Abstract
Hypokinetic gait is a common and very disabling symptom of Parkinson’s disease (PD). Repetitive transcranial magnetic stimulation (rTMS) over the motor cortex has been used with variable effectiveness to treat hypokinesia in PD. Preconditioning rTMS by transcranial direct current stimulation (tDCS) may enhance its effectiveness to treat hypokinetic gait in PD. Three-dimensional kinematic gait analysis was performed (1) prior to, (2) immediately after and (3) 30 min after low-frequency rTMS (1 Hz, 900 pulses, 80 % of resting motor threshold) over M1 contralateral to the more affected body side preconditioned by (1) cathodal, (2) anodal or (3) sham tDCS (amperage: 1 mA, duration: 10 min) in ten subjects with PD (7 females, mean age 63 ± 9 years) and ten healthy subjects (four females, mean age 50 ± 11 years). The effects of tDCS-preconditioned rTMS on gait kinematics were assessed by the following parameters: number of steps, step length, stride length, double support time, cadence, swing and stance phases. Our data suggest a bilateral improvement of hypokinetic gait in PD after 1 Hz rTMS over M1 of the more affected body side preceded by anodal tDCS. In contrast, 1 Hz rTMS alone (preceded by sham tDCS) and 1 Hz rTMS preceded by cathodal tDCS were ineffective to improve gait kinematics in PD. In healthy subjects, gait kinematics was unaffected by either intervention. Preconditioning motor cortex rTMS by tDCS is a promising approach to treat hypokinetic gait in PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) are promising non-invasive cortical stimulation techniques for an adjuvant treatment of movement disorders (Wu et al. 2008; Minks et al. 2011). Depending on stimulation parameters, rTMS over the primary motor cortex (M1) induces either an increase (high frequency stimulation) or a decrease (low frequency stimulation) of cortico-spinal excitability that outlasts the stimulation period (Pascual-Leone et al. 1994; Chen et al. 1997). In analogy, tDCS over M1 produces a lasting shift in cortico-spinal excitability depending on the direction of current flow (Nitsche and Paulus 1997). Preconditioning cortico-spinal excitability with tDCS can be used to enhance rTMS-induced cortical plasticity and shape the direction of rTMS-induced after-effects (Lang et al. 2004; Siebner et al. 2004). For example, a session of “inhibitory” 1 Hz rTMS applied over M1 after a “facilitatory” preconditioning with anodal tDCS causes a decrease in cortico-spinal excitability in healthy humans, while “inhibitory” 1 Hz rTMS preceded by a session of “inhibitory” cathodal tDCS induces an increase in cortico-spinal excitability (Siebner et al. 2004). The effects of tDCS preconditioning have been interpreted within a concept of “homeostatic plasticity”, meaning an adjustment of the direction and magnitude of cortical plasticity dependent upon the most recent history of postsynaptic activity to stabilize cortico-spinal excitability within a physiologically useful range (Huang et al. 1992; Kirkwood et al. 1996).
Several studies have applied rTMS (Siebner et al. 2000; Shimamoto et al. 2001; Lomarev et al. 2006; Ikegeuchi et al. 2003; Khedr et al. 2003; Lefaucheur et al. 2004; Filipović et al. 2010; Benninger et al. 2011, 2012) or tDCS (Fregni et al. 2006; Benninger et al. 2010) over the motor cortex in an attempt to improve the symptoms of PD. However, the efficiency of non-invasive brain stimulation as a therapeutic tool in PD remains highly controversial (Elahi et al. 2009; Arias et al. 2010; Benninger et al. 2011, 2012; Filipović et al. 2010). The effect size is generally small and the clinical effects are short living and variable even after serial application of either rTMS or tDCS over M1 (Benninger et al. 2011, 2012; Okabe et al. 2003; Ghabra et al. 1999; Tergau et al. 1999). Given the “homeostatic plasticity” concept preconditioning with tDCS may enhance the effectiveness of rTMS over M1. We recently observed positive effects of 1 Hz rTMS preconditioned by tDCS over M1 on finger and hand movements in PD (Grüner et al. 2010). The present study extends this earlier work and addresses the issue of whether a period of 1 Hz rTMS over M1 preconditioned by (1) cathodal, (2) anodal or (3) sham tDCS impacts on gait kinematics in PD.
Materials and methods
Patients
Ten PD patients (seven females, mean age 63 ± 9 years; disease duration 7 ± 6 years) participated. Clinical details regarding the patient cohort are given in Table 1. In the control group, ten healthy subjects (four females, mean age 50 ± 11 years) participated. Informed consent was obtained prior to testing and all procedures had been approved by the local Ethics Committee. Patients were tested on dopaminergic drugs and examined at the time of best motor response following administration of dopaminergic drugs during each experimental session. The motor subscore (part III) of the Unified PD rating scale (UPDRS) was assessed (Fahn et al. 1987) prior to experiments. None of the patients suffered akinesia or dyskinesia in any of the experimental sessions. The best motor response to dopaminergic medication was assessed serially prior to the experiments to assure that each patient was tested in the same clinical condition during each experimental session. The levodopa equivalent daily dose (LEDD) was calculated (Krack et al. 1998). All patients were tested with the Mini-Mental Status Examination to exclude cognitive decline (indicated by a score ≤24 points; Folstein et al. 1975).
Experimental procedures
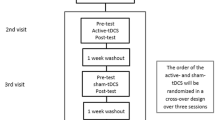
Three-dimensional kinematic gait analysis was performed (1) prior to (baseline condition), (2) immediately after and (3) 30 min after 1 Hz rTMS preconditioned by (a) sham tDCS, (b) anodal tDCS and (c) cathodal tDCS over M1 contralateral to the more affected body side in PD patients. Cortical stimulation was performed over the dominant hemisphere in healthy control subjects. The three experimental sessions were separated by 1 week. Earlier data report that the effects of a 10-min session of tDCS and a 15-min session of 1 Hz rTMS preconditioned by 10 min of tDCS on motor cortex excitability lasts about 1 h (Nitsche and Paulus 2001; Siebner et al. 2004). Thus, an interval of 1 week in between each experimental session should avoid any carry-over effects in between sessions. The order of sessions (preconditioned with sham, anodal or cathodal tDCS) was randomly assigned to each subject and counterbalanced across subjects. All participants were completely naïve to the experimental hypothesis and to the order of experimental sessions they were undergoing. All participants were informed about randomization and the study procedures (application of tDCS and rTMS, gait analysis) using a standard information sheet consented by the local Ethics committee.
Gait analysis
Gait analysis was conducted on a treadmill with a constant, steady-based walking speed (0.5 km/h). Movement kinematics was recorded using an ultrasonic motion analyzer (CMS HS, Zebris, Isny, Germany). Two ultrasound-emitting sensors were placed at a fixed distance of 2 m in the middle of the treadmill. Retro-reflective triplet markers were positioned bilaterally on the thigh and foot. Specific anatomical coordinates (anterior superior iliac spines, inner and outer knee joint centers and medial and lateral malleoli) were defined bilaterally by an ultrasound-based pointer. Pressure sensors were placed bilaterally beneath the heel and at the ball of the foot to determine swing and stance phase. The spatial coordinates were recorded at a frequency of 100 Hz. The total duration of gait assessment for each recording session was 60 s. The first and last 15 s were eliminated from the data set to eliminate possible effects of treadmill acceleration or deceleration on gait kinematics. For each stimulation session, three recording sessions were performed at (a) baseline, (b) 0 min and (c) 30 min after brain stimulation. The following spatio-temporal standard parameters (for a detailed description of these standard parameters see Kiss et al. 2004) were analysed using the application software WinGait (Zebris, Isny, Germany): (1) number of steps (n), (2) step and stride length (m); (3) cadence (steps/s), (4) double support time (s) and (5) stance and swing phase (% of gait cycle).
tDCS preconditioning of 1 Hz rTMS
Continuous tDCS of M1 was applied using a battery-driven DC stimulator (NeuroConn, Ilmenau, Germany). For effective tDCS of M1, a constant current flow of 1 mA was applied for 10 min through wet sponge electrodes (size 7 × 5 cm) positioned over the M1 contralateral to the more affected body side and the contralateral frontal pole (Fig. 1). The electrode placed over M1 was positioned at the optimal site for cortical magnetic stimulation to the contralateral first dorsal interosseus muscle (see below). The fronto-polar electrode was always placed over the eyebrow contralateral to the stimulated M1. The polarity of tDCS refers to the electrode placed over M1. For anodal (facilitatory) tDCS, the anode was placed over the M1, whereas for cathodal (inhibitory) tDCS the cathode was positioned over M1. For sham tDCS stimulation intensity faded off after 5 s of stimulation. That is no effective stimulation was applied over the 10-min intervention period.
Transcranial magnetic stimulation was performed using a 70-mm figure-of-eight coil and a Magstim Super Rapid stimulator (Magstim Company, Dyfed, UK). The coil was placed tangentially over the hand area of M1 of the hemisphere contralateral to the more affected body side at the optimal site for the response of the first dorsal interosseus muscle. This stimulation area was chosen, since previous works examining the effects of rTMS over the hand area of M1 in PD patients have shown a positive effect on gait disturbance (Lomarev et al. 2006; Lefaucheur et al. 2004). The optimal site was defined as the location where stimulation at a slightly supra-threshold intensity elicited the largest motor-evoked potential in the contralateral first dorsal interosseus muscle. Electromyographic activity was recorded using silver–silver–chloride electrodes positioned in a belly-tendon technique on the skin overlying the first dorsal interosseus muscle of the contralateral hand. The electromyographic signal was amplified, filtered (50–2,000 Hz) and digitized at a sampling rate of 5,000 Hz. The resting motor threshold was defined for each patient as the lowest stimulator output that elicited motor-evoked potentials with peak-to-peak amplitude of at least 50 μV in the contralateral first dorsal interosseus muscle in at least 5 of 10 trials. The average-resting motor thresholds are summarized for each patient in Table 1. RTMS was applied over M1 at a rate of 1 Hz, 80 % resting motor threshold for 15 min using a 70-mm figure-of-eight coil. RTMS immediately followed the application of tDCS.
Statistical analysis
Normal distribution of the outcome measures was verified using the Kolmogorov–Smirnov test. Repeated measures ANOVAs were calculated for each spatio-temporal parameter with the factors “group” [levels: (1) PD patients and (2) controls], “session” [levels: (1) 1 Hz rTMS preconditioned by sham tDCS, (2) 1 Hz rTMS preconditioned by anodal tDCS and (3) 1 Hz rTMS preconditioned by cathodal tDCS], “time” [levels: (1) baseline, (2) 0 min after rTMS application and (3) 30 min after rTMS application] and “side” [levels: (1) contralateral to stimulated hemisphere and (2) ipsilateral to stimulated hemisphere]. Post hoc pairwise comparisons between conditions were performed using t tests. A P value of 0.05 was considered significant after Bonferroni correction for multiple comparisons.
Results
All subjects tolerated 1 Hz rTMS preconditioned by tDCS well without side effects. The advantage of tDCS is that participants feel a tingling sensation only for the first few seconds of stimulation (Nitsche and Paulus 2001). Thus, none of the participants was able to discriminate sham from real tDCS. Table 2 presents mean data and standard deviations of each parameter assessed. Figure 2 provides average percentage changes from baseline for number of steps, step length and double support of the more (contralateral to stimulation) and less (ipsilateral to stimulation) affected body sides after 1 Hz rTMS applied to the M1 contralateral to the more affected body side preconditioned by (1) sham, (2) anodal and (3) cathodal tDCS. Data are provided for PD patients and control subjects directly after and 30 min after stimulation. Figure 3 shows the average percentage changes in relation to baseline of stride length and cadence.
Average percentage change from baseline of number of steps, step length and double support time of the more (contralateral) and less (ipsilateral) affected body side after 1 Hz rTMS applied to the M1 preconditioned by (1) sham, (2) anodal and (3) cathodal tDCS for PD patients and controls. Data are provided immediately after and 30 min after stimulation. *P ≤ 0.05; **P ≤ 0.01
Number of steps
After 1 Hz rTMS preconditioned by anodal tDCS, but not by sham or cathodal tDCS, PD patients decreased the number of steps compared to baseline immediately after and 30 min after stimulation. This was supported by a significant effect of the interaction “session” × “time” (F 4,36 = 3.3; P ≤ 0.01) as well as by the interaction “group” × “session” × “time” (F 4,36 = 3.6; P ≤ 0.05). This effect was evident both contralaterally and ipsilaterally to the stimulated hemisphere, which was demonstrated by a missing significance of the factor “side” (F 1,9 = 0; P > 0.05). In contrast, 1 Hz rTMS preconditioned by cathodal tDCS seems to increase the number of steps in PD subjects, regardless of the investigated body side. This implies a worsening of hypokinetic gait. However, the increase of number of steps does not reach level of significance. 1 Hz rTMS did not significantly modulate the number of steps in the control group, irrespective of the polarity of tDCS preconditioning, indicated by significant effect of “group” (F 1,9 = 14.6; P ≤ 0.01).
Step and stride length
Only 1 Hz rTMS preconditioned by anodal tDCS, but not by sham or cathodal tDCS, significantly increased step length in comparison to baseline (significant effect of the interaction “session” × “time”: F 4,32 = 2.8; P ≤ 0.05). This effect was evident at both body sides 30 min after, but not directly after stimulation. Consistently, stride length was significantly increased after 1 Hz rTMS preconditioned only by anodal tDCS, but not by sham or cathodal tDCS, 30 min after stimulation (significant effect of the interaction “session” × “time”: F 4,36 = 3.9; P ≤ 0.01). In the control group, 1 Hz rTMS did not significantly impact on step and stride length, this is evidenced by a significant effect of “group” (F 1,8 = 9.0; P ≤ 0.01 for step length, and F 1,9 = 10.6; P ≤ 0.01 for stride length).
Cadence
After 1 Hz rTMS preconditioned by anodal tDCS, but not by cathodal or sham tDCS, in PD patients cadence decreased in comparison to baseline immediately after and 30 min after stimulation. A significant effect of the interaction “group” × “session” × “time” (F 4,36 = 2.9; P ≤ 0.05) and “session” × “time” (F 18,4 = 3.9; P ≤ 0.01) underpins this observation. This effect reached significance in the post hoc tests after anodal, but not after sham tDCS preconditioning. In healthy subjects, 1 Hz rTMS preconditioned by anodal/cathodal or sham tDCS had no significant impact on cadence (significant effect of “group”: F 1,9 = 13.3; P ≤ 0.01).
Double support time
1 Hz rTMS preceded by anodal tDCS, but not by cathodal or sham tDCS, increased double support time compared to baseline bilaterally. This was indicated by a significant interaction “group” × “session” × “time” (F 4,16 = 2.9; P ≤ 0.05). Post hoc tests demonstrated a significant increase of double support time in relation to baseline only after 1 Hz rTMS preceded by anodal tDCS contralateral to the stimulated hemisphere. In the control group, 1 Hz rTMS preconditioned by anodal/cathodal or sham tDCS had no impact on double support time (significant group effect on “group” (F 1,4 = 39.1; P ≤ 0.01).
Stance and swing phases
TDCS-preconditioned rTMS had no significant effect on the swing and stance phases of the gait cycle in both PD patients and controls.
Discussion
This study was designed to investigate the effectiveness of low-frequency (1 Hz) rTMS preconditioned by sham, anodal and cathodal tDCS to improve the kinematics of hypokinetic gait in patients with PD. The protocol of non-invasive brain stimulation was chosen based on earlier data on motor cortex “homeostatic plasticity” showing that a session of “inhibitory” 1 Hz rTMS applied over M1 after a session of tDCS modulates cortico-spinal excitability in dependence of the type of tDCS stimulation (Siebner et al. 2004). The idea was that low-frequency (1 Hz) rTMS may shift the direction of cortico-spinal excitability towards facilitation after a period of tDCS—as observed in healthy subjects (Siebner et al. 2004)—and thereby improves motor function in PD patients. Our data extend the current knowledge regarding the effectiveness of non-invasive brain stimulation in PD by the following aspects: (1) 1 Hz rTMS preconditioned by anodal tDCS applied over M1 contralateral to the more affected side has beneficial effects on hypokinetic gait in PD, whereas 1 Hz rTMS preconditioned by sham or cathodal tDCS has not and (2) the beneficial effects of 1 Hz rTMS preconditioned by anodal tDCS on hypokinetic gait patterns in PD develop primarily at the body side contralateral to the stimulated hemisphere. Gait analysis is an important tool to evaluate the therapeutic effects of motor rehabilitation in PD (Peppe et al. 2007). Hypokinetic parkinsonian gait is characterized by an increase in the number of steps, an increase of double support times and stance phases and a reduction of step and stride length, cadence and swing phases of the gait cycle (Knutsson 1972; Morris et al. 1994). Our data show a decrease in the number of steps and an increase in step length and stride length after 1 Hz rTMS preconditioned by anodal tDCS. At the same time, we observed an increase of double support time after 1 Hz rTMS preconditioned by anodal tDCS. However, the present stimulation protocol influenced the clinical syndrome, and caused improvement of gait in PD remains a matter of speculation. The clinical effect of rTMS is frequently described as an enhancement of movement speed, e.g., speed of gait (Lefaucheur et al. 2004; Siebner et al. 2000), which means an improvement of bradykinesia rather than a change in muscular rigidity. Some authors have related such improvement to an increase in dopamine release (Strafella et al. 2003).
Based on the pertinent literature it appears that high-frequency (5, 10, 20, 50 Hz) rTMS over motor cortex is more effective (Siebner et al. 2000; Lomarev et al. 2006; Ikegeuchi et al. 2003; Khedr et al. 2003, 2006, but see Ghabra et al. 1999; Tergau et al. 1999; Benninger et al. 2011, 2012) than low-frequency (0.2, 0.5, 1 Hz) rTMS to improve motor disability in PD (Tergau et al. 1999; Okabe et al. 2003; Arias et al. 2010; Filipović et al. 2010, but see Lefaucheur et al. 2004). This suggests that an increase, but not a decrease, in cortico-spinal excitability ameliorates motor dysfunction in PD. However, the beneficial effect of high-frequency rTMS over M1 to improve motor symptoms in PD is less consistently found than widely held and some authors even documented no effect at all (Ghabra et al. 1999; Tergau et al. 1999; Benninger et al. 2011, 2012). Consequently, we intended to enhance the effectiveness of motor cortex rTMS by preconditioning with tDCS. Levodopa normalizes increased cortico-spinal excitability in PD (Lou et al. 2003) probably by increasing short- and long-intracortical inhibition (Bäumer et al. 2009; Fierro et al. 2008). In addition, D1- and D2-receptor activity contributes to the consolidation of the NMDA receptor-dependent tDCS effect on cortico-spinal excitability (Nitsche et al. 2006, 2009). For these reasons, we tested our patient cohort on dopaminergic drugs.
In healthy subjects, a session of inhibitory 1 Hz rTMS applied over M1 after an “inhibitory” preconditioning with cathodal tDCS induces an increase in cortico-spinal excitability, while “facilitatory” preconditioning with anodal tDCS causes a decrease in cortico-spinal excitability (Siebner et al. 2004). Transferred to our experimental design, the application of a session of inhibitory 1 Hz rTMS after a “facilitatory” preconditioning with anodal tDCS should cause a decrease in cortico-spinal excitability. Several studies using different brain imaging techniques, such as positron emission tomography and functional magnetic resonance imaging, have shown that the subcortical striato-nigral deficit in PD modifies neural activity within the cortical motor network (Sabatini et al. 2000). These changes in neural activation in PD include down-regulation of the supplementary motor area (Rascol et al. 1992) and up-regulation of the parietal and lateral prefrontal cortices (Samuel et al. 1997). Concerning M1 there is evidence of a “compensatory” up-regulation of neural activity in PD (Sabatini et al. 2000). Consequently, down-regulation of pathological overactivity of M1—as probably obtained by 1 Hz rTMS preconditioned by anodal tDCS in our study sample—might be a useful strategy to improve hypokinetic gait in PD. We did, however, not assess electrophysiological measures to probe the effects of tDCS-preconditioned rTMS on motor cortex excitability in PD, which is a limitation of the present set of data.
Earlier studies applied rTMS (inhibitory and facilitatory) to the hand area of M1 and found a positive effect on hypokinetic gait in PD (Lefaucheur et al. 2004; Lomarev et al. 2006). Combining facilitatory (5 Hz) rTMS over the leg area of M1 with a 30-min period of treadmill walking for 12 daily sessions modulated motor cortex excitability and improved walking performance in PD (Yang et al. 2013; Mak 2013). A recent study showed improvements of motor, but not non-motor, symptoms and signs of PD after inhibitory (1 Hz) rTMS over the supplementary motor area (Shirota et al. 2013). We followed the approach to apply rTMS to the hand area of M1 (Lefaucheur et al. 2004) after a period of preconditioning with tDCS. It is tempting to target rTMS to the hand area of M1 as it is easily accessible, whereas rTMS to the leg area of M1 is technically more challenging (Mak 2013; Yang et al. 2013). In addition, we observed positive effects of tDCS-preconditioned 1 Hz rTMS over M1 hand area on finger and hand movements in a different group of PD subjects (Grüner et al. 2010). Why modulation of cortical excitability within the hand area of M1 or the supplementary motor area impacts on gait performance remains a matter of speculation. Recent studies have suggested that the effects of rTMS on neural activity of M1 are not limited to the site of stimulation, but spread to other distinct motor areas. RTMS over the hand area of M1 caused a widespread neural activation within a network of primary and secondary cortical motor regions including M1/S1, supplementary motor area, dorsal premotor cortex, cingulate motor area, the putamen and thalamus both in healthy subjects and those with Parkinson’s disease as probed by functional magnetic resonance imaging (Bestmann et al. 2004; González-García et al. 2011). Similar, tDCS over M1 exhibited remote effects in cortical, as well as subcortical areas, including dorsal and ventral premotor cortices, thalamus, putamen and caudate (Lang et al. 2005). Based on these findings, a possible explanation for the effectiveness of tDCS-preconditioned rTMS over M1 hand area on gait kinematics in PD may be co-activation of M1 leg area and/or other motor areas relevant for gait performance. In addition, the effects of motor cortex stimulation may be facilitated by changes in striatal dopamine content. For example, a session of rTMS over M1 caused an increase of striatal dopamine binding in healthy humans (Strafella et al. 2003) and subjects with Parkinson’s disease (Strafella et al. 2005). A direct interference of rTMS with cortical excitability within the leg area of M1 is very unlikely based on the limited depth of current induction to be achieved with the current stimulation intensity (80 % of the resting motor threshold). However, we cannot exclude that tDCS may have modified cortical processing within a larger area surrounding M1 hand area.
Compared to sham stimulation, low-frequency (0.5 Hz, 600 pulses) rTMS applied over the hand area of the left M1 reduced upper limb bradykinesia bilaterally and improved walking performance in PD patients off dopaminergic drugs (Lefaucheur et al. 2004). Interestingly, 1 Hz rTMS alone (preconditioned by sham tDCS) did not show any effect on parkinsonian gait in the present study. A possible explanation for this apparent difference to earlier data may be the fact that we tested our patients on dopaminergic drugs, whereas Lefaucheur et al. (2004) did not. On the other hand the number of studies, which document no significant effect of 1 Hz rTMS over M1 on motor disability in PD, is rising (Tergau et al. 1999; Okabe et al. 2003; Arias et al. 2010; Filipović et al. 2010). We found that 1 Hz rTMS preconditioned by anodal tDCS was superior to preconditioning with cathodal tDCS or 1 Hz rTMS alone, in achieving a significant amelioration of several gait parameters. This behavioural effect was present at both hemi-bodies and lasted for at least 30 min after the intervention. Earlier investigators have explained the effect of tDCS preconditioning by a reduction of the motor cortex threshold for subsequent rTMS causing a lasting change in cortico-spinal excitability (Siebner et al. 2004; Lang et al. 2005). Using a very similar stimulation protocol, Siebner et al. (2004) showed that the effect of 15 min of 1 Hz rTMS over the hand area of M1 (80 % of the resting motor threshold) preconditioned by 10 min of tDCS, lasted about 1 h. We did not measure the cortico-spinal excitability, and therefore cannot comment upon the changes of cortical plasticity induced by the interventions.
Essentially, gait kinematics remained unchanged by either intervention among healthy subjects tested in the present study. Several earlier studies reported changes in individual finger and grasping performance of the ipsilateral hand after unilateral inhibitory rTMS of M1 (Avanzino et al. 2008; Dafotakis et al. 2008; Kobayashi 2010). For example, 1 Hz rTMS applied over M1 of either the right or the left hemisphere in right-handed healthy subjects improved movement kinematics of finger and hand tapping as well as grasping with the ipsilateral hand (Dafotakis et al. 2008). This effect was most pronounced after stimulation of the left M1 and was interpreted within the context of interhemispheric competition in a way that 1 Hz rTMS inhibits ipsilateral M1, thereby reduces transcallosal inhibition of contralateral M1 and facilitates motor performance at the ipsilateral hand. The present data set implies that gait performance is less prone to the modulatory effects of hand motor cortex stimulation as compared to upper limb motor performance in healthy subjects. Different to the situation in PD, the changes in cortical excitability are not sufficient to impact on lower limb motor activity when tDCS and rTMS are applied to the upper limb motor area of M1 in healthy subjects. It should be noted, however, that in the present study the group of healthy controls was not accurately age-matched to the group of parkinsonian patients (PD patients: 63 ± 9 years; control group, 50 ± 11 years), which may have influenced the effects of brain stimulation. Further work is needed to evaluate the effects of non-invasive brain stimulation on gait in younger compared to older subjects.
In conclusion, our data suggest a bilateral improvement of the kinematics of hypokinetic gait in PD after 1 Hz rTMS preceded by anodal tDCS. 1 Hz rTMS alone (sham tDCS) and 1 Hz rTMS preceded by cathodal tDCS were ineffective to improve gait kinematics. Our results show that tDCS-preconditioned 1 Hz rTMS over M1 is a safe and promising adjunct approach to improve gait kinematics in PD.
References
Arias P, Vivas J, Grieve KL, Cudeiro J (2010) Controlled trial on the effect of 10 days low-frequency repetitive transcranial magnetic stimulation (rTMS) on motor signs in Parkinson’s disease. Mov Disord 25:1830–1838
Avanzino L, Bove M, Trompetto C, Tacchino A, Ogliastro C, Abbruzzese G (2008) 1-Hz repetitive TMS over ipsilateral motor cortex influences the performance of sequential finger movements of different complexity. Eur J Neurosci 27:1285–1291
Bäumer T, Hidding U, Hamel W, Buhmann C, Moll CK, Gerloff C, Orth M, Siebner HR, Münchau A (2009) Effects of DBS, premotor rTMS, and levodopa on motor function and silent period in advanced Parkinson’s disease. Mov Disord 24:672–676
Benninger DH, Berman BD, Houdayer E, Pal N, Luckenbaugh DA, Schneider L, Miranda S, Hallett M (2011) Intermittent theta-burst transcranial magnetic stimulation for treatment of Parkinson’s disease. Neurology 76:601–609
Benninger DH, Iseki K, Kranick S, Luckenbaugh DA, Houdayer E, Hallett M (2012) Controlled study of 50-Hz repetitive transcranial magnetic stimulation for treatment of Parkinson’s disease. Neurorehabil Neural Repair 26:1096–1105
Benninger DH, Lomarev M, Lopez G, Wassermann EM, Li X, Considine E, Hallett M (2010) Transcranial direct current stimulation for the treatment of Parkinson’s disease. J Neurol Neurosurg Psychiatry 81:1105–1111
Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J (2004) Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur J Neurosci 19:1950–1962
Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG (1997) Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 48:1398–1403
Dafotakis M, Grefkes C, Wang L, Fink GR, Nowak DA (2008) The effects of 1 Hz rTMS over the hand area of M1 on movement kinematics of the ipsilateral hand. J Neural Transm 115:1269–1274
Elahi B, Elahi B, Chen R (2009) Effect of transcranial magnetic stimulation on Parkinson motor function-systematic review of controlled clinical trials. Mov Disord 24:357–363
Fahn S, Elton RL, Members of the UPDRS Development Committee (1987) The unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M (eds) Recent developments in Parkinson’s disease, vol 2. McMillan Healthcare Information, Florham Park, pp 153–163
Fierro B, Brighina F, D’Amelio M, Daniele O, Lupo I, Ragonese P, Palermo A, Savettieri G (2008) Motor intracortical inhibition in PD: l-DOPA modulation of high-frequency rTMS effects. Exp Brain Res 184:521–528
Filipović SR, Rothwell JC, Bhatia K (2010) Low-frequency repetitive transcranial magnetic stimulation and off-phase motor symptoms in Parkinson’s disease. J Neurol Sci 291(1–2):1–4
Folstein MF, Folstein SE, McHugh PR (1975) Mini-Mental State (a practical method for grading the state of patients for the clinician). J Psychiatr Res 12:189–198
Fregni F, Boggio PS, Santos MC, Lima M, Vieira AL, Rigonatti SP, Silva MT, Barbosa ER, Nitsche MA, Pascual-Leone A (2006) Noninvasive cortical stimulation with transcranial direct current stimulation in Parkinson’s disease. Mov Disord 21:1693–1702
Ghabra MB, Hallett M, Wassermann EM (1999) Simultaneous repetitive transcranial magnetic stimulation does not speed fine movement in PD. Neurology 52:768–770
González-García N, Armony JL, Soto J, Trejo D, Alegría MA, Drucker-Colín R (2011) Effects of rTMS on Parkinson’s disease: a longitudinal fMRI study. J Neurol 258:1268–1280
Grüner U, Eggers C, Ameli M, Sarfeld AS, Fink GR, Nowak DA (2010) 1 Hz rTMS preconditioned by tDCS over the primary motor cortex in Parkinson’s disease: effects on bradykinesia of arm and hand. J Neural Transm 117:207–216
Huang YY, Colino A, Selig DK, Malenka RC (1992) The influence of prior synaptic activity on the induction of long-term potentiation. Science 255:730–733
Ikegeuchi M, Touge T, Nishiyama Y, Takeuchi H, Kuriyama S, Ohkawa M (2003) Effects of successive repetitive transcranial magnetic stimulation on motor performances and brain perfusion in idiopathic Parkinson’s disease. J Neurol Sci 209:41–46
Khedr EM, Farweez HM, Islam H (2003) Therapeutic effect of repetitive transcranial magnetic stimulation on motor function in Parkinson’s disease patients. Eur J Neurol 10:567–572
Khedr EM, Rothwell JC, Shawky OA, Ahmed MA, Hamdy A (2006) Effect of daily repetitive transcranial magnetic stimulation on motor performance in Parkinson’s disease. Mov Disord 21:2201–2205
Kirkwood A, Rioult MC, Bear MF (1996) Experience-dependent modification of synaptic plasticity in visual cortex. Nature 381:526–528
Kiss RM, Kocsis L, Knoll Z (2004) Joint kinematics and spatial–temporal parameters of gait measured by an ultrasound-based system. Med Eng Phys 26:611–620
Knutsson E (1972) An analysis of Parkinsonian gait. Brain 95:475–486
Kobayashi M (2010) Effect of slow repetitive TMS of the motor cortex on ipsilateral sequential simple finger movements and motor skill learning. Restor Neurol Neurosci 28:437–448
Krack P, Pollak P, Limousin P, Hoffmann D, Xie J, Benazzouz A (1998) Subthalamic nucleus or internal pallidal stimulation in young onset Parkinson’s disease. Brain 121:141–147
Lang N, Siebner HR, Ernst D, Nitsche MA, Paulus W, Lemon RN, Rothwell JC (2004) Preconditioning with transcranial direct current stimulation sensitizes the motor cortex to rapid-rate transcranial magnetic stimulation and controls the direction of after-effects. Biol Psychiatry 56:634–639
Lang N, Siebner HR, Ward NS et al (2005) How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur J Neurosci 22:495–504
Lefaucheur JP, Drouot X, Von Raison F, Ménard-Lefaucheur I, Cesaro P, Nguyen J-P (2004) Improvement of motor performance and modulation of cortical excitability by repetitive transcranial magnetic stimulation of the motor cortex in Parkinson’s disease. Clin Neurophysiol 115:2530–2541
Lomarev MP, Kanchana S, Bara-Jimenez W, Iyer M, Wassermann EM, Hallett M (2006) Placebo-controlled study of rTMS for the treatment of Parkinson’s disease. Mov Disord 21:325–331
Lou JS, Benice T, Kearns G, Sexton G, Nutt J (2003) Levodopa normalizes exercise related cortico-motoneuron excitability abnormalities in Parkinson’s disease. Clin Neurophysiol 114:930–937
Mak MK (2013) Repetitive transcranial magnetic stimulation combined with treadmill training can modulate corticomotor inhibition and improve walking performance in people with Parkinson’s disease. J Physiother 59:128
Minks E, Mareček R, Pavlík T, Ovesná P, Bareš M (2011) Is the cerebellum a potential target for stimulation in Parkinson’s disease? Results of 1-Hz rTMS on upper limb motor tasks. Cerebellum 10:804–811
Morris ME, Iansek R, Matyas TA, Summers JJ (1994) The pathogenesis of gait hypokinesia in Parkinson’s disease. Brain 117:1169–1181
Nitsche MA, Kuo MF, Grosch J, Bergner C, Monte-Silva K, Paulus W (2009) D1-receptor impact on neuroplasticity in humans. J Neurosci 29:2648–2653
Nitsche MA, Lampe C, Antal A, Liebetanz D, Lang N, Tergau F, Paulus W (2006) Dopaminergic modulation of long-lasting direct current-induced cortical excitability changes in the human motor cortex. Eur J Neurosci 23:1651–1657
Nitsche MA, Paulus W (1997) Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 15:633–639
Nitsche MA, Paulus W (2001) Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57:1899–1901
Okabe S, Ugawa Y, Kanazawa I (2003) Effectiveness of rTMS on Parkinson’s Disease Study Group. 0.2-Hz repetitive transcranial magnetic stimulation has no add-on effects as compared to a realistic sham stimulation in Parkinson’s disease. Mov Disord 18:382–388
Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M (1994) Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain 117:847–858
Peppe A, Chiavalon C, Pasqualetti P, Crovato D, Caltagirone C (2007) Does gait analysis quantify motor rehabilitation efficacy in Parkinson’s disease patients? Gait Posture 26:452–462
Rascol O, Sabatini U, Chollet F, Celsis P, Montastruc JL, Marc-Vergnes JP, Rascol A (1992) Supplementary and primary sensory motor area activity in Parkinson’s disease. Regional cerebral blood flow changes during finger movements and effects of apomorphine. Arch Neurol 49:144–148
Sabatini U, Boulanouar K, Fabre N, Martin F, Carel C, Colonnese C, Bozzao L, Berry I, Montastruc JL, Chollet F, Rascol O (2000) Cortical motor reorganization in akinetic patients with Parkinson’s disease: a functional MRI study. Brain 123:394–403
Samuel M, Ceballos-Baumann AO, Blin J, Uema T, Boecker H, Passingham RE, Brooks DJ (1997) Evidence for lateral premotor and parietal overactivity in Parkinson’s disease during sequential and bimanual movements. A PET study. Brain 120:963–976
Shirota Y, Ohtsu H, Hamada M, Enomoto H, Ugawa Y (2013) Research committee on rTMS treatment of Parkinson’s disease supplementary motor area stimulation for Parkinson’s disease: a randomized controlled study. Neurology 80:1400–1405
Shimamoto H, Takasaki K, Shigemori M, Imaizumi T, Ayabe M, Shoji H (2001) Therapeutic effect and mechanism of repetitive transcranial magnetic stimulation in Parkinson’s disease. J Neurol 248:48–51
Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, Rothwell JC (2004) Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. J Neurosci 24:3379–3385
Siebner HR, Rossmeier C, Mentschel C, Peinemann A, Conrad B (2000) Short-term motor improvement after sub-threshold 5-Hz repetitive transcranial magnetic stimulation of the primary motor hand area in Parkinson’s disease. J Neurol Sci 178:91–94
Strafella AP, Ko JH, Grant J, Fraraccio M, Monchi O (2005) Corticostriatal functional interactions in Parkinson’s disease: a rTMS/[11C]raclopride PET study. Eur J Neurosci 22:2946–2952
Strafella AP, Paus T, Fraraccio M, Dagher A (2003) Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain 126:2609–2615
Tergau F, Wassermann EM, Paulus W, Ziemann U (1999) Lack of clinical improvement in patients with Parkinson’s disease after low and high frequency repetitive transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol Suppl 51:281–288
Yang YR, Tseng CY, Chiou SY, Liao KK, Cheng SJ, Lai KL, Wang RY (2013) Combination of rTMS and treadmill training modulates corticomotor inhibition and improves walking in Parkinson’s disease: a randomized trial. Neurorehabil Neural Repair 27:79–86
Wu AD, Fregni F, Simon DK, Deblieck C, Pascual-Leone A (2008) Noninvasive brain stimulation for Parkinson’s disease and dystonia. Neurotherapeutics 5:345–361
Conflict of interest
None of the authors has any financial disclosures regarding the present manuscript.
Full financial disclosures of all authors for the past year:
Stock Ownership in medically related fields: Ameli: none; Fisse: none; Sarfeld: none; Fink: none; Nowak: none.
Intellectual Property Rights: Ameli: none; Fisse: none; Sarfeld: none; Fink: none; Nowak: none.
Consultancies: Ameli: none; Fisse: none; Sarfeld: none; Fink: none; Nowak: none.
Expert Testimony: Ameli: none; Fisse: none; Sarfeld: none; Fink: none; Nowak: none.
Advisory Boards: Ameli: none; Fisse: none; Sarfeld: none; Fink: none; Nowak: none.
Employment: Ameli: none; Fisse: none; Sarfeld: none; Fink: none; Nowak: none.
Partnerships: Ameli: none; Fisse: none; Sarfeld: none; Fink: none; Nowak: none.
Contracts: Ameli: none; Fisse: none; Sarfeld: none; Fink: none; Nowak: none.
Honoraria: Ameli: none; Fisse: none; Sarfeld: none; Fink: none; Nowak: none.
Royalties: Ameli: none; Fisse: none; Sarfeld: none; Fink: none; Nowak: none.
Grants: Ameli: none; Fisse: none; Sarfeld: none; Fink: none; Nowak: none.
Other: Ameli: none; Fisse: none; Sarfeld: none; Fink: none; Nowak: none.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mitra von Papen and Mirabell Fisse have contributed equally.
Rights and permissions
About this article
Cite this article
von Papen, M., Fisse, M., Sarfeld, AS. et al. The effects of 1 Hz rTMS preconditioned by tDCS on gait kinematics in Parkinson’s disease. J Neural Transm 121, 743–754 (2014). https://doi.org/10.1007/s00702-014-1178-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-014-1178-2