Abstract
Previous metagenomic analysis indicated that numerous insect viruses exist in bat guano. In this study, we isolated a novel double-stranded RNA virus, a tentative member of the family Totiviridae, designated Tianjin totivirus (ToV-TJ), from bat feces. The virus is an icosahedral particle with a diameter of 40-43 nm, and it causes cytopathic effect in Sf9, Hz, and C6/36 cell lines. Full-length genomic sequence analysis showed that ToV-TJ shares high similarity with the totivirus OMRV-AK4, which was recently isolated from mosquitoes in Japan. The full-length genome of the ToV-TJ was 7611 bp and contained two predicted non-overlapping open reading frames (ORFs): ORF1, encoding the capsid protein (CP), and ORF2, encoding an RNA-dependent RNA polymerase. Bioassay of ToV-TJ by feeding on the larvae of Spodoptera exigua and Helicoverpa armigera (Hubner) suggests that this virus is not infectious for these two larvae in vivo. Sequences similar to that of ToV-TJ have been detected in bat feces sampled in Yunnan and Hainan Provinces, suggesting that this virus is widely distributed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bats are the only flying mammals. They represent one of the most diverse groups of mammals on earth and play important roles in many ecosystems [18]. More than two thirds of bat species hunt insects and serve as pest control. Nectar-feeding bats are critical pollinators for a wide variety of plants and seed dispersers [1, 13]. In recent years, bats have been identified as natural reservoirs of a number of emerging viral pathogens that have caused disease outbreaks in humans [2, 17]. Metagenomic analysis has also indicated that bats carry a large diversity of viruses that are able to infect bacteria, fungi, insects, plants, and vertebrates [3, 10]. In our previous studies, we analyzed the virome in feces collected from mixed insectivorous bat populations and found a large proportion of genomic sequences that are homologous to those of insect viruses (unpublished data). These results indicated that bat feces may be a rich source of insect viruses.
In this study, we used insect cell lines to isolate a virus from Myotis ricktti bat feces, and this virus, which was designated Tianjin totivirus-like virus (ToV-TJ), tentatively belongs to the family Totiviridae. The genomic sequence of this virus shares high similarity with a recently characterized novel totivirus, Omono River virus (OMRV), which was isolated from mosquitoes in Japan [9]. Characterization of ToV-TJ showed that it could infect a variety of insect cell lines and may have a wide geographical distribution.
Materials and methods
Sample collection
Bat fecal samples were collected from six locations: Tianjin city in 2010 (10TJ), Xianning city in Hubei Province in 2009 and 2010 (09 XN and 10XN), Xiangyun County in Yunnan Province in 2009 (09XY), Haikou in Hainan Province in 2007 (07HK), and Yuncheng in Shanxi Province in 2006 (06YC). The dominant bat species included Myotis ricketti for 10TJ and 06YC, Scotophilus kuhli for 07HK, Hipposideros for 09 XN and 09XY, Rhinolophus and Myotis 10XN. To collect bat fecal sample, clean plastic sheets measuring 2.0 × 2.0 m were placed under known bat roosting sites before sunset (6:00 pm). Relatively fresh fecal samples were collected the next morning (5:30-6:00 am) and stored in liquid nitrogen as soon as possible. Samples were transported to the laboratory and stored at –80 °C [11].
Cell cultures
The cell lines used in this study were Sf9, HzAM1 and C6/36 from insects, Vero and BK (Myotis davidii primary kidney cells) from mammals. The Sf9 and HzAM1 cell lines were cultured in Grace’s insect medium, the C6/36 cell lines in 60% Dulbecco’s modified Eagle medium (DMEM) and 30% RPMI medium 1640, the Vero cell lines in DMEM, and the BK primary cells in RPMI medium 1640. All media were supplemented with 10% FBS. The insect and mammal cell lines were grown at 28°C and 37°C, respectively, in a humidified 5% CO2 atmosphere. The isolation process was described previously [11]. Briefly, an aliquot of 100 mg feces was homogenized with the addition of 500 μL phosphate-buffered saline (PBS) and centrifuged at 5,000 × g (Sigma 1-15, Germany) for 5 min. The supernatant was diluted 1:10 in PBS and filtered through a 0.45-μm filter (Millipore, USA). An aliquot of 200 μL diluted supernatant was mixed with 20 U penicillin and 20 U streptomycin and added to Sf9 cells in a 24-well plate. After incubation at 28 °C for 1 h, the inoculum was removed and replaced with fresh Grace’s medium supplemented with 2% fetal bovine serum (FBS). Cell cultures were observed daily for cytopathic effect (CPE). Culture media collected after at least three blind passages were used as viral stocks and stored at –80 °C.
Virus isolation, purification, and examination by electron microscopy
Infected cells were harvested in cultured medium at 72 h postinfection, when strong CPE appeared. After three freeze-thaw cycles, lysates were clarified by centrifugation at 3,000 × g for 5 min and filtered through a 0.45-μm filter (Millipore). Viruses in the supernatant were purified by ultracentrifugation through a 30% (w/v) sucrose cushion in PBS at 184,000 × g for 2 h using a Ty70 rotor (Beckman). The pelleted viruses were resuspended in 200 μL PBS and stored in aliquots at −80 °C. Purified viruses were checked by electron microscopy using Formvar resin and carbon-coated copper grids (200 mesh), negatively stained with 2% phosphotungstic acid (pH 7.0), and examined at 200 kV in a Tecnai transmission electron microscope.
Full genome sequencing
A procedure to amplify the unknown viral genome (covering DNA and RNA viruses) was employed [5, 11]. Briefly, purified virus was treated with 1.4 ng ribonuclease A (Takara, China) and 40 U RQ1 DNase (Promega, USA) in a total volume of 140 μL at 37 °C for 30 min. The viral nucleic acid was extracted using a QIAamp Viral RNA Mini Kit (QIAGEN, Germany) according to the manufacturer’s protocol. Viral cDNA synthesis was performed by incubation of the extracted viral nucleic acid at 90 °C for 5 min, followed by quenching on ice for 2 min. A 20-μL reaction mixture containing the following was prepared: 10 pmol universal primer-dN6 (5′-GCCGGAGCTCTGCAGAATTCNNNNNN-3′) [5], 10 μL denatured nucleic acid, 0.6 mM each deoxynucleoside triphosphate, 20 U RNase inhibitor, and 200 U M-MLV reverse transcriptase (Promega). To synthesize second-strand cDNA/DNA, the reaction mixture was boiled for 2 min and rapidly cooled on ice, and this was followed by incubation at 37 °C for 30 min in the presence of 5 U Klenow fragment (New England Biolabs, USA) and 10 pmol universal primer-dN6. Polymerase chain reaction (PCR) was conducted using a universal primer (5′-GCCGGAGCTCTGCAGAATTC-3′). PCR products >500 bp were purified using an E.Z.N.A Gel Extraction Kit (Omega Bio-Tek, USA) and cloned into pGEM-T Easy Vector (Promega) after adding adenine (A) to the 3′-terminus of the PCR products using Taq polymerase. Recombinant plasmids were sequenced using the M13 forward and reverse primers, and the product was analyzed on an ABI Prism 3730 DNA analyzer (Applied Biosystems, USA). The full-length sequence of the viral genome was completed by PCR using specific primers designed based on the sequences obtained by random PCR. The 5′- and 3′-end sequences of the genome were obtained using a 5′-Full RACE kit (Takara). Routine sequence analysis was performed using DNAStar (DNAStar, USA). Identification of open reading frames (ORFs) was performed by translated BLAST search (BLASTX; http://www.ncbi.nlm.nih.gov/blast/Blast.cgi). Protein comparisons were also performed using BLAST results.
Cell susceptibility study
The 50 % tissue culture infectious dose (TCID50) was determined as descried previously [16]. The susceptibility of cell lines to the isolated virus was tested with HzAM1, C6/36, BK and Vero cells in 24-well plates. Approximately 1.2 × 105 cells per well were inoculated with 8000 TCID50 of the newly isolated ToV-TJ. After 1 h adsorption, the inoculum was removed and replaced with fresh medium containing 2% FBS. Cell cultures were checked daily for CPE. Infection was further confirmed by PCR using primers derived from the viral genomic sequence. Amplicons of expected size were sequenced for further identification.
Bioassay
Early second-instar healthy larvae of Spodoptera exigua and Helicoverpa armigera (Hubner) were used for virus infection. The infection experiments were divided into four groups: one negative group and three infected groups. The larvae were kept under starvation treatment overnight at 27 °C in a light incubator (LRH-250-G, China) and then transferred to a large plate and fed droplets consisting of 4% sucrose, 1 mg/mL food blue and diluted virus preparation. The virus concentrations for the four groups were 0, 6, 12 and 60 TCID50/μL, respectively, which were determined based on the original titer of viruses and the volume consumed by each individual insect. The number of H. armigera (Hubner) in each group was 22, 59, 67 and 61, respectively. The number of S. exigua larvae in the infected group was 40, while the negative group contained 20. The larvae became blue upon feeding on the inoculum and were considered to be inoculated with the virus. Larvae were observed daily.
Geographical distribution of the ToV-TJ
Specific primers for ToV-TJ, TJ-CP-F1 (5′- ATCCAGCTACGTGGAACAAGGT-3′), TJ-CP-R1 (5′-AAGTAGCTGCTGTTGTCAGC-3′), TJ-RDRP-F1 (5′-TCCAAGAGATTGCGCTCAAAGG-3′), and TJ-RDRP-R1 (5′-GCTGTGTAAGTGGTAGCTTAC-3′) were designed and used for PCR detection. PCR products of expected size were confirmed by sequencing.
Phylogenetic analysis
The sequence alignment was performed using ClustalX [20]. Phylogenetic trees were constructed using the neighbor-joining (NJ) method using the pairwise deletion parameter and the P-distance model with 1000 bootstrap replicates in the MEGA program (version 4.1) [19]. Putative members of the family Totiviridae include penaeid shrimp infectious myonecrosis virus (IMNV, YP_529549.1), Armigeres subalbatus virus SaX06-AK20 (AsTV, YP_003934934.1), Drosophila melanogaster totivirus SW-2009a (DTV, YP_003289293.1), piscine myocarditis virus AL V-708 (PMCV, YP_004581250), Omono River virus-AK4 (OMRV, BAJ21511.1) and OMRV-Y61 (BAJ21513.1). Helminthosporium victoriae virus 190S (NP_619670.2), a member of the genus Victorivirus, was used as un outgroup.
Results
Isolation and identification of an unknown virus
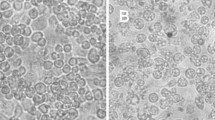
The infectious agent isolated from bat feces collected in Tianjin induced a severe CPE in Sf9 cells at the ninth day of the third passage of infection (Fig. 1B). The infected cells shrank, disintegrated and formed plaques. The purified viral particles were observed by electron microscopy, in which they showed icosahedral symmetry and has a diameter of 40-43 nm (Fig. 2A). The virus was able to cause evident CPE in HzAM1 cells (Fig. 1D) and C6/36 cells (Fig. 1F) but not in Vero and BK cells (data not shown). PCR results further confirmed virus replication in HzAM1 and C6/36 cells. Interestingly, the virus morphology differed for infections in the cell lines Sf9 and HzAM1, where a high ratio of empty particles was produced in Sf9 (Fig. 2A) compared with HzAM1 (Fig. 2B). This suggests that the Sf9 cells may not be suitable for ToV-TJ replication and packaging.
Sequence analysis of the ToV-TJ genome
Random-PCR and sequencing results indicated that the viral genomic sequence had a high nucleotide sequence similarity to OMRV-AK4 (93%), which was recently isolated from mosquitoes in Japan [9]. The virus isolated in the current study was designated Tianjin Totovirus (ToV-TJ) after the sampling location. The genome of the ToV-TJ was 7611 bp in length (GenBank accession number: JN391187) and contained two non-overlapping ORFs. The 5′-proximal ORF (ORF1, nt 7-5064) encoded a polypeptide of 1685 amino acids (aa), while the 3′-proximal ORF (ORF2, nt 5319-7535) encoded the RNA-dependent RNA polymerase (RdRP) and was 39 aa shorter than ORF2 of the OMRV-AK4 at the N-terminal end (ORF2, nt 5202-7535). The 5′ and 3′ untranslated regions of ToV-TJ were 6 and 79 nt long, respectively. The putative ORF1 contains two 2A or 2A-like sequence motifs (EGVEPNPGP) at aa positions 88-96 and 500-508, that are identical to those in OMRV-AK4. A dsRNA-binding domain between aa 404 and 474 was found in ORF1 of the ToV-TJ genome. The first predicted start codon (AUG, 5319-5321) in ORF2 of ToV-TJ was found 255 nt downstream of the ORF1 stop codon, suggesting a potential ribosomal -1 translational frameshift in this virus. A heptamer UUUUUUA was found at nt 4893-4899, followed by a pseudoknot (4900-4959) (http://bibiserv.techfak.uni-bielefeld.de/knotinframe/) in the ToV-TJ genomic sequence.
Bioassay in insects
The infected larvae of S. exigua and H. armigera (Hubner) showed no symptoms of disease until they became pupae. Likewise, PCR did not show any evidence of infection in these insects. These results indicated that ToV-TJ could not infect S. exigua or H. armigera (Hubner) per os.
Prevalence of ToV-TJ in bat feces
PCR targeting ToV-TJ-CP (621 bp) and ToV-TJ-RdRP (421 bp) showed that the bat feces collected from 09XY and 07HK were positive for ToV-TJ (data not shown). Three virus variants were detected by sequencing the partial capsid protein (CP) and RdRP sequences from the feces collected from 09XY, while only one variant was detected from 07HK (Table 1). The amplified partial CP sequences from 07HK shared 98% nucleotide similarity with OMRV-Y61, while those from 09XY showed a high degree of similarity (86-97%) with ToV-TJ and OMRV-AK4. Similarly, the amplified partial RdRP sequences from 07HK had 98% nt identity to OMRV-Y61, while those from 09XY had 91-99% identity to ToV-TJ and OMRV-AK4.
Phylogenetic analysis of ToV-TJ
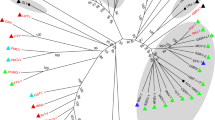
To study the evolutionary relationship of ToV-TJ to other viruses in the family Totiviridae, RdRP amino acid sequences were used for construction of a phylogenetic tree. The ToV-TJ RdRp showed a low similarity (ranging 3 to 8%) to the classified viruses of the family Totiviridae and a higher similarity (ranging 39 to 56%) to the unclassified viruses IMNV, AsTV and DTV. The highest similarity of 94% and 80%, respectively, was detected with OMRV-AK4 and OMRVY61. The phylogenetic analysis showed that ToV-TJ was distantly related to a representative member of the family Totiviridae and formed a distinct cluster with the unclassified members of the family Totiviridae (Fig. 3).
Phylogenetic analysis of RdRp amino acid sequences from ToV-TJ. The tree was constructed using the neighbor-joining method with 1,000 bootstrap replicates under the parameter of pairwise deletion and P-distance model. Bar, 0.2 amino acid substitutions per per site. HvV190S, Helminthosporium victoriae virus 190S (NP_619670.2); IMNV, penaeid shrimp infectious myonecrosis virus (YP_529549.1); AsTV, Armigeres subalbatus virus SaX06-AK20 (YP_003934934.1); DTV, Drosophila melanogaster totivirus SW-2009a (YP_003289293.1); PMCV, piscine myocarditis virus AL V-708 (YP_004581250); OMRV-AK4, Omono River virus-AK4 (BAJ21511.1); OMRV-Y61, Omono River virus-Y61 (BAJ21513.1)
Discussion
The insects eaten by insectivorous bats may include members of 19 or more families [4, 22]. When bats feed, they not only ingest the insects but also the microorganisms associated with the insects, such as bacteria and viruses. Undigested viruses are likely to be disseminated during bat migration and transportation of bat feces. Metagenomic analyses have revealed abundant insect viral sequences in insectivorous bat feces, where most have no or low similarity to known viral sequences [3, 10]. In this report, we successfully isolated one totivirus-like particle from insectivorous bat guano, demonstrating that insectivorous bat feces could be a good resource for the isolation of novel insect viruses.
ToV-TJ was 93% identical in genomic sequence to OMRV-AK4, a virus isolated from mosquitoes in Japan [9], suggesting that this virus may be a pathogen of mosquitoes. The biggest difference observed between ToV-TJ and OMRV-AK4 was that the putative RdRP of ToV-TJ was truncated by 39 amino acids at N-terminal end compared with the RdRP of OMRV-AK4. A phylogenetic tree based on deduced RdRP aa sequences suggests that ToV-TJ belongs to a unclassified group that includes all recently reported totivirus-like viruses that infect shrimp, mosquitoes, and Atlantic salmon [8, 9, 12, 14, 15, 23]. The current study used specific primers targeting the ToV-TJ genomic sequence and detected the presence of similar viruses in other sampling locations that include Yunnan and Hainan Province. The ToV-TJ and Yunnan ToV-TJ-like sequences were also determined to be highly genetically similar to OMRV-AK4, while the virus detected in Hainan was more genetically similar to OMRV-Y61, indicating the genetic diversity and wide distribution of this virus.
ToV-TJ was shown to infect Sf9, HzAM1 and C6/36 cell lines productively with evident cytopathic changes. Morphologically, the virions produced in Sf9 culture had a higher ratio of empty particles than those observed for HzAM1 cultures. However, the TCID50 assay on Sf9 and HzAM1 cells showed no significant difference in infectious virus yield. Unfortunately, bioassays on the larvae of S. exigua and H. armigera (Hubner) demonstrated that this virus could not infect these two insects per os, demonstrating that the ToV-TJ is not a pathogen for these two insect species. These results, together with the high genomic similarity to OMRV, further support the hypothesis that ToV-TJ originated from mosquitoes, a major component of the diet of Myotis ricketti.
Viruses in the family Totiviridae are remarkably diverse in the wide range of hosts they are able to infect, from fungi to protozoa [21]. Five genera are currently recognized: Giardiavirus, Leishmaniavirus, Totivirus, Victorivirus [6] and Trichomonasvirus [7]. The members of the genera Totivirus and Victorivirus infect fungi, and those of the genus Giardiavirus, Leishmaniavirus and Trichomonasvirus infect protozoa. Some recently identified viruses in shrimp, fish and mosquitoes have similar genomic structures and morphology but low similarity to members of other genera of the family Totiviridae [8, 9, 12, 14, 15, 23]. Phylogenetic analysis in our study also showed that the unclassified totiviruses identified from invertebrates form a cluster that is distinct from other members of the family Totiviridae, and they should be considered members of a proposed new genus, Artivirus, as suggested by Zhai et al. [23]. Based on the criteria previously adopted for totivirus species (an aa sequence identity of <50%)(21), ToV-TJ should belong to the same species as OMRV.
References
Altringham JD (1996) Bats: biology and behaviour. Oxford University Press, New York
Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T (2006) Bats: important reservoir hosts of emerging viruses. Clin Microbiol Rev 19:531–545
Donaldson EF, Haskew AN, Gates JE, Huynh J, Moore CJ, Frieman MB (2010) Metagenomic analysis of the viromes of three North American bat species: viral diversity among different bat species that share a common habitat. J Virol 84:13004–13018
Freeman PW (1981) Correspondence of Food-Habits and Morphology in Insectivorous Bats. J Mammal 62:166–173
Froussard P (1993) rPCR: a powerful tool for random amplification of whole RNA sequences. PCR Methods Appl 2:185–190
Ghabrial SA, Nibert ML (2009) Victorivirus, a new genus of fungal viruses in the family Totiviridae. Arch Virol 154:373–379
Goodman RP, Ghabrial SA, Fichorova RN, Nibert ML (2011) Trichomonasvirus: a new genus of protozoan viruses in the family Totiviridae. Arch Virol 156:171–179
Haugland O, Mikalsen AB, Nilsen P, Lindmo K, Thu BJ, Eliassen TM, Roos N, Rode M, Evensen O (2011) Cardiomyopathy syndrome of atlantic salmon (Salmo salar L.) is caused by a double-stranded RNA virus of the Totiviridae family. J Virol 85:5275–5286
Isawa H, Kuwata R, Hoshino K, Tsuda Y, Sakai K, Watanabe S, Nishimura M, Satho T, Kataoka M, Nagata N, Hasegawa H, Bando H, Yano K, Sasaki T, Kobayashi M, Mizutani T, Sawabe K (2011) Identification and molecular characterization of a new nonsegmented double-stranded RNA virus isolated from Culex mosquitoes in Japan. Virus Res 155:147–155
Li L, Victoria JG, Wang C, Jones M, Fellers GM, Kunz TH, Delwart E (2010) Bat guano virome: predominance of dietary viruses from insects and plants plus novel mammalian viruses. J Virol 84:6955–6965
Li Y, Ge X, Zhang H, Zhou P, Zhu Y, Zhang Y, Yuan J, Wang LF, Shi Z (2010) Host range, prevalence, and genetic diversity of adenoviruses in bats. J Virol 84:3889–3897
Lovoll M, Wiik-Nielsen J, Grove S, Wiik-Nielsen CR, Kristoffersen AB, Faller R, Poppe T, Jung J, Pedamallu CS, Nederbragt AJ, Meyerson M, Rimstad E, Tengs T (2010) A novel totivirus and piscine reovirus (PRV) in Atlantic salmon (Salmo salar) with cardiomyopathy syndrome (CMS). Virol J 7:309
Muchhala N (2009) Going to great lengths bats and flowers stage an evolutionary race. BATS 27:1–3
Nibert ML (2007) ‘2A-like’ and ‘shifty heptamer’ motifs in penaeid shrimp infectious myonecrosis virus, a monosegmented double-stranded RNA virus. J Gen Virol 88:1315–1318
Poulos BT, Tang KF, Pantoja CR, Bonami JR, Lightner DV (2006) Purification and characterization of infectious myonecrosis virus of penaeid shrimp. J Gen Virol 87:987–996
Reed LJ, Muench H (1938) A simple method of estimating fifty percent endpoints. Am J Hygiene 27:493–497
Shi Z (2010) Bat and virus. Protein Cell 1:109–114
Simmons NB (2005) Order Chiroptera. In: Wilson DE, Reeder DM (eds) Mammal species of the world: a taxonomic and geographic reference, vol 2. John Hopkins University Press, Maryland, pp 312–529
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biol Evol 24:1596–1599
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Wickner RB, Wang CC, Patterson JL (2005) Family Totiviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA (eds) Virus taxonomy eighth report of the international committee on taxonomy of viruses. Elsevier/Academic Press, London, pp 571–580
Zeale MR, Butlin RK, Barker GL, Lees DC, Jones G (2011) Taxon-specific PCR for DNA barcoding arthropod prey in bat faeces. Mol Ecol Resour 11:236–244
Zhai Y, Attoui H, Mohd Jaafar F, Wang H, Chao Y, Fan S, Sun Y, Liu L, Mertens P, Meng W, Wang D, Liang G (2010) Isolation and full-length sequence analysis of Armigeres subalbatus totivirus, the first totivirus isolate from mosquitoes representing a proposed novel genus (Artivirus) of the family Totiviridae. J Gen Virol 11:2836–2845
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, X., Zhang, Y., Ge, X. et al. A novel totivirus-like virus isolated from bat guano. Arch Virol 157, 1093–1099 (2012). https://doi.org/10.1007/s00705-012-1278-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-012-1278-y