(1H)-ones includes condensation of isatoic anhydride, aldehydes, and primary amines in the presence of appropriate catalyst. A number of catalysts have been reported including amberlyst-15 [27], silica sulfuric acid [28], p-TSA [29], acetic acid [30], Ga(OTf)3 [31], KAl(SO4)2 [32], etc. However, some of these catalysts are associated with certain drawbacks such as poor recoverability and biodegrability, toxicity, and corrosivity.
With powdered DEAEC in hand, we readily prepared a novel solid supported Brønsted acid through the acid–base interaction between DEAEC and phosphotungstic acid (PTA). This material (PTA supported on DEAEC, PTA@DEAEC) was found to be an efficient catalyst for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones (Scheme 1). Results show that the green and recyclable catalyst possessed excellent catalytic performance in the synthesis of desired products in good to excellent yields. In addition, the catalyst can be easily recovered by simple filtration and reused at least 5 times without significant loss of its catalytic activity.

Results and discussion
In our investigation, NaOH/urea aqueous solution was chosen due to its inexpensive and green features. Our method involves the dissolution of DEAEC in NaOH/urea aqueous solution followed by treating in an acidic coagulation bath under vigorous stirring. It was found that the agitation intensity appears to be of a particular importance for the formation of powdered product in regeneration step. In the cases under less vigorous stirring, fibrous products were regenerated rather than powdered ones. Moreover, the use of inner baffles is also in favor of the formation of powdered products. When the viscous solution of DEAEC was dropped into a stirred acidic coagulation bath under laminar flow condition, it tends to afford fibrous products due to the regular streamline. The inner baffles block the liquid flow, changing laminar flow into turbulent flow by which the streamline was disorganized, while the mass transfer was improved remarkably.
The morphologies of DEAEC before and after dissolution–regeneration process were observed by FE-SEM (field emission scanning electron microscopy). From the images (Fig. 1a, b), it can be found that unprocessed DEAEC keeps the original morphology of fibrous raw feedstock. Large-scale and enlarged FE-SEM images of regenerated DEAEC powder reveal that the morphology of as-prepared powder was granular as well as the particles have an average diameter of 5 μm (Fig. 1c, d). It clearly demonstrates the effect in particle size reduction (micronization) by the present method.
The regenerated DEAEC powder was then treated with PTA to afford PTA@DEAEC, which was characterized with FT-IR, energy-dispersive X-ray spectroscopy (EDXS), inductively coupled plasma atomic emission spectrometry (ICP-AES), and acid–base titration. The chemical structures and functionalities of DEAEC, powdered DEAEC, and PTA@DEAEC were characterized by FT-IR spectra. In the FT-IR spectra of DEAEC and powdered DEAEC (Fig. 2a, b), the absorbance at 3100–3500 cm−1 is assigned the O–H stretching vibration, broad band at 2910 cm−1 is assigned to aliphatic C-H stretching vibration, broad band at around 1380 cm−1 is assigned to aliphatic C-N stretching vibration, and the absorption at 1115 cm−1 originates from C–O–C contributes to glycosidic linkages. The characteristic peak centered at around 1635 cm−1 is attributed to uronic acid as a consequence of mild degradation [33]. The parent PTA Keggin structure shows characteristic P-Oa of tetrahedral PO4 stretching at 1081 cm−1, W=Od stretching at 988 cm−1, stretching of W-Oc-W inter bridges between corner-sharing WO6 octahedra at 895 cm−1, stretching of W-Ob-W intrabridges between edge-sharing WO6 octahedra at 812 cm−1, and P-O bending about 596 cm−1 [34], hence these peaks indicated the existence of Keggin heteropolyacid (Fig. 2c). These results clearly show that the regenerated DEAEC powder still keeps the intrinsical properties, while PW12O40 3− Keggin units has grafted on the matrix of DEAEC.
The loading of PTA on DEAEC was further confirmed by EDXS analysis (Fig. 3). The characteristic peaks of tungsten and phosphorus were observed from EDXS spectra of PTA@DEAEC. ICP-AES analysis was carried out to determine the loading capacity of PTA supported on DEAEC. The content of tungsten was calculated as 1.59 mmol g−1. The number of H+ sites of PTA@DEAEC was also determined to be 0.94 mmol g−1 by acid–base titration. This result also matched the value estimated based on tungsten elemental analysis.
The catalytic activity of PTA@DEAEC was demonstrated by the synthesis of 2,3-dihydroquinazolin-4(1H)-ones from isatoic anhydride, aldehydes, and primary amines (or ammonium carbonate). To optimize the reaction condition, initially, the reaction of isatoic anhydride (1.0 mmol), benzaldehyde (1.0 mmol), and aniline (1.2 mmol) in 5 cm3 of ethanol was chosen as a model. The effects of catalyst amount (molar ratio) and temperature were investigated. The results of this study are summarized in Table 1. It can be seen that the best result was determined by using 8 mol % (H+) of the catalyst in reflux ethanol (Table 1 entry 5). The model reaction using PTA as a homogeneous catalyst was also conducted (Table 1 entry 9). Homogeneous PTA shown higher catalytic activity then supported PTA, however, PTA@DEAEC is superior to PTA due to its satisfactory recoverability and reusability. Blank experiment was carried out in the presence of support DEAEC and gave negative result in the test (Table 1 entry 10).
Next, we applied the optimal protocol to a range of primary amines (or ammonium carbonate as ammonia source) and aldehydes to explore the scope of this reaction for preparation of various 2,3-dihydroquinazolin-4(1H)-one derivatives. As shown in Table 2, the three-component reactions worked well using aldehydes and amines with either electron-withdrawing or electron-donating groups, while the desired compounds were obtained in satisfactory to excellent yields. The experimental results showed the electronic effects of phenyl ring substituents had only a slight influence on the yields. It could be found that the reaction is sensitive to steric hindrance. Isolated yields of ortho-substituted and meta-substituted aldehydes or aromatic amines were always little lower than non-substituted or para-substituted, especially for aldehydes (Table 2 entries c and q).
To check the reusability of the catalyst, after initial experimentation PTA@DEAEC was collected by filtration, washed with hot ethanol and subjected to a second run by charging with the same substrates (Table 3). The results of this experiment and four subsequent experiments were almost consistent in yields (75, 76, 78, 75, 74 %). Although slightly more time was required to complete the reaction in the fifth run, the yields are comparable to those seen earlier.
In conclusion, we have developed a dissolution–regeneration method for the preparation of powdered DEAEC from commercially available fibrous feedstock. A novel solid Brønsted acid was obtained through the acid–base interaction between DEAEC and PTA, which could be employed as an efficient catalyst for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones. This celluloses-derived recyclable catalyst possessed excellent catalytic performance to afford desired products in satisfactory to excellent yields. In addition, the catalyst can be easily recovered by simple filtration and reused at least 5 times without significant loss of its catalytic activity.
Experimental
All chemicals were purchased from commercial suppliers (Aldrich or Shanghai Chemical Company, China) and used without further purification. Phosphotungstic acid (99 %) was purchased from Shanghai Han Si Chemical Co. Ltd (Shanghai, China). Diethylaminoethyl cellulose (CAS: 9013-34-7; FCP, nitrogen content 14 mg/g) was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) and dried under vacuum at 80 °C for 12 h. Isatoic anhydride (97 %) was supplied by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Melting points were recorded on a Büchi B-540 apparatus. FT-IR spectra of composite were taken on a Thermo Nicolet 6700 FT-IR spectrophotometer using KBr pellets. NMR spectra were measured on a Bruker AC 400 instrument in CDCl3 using TMS as an internal standard. Mass spectra were recorded on a Shimadzu QP 1100 EX mass spectrometer with 70 eV ionization potential. The morphology of the composite was observed using a JSM-6360LV scanning electron microscope. The loading of phosphotungstic acid was determined on an Agilent 725ES inductively coupled plasma atomic emission spectrophotometer and a JSM-6360LV energy-dispersive X-ray spectrophotometer.
Preparation of powdered DEAEC
In a typical procedure, 100 g aqueous solution containing NaOH/urea/H2O of a 7:12:81 by weight ratio was pre-cooled to below −12 °C for 3 h, 3.5 g DEAEC was then added at ambient temperature under vigorous stirring. The solids completely dissolved within 30 min to afford a transparent cellulose dope. The spinning dope was spun into a coagulation bath composed of 8 wt% H2SO4/10 wt% Na2SO4 aqueous solution at about 10 °C under vigorous stirring (3000 rpm). The suspension was subjected to centrifugation (4000 rpm, 20 min), while resulted powder was washed with water (30 cm3 × 6) and dried at 80 °C for 2 h.
Preparation of powdered PTA@DEAEC
In 25 cm3 round-bottom flask with inner baffles, a mixture of 2.00 g DEAEC powder, 5.9 g PTA (2 mmol), and 15 cm3 absolute methanol was magnetically stirred for 6 h at room temperature. The supported catalyst was collected by filtration, washed with hot methanol (10 cm3 × 6), and dried at 100 °C for 2 h.
General experimental procedure for the synthesis of 2,3-dihydroquinazolin-4(1H)-one derivatives in the presence of PTA@ DEAEC
To a 25 cm3 round-bottom flask charged with isatoic anhydride (1.0 mmol), aldehyde (1.0 mmol), primary amine (1.2 mmol) or (NH4)2CO3 (0.7 mmol), and 5 cm3 ethanol was added catalytic amount of PTA@DEAEC (0.085 g equal to 0.080 mmol of H+). The mixture was refluxed for appropriate time as listed in Table 1 (monitored by TLC, ethyl acetate: petroleum ether = 1:2, v/v). After completion of the reaction, the solid catalyst was separated by filtration and washed with hot ethanol (3 cm3 × 2). The combined ethanol solution was added 2 cm3 of water and the precipitate was collected by filtration. The crude product could be purified by further recrystallization from ethanol.
2-(4-Methyl-1,2,3-thiadiazol-5-yl)-3-(4-methylphenyl)-2,3-dihydroquinazolin-4(1H)-one (4q, C18H16N4OS)
Light yellow solid; m.p.: 174–176 °C; R f = 0.33 (ethyl acetate/petroleum ether 1:2, v/v); 1H NMR (400 MHz, CDCl3): δ = 7.85 (d, J = 4.0 Hz, 1H, Ar–H), 7.25-7.29 (m, 1H, Ar–H), 7.01–7.03 (d, J = 8.0 Hz, 2H, Ar–H), 6.85–6.89 (m, 3H, Ar–H), 6.50 (d, J = 8.0 Hz, 1H, Ar–H), 6.22 (s, 1H, CH), 4.85 (s, 1H, NH), 2.22 (s, 3H, CH3), 2.14 (s, 3H, CH3) ppm; 13C NMR (100 MHz, CDCl3): δ = 161.89, 156.42, 149.07, 144.30, 138.02, 136.77, 134.55, 130.20, 129.08, 126.94, 120.92, 116.57, 116.04, 67.94, 21.08, 12.24 ppm; MS (EI, 70 eV): m/z (%) = 336.1 (M+, 10), 275.1 (18), 237.1 (100), 221.1 (12), 207.1 (15), 170.1 (17), 130 (13), 120.0 (34), 91.1 (21), 65 (11).
References
Gandini A (2011) Green Chem 13:1061
Salimi H, Rahimi A, Pourjavadi A (2007) Monatsh Chem 138:363
Schwarzinger C, Pfeifer A, Schmidt H (2002) Monatsh Chem 133:1
Rose M, Palkovits R (2011) Macromol Rapid Commun 32:1299
Yang L, Zhou D, Qu C, Cui Y (2012) Catal Lett 142:1405
Keshipour S, Shojaei S, Shaabani A (2013) Cellulose 20:973
Kulshrestha Y, Husain Q (2006) Enzyme Microb Technol 38:470
Tasdelen C, Aktas S, Acma E, Guvenilir Y (2009) Hydrometallurgy 96:253
Norris MD, Stewart BW (1988) FEBS Lett 228:223
Khalili NR, Rahimi R, Rabbani M (2013) Monatsh Chem 144:597
Javid A, Heravi MM, Bamoharram FF (2012) Monatsh Chem 143:831
Prusov AN, Zheleznov KN, Alekseeva OV, Padokhin VA, Rozhkova OV (2002) Colloid J 64:601
Clarkin S, Clesceri L (2002) Appl Microbiol Biotechnol 60:485
Heinze T, Koschella A (2005) Polímeros 15:84
Bodirlau R, Teaca C-A, Spiridon I (2010) Monatsh Chem 141:1043
Gao SS, Wang JQ, Jin ZW (2012) Carbohydr Polym 87:1020
Pinkert A, Marsh KN, Pang S, Staiger MP (2009) Chem Rev 109:6712
Cai J, Zhang L, Zhou J, Qi H, Chen H, Kondo T, Chen X, Chu B (2007) Adv Mater 19:821
Zhang L, Ruan D, Zhou J (2001) Ind Eng Chem Res 40:5923
Cai J, Zhang L, Zhou J, Li H, Chen H, Jin H (2004) Macromol Rapid Commun 25:1558
Zhang D, Xie J, Yu P, Huang X, Yang M, Liu H (2012) Cellulose 19:189
Isobe N, Noguchi K, Nishiyama Y, Kimura S, Wada M, Kuga S (2013) Cellulose 20:97
Li B, Zhang M, Yan X, Peng Y (2010) J Chin Chem Soc 57:1243
Parish HA Jr, Gilliom RD, Purcell WP, Browne RK, Spirk RF, White HD (1982) J Med Chem 25:98
Wang M, Zhang TT, Liang Y, Gao JJ (2012) Monatsh Chem 143:835
Kikuchi H, Tasaka H, Hirai S, Takaya Y, Iwabuchi Y, Ooi H, Oshima Y (2002) J Med Chem 45:2563
Surpur MP, Singh PR, Patil SB, Samant SD (2007) Synth Commun 37:1965
Salehi P, Dabiri M, Zolfigol MA, Baghbanzadeh M (2005) Synlett 7:1155
Baghbanzadeh M, Salehi P, Dabiri M, Kozehgary G (2006) Synthesis 2:344
Karimi-Jaberi Z, Arjmandi R (2011) Monatsh Chem 142:631
Chen J, Wu D, He F, Liu M, Wu H, Ding J, Su W (2008) Tetrahedron Lett 49:3814
Dabiri M, Salehi P, Otokesh S, Baghbanzadeh M, Kozehgarya G, Mohammadi AA (2005) Tetrahedron Lett 46:6123
Chen SY, Mochizuki T, Abe Y, Toba M, Yoshimura Y (2014) Appl Catal B Environ 148:344
Janik MJ, Campbell KA, Bardin BB, Davis RJ, Neurock M (2003) Appl Catal A Gen 256:51
Zhang ZH, Lü HY, Yang SH, Gao JW (2010) J Comb Chem 12:64
Darvatkar NB, Bhilare SV, Deorukhkar AR, Raut DG, Salunkhe MM (2010) Green Chem Lett Rev 3:301
Ghorbani-Choghamarani A, Taghipour T (2011) Lett Org Chem 8:470
Niknam K, Mohammadizadeh MR, Mirzaee S (2011) Chin J Chem 29:1417
Wang LM, Hu L, Shao JH, Yu J, Zhang L (2008) J Fluorine Chem 129:1139
Majid G, Kobra A, Hamed MP, Hamid Reza S (2011) Chin J Chem 29:1617
Acknowledgments
Financial support from National Key Technology R&D Program (2011BAE06B02) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, S., Zhang, Q. & Peng, Y. Powdered diethylaminoethyl cellulose as biomass-derived support for phosphotungstic acid: new solid acidic catalyst for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones. Monatsh Chem 146, 1859–1864 (2015). https://doi.org/10.1007/s00706-015-1475-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-015-1475-y