Abstract
In this work we report the fabrication of cellulose-based humidity responsive material with antifungal activity. The quaternized cellulose (QC) derivatives with low degree of substitution (DS) values of 0.08–0.37 were synthesized in NaOH/urea aqueous solution. Water insoluble QC membranes (c-QCM) were prepared by casting from QC aqueous solutions, followed by crosslinking with glutaraldehyde. The c-QCMs were disintegrated in acid solutions, but were able to keep membrane shape in neutral and mild basic solutions with pH value of 7.2 and 9.7. The equilibrium water adsorption ratios of c-QCMs were in the range of 66–98%, depending on the DS values of quaternary ammonium groups and the pH value of the aqueous solutions. The antifungal activity of QC was evaluated and found that QC could effectively inhibit the reproduction of Rhizopus stolonifer, Aspergillus flavus and Penicillium digitatum, with minimum inhibitory concentration of 5, 10, and 7.5 mg/mL, respectively. The resistivity of the c-QCM changed for about 65–134 times corresponding to the change of environmental relative humidity from 20 to 99%; and the performance of c-QCM as a resistive-type humidity responsive material was consistent in the cycling of relative humidity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulose is the most abundant natural polymer on earth. Due to its good mechanical properties, biocompatibility and biodegradability (Cunha and Gandini 2010; Schurz 1999), cellulose has been developed into many types of environmentally friendly materials such as fibers, films and powders, with wide applications in areas of textiles, filtration, packaging, food additives and cosmetics, etc. In this work, a novel application as a humidity responsive material with antifungal activity was developed for quaternized cellulose derivative.
Contamination and diseases caused by moulds are of great concern in many areas, such as food packaging, food storage, building materials and so on. The conventional antifungal agents such as sorbic acid, potassium sorbate, imazalil and sodium ortho-phenylphenate, have been broadly applied as fungicides for fruits (Mehyar et al. 2011; Cabras et al. 1999; Montesinos-Herrero et al. 2011). However, significant concerns about the impact of these small molecule antifungal agents on the human health and the environment, and low durability are remained (Montesinos-Herrero et al. 2011). Because of the lower toxicity and long antifungal activity, antifungal polymers like polymeric quaternary ammonium compounds and polymeric N-halamine have been developed. For example, polymeric N-halamines were synthesized and used in antimicrobial paints, which provided antimicrobial efficiency against bacteria, fungi, and viruses (Cao and Sun 2009). The main concerns of polymeric N-halamines are its non-biodegradability after end use and the recontamination problem by the halogen (Hogan et al. 1979).
Due to the abundance, relative low cost, few allergy problems and biodegradability, naturally-occurring polymers such as chitosan and cellulose based antibacterial agents have been attracted many research focuses, especially in the age of seeking alternative sources of fossil. For example, quaternized chitosan derivatives show strong antibacterial efficacy against E. coli and S. aureus (Sajomsang et al. 2009; Ignatova et al. 2007). Sun et al. grafted 2-amino-4-chloro-6-hydroxy-s-triazine (ACHT) and methacrylamide (MAA) onto cotton cellulose (CC) and then the ACHT-immobilized and MMA-grafted cotton fabrics were chlorinated. The functionalized CC showed effective antimicrobial activities (Chen et al. 2007a; Luo and Sun 2006). Zhou et al. demonstrated that quaternized cellulose (QC) showed antimicrobial activity against Gram-negative bacteria (E. coli) and Gram-positive (S. aureus). The minimum inhibitory concentration (MIC) values of QC with DS value of 0.74 were 0.025 and 0.0125 wt% against E. coli and S. aureus, respectively (Song et al. 2010, 2008). However, the antifungal performance of these functional cellulose and chitosan derivatives has been rarely reported. In this study, QC is developed as a novel polymeric fungicide to inhibit the reproduction of the fungi Aspergillus flavus (A. flavus), Rhizopus stolonifer (R. stolonifier) and Penicillium digitatum (P. digitatum). The QC has stable antifungal functional groups, good water solubility, biocompatibility and biodegradability (Rodríguez et al. 2001). Hence it is a very promising green antifungal agent or component in antifungal paints, food packaging and food storage.
Polymers containing quaternary ammonium groups have been fabricated into potential humidity sensor materials. Lv et al. (2009) applied UV irradiated crosslinking approach to obtain a water-resistant resistive-type humidity sensitive film of poly(dimethyl-butyl-(ethyl-methacrylate)ammonium bromide) with high sensitivity (impedance of 103–107 Ω corresponding to the humidity range of 22–97%). Another humidity sensor prepared from an interpenetrating network of poly(dimethylaminoethyl methacrylate) and poly(glycidyl methacrylate) (Li et al. 2007) displayed impedance of 103–106 Ω corresponding to a humidity range of 20–97% and quick response time (<30 s). The quaternized cellulose is a quaternary ammonium salt and thus it may also display humidity responsive ability. Compared to other quaternary ammonium cationic polyelectrolytes synthesized by a bottom-up polymerization method in organic solvents (Lv et al. 2009; Li et al. 2007), the QC is easily fabricated by introducing quaternary ammonium groups onto cellulose in NaOH/urea aqueous solution under mild conditions. The QC is crosslinked by glutaraldehyde at 25 °C to obtain a water-insoluble resistive-type humidity responsive material. In combination of the antifungal and antibacterial efficiency, humidity sensitivity, and facile synthesis in an aqueous solution, the applications of the cellulose derivative QC will definitely be expanded to more fields.
Experimental section
Materials
The following materials were used: cellulose powder (CF11, Mw: 3.46 × 104, Whatman Ltd., UK); 3-chloro-2-hydroxypropyltrimethylammonium chloride powder (CHPTAC, Shanxi Dasheng Chemical Co., Ltd., Shanxi, China) was used without further purification; glutaraldehyde (25 wt% aqueous solution, Tianjin Fuchen Chemical Agent Co., Ltd., Tianjin, China); dialysis bag (MWCO: 7000, Shanghai Green Bird Science and Technology Development Co., Ltd., Shanghai, China). The reagents were analytical grade and used as received. The fungi A. flavus, R. stolonifer and P. digitatum were kindly provided by College of Life Science of Fujian Normal University, Fujian, China.
Quaternized cellulose derivatives by chemical modification
Cellulose/NaOH–urea–H2O solution was prepared according to the method reported by Zhang et al. (Cai and Zhang 2005a, b). Briefly, the solvent NaOH/urea aqueous solution (NaOH:urea:H2O = 7:12:81 by weight) was pre-frozen to −12 °C. Cellulose powder was swiftly added into it, followed by vigorous stirring for 10 min to get a transparent 5 wt% cellulose/NaOH–urea–H2O solution. Then CHPTAC was introduced into this solution. The quaternization reaction was performed at 19 °C for 24 h under stirring. The mixture was neutralized with 5 wt% HCl solution before filled into dialysis bags, and then dialyzed against distilled water for 6 days. The solution was collected and concentrated with a vacuum rotatory evaporator. Finally, the solution was freeze-dried with a lyophilizer (FD-1C-50, Shanghai Boyikang Experimental Instrument Co. Ltd., Shanghai, China). By adjusting the mole ratio of CHPTAC to anhydroglucose (AHG) unit of cellulose, five QCs were prepared (Table 1). The chemical structure of QC is shown in Scheme 1.
Preparation of crosslinked QC membranes
0.5 g of QC was added into 14.5 g of distilled water to make a 3.3 wt% QC aqueous solution. The solution was cast on a glass plate, and was air-dried at 26 °C to obtain the QC membrane (QCM).
To fabricate a crosslinked QCM, the QCM was immersed into a 7 wt% glutaraldehyde methanol solution with a composition: 7 wt% glutaraldehyde, 1 wt% hydrochloric acid, and 92 wt% methanol. The crosslinking reaction was performed at 25 °C for 4 h on a shaking bath. Then the QCM was thermally treated in an oven at 50 °C for 10 h to obtain the crosslinked QC membrane coded as c-QCM. The c-QCM was rinsed with distilled water to completely remove the uncrosslinked QC and the residual reactants. It was then dried in a 80 °C vacuum oven for 8 h, and stored in a desiccator before use.
Characterizations
13C-NMR of QC in D2O was recorded on an AVANCE III spectrometer at 25 °C operating at 500 MHz. 1H-NMR of QC in D2O was carried out on a Bruker-ARX 500 spectrometer at 22 °C operating at 400 MHz. Thermal gravimetric analysis (TGA) was performed on a TA5200/MDSC291 thermogravimetric analyzer at a heating rate of 10 °C min−1 from 30 to 600 °C in a nitrogen atmosphere. FTIR spectra in KBr form were performed on a Nicolet 5700 fourier transform infrared spectrometer. Nitrogen and carbon contents of QC and natural cellulose were measured on an elemental analyzer (Vario EL III, Hanau, Germany). The degree of substitution (DS) value of quaternary ammonium group of QC was calculated according to the following equation:
where N% is the nitrogen content of QC. The DS values are listed in Table 1.
Antifungal activity of QC
The fluid nutrient medium method was used to study the antifungal efficiency of QC. Firstly, a potato was peeled and shredded. 150 g of potato fragments were put into 500 mL distilled water and boiled for 2 h. The supernatant was collected by filtration. Into it 5 g of glucose was added and the solution was boiled for 1 h. The fluid nutrient medium (FNM) was obtained. Secondly, into FNM, varied volume of 1 wt% QC aqueous solution was added to reach QC concentration of 2.5, 5.0, 7.5, and 10.0 mg/mL. In such a way, the antifungal fluid nutrient medium (AFNM) was obtained. Thirdly, 20 μL of 1–2 × 104 colony-forming unit (CFU)/mL fungal aqueous suspension was added into 1.5 mL finger tub, followed by adding AFNM to reach a total volume of 1.5 mL. After inoculation, the fungal was incubated at 26.5 °C for 30 h in an incubator (Valencia-Chamorro et al. 2008; Clausen and Yang 2007). Inoculation of 1–2 × 104 CFU/mL fungal aqueous suspensions in FNM was run as blank control. After a certain time interval of incubation, the fungal proliferation on FNM was observed on a light microscopy(OLYMPUSBX51-DP70). From the hyphae filament density on the FNM, the antifungal efficiency can be determined. MIC is defined as the lowest QC concentration required to inhibit the reproduction of a fungal, at this concentration no obvious hyphae filaments are observed. The colonies were counted and the MIC values were obtained. Each sample was tested in triplicates.
Water absorption ratio of c-QCMs
The pre-weighted c-QCMs were soaked in pH = 7.2 and 9.7 aqueous solutions. After certain time intervals, the c-QCMs were taken out and the water on the surface was removed by filter papers, then the membranes were re-weighted. The water absorption ratio in acid solution was not measured because the acetal structure, which is formed between the hydroxyl groups of cellulose and the aldehyde groups of glutaraldehyde, can be easily hydrolyzed. The water absorption ratio (W a ) was calculated according to the following equation.
where m b and m a are the weight of c-QCMs before and after soaking, respectively.
Measurement of humidity response of c-QCM
The impedance response of c-QCM to relative humidity (RH) were measured on an electrochemical workstation (CHI660C, Shanghai Chenhua instrument Co. Ltd., Shanghai, China) at 0.05 V and 964 Hz. The setup is shown in Scheme 2. A cycle of adsorption (RH increases from 20 to 99%) and desorption (RH decreases from 99 to 20%) was performed to evaluate the consistent responding behavior of c-QCM. The humidity was controlled by saturated salt solutions in their equilibrium state in a sealed 1,000 mL jar. For example, saturated MgCl2 and Na2SO4 aqueous solutions provide 33 and 99% RH, respectively. The humidity was measured with a commercial hygrometer. All measurements were carried out at 17 ± 0.5 °C. The resistivity of c-QCM was calculated according to the following equation:
where R, w, t, l are the effective resistance, width, thickness and length of c-QCM membranes, respectively.
Results and discussion
Structure analysis
Figure 1 shows a typical 1H-NMR spectrum of QC-5 in D2O. Its signals were labeled: δ 2.96 ppm: protons of C2 on AHG rings; δ 3.15 ppm: protons of –CH3 connected to N+ (–N+(CH3)3); δ 3.28–4.44 ppm: the other protons on AHG rings (Burchard 2003; Heinze et al. 2003). Figure 2 displays a typical 13C-NMR spectrum of QC-5 in D2O. Its peaks were assigned as follows. δ 54.2 ppm: C10; δ 73.0 ppm: C7; δ 68.1 ppm, C9; δ 65.0 ppm: C8; δ 59.7 ppm: C6. The primary hydroxyl groups bearing quaternary ammonium groups would exhibit a new signal at δ 68.1 ppm (C6′), which was overlapped by the signal of C9 (Wang et al. 2010; Song et al. 2008). The peaks at 102.8 and 102.1 ppm were assigned to C1 and C1′, respectively (Zhang et al. 2010). The latter represented the substitution of C2–OH adjacent to C1. The remaining carbon atoms on AHG rings were presented in the range of 70–85 ppm. Therefore, the NMR spectrum confirmed that a quaternized cellulose derivative was synthesized.
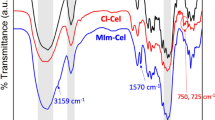
Figure 3 shows FTIR spectra of cellulose and QCs. The peak at 3,400–3,500 cm−1 was the stretching vibration of –OH. The stretching vibration of –CH2– positioned at 2,901, 1,372 and 1,058 cm−1 (Heinze et al. 2003). Compared to native cellulose, QCs displayed two new peaks at 1,481 and 1,417 cm−1. The former was assigned to the methyl groups of the substituted quaternary ammonium, while the latter was attributable to the stretching vibration of C–N (Kačuráková et al. 1994), further confirming the successful introduction of quaternary ammonium groups onto cellulose molecules.
Thermal stability
Figure 4 shows the thermogravimetric (TG) and derivative thermogravimetric (DTG) curves of native cellulose and QCs. Cellulose underwent ~5% weight loss in the range of 25–300 °C, which was caused by the evaporation of absorbed moisture. It decomposed rapidly at 365.4 °C. QCs lost ~10% weight in temperature range of 25–225 °C, which was also due to the moisture loss. The sharp weight loss of QCs occurred at ~295 °C, which was ~70 °C less than that of native cellulose, suggesting that QCs were less thermal stable. Figure 4 also indicates that the residue of QCs was more than that of cellulose. This is because the carbon content of QC is higher than that of cellulose (Table 1), resulting in the generation of more char during thermal degradation in the TG measurement.
Antifungi activity of QC
The antifungi activity of QC-5 against R. stolonifer, P. digitatum and A. flavus is shown in Fig. 5. In Fig. 5a0, b0 and c0, the hyphae were dense and long. The amount and length of fungi hyphae filaments substantially decreased with increasing QC-5 concentration. Therefore, QC-5 displayed effective antifungal activity against R. stolonifer, P. digitatum and A. flavus. The hyphae filaments disappeared in Fig. 5a2, b4 and c3. Thus the MIC of QC-5 were 5, 7.5 and 10 mg/mL against R. stolonifer, P. digitatum and A. flavus, respectively. Among the three fungi, the QC-5 exhibited the most effective inhibition against R. stolonifer. This is attributed to the different structure of their hyphae. The reproduction of these filamentous fungi relies mainly on asexual or sexual spores, which are produced by all kinds of fungi. The three fungi have the same composition of hyphae cell wall, but the hyphae of R. stolonifer is formed by one cell, and that of A. flavus and P. digitatum are formed by multiple cells (Alexopoulos et al. 1996). The QC molecules enter the hyphae cells and destroy them to inhibit spore reproduction. Therefore, less QC is needed to destroy the hyphae cells of R. stolonifer, while higher concentrations of QC are necessary for the killing of the hyphae cells of A. flavus and P. digitatum. The measurements of the antifungi activities of the other QCs with lower DS values demonstrated that their MIC values were much higher than that of QC-5.
Similar to the antimicrobial mechanism of quaternary ammonium compounds (Franklin and Snow 1981), the antifungal mechanism of QC compounds is supposed to be triggered by the adsorption of QC onto the fungal cell walls under the attracting forces such as electrostatic force (positive QC-negative fungal pairs), hydrogen bonding (QC-fungal proteins pairs) and hydrophobic interaction (QC-fungal proteins pairs). This binding to the cytoplasmic membrane blocks the permeable channels for the mass transportation therefore inhibiting the reproduction of fungal cells. It has been proved that high positive charge density on the antibacterial polymers may enhance the driving force for their binding to cytoplasmic membrane, and hence strengthening their antibacterial activity (Chang et al. 2010). This well explains that QC-5 with the highest DS of quaternary ammonium group shows the best antifungi activity. With respect to the renewability and biodegradability, QC based fungicide would be more favorable over petroleum based ones like quaternary ammonium polystyrenes (Chen et al. 2007b).
Water absorption ratio of c-QCM
QC is a water soluble cationic polyelectrolyte. The cross-linking by glutaraldehyde makes it water insoluble, but it still swells in aqueous solutions because of the presence of quaternary ammonium and hydroxyl groups on its molecules. Figure 6 shows the water absorption ratio of c-QCMs as a function of time in solutions of pH = 7.2 and 9.7. It took 100–280 min to reach equilibrium water absorption, which highly depended on the DS values of the quaternary ammonium group and pH values of the solutions. For example, in solution of pH = 9.7, the equilibrium water absorption time was 280, 150 and 100 min for c-QCM-2 (DS = 0.18), -3 (DS = 0.25), and -5 (DS = 0.37), respectively, suggesting c-QCM with higher DS showed shorter equilibrium absorption time. In both solutions, the water absorption ratio increased with increasing DS values. The equilibrium water absorption ratio of c-QCM-2, 3, 5 were 77.3, 84.6, 98.3%, in pH = 7.2 solution, and were 66.4, 72.1, 96.4% in pH = 9.7 solution, respectively. However, they deceased with increasing pH values for the c-QCM with same DS value. This should be because the shielding effect of OH− anions weakens the electrostatic repulsion among N+ cations of quaternary ammonium groups in the QC molecular chains, resulting in less swelling ability. The durability (swelling without disintegration) of cross-linked QC membranes in neutral and basic environments makes them a promising humidity responsive material because of the presence of quaternary ammonium groups.
Humidity sensitivity of c-QCM
Figure 7 shows the effect of DS value of c-QCM on the resistivity response to the humidity. It can be seen that the resistivity of the c-QCMs changed about 65–134 times over a wide RH range (20–99%), depending on the DS values. For instance, the resistivity is: c-QCM-2 (DS = 0.18): 1,644.4 and 19.8 Ωm; c-QCM-3 (DS = 0.25): 695.7 and 5.19 Ωm; c-QCM-5 (DS = 0.37): 189.2 and 2.9 Ωm, in RH of 20 and 99%, respectively. The results indicate that the c-QCMs are sensitive to humidity. For all three c-QCMs, no obvious hysteresis is shown for the two curves of adsorption and desorption processes, suggesting that the performance of c-QCM as an impedance-type humidity responsive material is stable in the RH range of 20–99%. When the DS value increased from 0.18 to 0.37, the resistivities of c-QCM decreased greatly at the same RH. The results are due to the following reasons. In the c-QCM membranes, the chloride ions and ammonium salt units contribute to the ionic conduction in humidified environments (Lv et al. 2009), so the resistivity of c-QCM membranes is highly dependent on the amount of chloride ions and ammonium salt units. The QC with higher DS value contains more Cl− and N+ ions, leading to lower resistivity.
The resistivity responses of c-QCM membranes with time are shown in Fig. 8, when the RH changed from 33 to 20%. The resistivity increased rapidly in the initial 6 h, and then almost steadied thereafter. Although c-QCM-2 displayed the highest resistivity of 1,644.4 Ωm at RH of 20%, its resistivity response style was similar to c-QCM-3 and c-QCM-5 with lower resistivities. This is because the structure of c-QCM-2 is more robust than the others. Compared to c-QC-3 and c-QC-5, the c-QC-2 has the lowest DS value of 0.18, and hence the highest amount of hydroxyl groups is remained, resulting in the strongest hydrogen bonding (a type of crosslinking). This would largely restrict the movement of QC chains in response to the change of RH. Both the concentration of ions and the crosslinking degrees show great effects on the humidity sensing properties of polyelectrolyte based humidity sensors (Gong et al. 2002; Lee et al. 2003, 2004). Obviously, the resistivity responses of all the c-QCMs were slow. This is highly due to the low amount of ammonium salt units on the c-QCMs. Lv et al. (2009) reported a polyelectrolyte humidity with an ammonium salt unit in each repeating unit showed a fast response time of ∼9 and 32 s for adsorption and desorption between 33% RH and 97% RH, respectively. The study to increase DS values of quaternary ammonium group on the QC chains is ongoing to try to improve the resistivity response of c-QCM. It should be pointed out that many other polyelectrolyte based humidity sensors are prepared by thermal cross-linking at high temperature (usually over 100 °C) and long time (over 10 h) in order to achieve water durability (Lee et al. 2003), whereas our c-QCM was crosslinked at 25 °C for 4 h. Thus the fabrication of c-QCM is more cost- and energy-saving.
Conclusion
The quaternized cellulose (QC) derivatives with low DS values in the range of 0.08–0.37 were synthesized in NaOH/urea aqueous solution. The QC samples displayed effective antifungal activities against R. stolonifer, P. digitatum and A. flavus, with minimum inhibitory concentration of 5, 10 and 7.5 mg/mL, respectively. QCs were crosslinked with glutaraldehyde to render them water-resistant. The membrane morphology of crosslinked QC membranes was maintained in neutral and mild basic solutions, but their water adsorption ratios were up to 166–198% of their original weight. The equilibrium water adsorption ratios were highly dependant on the DS values of the quaternary ammonium group and the pH values of the aqueous solutions. The crosslinked QC membranes were fabricated into resistive-type humidity responsive materials. They showed stable performance and high sensitivity to the environmental humidity. For example, the impedance of QC (DS = 0.25) decreased from 695.7 to 5.2 Ωm as the humidity increased from 20 to 99%. Due to the low DS value of quaternary ammonium groups, the current QCs showed slow response.
References
Alexopoulos CJ, Mims CW, Blackwell M (1996) Introductory mycology, 4th edn. Wiley, New York
Burchard W (2003) Solubility and solution structure of cellulose derivatives. Cellulose 10(3):213–225
Cabras P, Schirra M, Pirisi FM, Garau VL, Angioni A (1999) Factors affecting imazalil and thiabendazole uptake and persistence in citrus fruits following dip treatments. J Agric Food Chem 47(8):3352–3354
Cai J, Zhang L (2005a) Rapid dissolution of cellulose in LiOH/urea and NaOH/urea aqueous solutions. Macromol Biosci 5(6):539–548
Cai J, Zhang L (2005b) Unique gelation behavior of cellulose in NaOH/urea aqueous solution. Biomacromolecules 7(1):183–189
Cao Z, Sun Y (2009) Polymeric N-Halamine latex emulsions for use in antimicrobial paints. ACS Appl Mater Inter 1(2):494–504
Chang H-I, Yang M-S, Liang M (2010) The synthesis, characterization and antibacterial activity of quaternized poly(2,6-dimethyl-1,4-phenylene oxide)s modified with ammonium and phosphonium salts. Reac Funct Polym 70(12):944–950
Chen Y, Wang L, Yu H, Shi Q, Dong X (2007a) Synthesis, characterization, and antibacterial activities of novel N-halamine copolymers. J Mater Sci 42(11):4018–4024
Chen Z, Luo J, Sun Y (2007b) Biocidal efficacy, biofilm-controlling function, and controlled release effect of chloromelamine-based bioresponsive fibrous materials. Biomaterials 28(9):1597–1609
Clausen CA, Yang V (2007) Protecting wood from mould, decay, and termites with multi-component biocide systems. Int Biodeter Biodegr 59(1):20–24
Cunha A, Gandini A (2010) Turning polysaccharides into hydrophobic materials: a critical review. Part 1. Cellulose. Cellulose 17(5):875–889
Franklin TJ, Snow GA (1981) Biochemistry of Antimicrobial Action. Chapman & Hall, London, p 58
Gong MS, Joo SW, Choi BK (2002) Humidity sensor using mutually reactive copolymers containing quaternary ammonium salt and reactive function. Sensor Actuat B Chem 86(1):81–87
Heinze T, Liebert TF, Pfeiffer KS, Hussain MA (2003) Unconventional cellulose esters: synthesis, characterization and structure-property relations. Cellulose 10(3):283–296
Hogan M, Chi P, Hoel D, Mitchell T (1979) Association between chloroform levels in finished drinking water supplies and various site-specific cancer mortality rates. J Environ Pathol Toxicol 2(3):873
Ignatova M, Manolova N, Rashkov I (2007) Novel antibacterial fibers of quaternized chitosan and poly (vinyl pyrrolidone) prepared by electrospinning. Eur Polym J 43(4):1112–1122
Kačuráková M, Ebringerová A, Hirsch J, Hromádková Z (1994) Infrared study of arabinoxylans. J Sci Food Agric 66(3):423–427
Lee C-W, Kim Y, Joo S-W, Gong M-S (2003) Resistive humidity sensor using polyelectrolytes based on new-type mutually cross-linkable copolymers. Sensor Actuat B Chem 88(1):21–29
Lee C-W, Choi B-K, Gong M-S (2004) Humidity sensitive properties of alkoxysilane-crosslinked polyelectrolyte using sol–gel process. Analyst 129(7):651–656
Li Y, Chen Y, Zhang C, Xue T, Yang M (2007) A humidity sensor based on interpenetrating polymer network prepared from poly (dimethylaminoethyl methacrylate) and poly(glycidyl methacrylate). Sensor Actuat B Chem 125(1):131–137
Luo J, Sun Y (2006) A cyclic N-halamine-based fibrous materials: preparation, characterization, and biocidal functions. J Polym Sci Pol Chem 44(11):3588–3600
Lv X, Li Y, Li P, Yang M (2009) A resistive-type humidity sensor based on crosslinked polyelectrolyte prepared by UV irradiation. Sensor Actuat B Chem 135(2):581–586
Mehyar GF, Al-Qadiri HM, Abu-Blan HA, Swanson BG (2011) Antifungal effectiveness of potassium sorbate incorporated in edible coatings against spoilage molds of apples, cucumbers, and tomatoes during refrigerated storage. J Food Sci 76(3):M210–M217
Montesinos-Herrero C, Smilanick JL, Tebbets JS, Walse S, Palou L (2011) Control of citrus postharvest decay by ammonia gas fumigation and its influence on the efficacy of the fungicide imazalil. Postharvest Biol Technol 59(1):85–93
Rodríguez R, Alvarez-Lorenzo C, Concheiro A (2001) Rheological evaluation of the interactions between cationic celluloses and carbopol 974P in water. Biomacromolecules 2:886–893
Sajomsang W, Tantayanon S, Tangpasuthadol V, Daly WH (2009) Quaternization of N-aryl chitosan derivatives: synthesis, characterization, and antibacterial activity. Carbohyd Res 344(18):2502–2511
Schurz J (1999) ‘Trends in polymer science’: a bright future for cellulose. Prog Polym Sci 24(4):481–483
Song Y, Sun Y, Zhang X, Zhou J, Zhang L (2008) Homogeneous quaternization of cellulose in NaOH/urea aqueous solutions as gene carriers. Biomacromolecules 9(8):2259–2264
Song Y, Zhang J, Gan W, Zhou J, Zhang L (2010) Flocculation properties and antimicrobial activities of quaternized celluloses synthesized in NaOH/urea aqueous solution. Ind Eng Chem Res 49(3):1242–1246
Valencia-Chamorro SA, Palou L, del Rio MA, Perez-Gago MB (2008) Inhibition of penicillium digitatum and penicillium italicum by hydroxypropyl methylcellulose–lipid edible composite films containing food additives with antifungal properties. J Agric Food Chem 56(23):11270–11278
Wang Z-M, Xiao K-J, Li L, Wu J-Y (2010) Molecular weight-dependent anticoagulation activity of sulfated cellulose derivatives. Cellulose 17(5):953–961
Zhang K, Brendler E, Fischer S (2010) FT Raman investigation of sodium cellulose sulfate. Cellulose 17(2):427–435
Acknowledgments
This work is supported by the National Basic Research Program of China (2010CB732203), the National Natural Science Foundation of China (No. 50973019, 50843030), the Natural Science Foundation of Fujian Province (2010J06017).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, D., Xie, J., Yu, P. et al. Antifungal activity and humidity sensitivity of quaternized cellulose synthesized in NaOH/urea aqueous solution. Cellulose 19, 189–198 (2012). https://doi.org/10.1007/s10570-011-9626-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-011-9626-8