Abstract
Background
Obstruction is a common cause of ventriculo-peritoneal shunt failure. Head computed tomography and plain x-ray examinations of shunt tubing (“shunt series”) are routinely used in patients readmitted for reemerging symptoms but are of limited value. The validity of shunt series can be improved by applying contrast agent into the system (contrast-enhanced shunt series, a.k.a. a “shuntogram” or “shuntography”). We hypothesized that contrast-enhanced shunt series have a high predictive value for shunt revision surgeries.
Methods
We retrospectively re-evaluated 107 contrast-enhanced shunt series and reviewed the patient histories. We defined outcome parameters for calculating the utility of a pathological contrast-enhanced shunt series in predicting revision surgery.
Results
Of 107 contrast-enhanced shunt series, 41 examinations were positive for obstruction, mainly of the ventricular (36.5 %) and the peritoneal catheter (48.8 %). Within 30 days, 35 successful revision surgeries and 3 revision surgeries without resolution of symptoms were performed. In two cases the shunt tubing was found to be patent. Sixty-six negative examinations resulted in two revision surgeries, in addition to ten surgeries not attempting to restore patency. After 30 days, the specificity of contrast-enhanced shunt series for shunt failure identification was calculated at 92.8 %, the sensitivity at 94.7 %, the positive predictive value at 87.8 %, and the negative predictive value at 97.0 %.
Conclusions
The contrast-enhanced shunt series method is a highly specific examination with a negative predictive value exceeding that of head computed tomography and plain shunt series. Compared to radionuclide marker studies, contrast-enhanced shunt series demonstrate better spatiotemporal resolution, enabling focused local surgical repair.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ventriculo-peritoneal shunts are the mainstay of treatment for most forms of hydrocephalus (HCP). Despite technological advances, readmission and reoperation rates remain high. Up to 11 % of patients dismissed from the hospital after a shunt implantation procedure will be readmitted within 30 days, most likely for malfunction, less likely for infection [1]. A recent review of the literature on adverse events in shunt surgery compared rates of failure due to infection or mechanical obstruction. The frequencies of mechanical adverse events in the pediatric studies range from 35 % to 64 %, while those in mixed and adult series were somewhat lower (8 % to 47 %). Shunt infection is the second most common complication and is estimated to occur in 3 % to 12 % in patients [2]. Possible mechanical reasons for shunt revisions include overdrainage (40 %), underdrainage (45 %), disconnection (9 %), migration (3 %), and fracture (2 %) [3]. Accurate and timely evaluation of suspected shunt malfunction is of the essence to avoid harmful elevations in intracranial pressure. Furthermore, accurate identification of the site of shunt failure will facilitate prompt and accurate surgical repair.
Most commonly in this context, plain radiographs are routinely performed along the tubing of the skull, chest, and abdomen (“shunt series”), as well as head computed tomography (HCT). In studies analyzing the diagnostic yield of these modalities, the sensitivity of shunt series for detecting a shunt malfunction subsequently undergoing revision was found to be very low [4–6]; in a large review the positive predictive value of shunt series was calculated at 72.3 %, the negative predictive value at 75.1 % [7]. The reliability of alterations of the ventricular system on HCT scans used to rule out shunt malfunction in pediatric patients has been questioned [8]. This corresponds to our clinical experience in patients with long-standing HCP and normal pressure hydrocephalus (NPH), where clinical shunt malfunction does not always go along with changes of the ventricular system. Clinical pathways in pediatric emergency rooms have been proposed, implemented, and evaluated for patients returning after a shunt operation [9].
We routinely use contrast-enhanced shunt series (also referred to as “shuntogram” or “shuntography”) as an adjunct diagnostic procedure when a standard workup fails to show a structural explanation for a clinically suspected shunt malfunction. In this article, we will review our experience of contrast-enhanced shunt series and provide an assessment of its utility in managing patients with suspected shunt failure. Furthermore, we will discuss pitfalls and complication rates of this invasive diagnostic procedure.
Material and methods
Indication and technique of contrast-enhanced shunt series
The rationale for performing a dynamic contrast-enhanced shunt series was based on clinical grounds given signs of underdrainage unexplained by plain shunt series and cranial cross-sectional imaging [HCT or magnetic resonance imaging (MRI)] (Table 1). The individual decision to perform a contrast-enhanced shunt series, including the consequences thereof, was at the discretion of the surgeon on call.
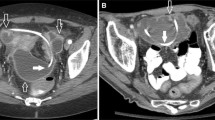
Contrast-enhanced shunt series are carried out in the angiography suite by a neurosurgeon and a neuroradiologist. Patients are examined in a supine position with the head rotated to the contralateral side of the implanted shunt system. Limited shaving directly over the prechamber is followed by thorough local disinfection. The prechamber is punctured with a 23-gauge (0.65-mm-diameter) injection needle. Spontaneous outflow or careful aspiration of cerebrospinal fluid (CSF) confirms correct needle position, while a small amount of CSF is collected for a routine cell count and microbiological testing. For contrast-enhanced demonstration of patency in the proximal catheter, 1 to 3 ml of the water-soluble nonionic iodinated contrast agent iopamidol (Solutrast 200 M®, Bracco Inc., Italy) is slowly injected into the prechamber under gentle application of manual pressure to the skin overlying the distal catheter (Fig. 1). When evaluating the patency of the distal system, gentle compression is applied to the tubing proximal to the reservoir while injecting. Injection, tube filling, and contrast outflow at the proximal (i.e., ventricular) and distal (i.e., atrial or peritoneal) catheter end are documented using plain radiography with a frame rate of four pictures per second on a biplane angiography suite (Artis Q, Siemens Medical Solutions, Forchheim, Germany). Images are then saved to the picture archive and communication system (PACS) for review and evaluation.
Normal finding in a contrast-enhanced shunt series of the head exhibiting retrograde filling of the ventricular catheter. While the tubing between the prechamber (c) and the programmable differential pressure valve (d) is occluded by gentle pressure to the overlying skin, contrast agent is injected percutaneously into the prechamber (c) and exits the openings of the ventricular catheter (a) in a retrograde manner. Thus, an unobstructed proximal part of the shunt system is shown. Part (e) denotes the gravity-adjusted valve of the proGAV valve system (Miethke Inc.), part (b) denotes tubing fixation and bur hole cover. The distal catheter still shows the double contour of tubing not filled with contrast agent
Selection of patient study population
We retrieved 133 consecutive contrast-enhanced shunt series on 110 patients from our local PACS server over a period ranging from 2008 to the first half of 2014 and reviewed the patient histories, implications, assessments, and treatment consequences corresponding to this diagnostic procedure. During that time period this institution performed 1085 shunt procedures. We excluded patients with a follow-up of less than 30 days (n = 23) and those with technically insufficient procedures (n = 3). The study was approved by the Institutional Review Board (protocol no. 17/10/15An) and conducted in accordance with the 1964 Helsinki Declaration and its later amendments. For this type of study, formal consent is not required.
Evaluation of contrast-enhanced shunt series and definition of positive and negative findings
The contrast-enhanced shunt series were reevaluated. Negative testing was defined as complete contrast filling of the shunt tubing and free contrast outflow through the proximal catheter perforations as well as the distal catheter opening. Reduced outflow of the proximal catheter, discontinuation or leakage within the tubing route, as well as pooling or diminished outflow at the distal catheter ending were criteria that suggested malfunction. In these cases, contrast-enhanced shunt series were interpreted as positive.
Definitions used to calculate statistical test characteristics
In a recent evaluation of technetium-99 (99Tc) enhanced shunt series, Thompson et al. [10] suggested that a shunt series could only be declared truly negative if a 30-day follow-up revealed no further clinical events. We adapted this definition for our evaluation of the reliability of contrast-enhanced shunt series (Table 2). A negative contrast-enhanced shunt series was considered a true negative if no further invasive test and no shunt revision surgery aiming at restoring patency were performed for 30 days or when a revision was performed without pertinent intraoperative findings. Revision surgery aiming to correct the shunt system’s pressure setting, such as the addition of an anti-siphon device, was allowed. A negative test was labeled as a false negative if revision surgery was performed and an occlusion was found. Even without proper identification of any occlusion, the test was still considered a false negative if the clinical course was unremarkable for 30 days after revision surgery, assuming that the previously obstructed flow had been restored. On the other hand, a positive contrast-enhanced shunt series was a true positive if revision surgery took place with intraoperative findings as expected or with an unremarkable clinical course for 30 days. Lastly, a positive test was labeled as false positive if no intraoperative occlusion was found or if additional invasive tests or further surgeries took place within 30 days.
Results
Baseline characteristics
We evaluated 133 contrast-enhanced shunt series on 110 patients over a 6.5-year period. After excluding 23 examinations for a follow-up of less than 30 days and of 3 examinations for technically insufficient execution (e.g., misplaced cannula), we were left with n = 107 examinations in 89 patients (45 male, 44 female). Mean age at evaluation was 44.1 years (SD 27.5 years); 23 examinations were (21.5 %) performed on patients under 16 years of age by the pediatric neurosurgery team; 28 examinations (26.2 %) were performed on patients over the age of 70 years. The implantation diagnosis was pseudotumor cerebri/idiopathic intracranial hypertension in 5 patients, obstructive HCP in 10 patients, normal pressure hydrocephalus (NPH) in 22 patients, and other forms of communicating HCP in 52 patients (Fig. 2). All shunts evaluated were ventriculo-peritoneal shunts except for two ventriculo-atrial shunts (initial diagnosis: NPH; communicating HCP), one ventriculo-pleural shunt (communicating HCP), and one subduro-peritoneal shunt (communicating HCP). The contrast-enhanced shunt series examination was performed at a mean of 38.9 months (minimum 2 days, maximum 34.9 years) after shunt implantation or the latest shunt revision. Implanted valves evaluated were Hakim-Medos valves in 58 cases (54.2 %), proGAV systems in 30 cases (28.0 %), proSA systems in 10 cases (9.3 %), Pudenz-Schulte valves in 5 cases (4.7 %), and other shunt systems in the remaining cases. Mean follow-up was 24.9 months (minimum 35 days, maximum 69.4 months); nine cases had a follow-up of less than 3 months.
Pie chart showing diagnosis at the time of the primary CSF diversion procedure of patients undergoing contrast-enhanced shunt series. Numbers given are absolute patient numbers (n = 89). On the left, the pediatric patient population is given (≤15 years of age at time of initial CSF diversion procedure), on the right the adult patient population
Radiation dosage required
The applied radiation dose was low in the majority of cases (median dose area product 182.6 μGy × m2). The lowest dose required was 2 μGy × m2 in a 3-month-old infant. However, the radiation dose cumulated to relevant exposures in extremely obese patients when additional images using the digital subtraction technique were required; the highest dose area product applied was 8214 μGy × m2 in a 75-year-old patient.
Patients with negative findings in contrast-enhanced shunt series
Of 107 contrast-enhanced shunt series examinations, 66 (61.7 %) were read as normal, showing no signs of obstruction or other pathological findings (Fig. 3). Within 30 days, 12 patients underwent surgery, 8 of those to change the valve type or to address issues other than obstruction. In two patients, intraoperative shunt testing was performed without evidence of obstruction and hence no need for revision. However, in another two patients, intraoperative testing revealed obstruction in spite of an inconspicuous contrast-enhanced shunt series. These two patients underwent shunt revision surgery on clinical grounds only at day 4 and 7 after the contrast-enhanced shunt series (2 cases of a false-negative examination at 30 days). One of these patients was lost to follow-up at day 35; the other patient underwent placement of a ventriculo-atrial shunt 5 days after the first revision. Within 90 days, seven additional patients underwent surgery, three to change the valve type or to resolve issues other than obstruction. Two patients were found to have an infection and underwent externalization of the shunt catheter. However, another two patients underwent revision surgery of their shunt system at day 31 and 50 after contrast-enhanced shunt series, in one case upon clinical judgment only, in one case after a second contrast-enhanced shunt series demonstrating possible distal obstruction. Both patients did well for at least 6 months after the revision (total of 4 cases of a false-negative examination at 90 days).
Flow chart depicting results of contrast-enhanced shunt series and false-negative and false-positive results. Boxes in the fourth row indicate results from 30-day follow-up; boxes in the bottom row indicate results from 90-day follow-up. Dash-dot-line boxes (·-·) show false-positive results; dashed-line (---) boxes show false-negative counts. From these numbers, the following test parameters are calculated for the 30-day follow-up: specificity 92.8 %, false-positive rate/type I error 7.2 %; sensitivity 94.7 %, false-negative rate/type II error 5.3 %; positive predictive value 87.8 %; negative predictive value 97.0 %
Forty-seven patients with normal examinations and without revision surgery within their follow-up or within 90 days were followed over a mean of 22.0 months. Eleven of these patients underwent shunt-related surgery at a later date; the remaining 36 patients did not undergo any shunt-related procedure during their follow-up. One patient received a repeat examination 3 days after the first, again demonstrating patency, while an additional eight patients had repeat examinations an average of 17.1 months after the initial examination with different findings.
Patients with positive findings for shunt obstruction in contrast-enhanced shunt series
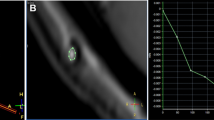
Forty-one examinations were evaluated as positive. The findings included complete or partial central catheter occlusion (m = 15; 36.6 %; e.g., Fig. 4a), valve level occlusion (2; 4.9 %), distal catheter occlusion or peritoneal pooling (e.g., Fig. 4b) and retrograde flow along the catheter (e.g., Fig. 4c) (20; 48.8 %), and leakage with or without disconnection that was not observed in the plain shunt series (4; 17 %; e.g., Fig. 4d). All patients underwent surgery an average of 3 days after the index examination. Five patients had a complete revision of their system, and four patients underwent a shunt system exchange (e.g., ventriculo-atrial placement). Revision surgery of 29 patients was targeted and of only a limited approach to restore shunt patency, guided by the contrast-enhanced shunt series (revision of the central catheter in 12 cases, revision of the valve in 1 case, revision of a kinked tube in 1 case, and revision of the distal catheter in 15 cases). In one patient we detected a shunt infection, and the shunt was externalized. However, two patients were found to have a patent shunt system intraoperatively, so no revision surgery was performed. These patients were followed for over 2 years; one received an anti-siphon device 13 days after the exploratory surgery as the only procedure. Of the 38 patients undergoing revision surgery, three patients had to be reoperated to restore shunt patency within 30 days, two for recurrent distal occlusion, one for recurrent central occlusion. Another four patients underwent revision surgery for distal occlusion within 90 days of the index examination (5 cases of a false-positive examination at 30 days, a total of nine cases of a false-positive examination at 90 days).
Contrast-enhanced shunt series with typical pathological findings. a Occluded central ventricular catheter with jet-like retrograde outflow of contrast agent through only two openings and double contour of the most proximal tip of the catheter. b Peritoneal pooling of contrast medium suggestive of outflow occlusion due to peritoneal pseudocyst formation. c Retrograde filling of a connective tissue sleeve along the most distal tip of the peritoneal catheter, suspicious of peritoneal malabsorption due to catheter scarring. d Catheter leakage at the peritoneal entry point with occluded distal catheter
Complications of contrast-enhanced shunt series
During contrast-enhanced shunt series one patient experienced an allergic reaction with nausea and a rash that resolved after administration of steroids. No patients suffered from seizures or other neurological sequelae; no infectious complications were observed during the follow-up period.
Discussion
Standard shunt series x-ray examinations only demonstrate the integrity or disconnection of radio-opaque shunt tubing; hence, shunt failures caused by occlusion along the tubing including the valve will not be visible. In our retrospective analysis of 107 contrast-enhanced shunt series, we were able to show both a high positive and a high negative predictive value for revision surgery. We found that contrast-enhanced shunt series offer an excellent addition to the diagnostic armamentarium for managing shunt failure. In addition, contrast-enhanced shunt series allow for precise and focused shunt revision surgeries, since it is possible to accurately determine the site of occlusion. It has previously been pointed out that with non-radio-opaque shunt tubing, contrast injection is essential to rule out disconnection [11].
Larger retrospective case series of this simple diagnostic measure are elusive. In a report on 23 examinations in 15 adult patients, 4 studies were evaluated as normal. None of the patients with normal studies required surgical revision [12]. No other studies exist with a higher number of patients. No prospective case series have been published.
Differences of contrast-enhanced shunt series, radionuclide studies, and other imaging modalities
There are a number of studies regarding diagnostic shunt examinations requiring the injection of radioactive markers (99Tc) into the prechamber or into the valve. Marker accumulation in the ventricles and the peritoneal cavity is then assessed; various methods for defining “normal” marker accumulation have been described [10, 13]. It has been indicated that a 99Tc study can reliably demonstrate persistent fibrous tracts in patients with broken or disconnected catheters [14]. However, in a large study comparing the diagnostic yield of standard shunt series, HCT, and radionuclide studies, the positive predictive value for shunt revision was found to be 55 % for HCT and only 62.5 % for 99Tc scans [5]. These low predictive values are contradicted by a study of 85 pediatric patients, where a sensitivity of 96 % and a specificity of 89 % were found [15]. Furthermore, a review of 115 negative 99Tc scans found a false-negative rate of 14 % [16]. During a very detailed analysis of the different parameters of marker accumulation for defining a “normal” 99Tc scan, sensitivity fell between 37.5 % and 87.5 %, specificity between 51.4 % and 97.2 %, positive predictive value between 39.2 % and 82.8 %, and negative predictive value between 81.3 % and 92.0 % [10]. Finally, in a retrospective study on 68 99Tc shunt series, a high false-negative rate of 25 % was found [17]. Contrast-enhanced shunt studies in our series compare at least similarly, if not favorably, to all of the statistical parameters given in the aforementioned studies of 99Tc shunt series. We would have expected that sensitivity (and therefore false-negative rates) would improve with radioisotope studies, since small amounts of tracer are easily detected with gamma cameras. However, this is not the case, possibly due to the fact that a shunt system patent for a small amount of CSF yields only clinically insufficient drainage.
A number of typical complications of the peritoneal catheter, such as misplacement and pseudocyst formation, can be assessed reliably using abdominal computed tomography (CT) and ultrasound examination. In reviewing 70 abdominal CT scans, pathological findings were seen in 16 patients (22.9 %) [18]. Nevertheless, this examination should only be reserved for patients with an unclear abdominal pathology within the context of a specific issue and does not play a role in routine diagnostics. However, an abdominal CT subsequently performed after a contrast-enhanced shunt series will accurately demonstrate pseudocyst filling. Abdominal ultrasound offers a readily available technique for ruling out pseudocyst formation, especially in children [19].
Very few studies have focused on the role of MRI in diagnosing ventriculo-peritoneal shunt malfunction. In a prospective study comparing asymptomatic shunt patients with patients already diagnosed with shunt obstruction, significantly different cerebrospinal fluid (CSF) velocities were observed in the intracranial catheter [20]. As with a head or abdominal CT, MR imaging of the ventricular catheter will only point indirectly toward shunt obstruction.
A contrast-enhanced shunt series examination is fairly inexpensive. According to the internal cost allocation of our institution, the charges are €45.60 including the contrast agent. In comparison, a 99Tc scan with SPECT and tracer totals €2000.00.
Pitfalls in performing contrast-enhanced shunt series
While all studies conducted during the index period were included in the above evaluation, we also experienced some difficulties in performing and interpreting contrast-enhanced shunt series. While virtually all shunt systems on the market today feature the option of percutaneous puncture to draw CSF in order to exclude shunt infection, some shunt systems (e.g., Miethke® ProGAV system with control reservoir) include a no-return valve in the prechamber, therefore making retrograde contrast filling of the ventricular catheter impossible. This should not be misinterpreted as ventricular catheter occlusion; ventricular catheter patency testing must rely on CSF aspiration from the prechamber in these cases.
Some have alluded to the possibility of damaging the sensitive valve mechanism when the contrast agent is injected too forcefully [Aschoff A, oral communication, DGNC Düsseldorf, May 29, 2013]. We agree that injection has to be performed gently and have used a 10-ml syringe with a 23-gauge butterfly needle to lower the pressure impact on the valve. In our series, we are not aware of any valve damage.
When the injection cannula is misplaced or becomes disengaged before injection, filling might occur in the connective tissue sleeve alongside the catheter, possibly mimicking patency of the peritoneal catheter (“extravasation”). However, if careful observation is given to the filling of the prechamber and a loss of double contour in the tubing (Fig. 1), the examiner will readily be aware of correct filling.
Limitations of the study
There are a number of limitations of this study. First and foremost, the design is retrospective. Although we re-evaluated the images saved in the picture archive and communication system (PACS) and were thus able to exclude false or incomplete interpretation of films, a prospective data collection would have offered the possibility of predefined documentation of intraoperative findings. Furthermore, findings and complications below the threshold of clinically necessary documentation might be underreported.
Second, the population is very heterogeneous and comprises different age groups, including pediatric patients, different pathologies, as well as different shunt and valve types. However, the technique presented here and its potential and limitations as discussed are likely independent from the demographic factors given. We believe that a detailed subgroup analysis would not yield more relevant results.
Evaluation of single radiographs and short sequences of films taken in the angiography suite is subjective. We have neither objectively measured nor calculated the flow of the contrast agent administered in different sections of the shunt tubing. An additional objective measurement, such as the reading of an added manometer to the injection cannula, would possibly enhance the validity of contrast-enhanced shunt series.
Lastly, it should be mentioned that use of iopamidol is off-label for contrast-enhanced shunt series. However, iopamidol (Solutrast 200 M®, Bracco Inc., Italy) is approved for intrathecal injections and myelograms.
Conclusion
Contrast-enhanced shunt series have a better sensitivity and specificity for detection of obstructive shunt malfunction than plain shunt series alone. Statistical tests compare favorably to those of radionuclide tracer shunt series (99Tc). We recommend performing contrast-enhanced shunt series instead of radionuclide tracer series in any patient with signs and symptoms of underdrainage and an unremarkable plain shunt series. In order to minimize the risk of shunt infection, the prechamber should only be assessed once; CSF should be withdrawn for microbiology testing as a routine part of contrast-enhanced shunt series.
Abbreviations
- CSF:
-
cerebrospinal fluid
- HCP:
-
hydrocephalus
- HCT:
-
head computed tomography
- NPH:
-
normal pressure hydrocephalus
- PACS:
-
picture archive and communication system
References
Buchanan CC, Hernandez EA, Anderson JM, Dye JA, Leung M, Buxey F, Bergsneider M, Afsar-Manesh N, Pouratian N, Martin NA (2014) Analysis of 30-day readmissions among neurosurgical patients: surgical complication avoidance as key to quality improvement. J Neurosurg 121(1):170–175
Wong JM, Ziewacz JE, Ho AL, Panchmatia JR, Bader AM, Garton HJ, Laws ER, Gawande AA (2012) Patterns in neurosurgical adverse events: cerebrospinal fluid shunt surgery. Neurosurg Focus 33(5):E13
Johanson CE (2011) Clinical Evaluation of adult hydrocephalus. In: Winn HR (ed) Youmans neurological surgery. Elsevier, Philadelphia, pp 487–514
Griffey RT, Ledbetter S, Khorasani R (2007) Yield and utility of radiographic “shunt series” in the evaluation of ventriculo-peritoneal shunt malfunction in adult emergency patients. Emerg Radiol 13(6):307–311
Lehnert BE, Rahbar H, Relyea-Chew A, Lewis DH, Richardson ML, Fink JR (2011) Detection of ventricular shunt malfunction in the ED: relative utility of radiography, CT, and nuclear imaging. Emerg Radiol 18(4):299–305
Vassilyadi M, Tataryn ZL, Alkherayf F, Udjus K, Ventureyra EC (2010) The necessity of shunt series. J Neurosurg Pediatr 6(5):468–473
Mater A, Shroff M, Al-Farsi S, Drake J, Goldman RD (2008) Test characteristics of neuroimaging in the emergency department evaluation of children for cerebrospinal fluid shunt malfunction. CJEM 10(2):131–135
Iskandar BJ, McLaughlin C, Mapstone TB, Grabb PA, Oakes WJ (1998) Pitfalls in the diagnosis of ventricular shunt dysfunction: radiology reports and ventricular size. Pediatrics 101(6):1031–1036
Chern JJ, Macias CG, Jea A, Curry DJ, Luerssen TG, Whitehead WE (2010) Effectiveness of a clinical pathway for patients with cerebrospinal fluid shunt malfunction. J Neurosurg Pediatr 6(4):318–324
Thompson EM, Wagner K, Kronfeld K, Selden NR (2014) Using a 2-variable method in radionuclide shuntography to predict shunt patency. J Neurosurg 121(6):1504–1507
Blair K, AuCoin R, Kloiber R, Molnar CP (1989) The complementary role of plain radiographs and radionuclide shuntography in evaluating CSF-VP shunts. Clin Nucl Med 14(2):121–123
Bartynski WS, Valliappan S, Uselman JH, Spearman MP (2000) The adult radiographic shuntogram. AJNR Am J Neuroradiol 21(4):721–726
Chiewvit S, Nuntaaree S, Kanchaanapiboon P, Chiewvit P (2014) Assessment lumboperitoneal or ventriculoperitoneal shunt patency by radionuclide technique: a review experience cases. World J Nucl Med 13(2):75–84
Clyde BL, Albright AL (1995) Evidence for a patent fibrous tract in fractured, outgrown, or disconnected ventriculoperitoneal shunts. Pediatr Neurosurg 23(1):20–25
May CH, Aurisch R, Kornrumpf D, Vogel S (1999) Evaluation of shunt function in hydrocephalic patients with the radionuclide 99mTc-pertechnetate. Childs Nerv Syst 15(5):239–244, discussion 245
O’Brien DF, Taylor M, Park TS, Ojemann JG (2003) A critical analysis of ‘normal’ radionucleotide shuntograms in patients subsequently requiring surgery. Childs Nerv Syst 19(5-6):337–341
Vassilyadi M, Tataryn ZL, Matzinger MA, Briggs V, Ventureyra EC (2006) Radioisotope shuntograms at the Children’s Hospital of Eastern Ontario. Childs Nerv Syst 22(1):43–49
Chung JJ, Yu JS, Kim JH, Nam SJ, Kim MJ (2009) Intraabdominal complications secondary to ventriculoperitoneal shunts: CT findings and review of the literature. AJR Am J Roentgenol 193(5):1311–1317
Briggs JR, Hendry GM, Minns RA (1984) Abdominal ultrasound in the diagnosis of cerebrospinal fluid pseudocysts complicating ventriculoperitoneal shunts. Arch Dis Child 59(7):661–664
Kurwale NS, Agrawal D (2011) Phase-contrast magnetic resonance imaging of intracranial shunt tube: a valuable adjunct in the diagnosis of ventriculoperitoneal shunt malfunction. Clin Neurosurg 58:138–142
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements) or non-financial interest (such as personal or professional relationships, affiliations, knowledge or belief) in the subject matter or materials discussed in this manuscript.
Ethical approval
For this type of study formal consent is not required.
Additional information
Comments
Shunting dysfunctions represent very challenging issues in neurosurgery because of the heterogeneity of patients and hardware. Neurosurgeons are often required to solve life-treating situations at the last minute, frequently without the necessary information. However, the matter is underrepresented in the contemporary literature. Alternative methods, like injection of radionuclide markers or MRI or x-ray examinations, are penalized by low predictive values. In this context, the presented manuscript is welcome in order to present the authors’ results using an easy, cheap, and efficient method to identify the cause of shunting dysfunction, the so-called shuntography.
The authors retrospectively evaluated 133 contrast-enhanced shunt series over a 6.5-year time period. The study presents the typical heterogeneity of these patients: different pathologies, different shunts and valve types, and different age groups. Nevertheless, the authors performed a very exemplary analysis of the data resulting in four cases with false -egative and nine case of false-positive results; moreover, one patient experienced an allergic reaction in all probability caused by iopamidol, off-label for contrast-enhanced shunt series.
Very valuable are the three described pitfalls, the no-return valve in the prechamber, the risk of damaging the valve through forceful injection of the contrast agent, and the latency-mimicking risk.
In conclusion, the authors recommend performing contrast-enhanced shunt series instead of radionuclide tracer series, which reflects our clinical experience too. Overall, the authors should be congratulated for this straightforward study.
Lehel Török Neuruppin
Alex Alfieri Neuruppin
Rights and permissions
About this article
Cite this article
von Eckardstein, K.L., Kallenberg, K., Psychogios, MN. et al. Contrast-enhanced shunt series (“shuntography”) compare favorably to other shunt imaging modalities in detecting shunt occlusion. Acta Neurochir 159, 63–70 (2017). https://doi.org/10.1007/s00701-016-3007-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-016-3007-x